Abstract
Ribavirin is an antiviral agent used in the treatment of chronic hepatitis C virus infection. One of the limitations associated with the use of ribavirin is a reversible anemia caused by its accumulation in erythrocytes. Therefore, it is of interest to determine ribavirin levels in erythrocytes, as well as in plasma, as these measurements may be predictive of hematotoxicity. In the present study, we describe a high-performance liquid chromatographic (HPLC) assay for ribavirin in whole blood to estimate concentrations of free ribavirin and phosphorylated anabolites in erythrocytes. Since ribavirin exists primarily as phosphorylated anabolites (mono-, di-, and triphosphates) in erythrocytes, whole-blood extracts were initially dephosphorylated with acid phosphatase. The enzyme-treated samples were subjected to phenyl boronic acid column extraction for cleanup. The purified fraction was analyzed by reversed-phase HPLC, which was optimized for determination of ribavirin levels in whole blood. The recoveries of ribavirin from whole blood ranged from 63.1 to 90.7% at concentrations ranging from 1.67 to 40.0 μM. Intra- and interassay variations estimated at these concentrations were 3.2 to 10.4 and 4.7 to 11.7%, respectively. This method was used to quantitate ribavirin in samples both treated and untreated with acid phosphatase to estimate the extent of intracellular phosphorylation in erythrocytes. The method was also used to evaluate the effects of dipyridamole, a nucleoside transporter inhibitor, on ribavirin disposition in erythrocytes in in vitro experiments.
Recent interest in the treatment of hepatitis C virus (HCV) infection has focused on the combined use of alpha interferon and ribavirin, with available results revealing antiviral synergism against HCV (1, 2, 12, 14). Early studies of patients with HCV infection showed that 48% of subjects receiving combination therapy had had HCV RNA eradicated from their serum. In addition, improvement of liver function (aminotransferases) was observed in all patients randomized to both drugs (1). With this improvement, increased clinical use of ribavirin has occurred.
Intracellular phosphorylation is required for ribavirin to exert its pharmacological activity. Antiviral activity is presumed to arise from the inhibition of viral RNA polymerase or from 5′ capping of viral mRNA by ribavirin triphosphate (3).
One of the complications associated with the use of ribavirin is hemolytic anemia, which sometimes necessitates cessation of therapy (1). The anemia is likely due to accumulation of ribavirin in erythrocytes. Once transported into erythrocytes, ribavirin is phosphorylated to its active form and sequestered within the cells at concentrations estimated to be up to ninefold higher than those in plasma (7). Although the mechanism has not been fully clarified, it is believed that ribavirin phosphates impair ATP-dependent transport systems by competing with ATP located on the erythrocyte cell membrane, resulting in membrane destabilization.
Several assays for ribavirin have been developed; these use high-performance liquid chromatography (HPLC) and radioimmunoassay (RIA) techniques for pharmacokinetic study of human biological fluids such as plasma, urine, and cerebrospinal fluid (4, 7, 8, 10). An attempt to quantitate ribavirin in erythrocytes using RIA has been reported by Lertora et al. Ribavirin levels in plasma and erythrocytes were compared in patients with AIDS, and the results indicated that ribavirin was highly concentrated in erythrocytes (8). How extensively phosphorylated anabolites accumulated in erythrocytes could not be estimated, as information about the cross-reactivity of the antiribavirin antibody used for analysis with the phosphorylated anabolites is lacking. Further, results were not obtained and compared following sample treatment with acid phosphatase to differentiate phosphorylated ribavirin from the unchanged form. An alternative approach for overcoming such limitations in determining the concentrations of phosphorylated compounds is to use chromatographic techniques.
The purpose of the present study was to use HPLC for the determination of unchanged and phosphorylated ribavirin levels in whole blood. The level of phosphorylated ribavirin can be estimated by comparing the difference between the total and the unchanged levels, which are measured in samples with and without dephosphorylation, respectively. In addition, concentrations of total (unchanged plus phosphorylated) and unchanged drug in erythrocytes can be estimated from the corresponding plasma and whole-blood levels, with knowledge of each subject’s hematocrit. Thus, the method described here is an indirect one based on the conversion of ribavirin phosphates to ribavirin, with quantitation of ribavirin. Such a method may be useful for predicting drug toxicity or evaluating potential drug interactions. As an example, this HPLC method was used to explore the in vitro effects of dipyridamole, a nucleoside transport inhibitor, on ribavirin disposition in plasma and erythrocytes.
MATERIALS AND METHODS
Chemicals and instruments.
