Abstract
This study investigated the antioxidant, antimicrobial, anticancer, and phytochemical profiling of extracts from the leaves and stem/root of Acanthus ebracteatus (AE). The total phenolic content (TPC), total flavonoid content (TFC), 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) radical-scavenging activity, 2, 2′-azino-Bis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical-scavenging activity, metal chelating activities (MCA), ferric reducing antioxidant power (FRAP) and oxygen radical antioxidant capacity (ORAC) were used for antioxidant assessment. The ethanolic extracts of the leaves (AEL-nor) and stem/root (AEWP-nor) without chlorophyll removal and those with chlorophyll removal, using sedimentation process (AEL-sed and AEWP-sed), were prepared. Generally, AEL-sed showed the highest antioxidant activity (FRAP: 1113.2 µmol TE/g; ORAC: 11.52 µmol TE/g; MCA: 47.83 µmol EDTA/g; ABTS 67.73 µmol TE/g; DPPH 498.8 µmol TE/g; TPC: 140.50 mg/GAE g and TFC: 110.40 mg/CE g) compared with other extracts. Likewise, AEL-sed also showed the highest bacteriostatic (MIC) and bactericidal (MBC) effects, as well as the highest anticancer and antiproliferative activity against oral squamous carcinoma (CLS-354/WT) cells. UPLC-ESI-QTOF/MS analysis of AEL-sed and AEWP-sed tentatively identified several bioactive compounds in the extracts, including flavonoids, phenols, iridoids, and nucleosides. Our results provide a potentially valuable application for A. ebracteatus, especially in further exploration of the plant in oxidative stress-related disorders, as well as the application of the plant as potential nutraceuticals and cosmeceuticals.
Keywords: Acanthus ebracteatus Vahl, antioxidant, antibacterial, anticancer, polyphenols
1. Introduction
Medicinal plants have played a pivotal role in primary health care over the past few decades, especially in low- and middle-income countries. Several medicinal plants have been the building blocks for the successful discovery of bioactive medicinal agents currently used in the treatment of a wide range of diseases. Furthermore, medicinal plants are generally perceived as safer substituents for the treatment of devastating diseases, including diabetes, cancer, cardiovascular disorders, and neurodegenerative diseases amongst others [1,2,3]. The roles of these natural endowments in oxidative stress-related diseases have been extensively explored. The display of excellent antioxidant activities by plant extracts is directly correlated to the existence of bioactive constituents, notably polyphenolic compounds, which make these medicinal plant extracts display properties indicating they are significant antidiabetic, anti-inflammatory, antiaging, and anticancer agents [3,4].
Acanthus ebracteatus Vahl. (Sea Holly) is a multipurpose mangrove medicinal plant belonging to the Acanthaceae family that grows in several southeast Asian countries including Thailand, Malaysia, Indonesia, the Philippines, and Vietnam [5,6]. A. ebracteatus has several traditional folk medicinal uses, especially in the treatment of rheumatism, cough, snake-bite, chronic fever, asthma, hepatitis, intestinal worms, preventing hair loss, herpes zoster, leucorrhea, wound, menstrual disorders, rash, and skin diseases [5]. Although there are few reports regarding the phytochemical richness of A. ebracteatus, previous studies have reported the presence of aliphatic alcohol, aliphatic glycosides, phenolic glycosides, terpenes, megastigmane glycosides, flavonoids, and lignan glycosides [6,7]. A. ebracteatus has been pharmacologically reported to show anti-inflammatory, neuroprotection and wound healing effects [5,6,8,9]. However, none of these reports provided detailed information regarding the phytochemical and pharmacological profiles of the leaves and the stem/root extracts of this species. As such, this work evaluated the chemical composition, antioxidant, antibacterial and cytotoxic activities of A. ebracteatus leaves and stem/root extracts.
2. Results
2.1. Evaluation of the Antioxidant Activity
The antioxidant activities of the leaves and stem/root extracts of A. ebracteatus prepared without chlorophyll removal and with the sedimentation chlorophyll removal method described in Section 4.2 were examined using several in vitro assays (DPPH, ABTS, FRAP, MCA, and ORAC). It was observed from the results that the leaves extract (AEL) showed better antioxidant activity in all the assays compared to the extract from the stem/root (AEWP). The leaves extract from the sedimentation process (AEL-sed) showed the highest antioxidant activity in the FRAP (1113.2 ± 4.2 µmol TE/g), ORAC (11.52 ± 0.3 µmol TE/g), MCA (47.83 ± 0.01 µmol EDTA/g), ABTS (67.73 ± 0.5 µmol TE/g) and DPPH (498.8 ± 0.4 µmol TE/g) assays (Table 1).
Table 1.
Antioxidant activity of different extracts from A. ebracteatus.
| Sample/Assay | AEWP-nor | AEWP-sed | AEL-nor | AEL-sed |
|---|---|---|---|---|
| TPC (mg GAE/g dry extract) | 30.49 ± 0.10 e | 36.88 ± 0.10 d | 138.20 ± 0.10 b | 140.50 ± 0.10 a |
| TFC (mg CE/g dry extract) | 20.24 ± 0.20 e | 28.82 ± 0.10 d | 107.60 ± 0.02 b | 110.40 ± 0.50 a |
| DPPH-RSA (µmol TE/g dry extract) | 91.90 ± 0.40 f | 104.90 ± 0.08 e | 448.10 ± 1.20 b | 498.80 ± 0.40 a |
| ABTS-RSA (µmol TE/g dry extract) | 44.01 ± 0.10 d | 48.59 ± 0.08 c | 57.05 ±0.10 b | 67.73 ± 0.50 a |
| FRAP (µmol TE/g dry extract) | 182.80 ± 0.40 c | 223.01 ± 0.40 b | 1098.20 ± 7.1 a | 1113.20 ± 4.20 a |
| MCA (µmol EDTA/g dry extract) | 36.32 ± 0.10 e | 37.29 ± 0.05 d | 46.87 ± 0.20 b | 47.83 ± 0.01 a |
| ORAC (µmol TE/g dry extract) | 3.22 ± 0.20 b | 3.32 ± 0.60 b | 11.51 ± 0.50 a | 11.52 ± 0.30 a |
Different lowercase superscripts within the same column indicate significant difference (p < 0.05). Mean (n = 3); AEWP-nor: extract from the stem/root without dechlorophyllization; AEWP-sed: extract from the stem/root after the dechlorophyllization process using the sedimentation process; AEL-nor: extract from the leaves without dechlorophyllization; AEL-sed: extract from the leaves after the dechlorophyllization process using the sedimentation process.
2.2. Total Phenolic and Flavonoid Content
Likewise, in the total phenolic (TFC) and total flavonoid content (TFC) quantification, AEL extract also showed higher phenolic and flavonoid contents (AEL-sed; TFC: 110.4 ± 0.5 mg CE/g; TPC: 140.5 ± 0.1 mg GAE/g; AEL-nor; TFC: 107.6 ± 0.02 mg CE/g; TPC: 138.2 ± 0.1 mg GAE/g) compared to AEWP (AEL-sed; TFC: 28.82 ± 0.1 mg CE/g; TPC: 136.88 ± 0.1 mg GAE/g; AEL-nor; TFC: 20.24 ± 0.2 mg CE/g; TPC: 30.49 ± 0.1 mg GAE/g) (Table 1).
2.3. Evaluation of Antimicrobial Activity
The antimicrobial activity (MIC and MBC) of the extracts against E. coli and L. monocytogenes is shown in Table 2. The results showed that the AEL extract displayed more potent antibacterial activity compared to AEWP, with MIC values ranging from 0.25–0.5 mg/mL and MBC values of 0.5–1.0 mg/mL (Table 2).
Table 2.
Antibacterial activity of different extracts from A. ebracteatus.
| Samples | MIC | MBC | ||
|---|---|---|---|---|
| EC | LM | EC | LM | |
| AEWP-nor | 1.00 a | 1.00 a | 2.00 a | 2.00 a |
| AEWP-sed | 1.00 a | 1.00 a | 2.00 a | 2.00 a |
| AEL-nor | 0.25 c | 0.50 b | 0.50 c | 1.00 b |
| AEL-sed | 0.25 c | 0.50 b | 0.50 c | 1.00 b |
EC, Escherichia coli; LM, Listeria monocytogenes; MIC, minimum inhibitory concentration; MBC, minimum bactericidal concentration. Different lowercase superscripts within the same column indicate significant difference (p < 0.05). Mean (n = 3).