All chemicals were of HPLC or reagent grade and were obtained from Fisher Scientific (Fair Lawn, N.J.) or Sigma (St. Louis, Mo.). Ribavirin (1-β-d-ribofuranosyl-1H-1,2,4-triazole-3-carboxamide), 3-methylcytidine methosulfate (as an internal standard), dipyridamole, and acid phosphatase type IV from sweet potato were purchased from Sigma. Phenyl boronic acid (PBA) cartridges (Bond Elute PBA [100 mg]) used for solid-phase extraction were obtained from Varian (Harbor City, Calif.).
HPLC apparatus and conditions.
The HPLC system used in this study consisted of a Waters M-510 HPLC pump (Waters Associates, Milford, Mass.), a model 717 Plus auto sampler, a C18 reversed-phase column (Novapak [3.9 by 300 mm]), a Spectroflow 783 UV detector (Kratos Analytical, Ramsey, N.J.), and an HP integrator, model 3396A (Hewlett-Packard, Avondale, Pa.). The wavelength was fixed at 235 nm, and the sensitivity was 0.002 to 0.01 absorbance units, full scale. As the mobile-phase solvent, 10 mM ammonium phosphate buffer (pH 6.5) was used at a flow rate of 0.7 ml/min for analysis of enzyme-treated samples. For nontreated samples, the pH of the mobile phase was adjusted to 2.5.
Enzyme digestion and PBA column extraction.
A stock solution of ribavirin was prepared at a concentration of 4.1 mM in distilled water and stored at −20°C. Reference samples containing 0.5, 1, 5, 10, 20, and 50 μM ribavirin for calibration curves and at 1.67, 10, and 40 μM concentrations for controls were prepared by diluting the stock solution with drug-free whole blood, which had been collected from a healthy male subject. Four hundred microliters of the sample was precipitated by adding 80 μl of 2.3 M perchloric acid and then centrifuged at 1,500 × g for 5 min. The supernatant was neutralized with 10 M KOH and then was divided into two portions, one for enzyme digestion and the other without enzyme treatment. The resulting mixtures were stored at −20°C until analysis. Preliminary study indicated that anabolites are stable for up to 1 month following precipitation.
Two hundred microliters of stored sample was treated with acid phosphatase to hydrolyze the phosphorylated anabolites, according to the method developed by Robbins et al. (13) with minor modification. The reaction mixture consisted of 200 μl of whole-blood extracts, 300 μl of 30 μM Tris, 25 μl of 1 M sodium acetate buffer (pH 4.0), and 2.5 μl of acid phosphatase solution (2.75 U). After incubation for 2 h at 37°C, the reaction was terminated by adding 2.5 μl of 10 N KOH. We confirmed the completion of dephosphorylation by monitoring the peak height of ribavirin at various incubation times—30, 60, 90, and 120 min—with a plateau level achieved at 90 min, corresponding to completion. To the resulting mixture, 25 μl of 3-methylcytidine methosulfate solution (100 μg/ml) was added as an internal standard. After vortexing, the mixture was diluted with 0.5 ml of 250 mM ammonium acetate buffer (pH 8.5) and vortexed again. This mixture was loaded onto the PBA cartridge, which had been pretreated with 1 ml of 100 mM formic acid followed by 5 ml of 250 mM ammonium acetate buffer (pH 8.5). After the cartridges were positioned on a 10-port vacuum elution manifold (Waters Associates), the sample was drained away and the cartridges were washed five times under reduced pressure with 1-ml aliquots of 250 mM ammonium acetate buffer (pH 8.5). Ribavirin and the internal standard were subsequently eluted with 1 ml of 100 mM formic acid into glass tubes. The effluents were dried under nitrogen and reconstituted in 200 μl of mobile phase. Twenty-microliter aliquots of reconstituted samples were injected onto the HPLC column.
In vitro effects of dipyridamole on ribavirin disposition.
Blood was drawn into heparinized tubes from a healthy subject. Six hundred thirty microliters of blood was preincubated with 70 μl of dipyridamole solution at 37°C for 30 min. The dipyridamole solution was prepared by dissolving dipyridamole in phosphate-buffered saline (PBS) containing 2% isopropanol. The final concentration of dipyridamole in the reaction mixture was 25 μM. The same volume of 2% isopropanol-PBS solution was used as a control. Each sample was further incubated with ribavirin (final concentration, 37 μM) with periodic gentle shaking at 37°C for 18 h. After incubation, samples were centrifuged at 1,500 × g for 15 min for determination of plasma ribavirin levels and were precipitated with perchloric acid immediately, as described above, for whole-blood ribavirin levels. Total and unchanged ribavirin levels in plasma and whole blood were determined with and without enzyme digestion, respectively. All measurements were performed in duplicate. The concentration of ribavirin in erythrocytes was calculated as Crbc = [Cw − Cp(1 − Hct)]/Hct, where Crbc is the concentration in erythrocytes, Cw is the concentration in whole blood, Cp is the concentration in plasma, and Hct is the study subject’s hematocrit.
RESULTS
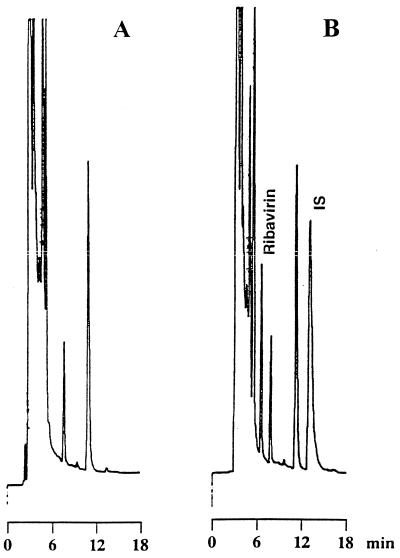
Typical chromatograms of whole-blood samples following enzyme digestion are shown in Fig. 1. Similar chromatograms were obtained from plasma samples (data not shown). The retention times of ribavirin and the internal standard were 6.5 and 13.1 min, respectively. Although minor interference was present at the retention time of the internal standard (Fig. 1), its influence on the quantitation of ribavirin was negligible. For analysis of non-enzyme-treated samples, the pH of the mobile phase solvent was lowered by 2.5 to separate the internal standard from an overlapping endogenous phosphorylated compound (data not shown). The detection limit of ribavirin was 40 pmol as injected on the column and yielded a signal-to-noise ratio of better than 3. This corresponds to a ribavirin concentration of 0.08 μM for plasma or 0.2 μM for whole blood.
FIG. 1.
Chromatograms of blank whole blood (A) and whole blood spiked with 37 μM ribavirin and an internal standard (IS) (B).
The linearity of the calibration curve was determined by plotting the peak height ratio of ribavirin to the internal standard against the ribavirin concentration in whole blood. A linear response was obtained for ribavirin concentrations from 0.5 to 50 μM. The equation of the line calculated by regression analysis was y = 0.0188x + 0.0524 (r = 0.9999), where y is the peak height ratio and x is the micromolar concentration.
The recovery of ribavirin from whole blood was examined by comparing the peak height ratios of ribavirin to the internal standard between control samples (1.67, 10, and 40 μM) and the corresponding reference samples. The reference samples were prepared by adding ribavirin standard to blank extracts, which were obtained from dephosphorylated whole blood by treatment with PBA column extraction. Although the mean recovery was 63.2% at the lower concentration (1.67 μM), recoveries at 10 and 40 μM (concentrations corresponding to the therapeutic range at steady state) were 85.9 and 90.7%, respectively (Table 1). A preliminary study indicated that the loss of ribavirin during acid precipitation was negligible. Recovery of the internal standard was 89.2% ± 2.6% (coefficient of variation [CV] = 2.9%).
TABLE 1.
Recovery of ribavirin from whole blood
| Added concn (μM) | Recovery rate (%)
|
|
|---|---|---|
| Mean ± SDa | CV | |
| 1.67 | 63.2 ± 6.1 | 9.6 |
| 10.0 | 85.9 ± 4.0 | 4.6 |
| 40.0 | 90.7 ± 5.4 | 5.9 |
n = 5.
The analytical precision for whole-blood ribavirin determinations was evaluated by intra- and interassay validation at concentrations of 1.67, 10, and 40 μM. For intra-assay precision, five sets of each control sample were assayed on the same day. For interassay precision, five samples of each concentration were assayed on five different days over 4 weeks. The CVs of intra- and interassay precision were greater than 10% at 1.67 μM, but less than 7% at 10 and 40 μM (Table 2).
TABLE 2.
Intra- and interassay precision
| Added concn (μM) | Observed concn (μM)
|
|||
|---|---|---|---|---|
| Intra-assay (n = 5)
|
Interassay (n = 5)
|
|||
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | |
| 1.67 | 1.85 ± 0.19 | 10.4 | 1.62 ± 0.23 | 11.7 |
| 10.0 | 10.0 ± 0.32 | 3.2 | 10.3 ± 0.48 | 4.7 |
| 40.0 | 39.1 ± 2.30 | 5.9 | 41.2 ± 2.45 | 6.0 |
We applied our method to in vitro experiments investigating disposition of ribavirin in plasma and erythrocytes, with preliminary data presented in the present work. As shown in Table 3, erythrocyte ribavirin concentrations were three times higher than plasma ribavirin levels when ribavirin was incubated with heparinized whole blood at a concentration of 37 μM. Seventy-four percent of erythrocyte ribavirin consisted of phosphorylated anabolites, whereas the anabolites were not detected at all in plasma. Dipyridamole, a nucleoside transport inhibitor, reduced erythrocyte ribavirin by 30%, resulting in a 40% decrease in phosphorylated ribavirin concentrations. A corresponding 69% increase in unchanged ribavirin in plasma was measured in the presence of dipyridamole. There was no change in total ribavirin levels in whole blood (Table 3).