2.4. Evaluation of Anticancer Activity
The effects of the extracts on the cellular viability of CLS-354 / WT cells were tested following a tetrazolium-based MTT assay. The results demonstrated that AEL-sed and AEWP-sed resulted in a significant (p < 0.001) reduction in cell viability of CLS-354/WT in a dose-dependent manner. The ED50 was 200 μg/mL and 400 μg/mL for AEL-sed and AEWP-sed, respectively (Figure 1).
Figure 1.
Epithelium-like phenotype oral squamous carcinoma cell (CLS-354/WT) death, in percent, treated with AEL-sed and AEWP-sed extracts (1600–12.5 μg/mL), using MTT assay. Data are expressed as mean ± SEM from at least three independent experiments and analyzed via one-way ANOVA with Dunnett’s test. * p < 0.05, *** p < 0.001 vs untreated control.
2.5. Evaluation of the Anti-Proliferative Effect
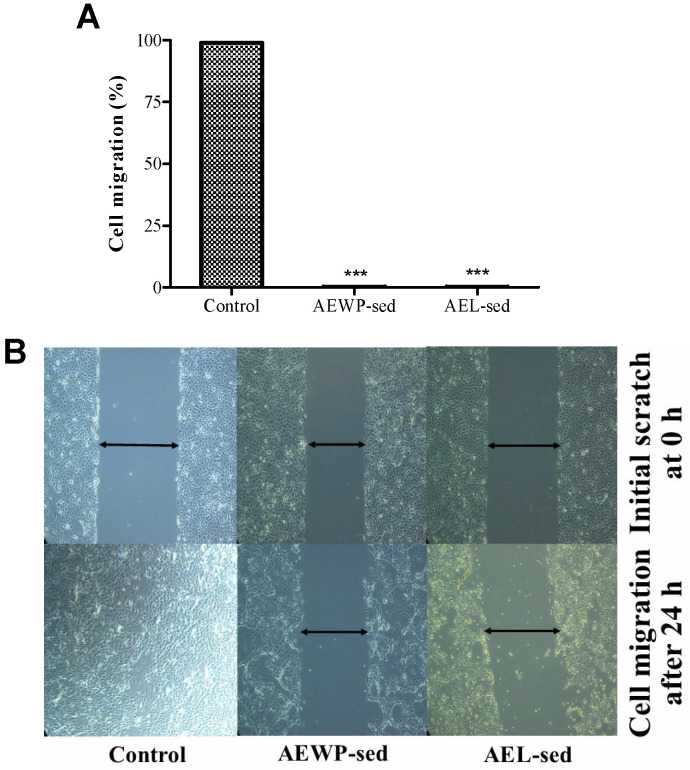
The AEL-sed and AEWP-sed extracts were further investigated for their anti-proliferative effects on CLS-354/WT. As shown in Figure 2, The results indicated that both extracts significantly (p < 0.001) inhibited the proliferation of cells. Moreover, the extracts also retarded cell migration. However, cell viability was not significantly affected. These results suggested a pronounced anti-proliferative effect of AEL-sed and AEWP-sed extract on CLS-354/WT at the tested concentration.
Figure 2.
Antiproliferative effects of AEL-sed and AEWP-sed extracts (800 μg/mL) in epithelium-like phenotype oral squamous carcinoma cell (CLS-354/WT) at 0 and 24 h (A) Percentage migration calculated using the length of cell migration obtained from microscopic image (B) Microscopic photographs of CLS-354/WT cells migration by scratch technique. Data are expressed as mean ± SEM from at least 3 independent experiments and analyzed by one-way ANOVA followed by Dunnett’s test. *** p < 0.001 vs. untreated control.
2.6. Identification of Compounds in AEL-sed and AEPW-sed
The profiling of the phytochemical constituents in AEL-sed and AEWP-sed was performed via UPLC-QTOF-MS analysis, in the negative ionization mode. The chromatogram of the peaks of the eluted compounds in AEL-sed and AEPW-sed showed several peaks (within 20 min), suggesting the presence of several constituents in the extract (Figure 3). The compounds with mass error <5 ppm and high relative abundance are presented in Table 3 and Table 4. The data shown in Table 3 indicated that the majority of the compounds tentatively identified in AEL-sed were glycosidic constituents, especially flavonoids and phenolic compounds. According to Table 3, simple phenolic and phenolic glycosides, including caffeic acid (Rt: 6.36 min), dihydroferulic acid 4-O-glucuronide (Rt: 5.52 min), 4-glucogallic acid (Rt: 5.532 min), chlorogenic acid (Rt: 7.038 min), kelampayoside A (Rt: 5.269 min) and hydrojuglone glucoside (Rt: 8.018 min) were tentatively identified in AEL-sed.
Figure 3.
Total ion chromatograms of A. ebracteatus extract using UHPLC-ESI-QTOF-MS in the negative electrospray ionization mode showing the chromatogram intensity against the acquisition time; (A) AEL-sed, (B) AEWP-sed.
Table 3.
Compounds tentatively identified in AEL-sed using UHPLC-ESI-QTOF-MS analysis.
| No | Rt (min) | Accurate Mass (m/z) | Calculated Mass (Da) | Score (DB) | Predicted Formula | Compound Identity |
|---|---|---|---|---|---|---|
| 1 | 2.997 | 337.0775 | 338.0848 | 98.14 | C12H18O11 | L-Ascorbic acid-2-glucoside |
| 2 | 3.311 | 225.0015 | 226.009 | 63.57 | C8H7ClN4S | 6-(2-Chloroallylthio)purine |
| 3 | 4.076 | 134.0471 | 135.0544 | 87.88 | C5H5N5 | Adenine |
| 4 | 4.604 | 330.119 | 331.1262 | 98.71 | C14H21NO8 | 5′-O-beta-D-Glucosylpyridoxine |
| 5 | 4.729 | 128.0351 | 129.0426 | 95.05 | C5H7NO3 | (R)-(+)-2-Pyrrolidone-5-carboxylic acid |
| 6 | 4.767 | 243.0624 | 244.0697 | 99.79 | C9H12N2O6 | Pseudouridine |
| 7 | 4.805 | 174.077 | 175.0843 | 99.74 | C7H13NO4 | Calystegine B5 |
| 8 | 4.854 | 180.0664 | 181.0736 | 99.62 | C9H11NO3 | 3-Amino-3-(4-hydroxyphenyl)propanoate |
| 9 | 5.005 | 282.0842 | 283.0915 | 98.4 | C10H13N5O5 | Guanosine |
| 10 | 5.055 | 150.042 | 151.0492 | 98.18 | C5H5N5O | 8-Hydroxyadenine |
| 11 | 5.256 | 405.1395 | 406.1468 | 98.28 | C17 H26O11 | Morroniside |
| 12 | 5.269 | 477.1608 | 478.1681 | 97.79 | C20H30O13 | Kelampayoside A |
| 13 | 5.356 | 108.0456 | 109.0529 | 98.09 | C6H7NO | 3-Hydroxy-2-Methylpyridine |
| 14 | 5.52 | 371.0979 | 372.1052 | 98.94 | C16H20O10 | Dihydroferulic acid 4-O-glucuronide |
| 15 | 5.532 | 331.0668 | 332.0741 | 99.44 | C13H16O10 | 4-Glucogallic acid |
| 16 | 5.959 | 355.1044 | 356.1116 | 95.68 | C16H20O9 | 1-O-2′-Hydroxy-4′-methoxycinnamoyl-b-D-glucose |
| 17 | 6.16 | 461.1665 | 462.1738 | 99.05 | C20H30O12 | Verbasoside |
| 18 | 6.26 | 403.1245 | 404.1318 | 96.86 | C17H24O11 | Oleoside 11-methyl ester |
| 19 | 6.336 | 341.0875 | 342.0947 | 83.78 | C15H18O9 | Glucocaffeic acid |
| 20 | 6.36 | 179.0349 | 180.0423 | 96.04 | C9H8O4 | Caffeic Acid |
| 21 | 6.637 | 339.0721 | 340.0794 | 99.12 | C15H16O9 | Aesculin |
| 22 | 7.038 | 353.088 | 354.0953 | 99.19 | C16H18O9 | Chlorogenic acid |
| 23 | 7.114 | 593.151 | 594.1583 | 98.71 | C27H30O15 | Saponarin |
| 24 | 7.164 | 387.1657 | 388.1729 | 97.14 | C18H28O9 | 2-[4-(3-Hydroxypropyl)-2-methoxyphenoxy]-1,3-propanediol-1-xyloside |
| 25 | 7.39 | 639.1929 | 640.2024 | 57.65 | C29H36O16 | beta-Hydroxyacteoside |
| 26 | 7.415 | 637.1046 | 638.1119 | 99.11 | C27H26O18 | Scutellarein-7-glucuronosyl-(1->2)-glucuronide |
| 27 | 7.54 | 563.1406 | 564.1476 | 97.71 | C26H28O14 | Luteolin 3′-methyl ether 7,4′-dixyloside |
| 28 | 7.565 | 415.1608 | 416.1682 | 95.16 | C19H28O10 | Phenylethyl primeveroside |
| 29 | 7.817 | 177.0195 | 178.0267 | 86.68 | C9H6O4 | Esculetin |
| 30 | 7.842 | 399.166 | 400.1734 | 95.33 | C19H28O9 | Corchoionoside B |
| 31 | 7.917 | 621.11 | 622.117 | 98.15 | C27H26O17 | Genistein 4′,7-O-diglucuronide |
| 32 | 7.942 | 383.0622 | 384.0693 | 97.99 | C16H16O11 | 2-O-Feruloylhydroxycitric acid |
| 33 | 7.967 | 353.0517 | 354.0588 | 98.05 | C15H14O10 | 2-O-p-Coumaroylhydroxycitric acid |
| 34 | 8.018 | 337.0932 | 338.1004 | 98.79 | C16H18O8 | Hydrojuglone glucoside |
| 35 | 8.369 | 461.0725 | 462.0797 | 98.96 | C21H18O12 | 3-Methylellagic acid 8-rhamnoside |
| 36 | 8.432 | 623.1996 | 624.2065 | 96.46 | C29H36O15 | Isoacteoside |
| 37 | 8.62 | 637.2143 | 638.2212 | 98.89 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone-4′-rhamnosyl-(1->6)-glucoside |
| 38 | 8.745 | 429.1764 | 430.1837 | 97.61 | C20H30O10 | Phenethyl rutinoside |
| 39 | 8.871 | 579.1728 | 580.1799 | 97.92 | C27H32O14 | Cascaroside F |
| 40 | 8.884 | 445.0774 | 446.0847 | 97.07 | C21H18O11 | Baicalin |
| 41 | 8.971 | 475.088 | 476.0953 | 98.09 | C22H20O12 | Diosmetin 7-O-beta-D-glucuronopyranoside |
| 42 | 9.147 | 665.2081 | 666.2159 | 88.16 | C31H38O16 | Tetramethylquercetin 3-rutinoside |
| 43 | 9.273 | 651.2289 | 652.2359 | 97.31 | C31H40O15 | (-)-Matairesinol-4′-[apiosyl-(1->2)-glucoside] |
| 44 | 9.373 | 413.2173 | 414.2247 | 95.11 | C21H34O8 | (4R,5S,7R,11S)-11,12-Dihydroxy-1(10)-spirovetiven-2-one 11-glucoside |
| 45 | 9.386 | 433.1498 | 434.1571 | 96.09 | C22H26O9 | Vestitone 7-glucoside |
| 46 | 9.624 | 431.1345 | 432.1419 | 95.75 | C22H24O9 | 4′-O-Methylglucoliquiritigenin |
| 47 | 9.8 | 591.2074 | 592.2147 | 96.83 | C29H36O13 | Osmanthuside B |
| 48 | 10.151 | 503.1552 | 504.1625 | 72.14 | C25H28O11 | Sergeolide |
| 49 | 10.277 | 473.1438 | 474.1511 | 92.54 | C24H26O10 | Luteolin 7,3′-dimethyl ether 5-glucoside |
| 50 | 10.327 | 275.092 | 276.0993 | 98.16 | C15H16O5 | 5-De-O-methyltoddanol |
| 51 | 10.478 | 285.0405 | 286.0478 | 98.52 | C15H10O6 | Luteolin |
| 52 | 10.955 | 329.1037 | 330.1107 | 85.17 | C18H18O6 | Isoamericanol A |
| 53 | 11.03 | 207.0666 | 208.0738 | 97.72 | C11H12O4 | 5-(3′,5′-Dihydroxyphenyl)-gamma valerolactone |
| 54 | 11.18 | 220.0613 | 221.0685 | 99.48 | C11H11NO4 | Methyl dioxindole-3-acetate |
| 55 | 11.193 | 329.2332 | 330.2404 | 98.67 | C18 H34O5 | 9S,10S,11R-trihydroxy-12Z-octadecenoic acid |
| 56 | 11.281 | 269.0455 | 270.0528 | 98.69 | C15H10O5 | Apigenin |
| 57 | 11.432 | 299.0563 | 300.0635 | 98.53 | C16 H12O6 | Diosmetin |
| 58 | 11.482 | 619.1446 | 620.1518 | 96.93 | C32H28O13 | Apigenin-7-(3″-acetyl-6″-E-p-coumaroylglucoside) |
| 59 | 11.934 | 268.0611 | 269.0682 | 97.25 | C15H11NO4 | Evoxanthidine |
| 60 | 13.088 | 307.1907 | 308.198 | 81.32 | C18H28O4 | Dihydrocapsiate |
| 61 | 13.289 | 675.358 | 676.3652 | 94.84 | C33H56O14 | Gingerglycolipid A |
| 62 | 13.44 | 293.1752 | 294.1825 | 98.16 | C17H26O4 | Myrsinone |
| 63 | 14.067 | 273.0765 | 274.0837 | 98.17 | C15 H14O5 | 2,3,4-Trihydroxy-4′-ethoxybenzophenone |
| 64 | 14.545 | 241.0866 | 242.0938 | 98.73 | C15H14O3 | Resveratrol 4′-methyl Ether |
| 65 | 15.8 | 291.0425 | 292.0498 | 98.05 | C15H13ClO4 | Chlorosesamone |
Table 4.
Compounds tentatively identified in AEWP-sed using UHPLC-ESI-QTOF-MS analysis.
| No | Rt (min) | Accurate Mass (m/z) | Calculated Mass (Da) | Score (DB) | Predicted Formula | Compound Identity |
|---|---|---|---|---|---|---|
| 1 | 2.251 | 629.1697 | 630.1768 | 77.85 | C27H34O17 | Leucodelphinidin-3-O-(beta-D-glucopyranosyl-(1->4)-alpha-L-rhamnopyranoside) |
| 2 | 2.302 | 191.0562 | 192.0635 | 99.67 | C7H12O6 | Quinic acid |
| 3 | 2.427 | 827.2658 | 828.2731 | 97.02 | C30H52O26 | Verbascose |
| 4 | 2.528 | 503.1612 | 504.1685 | 98.71 | C18H32 O16 | Nephritogenoside |
| 5 | 2.653 | 683.225 | 684.2323 | 80.52 | C37H36N2O11 | Citbismine C |
| 6 | 2.654 | 341.1091 | 342.1163 | 98.89 | C12 H22 O11 | 2-O-a-D-Galactopyranuronosyl-L-rhamnose |
| 7 | 3.156 | 290.0878 | 291.095 | 99.06 | C11H17NO8 | Sarmentosin epoxide |
| 8 | 3.193 | 665.2136 | 666.2208 | 97.88 | C24H42O21 | Fagopyritol A3 |
| 9 | 3.331 | 225.0016 | 226.009 | 65.47 | C8H7ClN4S | 6-(2-Chloroallylthio)purine |
| 10 | 3.381 | 203.0196 | 204.0269 | 99.89 | C7H8O7 | Daucic acid |
| 11 | 4.762 | 243.0623 | 244.0697 | 99.5 | C9H12N2O6 | Pseudouridine |
| 12 | 4.837 | 174.077 | 175.0843 | 99.62 | C7H13NO4 | Calystegine B5 |
| 13 | 5.038 | 282.0839 | 283.0913 | 98.71 | C10H13N5O5 | Guanosine |
| 14 | 5.264 | 477.1609 | 478.1681 | 98.24 | C20H30O13 | Kelampayoside A |
| 15 | 5.904 | 329.0876 | 330.0948 | 84.7 | C14H18O9 | 3′-Glucosyl-2′,4′,6′-trihydroxyacetophenone |
| 16 | 6.093 | 359.0981 | 360.1055 | 97.46 | C15H20O10 | 6′-Methoxypolygoacetophenoside |
| 17 | 6.168 | 461.1666 | 462.1738 | 99.21 | C20H30O12 | Verbasoside |
| 18 | 6.243 | 167.0348 | 168.0421 | 99.62 | C8H8O4 | Dihydroxyphenylacetic acid |
| 19 | 6.268 | 343.1027 | 344.1102 | 97.17 | C15H20O9 | 4′,6′-Dihydroxy-2′-methoxyacetophenone 6′-glucoside |
| 20 | 6.344 | 341.0875 | 342.0947 | 82.32 | C15H18O9 | Glucocaffeic acid |
| 21 | 6.368 | 403.1241 | 404.1315 | 97.32 | C17H24O11 | Oleoside 11-methyl ester |
| 22 | 6.469 | 179.035 | 180.0416 | 60.28 | C9H8O4 | Caffeic Acid |