TABLE 3.
Effects of dipyridamole on ribavirin disposition in peripheral blood
| Blood constituent | Ribavirin fraction | Ribavirin concn (μM)a
|
% Changeb | |
|---|---|---|---|---|
| Control | With dipyridamole | |||
| Plasma | Free | 17.6 ± 1.5 | 29.7 ± 2.8 | 68.8 |
| Phosphates | ND | ND | ||
| Total | 17.6 ± 1.5 | 29.7 ± 2.8 | 68.8 | |
| Whole blood | Free | 16.1 ± 1.7 | 23.0 ± 2.2 | 42.9 |
| Phosphates | 16.5 ± 0.7 | 9.9 ± 2.6 | −40.0 | |
| Total | 32.6 ± 1.8 | 32.9 ± 2.3 | 0.9 | |
| Erythrocytes | Free | 14.0 ± 5.8 | 14.0 ± 4.2 | 0.0 |
| Phosphates | 38.9 ± 1.5 | 23.3 ± 6.1 | −40.0 | |
| Total | 52.9 ± 5.6 | 37.2 ± 2.2 | −29.7 | |
Values are means ± SD. ND, not detected.
Change in ribavirin concentration as a result of dipyridamole treatment, with the control value set at 100%.
DISCUSSION
HPLC assays previously available for ribavirin quantitation cannot be applied to whole-blood samples. Paroni et al. described a method for quantitating serum ribavirin by using uridine as the internal standard (10). However, since endogenous uridine is also highly concentrated in erythrocytes in a phosphorylated form, it is not a suitable internal standard for determination of drug concentrations in erythrocytes. Granich et al. used 3-methoxycytidine methosulfonate as the internal standard (4). They successfully achieved the separation of ribavirin from other endogenous and exogenous contaminants in plasma, urine, and cerebrospinal fluids (not erythrocytes) by using a pair of μBondapak C18 (Waters Associates) analytical columns. However, the use of two analytical columns is not satisfactory, owing to extensive band spreading and the high pressure generated in the HPLC system. We optimized the analytical conditions to achieve sufficient peak separation on a single column. The use of Novapak C18 with a smaller particle size than that of μBondapak C18 provided good peak separation for non-enzyme-treated whole-blood samples. However, we observed that several endogenous peaks, which were coeluted with ribavirin or the internal standard, appeared after dephosphorylation. This problem was resolved by adjusting the pH of the mobile phase, resulting in markedly improved separation as shown in Fig. 1. The sensitivity, recovery, linearity of the calibration curve, and intra- and interassay precision, determined in the dephosphorylated whole blood sample, were comparable with those of previous studies using plasma sample analysis (4).
Applying the method, we examined the ribavirin disposition in plasma and erythrocytes via in vitro experiments. As suggested previously (9), we determined that ribavirin is highly concentrated in erythrocytes as phosphorylated anabolites (formed by cellular adenosine kinase). Also, limited application of this assay suggested that dipyridamole reduces the transport of ribavirin into erythrocytes. Our preliminary results further suggest a decrease in the formation of phosphorylated anabolites and an increase in unchanged levels in plasma. Since dipyridamole is an inhibitor of nucleoside transporters (5), it was suggested that ribavirin transport into erythrocytes is mediated at least partly via nucleoside transporters present on the cell surface. This hypothesis has been supported by kinetic studies using several probes for nucleoside transporters, such as nitrobenzylthioinosine and endogenous nucleosides (6). Further work has been carried out by Patil and colleagues, who have evaluated transport mechanisms at the level of the gastrointestinal tract (11).
In conclusion, an improved analytical method has been developed for quantitating total and unchanged ribavirin in whole blood and erythrocytes. Preliminary data suggest dipyridamole inhibits ribavirin transport into erythrocytes. Evaluation of this specific drug combination clinically may be warranted and may have implications for the penetration of ribavirin to other cells of interest linked to pharmacologic effects. The HPLC method described here will be useful for predicting drug toxicity and clarifying ribavirin disposition in plasma and erythrocytes in future pharmacokinetic investigations.