| 23 | 6.871 | 513.2184 | 514.2256 | 98.64 | C21H38O14 | 2-O-(beta-D-galactopyranosyl-(1->6)-beta-D-galactopyranosyl) 2S,3R-dihydroxynonanoic acid |
| 24 | 6.921 | 431.1556 | 432.1629 | 99.13 | C19H28O11 | Benzyl gentiobioside |
| 25 | 6.971 | 387.1293 | 388.1365 | 98.02 | C17H24O10 | Geniposide |
| 26 | 6.984 | 326.0886 | 327.0955 | 94.43 | C14H17NO8 | Blepharin |
| 27 | 7.047 | 457.1356 | 458.1427 | 98.51 | C20H26O12 | 7-Hydroxy-4-methylphthalide O-[arabinosyl-(1->6)-glucoside] |
| 28 | 7.122 | 593.1515 | 594.1587 | 98.85 | C27H30O15 | Saponarin |
| 29 | 7.222 | 293.124 | 294.1313 | 99.71 | C12H22O8 | Ethyl 3-O-beta-D-glucopyranosyl-butanoate |
| 30 | 7.272 | 785.2499 | 786.257 | 97.39 | C35H46O20 | Echinacoside |
| 31 | 7.461 | 639.193 | 640.2006 | 93.47 | C29H36O16 | beta-Hydroxyacteoside |
| 32 | 7.511 | 563.1404 | 564.1476 | 97.59 | C26H28O14 | Luteolin 3′-methyl ether 7,4′-dixyloside |
| 33 | 7.524 | 137.0247 | 138.0319 | 99.35 | C7H6O3 | 2,5-Dihydroxybenzaldehyde |
| 34 | 7.649 | 327.1085 | 328.1158 | 99.26 | C15H20O8 | Dihydromelilotoside |
| 35 | 7.787 | 581.224 | 582.2314 | 97.41 | C28H38O13 | (+)-Lyoniresinol 9-glucoside |
| 36 | 7.925 | 383.0619 | 384.0691 | 99.1 | C16H16O11 | 2-O-Feruloylhydroxycitric acid |
| 37 | 7.95 | 621.1096 | 622.1166 | 98.47 | C27H26O17 | Genistein 4′,7-O-diglucuronide |
| 38 | 7.975 | 353.0514 | 354.0586 | 99.36 | C15H14O10 | 2-O-p-Coumaroylhydroxycitric acid |
| 39 | 8.051 | 337.0925 | 338.0999 | 99.16 | C16H18O8 | Hydrojuglone glucoside |
| 40 | 8.377 | 551.2125 | 552.2198 | 97.21 | C27 H36O12 | Prupaside |
| 41 | 8.427 | 623.1992 | 624.2061 | 98.06 | C29H36O15 | Isoacteoside |
| 42 | 8.628 | 637.2133 | 638.2204 | 97.93 | C30H38O15 | 4′-Hydroxy-5,7,2′-trimethoxyflavanone 4′-rhamnosyl-(1->6)-glucoside |
| 43 | 9.055 | 563.1037 | 564.1109 | 98.35 | C25H24O15 | Larycitrin 3-(4″-malonylrhamnoside) |
| 44 | 9.18 | 533.093 | 534.1001 | 95.67 | C24H22O14 | 2′-Hydroxygenistein 7-(6″-malonylglucoside) |
| 45 | 9.256 | 665.2085 | 666.2156 | 98.23 | C31H38O16 | Tetramethylquercetin 3-rutinoside |
| 46 | 9.381 | 503.0817 | 504.089 | 92.63 | C23H20O13 | Gomphrenol 3-methylether 4′-glucuronide |
| 47 | 9.557 | 393.1545 | 394.1624 | 88.04 | C20H26O8 | Gibberellin A43 |
| 48 | 9.633 | 431.134 | 432.1416 | 86.12 | C22H24O9 | 4′-O-Methylglucoliquiritigenin |
| 49 | 9.833 | 144.0455 | 145.0528 | 87.81 | C9H7NO | 4-formyl Indole |
| 50 | 9.934 | 291.0871 | 292.0943 | 85.16 | C15H16O6 | trans-Grandmarin |
| 51 | 10.197 | 395.2066 | 396.214 | 94.7 | C21H32O7 | Isopetasoside |
| 52 | 10.335 | 275.0919 | 276.0993 | 97.91 | C15H16O5 | 5-De-O-methyltoddanol |
| 53 | 10.36 | 213.0917 | 214.099 | 98.96 | C14H14O2 | Ethyl 1-naphthylacetic acid |
| 54 | 10.436 | 285.0403 | 286.0476 | 99.79 | C15H10O6 | Luteolin |
| 55 | 10.586 | 721.2332 | 722.2405 | 43.96 | C34 H42O17 | Amorphigenol O-vicianoside |
| 56 | 10.687 | 135.0815 | 136.0889 | 94.23 | C9H12O | 2-(1-Pentenyl)furan |
| 57 | 11.038 | 207.0662 | 208.0734 | 99.66 | C11H12O4 | 5-(3′,5′-Dihydroxyphenyl)-gamma-valerolactone |
| 58 | 11.314 | 269.0454 | 270.0527 | 98.57 | C15H10O5 | Apigenin |
| 59 | 11.402 | 299.0558 | 300.0631 | 97.59 | C16H12O6 | Diosmetin |
| 60 | 11.515 | 619.1449 | 620.1521 | 96.73 | C32H28O13 | Apigenin 7-(3″-acetyl-6″-E-p-coumaroylglucoside) |
| 61 | 12.569 | 375.1447 | 376.1519 | 83.09 | C20H24O7 | alpha-Peroxyachifolide |
| 62 | 12.946 | 223.1338 | 224.1411 | 99.62 | C13H20O3 | Methyl jasmonate |
| 63 | 13.096 | 381.0973 | 382.1046 | 97.02 | C21H18O7 | Mollicellin B |
| 64 | 13.247 | 227.0709 | 228.0782 | 98.97 | C14H12O3 | 3,4′,5-Trihydroxystilbene |
| 65 | 13.373 | 283.0606 | 284.0679 | 84.78 | C16 H12O5 | Genkwanin |
| 66 | 13.398 | 305.1752 | 306.1825 | 84.31 | C18H26O4 | Capsiate |
| 67 | 13.448 | 309.2069 | 310.2141 | 84.83 | C18H30O4 | Auxin b |
| 68 | 13.448 | 293.1755 | 294.1828 | 95.67 | C17H26O4 | Myrsinone |
| 69 | 14.076 | 273.0764 | 274.0838 | 96.81 | C15H14O5 | 6′-Hydroxy-O-desmethylangolensin |
| 70 | 14.565 | 307.1912 | 308.1984 | 84.43 | C18H28O4 | Dihydrocapsiate |
| 71 | 15.08 | 487.3423 | 488.3494 | 95.88 | C30H48O5 | 21beta-Hydroxyhederagenin |
| 72 | 15.959 | 423.1805 | 424.1877 | 97.88 | C25H28O6 | 1,7-Dihydroxy-3,6-dimethoxy-2,8-diprenylxanthone |
| 73 | 16.737 | 407.1858 | 408.193 | 98.62 | C25H28O5 | 1-Hydroxy-3,5-dimethoxy-2,4-diprenylxanthone |
The several flavonoids and flavonoid glycosides tentatively identified in AEL-sed included luteolin (Rt: 10.478 min), apigenin (Rt: 11.281 min), diosmetin (Rt: 11.432 min), saponarin (Rt: 7.114 min), scutellarein 7-glucuronosyl-(1->2)-glucuronide (Rt: 7.415 min), luteolin 3′-methyl ether 7,4′-dixyloside (Rt: 7.54 min), genistein 4′,7-O-diglucuronide (Rt: 7.917 min), baicalin (Rt: 8.884 min), diosmetin 7-O-beta-D-glucuronopyranoside (Rt: 8.971 min), tetramethylquercetin 3-rutinoside (Rt: 9.147 min) and vestitone 7-glucoside (Rt: 8.386 min). Other flavonoids, including 4′-O-methylglucoliquiritigenin, luteolin 7,3′-dimethyl ether 5-glucoside, and apigenin 7-(3″-acetyl-6″-E-p-coumaroylglucoside were also identified in AEL-sed. Aside from the polyphenolic compounds identified in AEL-sed, the UPLC-QTOF-MS data revealed the presence of several other classes of compounds, such as nucleosides (adenine guanosine and 8-hydroxyadenine), alkaloids (calystegine B5, evoxanthidine and dihydrocapsiate), coumarins (aesculin, esculetin, and osmanthuside B), and quassinoids (sergeolide).
Table 4 summarizes the tentative chemical constituents identified in AEWP-sed. Likewise, the retention times, absorbance spectra, and the data of MS were used to determine the tentative chemical composition of AEWP-sed. The results also suggested that AEWP possesses several classes of compounds, including alkaloids, terpenoids, flavonoids, and phenols, similarly to AEL-sed. The phytochemical profile of AEWP-sed looked similar to that of AEL-sed. However, the compounds such as quinic acid (Rt: 2.302), citbismine C (Rt: 2.653 min), 3′-glucosyl-2′,4′,6′-trihydroxyacetophenone (Rt: 5.904 min), geniposide (Rt: 6.971 min), 7-hydroxy-4-methylphthalide O-[arabinosyl-(1->6)-glucoside] (Rt: 7.047 min), echinacoside (Rt: 7.272 min), dihydromelilotoside (Rt: 7.649 min), (+)-lyoniresinol 9-glucoside (Rt: 7.787 min), prupaside (Rt: 8.377 min), larycitrin 3-(4″-malonylrhamnoside) (Rt: 9.055 min), 2′-hydroxygenistein 7-(6″-malonylglucoside) (Rt: 9.18 min), gomphrenol 3-methylether 4′-glucuronide (Rt: 9.381 min), trans-grandmarin (Rt: 9.934 min), isopetasoside (Rt: 10.197 min), amorphigenol O-vicianoside (Rt: 10.586 min), alpha-peroxyachifolide (Rt: 12.569 min), mollicellin B (Rt: 13.096 min), 3,4′,5-trihydroxystilbene (Rt: 13.247 min), genkwanin (13.373 min), capsiate (Rt: 13.398 min), 6′-hydroxy-O-desmethylangolensin (Rt: 14.076 min) and 21-beta-hydroxyhederagenin (Rt: 15.08 min) identified in the QTOF-MS data of AEWP-sed, were absent in AEL-sed.
3. Discussion
In this study, the leaves and bark/root of A. ebracteatus were extracted with and without using a chlorophyll removal process, and the extracts were analyzed using UHPLC-QTOF-MS for their phytochemical profiles and their antioxidant, antimicrobial and anticancer properties. Free radicals are essential by-products generated during metabolic processes by the body. However, these radicals have the ability to form complexes through ionizing radiation, leading to oxidative stress, and they further attack biological molecules such as lipids, nucleic acid, and protein [10,11]. It has been widely acknowledged that reactive oxygen species and oxidative stress are extensively implicated in the pathophysiology of several diseases that plague humankind, including diabetes, cardiovascular diseases, and cancer [12,13]. The ability of a medicinal plant extract to exert any form of bioactivity is largely dependent on the phyto-constituents present in the plant. In addition, many natural products, including plant extracts or isolated bioactive compounds have displayed several pharmacological activities linked to their potential to modulate oxidative stress and exhibit antioxidant properties [13]. As such, the antioxidant activity of an extract plays a vital role in its pharmacological effects. In view of this, the antioxidant activities of A. ebracteatus extracts were evaluated using various established techniques, namely DPPH, ABTS, FRAP, MCA, and ORAC assays. Generally, the leaves extracts (AEL-sed and AEL-nor) exhibited the highest antioxidant properties. The ability of AEL-sed to scavenge DPPH (498.80 µmol TE/g) and ABTS (67.73 µmol TE/g) radicals, as well as reduce (FRAP: 1113.20 µmol TE/g) or chelate (MCA: 47.83 µmol EDTA/g) metal ions was of a greater extent compared to the extract from the stem/root. In a previous study, the ethanolic extract of A. ebracteatus was shown to exert DPPH (IC50: 0.12 ± 0.03 mg/L)-scavenging activity [9].
The results obtained from the total phenolics and flavonoids content indicated that AEL was rich in total phenolics (140.5 and 138.2 mg GAE/g for AEL-sed and AEL-nor, respectively) and flavonoids (110.4 and 107.6 mg CE/g for AEL-sed and AEL-nor, respectively). Earlier studies have indicated the presence of high levels of phenolics and flavonoids in A. ebracteatus [6]. The results obtained from our study confirmed the presence of phenolics and flavonoids. However, the TPC and TFC contents reported in our study were markedly higher [6]. The disparity in the phenolic and flavonoid contents may be attributed to the differences in plant origin, growth conditions, extraction methods, and the solvent employed for extraction.
Several bioactive molecules were detected in the A. ebracteatus extracts, including baicalin, apigenin, luteolin, glucocaffeic acid, caffeic acid, aesculin, diosmetin, genkwanin, saponarin, and hydrojuglone glucoside. These identified compounds could be responsible for the observed antioxidant properties since previous reports illustrated the antioxidant potential of these compounds through several mechanism in in vitro and in vivo models [12,14,15,16]. Prasansuklab and Tencomnao [6] reported the antioxidant potential of A. ebracteatus extract and suggested that its protective effects against oxidative stress injury were attributed to the presence of polyphenolic compounds in the extract (verbasoside, leucosceptoside A, isoverbascoside, and Vicenin-2). Furthermore, Ilori et al. [17] noted that polyphenolic compounds, such as verbascoside, leucosceptoside A, martynoside, β-hydroxyacteoside, pteleifoside G, magnolenin C, vecenin-2, shaftoside, luteolin-7-O-β-d glucuronide, and apigenin, which have several reported therapeutic activities such as antimicrobial, anticancer, wound healing, anti-inflammatory, anti-hair loss, and antioxidant properties, were reported to be present in A. ebracteatus [8,17].
The antimicrobial properties of the A. ebracteatus extracts could be obviously related to their high polyphenolic constituents. Pratoomsoot et al. [9] previously reported that extracts from A. ebracteatus showed significant antimicrobial activity against the A. baumannii DMST 10437, E. coli 4212, S. aureus DMST 8840, methicillin-resistant S. aureus DMST, S. epidermidis DMST 3547, S. epidermidis DMST 4343, and S. pyogenes DMST 30563 strains. The importance of controlling bacterial infections cannot be over-emphasized due to their prevailing negative effects in primary health care as well as the complications that arise from bacterial infections related to other diseases. An increasing number of reports illustrate the importance of medicinal plants in the treatment of bacterial infections [18]. The results indicated that A. ebracteatus extract showed significant antibacterial properties.
Cancer is a major cause of death globally and, unfortunately, there is no known cure for this dreaded disease [19]. As such, finding a cost-effective, alternate, and safer treatment for cancer is warranted. Medicinal plants have gained attention as alternative chemopreventive and therapeutic agents in recent years. In fact, numerous anticancer agents presently approved for cancer treatment or undergoing clinical trials as possible anticancer drugs have direct links to medicinal plants and are building blocks for the emergence of some synthetic anticancer agents [20]. Oral carcinogenesis is a multistep process that includes genetic events which lead to the disruption of the normal regulatory pathways that control cellular functions [21]. Oropharyngeal cancer and its treatment via chemotherapy causes several complications, including dysphagia, mucositis, pain, related infections, and bleeding [22]. Similar to chemotherapeutic agents, natural products such as phenethyl isothiocyanate [23], resveratrol [24], and curcumin [25] have been reported to have excellent anticancer efficacy with no or minimal side effects. The results from our study suggested that AEL-sed showed reasonable anticancer effects. Several phytochemicals, such as diosmetin, esculetin, isoacteoside, baicalin, isoamericanol A, luteolin, apigenin, and genkwanin, among several others identified in the extract, have been reported as promising anticancer agents in several in vitro and in vivo studies [26,27,28,29,30,31,32,33,34]. Therefore, A. ebracteatus extract contains several constituents with promising bioactivities that could be beneficial for the treatment of several disorders.