ACKNOWLEDGMENTS
We thank Assefa Gebremichael and Eralp Bellibas for useful suggestions.
REFERENCES
- 1.Bizollon T, Palazzo U, Ducerf C, Chevallier M, Elliott M, Baulieux J, Pouye M, Trepo C. Pilot study of the combination of interferon alfa and ribavirin as therapy of recurrent hepatitis C after liver transplantation. Hepatology. 1997;26:500–504. doi: 10.1002/hep.510260236. [DOI] [PubMed] [Google Scholar]
- 2.Dieterich D T, Purow J M, Rajapaksa R. Activity of combination therapy with interferon alfa-2b plus ribavirin in chronic hepatitis C patients co-infected with HIV. Semin Liver Dis. 1999;19(Suppl. 1):87–94. [PubMed] [Google Scholar]
- 3.Glue P. The clinical pharmacology of ribavirin. Semin Liver Dis. 1999;19(Suppl. 1):17–24. [PubMed] [Google Scholar]
- 4.Granich G G, Krogstad D J, Connor J D, Desrochers K L, Sherwood C. High-performance liquid chromatography (HPLC) assay for ribavirin and comparison of the HPLC assay with radioimmunoassay. Antimicrob Agents Chemother. 1989;33:311–315. doi: 10.1128/aac.33.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith D A, Javis S M. Nucleoside and nucleobase transport system of mammalian cells. Biochim Biophys Acta. 1996;1286:153–181. doi: 10.1016/s0304-4157(96)00008-1. [DOI] [PubMed] [Google Scholar]
- 6.Jarvis S M, Thorn J A, Glue P. Ribavirin uptake by human erythrocytes and the involvement of nitrobenzylthioinosine-sensitive (es)-nucleoside transporters. Br J Pharmacol. 1998;123:1587–1592. doi: 10.1038/sj.bjp.0701775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laskin O L, Longstreth J A, Hart C C, Scavuzzo D, Kalman C M, Connor J D, Roberts R B. Ribavirin disposition in high-risk patients for acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1987;41:546–555. doi: 10.1038/clpt.1987.70. [DOI] [PubMed] [Google Scholar]
- 8.Lertora J J L, Rege A B, Lacour J T, Ferencz N, George W J, VanDyke R B, Agrawal K C, Hyslop N E. Pharmacokinetics and long-term tolerance to ribavirin in asymptomatic patients infected with human immunodeficiency virus. Clin Pharmacol Ther. 1991;50:442–449. doi: 10.1038/clpt.1991.162. [DOI] [PubMed] [Google Scholar]
- 9.Page T, Conner J D. The metabolism of ribavirin in erythrocytes and nucleated cells. Int J Biochem. 1990;22:379–383. doi: 10.1016/0020-711x(90)90140-x. [DOI] [PubMed] [Google Scholar]
- 10.Paroni R, Sirtori C, Gallikienle M. High-performance liquid chromatographic determination of ribavirin in serum and urine and of its urinary metabolite 1,2,4-triazole-3-carboxamide. J Chromatogr. 1987;420:189–196. doi: 10.1016/0378-4347(87)80172-x. [DOI] [PubMed] [Google Scholar]
- 11.Patil S D, Ngo L Y, Glue P, Unadkat J D. Intestinal absorption of ribavirin is preferentially mediated by the Na+-nucleoside purine (N1) transporter. Pharm Res. 1998;15(6):950–952. doi: 10.1023/a:1011945103455. [DOI] [PubMed] [Google Scholar]
- 12.Reichard O, Norkrans G, Fryden A, Braconier J H, Sonnerborg A, Weiland O. Randomised, double-blind, placebo-controlled trial of interferon alpha-2b with and without ribavirin for chronic hepatitis C. The Swedish Study Group. Lancet. 1998;351:83–87. doi: 10.1016/s0140-6736(97)06088-1. [DOI] [PubMed] [Google Scholar]
- 13.Robbins B L, Waibel B H, Fridland A. Quantitation of intracellular zidovudine phosphates by use of combined cartridge-radioimmunoassay methodology. Antimicrob Agents Chemother. 1996;40:2651–2654. doi: 10.1128/aac.40.11.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thevenot T, Mathurin P, Moussalli J, Perrin M, Plassart F, Blot C, Opolon P, Poynard T. Effects of cirrhosis, interferon and azathioprine on adverse events in patients with chronic hepatitis C treated with ribavirin. J Viral Hepat. 1997;4:243–253. doi: 10.1046/j.1365-2893.1997.00051.x. [DOI] [PubMed] [Google Scholar]