4. Materials and Methods
4.1. Chemicals and Reagents
Dimethyl sulfoxide and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich Corp., (St. Louis, MO, USA). Fetal bovine serum was procured from Biochrom GmbH (Berlin, Germany). RPMI-1640, phosphate buffer saline, and penicillin/streptomycin (U/mL) were purchased from PAA Laboratories GmbH (Pasching, Austria). 2′,7′-dichlorodihydrofluorescein diacetate, 0.25 % trypsin-EDTA, and stable L-glutamine were purchased from Gibco Life Technologies (Carlsbad, CA, USA). Phenotype oral squamous carcinoma cells (CLS-354/WT) were obtained from the Research Institute for Health Sciences, School of Allied Health Sciences, Walailak University, Nakhon Si Thammarat, Thailand. All other chemicals used were of analytical grade and used as purchased.
4.2. Plant Material
The leaves, stem and root of A. ebracteatus were collected from Surat Thani Province, Thailand. The plant was authenticated at the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. The samples were powdered with a mechanical grinder (Jing Gongyi, JGY-800B, Yongkang, China) to fine particles and the powdered leaves and stem/roots were divided into two equal portions and extracted separately.
4.3. Preparation of A. ebracteatus Extracts
4.3.1. Classical Ethanol Extraction
The powdered leaves (200 g) and stem/roots (200 g) were extracted with 2 L of 70% ethanol at a solvent/solid ratio of 10:1 (v/w) on a shaker for 24 h. Subsequently, the extraction mixture was filtered, and the resulting filtrate was dried under reduced pressure with a rotary evaporator at 45 °C. The dried extract of the leaves (AEL-nor) and back/root (AEWP-nor) were stored at 4 °C until further use.
4.3.2. Extraction Using the Sedimentation Method
Likewise, 200 g of the powdered leaves and 200 g of the powdered stem/roots were subjected to 70% ethanol extraction at a solvent/solid ratio of 10:1 (v/w) on a shaker for 24 h. Thereafter, the solution obtained after filtration was concentrated to 30% of the initial volume, and the mixture was refrigerated at 4 °C for 24 h to sediment. Thereafter, the solution was decanted, and the top layer (without chlorophyll) was centrifuged (6000 rpm, 30 min at 4 °C). The supernatants obtained from the leaves extract (AEL-sed) and the stem/roots (AEWP-sed) were freeze-dried and stored until further use [35,36].
4.4. Total Phenolic and Flavonoid Content
The TPC and TFC of the extracts were determined based on previously reported protocol [37,38]. The TPC of the extracts was spectrophotometrically determined using the Folin–Ciocalteu method. Briefly, 0.1 mL of the extracts were added to 0.75 mL of 10% Folin–Ciocalteu reagent, and the mixture was allowed to stand for 5 min. Subsequently, 0.75 mL of a saturated solution of Na2CO3 was added, and the mixtures were incubated at room temperature for 3 h, while shaking randomly. Thereafter, the absorbance of the blue-colored solution was measured at 760 nm. TPC was expressed as mg gallic acid equivalent (GE)/g dry extract.
For the analysis of the TFC of the extracts, 800 µL of distilled water was mixed with 200 µL of the extract solution, 60 µL of 5% NaNO2 solution, and 60 µL of 10% AlCl3 solution. The mixture was allowed to stand for 5 min at room temperature and thereafter 400 µL of 1M NaOH solution was added. The mixture was made up to a volume of 2 mL with distilled water and thoroughly mixed. The absorbance of the solution was measured at 510 nm. TFC was calculated from the standard curve of catechin and expressed as mg catechin equivalent (CE)/g extract.
4.5. Antioxidant Activity
Measurements of the DPPH radical-scavenging activity (DPPH-RSA), ABTS radical-scavenging activity (ABTS-RSA), metal chelating activities (MCA), ferric reducing antioxidant power (FRAP), and oxygen radical antioxidant capacity (ORAC) of the extracts were performed using previously reported methods [37,38].
For ABTS-RSA, the stock solutions included 7.4 mM ABTS solution and 2.6 mM potassium persulfate solution. The working solution was prepared by mixing the two stock solutions in equal quantities. The mixture was allowed to react for 12 h at room temperature in the dark. The solution obtained (1 mL) was then diluted with 50 mL of distilled water to obtain an absorbance of 1.10 ± 0.02 units at 734 nm. The sample (150 μL) was mixed with 2850 μL of ABTS solution, and the mixture was left at room temperature for 1 h in the dark. The absorbance was then measured at 734 nm using a spectrophotometer. The blank was prepared in the same manner, except that distilled water was used instead of the sample. A standard curve of Trolox ranging from 50–600 μM was prepared. The activity was expressed as μmol Trolox equivalent (TE)/g solid.
The extracts sample (0.3 mL) was mixed with 2.7 mL of a methanolic solution containing DPPH (0.15 mM). The mixture was shaken vigorously and left to stand for 60 min in the dark (until stable absorption values were obtained) at room temperature (25 °C). The reduction of the DPPH-RSA was measured by continuously monitoring the decrease in absorbance at 517 nm. The DPPH scavenging activity was expressed as μmol Trolox equivalent (TE)/g solid.
The FRAP reagent was prepared by mixing acetate buffer (30 mM, pH 3.6) and 10 mM TPTZ solution in a 40 mM HCl and 20 mM iron (III) chloride solution in proportions of 10:1:1 (v/v). The sample solution (150 µL) was mixed with 2.85 mL of working FRAP reagent and incubated in dark conditions at room temperature for 30 min. The absorbance of the reaction mixture was read at 593 nm. The standard curve was prepared using Trolox ranging from 0–500 µM. The activity was expressed as µmol Trolox equivalent (TE)/g sample.
For MCA, 1 mL of extract was mixed with 3.7 mL of distilled water and the mixture was reacted with 0.1 mL of 2 mM FeCl2 and 0.2 mL of 5 mM ferrozine for 20 min. The absorbance was read at 532 nm. One milliliter of distilled water instead of the extract was used as a control. The chelating activity was expressed as μmol EDTA equivalent (EE)/g solid.
4.6. Antibacterial Activity
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) measurements of the extracts were performed against Listeria monocytogenes and Escherichia coli 0157, using the previously reported protocol [39].
4.7. Anticancer Efficacy Compounds
The anticancer efficacy of the extracts was tested against epithelium-like phenotype oral squamous carcinoma cell (CLS-354/WT) by an indirect method [40]. Briefly, carcinoma cells were cultured in RPMI-1640 supplemented with 10% fetal bovine serum, 1% v/v penicillin/streptomycin (U/mL), and 2mM stable ʟ-glutamine. Approximately, 1 × 104 (cells/mL) cells were seeded in 96-well plates and incubated in an incubator with 5% CO2 at 37 °C. The cells were allowed to form a 70% confluent monolayer and treated with the extract (1600–12.5 μg/mL) and supplemented fresh RPMI-1640 as a negative control, in triplicate. The percentage of cell death was analyzed using MTT assay. The insoluble formazan crystals were solubilized with 99.9% DMSO, and the absorbance was measured at 560 nm using a multi-mode plate reader (BioTek, Winooski, VT, USA). The percentage of cell death was calculated.
4.8. Anti-Proliferative Effect of Extract
The in vitro scratch assay was evaluated to quantify the anti-migration capabilities of cells treated with the extracts. Briefly, CLS-354/WT cells were seeded at a cell density at 3 x 104 cells/well in a 6-well plate. The confluent monolayer (70%) of the cells was scratched using a sterile pipette tip to create a wound of 1 mm width. Subsequently, the cells were washed with phosphate buffer (pH 7.4) to remove cellular debris and replaced with a fresh medium containing the extract above ED50 (50 % inhibition of cancer cell growth), or with RPMI-1640 medium as a negative control. Images of cell migration were captured at 0 and 24 h using a Carl Zeiss microscope Axio Vert. A1 (Konigsallee, Gottingen, Germany). The residual gap between the migrating cells was measured using Image J software (1.8.0_172).
4.9. UHPLC-ESI-QTOF-MS Profiling of the Extracts
The extracts (AEL-sed and AEWP-sed) with significant antioxidant and antimicrobial activities were selected for LCMS profiling. The experimental procedures and instrumental parameters were previously described by Eze and Tola [41]. The analysis was performed using an Agilent 1290 Infinity II LC System (Agilent Technologies, Santa Clara, CA, USA) equipped with an autosampler, a binary pump, a vacuum degasser, and a diode array detector. The extracts were separated on Agilent’s ZORBAX Eclipse Plus C18 column (150 × 2.1 mm, 1.8 µmm). The mobile phases consisted of (A) acidified Milli-Q water (0.1% formic acid) and (B) acetonitrile. The following parameters were employed for the elution: 0.50 min: 0% B; 16.50 min: 100% B; 17.50 min: 100% B; 20.00 min: 0.00% B; 22.00 min: 0.00% B; injection volume of 2.0 mL, flow rate of 0.2 µL min−1, and column temperature of 25 °C. The HPLC system was coupled to an Agilent 6545 LC/Q-TOF MS mass spectrometer equipped with a dual Agilent Jet Stream ESI negative mode, with a mass range of m/z 100 to 1500 at a scan rate of 1.00 spectrum per second. Accurate mass measurements by the instrument were ensured using an automated calibrant delivery system that continuously introduced a reference solution with a mass mix of m/z 112.985587 (TFA anion) to m/z 1033.988109 (HP-0921) in the ESI-negative mode, while a mass mix of m/z 121.050873 (purine) and m/z 922.009798 (HP-0921) were introduced in the ESI-positive mode. The parameters set for ESI-MS included: drying gas temperature: 325 °C; drying gas flow rate: 13 L minˉ1; nebulizer gas pressure: 35 psig; capillary voltage: 4000 V; fragmentor voltage: 175 V; radiofrequency voltage in the octupole: 750 V, and fixed collision energies of 10.00 eV, 20.00 eV and 40.00 eV. Data acquisition was performed on Mass Hunter Workstation Software Data Acquisition for Q-TOF, version B.08.00 (B8058.3 SP1) and QTOF Firmware, version 20.712.
4.10. Statistical Analysis
The results were expressed as mean ± standard error. Statistical analysis was determined by one-way ANOVA followed by Dunnett’s test using GraphPad Prism 6 (GraphPad Software Inc., La Jolla, CA, USA). Differences of p < 0.05 were considered significant.
5. Conclusions
In conclusion, the results from this study suggested that A. ebracteatus displayed promising antioxidant, antibacterial and anticancer activities. The leaves of the plant showed better activity in all the tested assays when compared to other extracts. Furthermore, UPLC-ESI-QTOF-MS analysis indicated that the plant is rich in polyphenolic compounds, including phenolic acids, flavonoids, iridoids, and o-glycosyl compounds. These results suggested that A. ebracteatus can be explored as a possible nutraceutical for the treatment of oxidative stress-related disorders. Further studies are needed to validate the in vivo pharmacological and activities, especially in unexplored and valuable aspects of A. ebracteatus.
Author Contributions
Conceptualization, O.J.O. and C.O.; methodology, O.O.O., T.J.J. and S.S. (Sudarshan Singh); formal analysis, O.O.O., S.S. (Sudarshan Singh) and O.J.O.; investigation, O.O.O., S.S. (Sudarshan Singh), S.N. and S.S. (Sasikarn Sripetthong); resources, W.C. and C.O.; data curation, O.O.O., S.S. (Sudarshan Singh) and O.J.O.; writing—original draft preparation, S.S. (Sudarshan Singh) and O.J.O.; writing—review and editing, C.O. and O.J.O.; supervision, C.O. and O.J.O.; project administration, C.O. and O.J.O.; funding acquisition, C.O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors are grateful for the grant support from Sura Thani Provincial Health Office (Contract No.361/2564.)
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Makinde E.A., Radenahmad N., Adekoya A.E., Olatunji O.J. Tiliacora triandra extract possesses antidiabetic effects in high fat diet/streptozotocin-induced diabetes in rats. J. Food Biochem. 2020;44:e13239. doi: 10.1111/jfbc.13239. [DOI] [PubMed] [Google Scholar]
- 2.Sinan K.I., Cádiz-Gurrea M.D.L.L., Leyva-Jiménez F.J., Fernández-Ochoa Á., Segura-Carretero A., Glamocilija J., Sokovic M., Nenadić M., Aktumsek A., Dall’Acqua S., et al. New insights on Phyllanthus reticulatus Poir. leaves and stem bark extracts: UPLC-ESI-TOF-MS profiles, and biopharmaceutical and in silico analysis. New J. Chem. 2021;45:21049–21065. doi: 10.1039/D1NJ03621A. [DOI] [Google Scholar]
- 3.Salehi B., Azzini E., Zucca P., Maria Varoni E., Anil Kumar N.V., Dini L., Panzarini E., Rajkovic J., Valere Tsouh Fokou P., Peluso I., et al. Plant-derived bioactives and oxidative stress-related disorders: A key trend towards healthy aging and longevity promotion. Appl. Sci. 2020;10:947. doi: 10.3390/app10030947. [DOI] [Google Scholar]
- 4.Forni C., Facchiano F., Bartoli M., Pieretti S., Facchiano A., D’Arcangelo D., Norelli S., Valle G., Nisini R., Beninati S., et al. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. BioMed Res. Int. 2019;2019:8748253. doi: 10.1155/2019/8748253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somchaichana J., Bunaprasert T., Patumraj S. Acanthus ebracteatusVahl. Ethanol Extract Enhancement of the Efficacy of the Collagen Scaffold in Wound Closure: A Study in a Full-Thickness-Wound Mouse Model. J. Biomed. Biotechnol. 2012;2012:1–8. doi: 10.1155/2012/754527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prasansuklab A., Tencomnao T. Acanthus ebracteatus leaf extract provides neuronal cell protection against oxidative stress injury induced by glutamate. BMC Complement. Altern. Med. 2018;18:278. doi: 10.1186/s12906-018-2340-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li M.-Y., Xiao Q., Pan J.-Y., Wu J. Natural products from semi-mangrove flora: Source, chemistry and bioactivities. Nat. Prod. Rep. 2009;26:281–298. doi: 10.1039/B816245J. [DOI] [PubMed] [Google Scholar]
- 8.Wisuitiprot V., Ingkaninan K., Chakkavittumrong P., Wisuitiprot W., Neungchamnong N., Chantakul R., Waranuch N. Effects of Acanthus ebracteatus Vahl. extract and verbascoside on human dermal papilla and murine macrophage. Sci. Rep. 2022;12:1–13. doi: 10.1038/s41598-022-04966-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pratoomsoot C., Wongkattiya N., Sanguansermsri D. Synergistic Antimicrobial and Antioxidant Properties of Coccinia grandis (L.) Voigt, Clerodendrum inerme (L.) Gaertn. and Acanthus ebracteatus Vahl. Extracts and Their Potential as a Treatment for Xerosis Cutis. Complement. Med. Res. 2020;27:410–420. doi: 10.1159/000507606. [DOI] [PubMed] [Google Scholar]
- 10.Awadelkareem A.M., Al-Shammari E., Elkhalifa A.E.O., Adnan M., Siddiqui A.J., Snoussi M., Khan M.I., Azad Z.R.A.A., Patel M., Ashraf S.A. Phytochemical and In Silico ADME/Tox Analysis of Eruca sativa Extract with Antioxidant, Antibacterial and Anticancer Potential against Caco-2 and HCT-116 Colorectal Carcinoma Cell Lines. Molecules. 2022;27:1409. doi: 10.3390/molecules27041409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physio-logical functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Eze F.N., Jayeoye T.J. Chromolaena odorata (Siam weed): A natural reservoir of bioactive compounds with potent an-ti-fibrillogenic, antioxidative, and cytocompatible properties. Biomed. Pharmacother. 2021;141:111811. doi: 10.1016/j.biopha.2021.111811. [DOI] [PubMed] [Google Scholar]
- 13.Song P., Sun C., Li J., Long T., Yan Y., Qin H., Makinde E.A., Famurewa A.C., Jaisi A., Nie Y., et al. Tiliacora triandra extract and its major constituent attenuates diabetic kidney and testicular impairment by modulating redox imbalance and pro-inflammatory responses in rats. J. Sci. Food Agric. 2021;101:1598–1608. doi: 10.1002/jsfa.10779. [DOI] [PubMed] [Google Scholar]
- 14.Zafirah A., Shiou A., Lee Y., Mohamed R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017;10:37–44. [Google Scholar]
- 15.Wang C., Liao Y., Wang S., Wang D., Wu N., Xu Q., Jiang W., Qiu M., Liu C. Cytoprotective effects of diosmetin against hydrogen peroxide-induced L02 cell oxidative damage via activation of the Nrf2-ARE signaling pathway. Mol. Med. Rep. 2018;17:7331–7338. doi: 10.3892/mmr.2018.8750. [DOI] [PubMed] [Google Scholar]
- 16.Wang X., Yu J.-Y., Sun Y., Wang H., Shan H., Wang S. Baicalin protects LPS-induced blood–brain barrier damage and activates Nrf2-mediated antioxidant stress pathway. Int. Immunopharmacol. 2021;96:107725. doi: 10.1016/j.intimp.2021.107725. [DOI] [PubMed] [Google Scholar]
- 17.Ilori N.T.O., Liew C.X., Fang C.M. The anti-inflammatory properties of Acanthus ebracteatus, Barleria lupulina and Clinacanthus nutans: A systematic review. Mol. Biol. Rep. 2020;47:9883–9894. doi: 10.1007/s11033-020-06025-x. [DOI] [PubMed] [Google Scholar]
- 18.Limsuwan S., Jarukitsakul S., Issuriya A., Chusri S., Joycharat N., Jaisamut P., Saising J., Jetwanna K.W.-N., Voravuthikunchai S.P. Thai herbal formulation ‘Ya-Pit-Samut-Noi’: Its antibacterial activities, effects on bacterial virulence factors and in vivo acute toxicity. J. Ethnopharmacol. 2020;259:112975. doi: 10.1016/j.jep.2020.112975. [DOI] [PubMed] [Google Scholar]
- 19.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 20.Adnan M., Siddiqui A.J., Hamadou W.S., Patel M., Ashraf S.A., Jamal A., Awadelkareem A.M., Sachidanandan M., Snoussi M., De Feo V. Phytochemistry, Bioactivities, Pharmacokinetics and Toxicity Prediction of Selaginella repanda with Its Anticancer Potential against Human Lung, Breast and Colorectal Carcinoma Cell Lines. Molecules. 2021;26:768. doi: 10.3390/molecules26030768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams H.K. Molecular pathogenesis of oral squamous carcinoma. Mol. Pathol. 2000;53:165–172. doi: 10.1136/mp.53.4.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thirayan V., Jameson M.B., Gregor R.T. Prophylactic versus reactive percutaneous endoscopic gastrostomy in oro-pharyngeal squamous cell carcinoma patients undergoing radical radiotherapy. N. Z. J. Surg. 2021;91:2720–2725. doi: 10.1111/ans.17159. [DOI] [PubMed] [Google Scholar]
- 23.Shoaib S., Tufail S., Islam N. Phenethyl isothiocyanate induces apoptosis through ROS generation and caspase-3 activation in cervical cancer cells. Front. Pharmacol. 2021;12:1651. doi: 10.3389/fphar.2021.673103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Da Costa P.S., Ramos P.S., Ferreira C., Silva J.L., El-Bacha T., Fialho E. Pro-Oxidant Effect of Resveratrol on Human Breast Cancer MCF-7 Cells is Associated with CK2 Inhibition. Nutr. Cancer. 2021:1–10. doi: 10.1080/01635581.2021.1977834. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X., Yang X., Lin J., Song F., Shao Y. Low curcumin concentration enhances the anticancer effect of 5-fluorouracil against colorectal cancer. Phytomedicine. 2021;85:153547. doi: 10.1016/j.phymed.2021.153547. [DOI] [PubMed] [Google Scholar]
- 26.Ning R., Chen G., Fang R., Zhang Y., Zhao W., Qian F. Diosmetin inhibits cell proliferation and promotes apoptosis through STAT3/c-Myc signaling pathway in human osteosarcoma cells. Biol. Res. 2021;54:40. doi: 10.1186/s40659-021-00363-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duan J., Shi J., Ma X., Xuan Y., Li P., Wang H., Fan Y., Gong H., Wang L., Pang Y., et al. Esculetin inhibits proliferation, migration, and invasion of clear cell renal cell carcinoma cells. Biomed. Pharmacother. 2020;125:110031. doi: 10.1016/j.biopha.2020.110031. [DOI] [PubMed] [Google Scholar]
- 28.Wu S.-T., Liu B., Ai Z.-Z., Hong Z.-C., You P.-T., Wu H.-Z., Yang Y.-F. Esculetin Inhibits Cancer Cell Glycolysis by Binding Tumor PGK2, GPD2, and GPI. Front. Pharmacol. 2020;11:379. doi: 10.3389/fphar.2020.00379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X., Guo F., Peng Q., Liu Y., Yang B. Suppression of in vitro and in vivo human ovarian cancer growth by isoac-teoside is mediated via sub-G1 cell cycle arrest, ROS generation, and modulation of AKT/PI3K/m-TOR signalling pathway. J. BUON. 2019;24:285–290. [PubMed] [Google Scholar]
- 30.Zhao F., Zhao Z., Han Y., Li S., Liu C., Jia K. Baicalin suppresses lung cancer growth phenotypes via miR-340-5p/NET1 axis. Bioengineering. 2021;12:1699–1707. doi: 10.1080/21655979.2021.1922052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katagi A., Sui L., Kamitori K., Suzuki T., Katayama T., Hossain A., Noguchi C., Dong Y., Yamaguchi F., Tokuda M. Inhibitory effect of isoamericanol A from Jatropha curcas seeds on the growth of MCF-7 human breast cancer cell line by G2/M cell cycle arrest. Heliyon. 2016;2:e00055. doi: 10.1016/j.heliyon.2015.e00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imran M., Rauf A., Abu-Izneid T., Nadeem M., Shariati M.A., Khan I.A., Imran A., Orhan I.E., Rizwan M., Atif M., et al. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019;112:108612. doi: 10.1016/j.biopha.2019.108612. [DOI] [PubMed] [Google Scholar]
- 33.Imran M., Aslam Gondal T., Atif M., Shahbaz M., Batool Qaisarani T., Hanif Mughal M., Salehi B., Martorell M., Sharifi-Rad J. Apigenin as an anticancer agent. Phytother. Res. 2020;34:1812–1828. doi: 10.1002/ptr.6647. [DOI] [PubMed] [Google Scholar]
- 34.Wang X., Song Z.-J., He X., Zhang R.-Q., Zhang C.-F., Li F., Wang C.-Z., Yuan C.-S. Antitumor and immunomodulatory activity of genkwanin on colorectal cancer in the APC Min/+ mice. Int. Immunopharmacol. 2015;29:701–707. doi: 10.1016/j.intimp.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y., Liu C., Song X., An M., Liu M., Yao L., Famurewa A.C., Olatunji O.J. Antioxidant and Anti-inflammatory Properties Mediate the Neuroprotective Effects of Hydro-ethanolic Extract of Tiliacora triandra Against Cisplatin-induced Neurotoxicity. J. Inflamm. Res. 2021;14:6735–6748. doi: 10.2147/JIR.S340176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Olatunde O.O., Della Tan S.L., Shiekh K.A., Benjakul S., Nirmal N.P. Ethanolic guava leaf extracts with different chlorophyll removal processes: Anti-melanosis, antibacterial properties and the impact on qualities of Pacific white shrimp during refrigerated storage. Food Chem. 2021;341:128251. doi: 10.1016/j.foodchem.2020.128251. [DOI] [PubMed] [Google Scholar]
- 37.Olatunde O.O., Benjakul S., Vongkamjan K., Amnuaikit T. Liposomal Encapsulated Ethanolic Coconut Husk Extract: Antioxidant and Antibacterial Properties. J. Food Sci. 2019;84:3664–3673. doi: 10.1111/1750-3841.14853. [DOI] [PubMed] [Google Scholar]
- 38.Olatunde O.O., Benjakul S., Vongkamjan K. Antioxidant and antibacterial properties of guava leaf extracts as affected by solvents used for prior dechlorophyllization. J. Food Biochem. 2018;42:e12600. doi: 10.1111/jfbc.12600. [DOI] [Google Scholar]
- 39.Odedina G.F., Vongkamjan K., Voravuthikunchai S.P. Potential Bio-Control Agent from Rhodomyrtus tomentosa against Listeria monocytogenes. Nutrients. 2015;7:7451–7468. doi: 10.3390/nu7095346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Utaipan T., Boonyanuphong P., Chuprajob T., Suksamrarn A., Chunglok W. A trienone analog of curcumin, 1,7-bis(3-hydroxyphenyl)-1,4,6-heptatrien-3-one, possesses ROS- and caspase-mediated apoptosis in human oral squa-mous cell carcinoma cells in vitro. Appl. Biol. Chem. 2020;63:7. doi: 10.1186/s13765-020-0491-8. [DOI] [Google Scholar]
- 41.Eze F.N., Tola A.J. Protein glycation and oxidation inhibitory activity of Centella asiatica phenolics (CAP) in glucose-mediated bovine serum albumin glycoxidation. Food Chem. 2020;332:127302. doi: 10.1016/j.foodchem.2020.127302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.