Background:
In the context of headache surgery, greater occipital nerve (GON) transection is performed when the nerve appears severely damaged, if symptoms are recurrent or persistent, and when neuromas are excised. Lesser occipital nerve (LON) excision is commonly performed during the primary decompression surgery. Advanced techniques to address the proximal nerve stump after nerve transection such as regenerative peripheral nerve interface (RPNI), targeted muscle reinnervation (TMR), relocation nerve grafting, and reset neurectomy have been shown to improve chronic pain and neuroma formation. These techniques have not been described in the head and neck region.
Methods:
This article describes RPNI, TMR, and reset neurectomy with GON autograft relocation to prevent chronic pain and neuroma formation after GON/LON transection.
Results:
RPNI and TMR are feasible options in patients undergoing GON/LON transection. Further, relocation nerve grafting with GON autograft relocation is a method that is beneficial in patients with diffuse nerve injury requiring proximal nerve division.
Conclusion:
Advanced nerve reconstruction techniques should be considered in headache surgery following GON/LON transection.
Takeaways
Question: Which surgical techniques can be used to address the proximal nerve stump after transection of the greater and lesser occipital nerves.
Findings: RPNI, TMR, and relocation nerve grafting are methods that can be considered in patients undergoing GON/LON transection.
Meaning: Advanced techniques to address the proximal nerve stump after nerve transection are available in the head and neck region after transection of sensory nerves.
INTRODUCTION
The anatomic basis, patient screening, technical details, and outcomes of greater occipital nerve (GON) and lesser occipital nerve (LON) decompression for neuropathic pain have been described in detail.1–8 In general, most headache surgery experts agree that decompression of the GON should be the first step in treatment of patients with GON pain. If primary decompression fails, most experienced surgeons will offer secondary transection of the GON.9 A minority of surgeons will consider GON excision during the first surgery if the nerve appears severely damaged (yellow discoloration, absence of vasa vasorum, and presence of significant scarring).10 Another clinical indication for GON neurectomy is patients presenting with painful neuromas of the GON after unintended iatrogenic or traumatic injury that require neuroma excision. On the contrary, the LON is considered a small sensory nerve with small innervation pattern, and transection is commonly performed during the initial surgery.11 Although GON and LON transection has been discussed in a small number of publications, limited information on techniques to address the proximal nerve stump is available.9
After transection of peripheral nerves, axons regenerate and grow in an unorganized fashion, resulting in formation of neuromas at the nerve stump.12 Symptomatic neuromas cause severe neuropathic pain, leading to significant morbidity for patients. Given the risk of formation of painful neuromas, surgical techniques to prevent unorganized growth of axons have been employed to reconstruct the proximal nerve stump.
Traditionally, burial of nerves in the soft tissues and bone was the preferred technique to reconstruct proximal nerve ends (Fig. 1A).13,14 However, this technique has yielded mixed clinical results. Therefore, newer techniques to prevent neuroma formation and chronic pain have been described and investigated.13,15–21
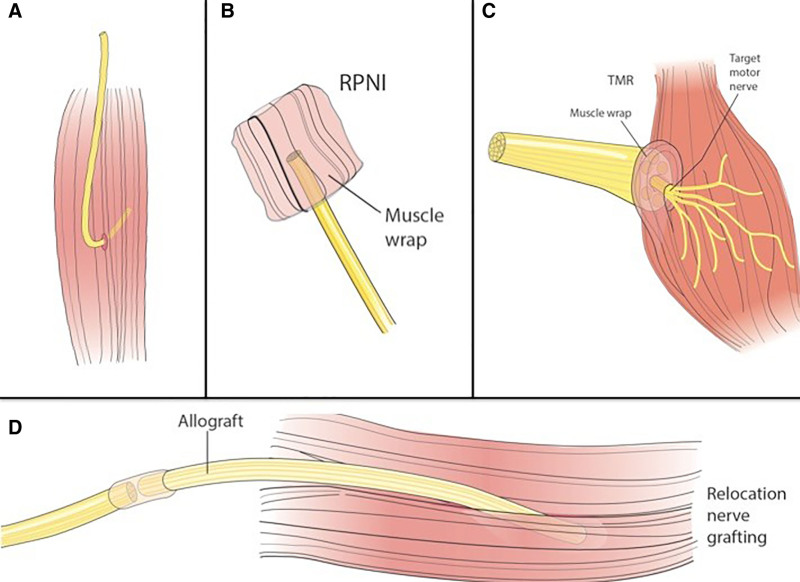
Fig. 1.
Nerve reconstruction techniques after nerve transection. Several different techniques for nerve reconstruction have been described, including muscle burial (A), RPNI (B), TMR (C), and relocation nerve grafting (D).
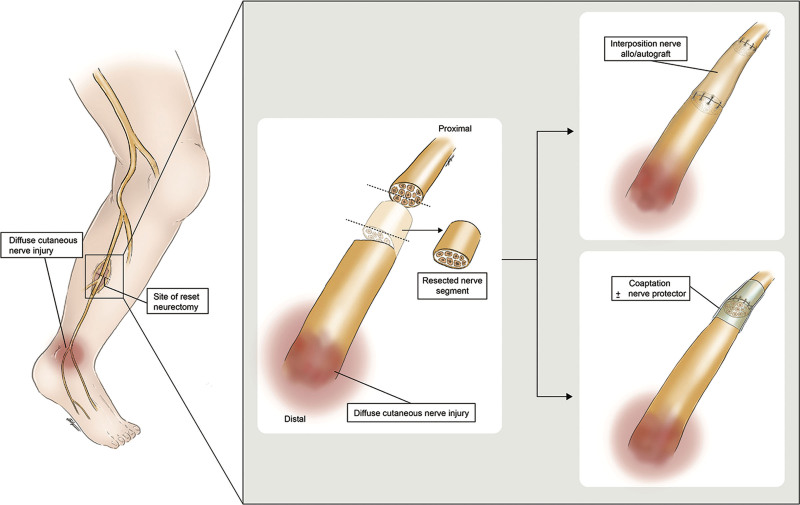
Several procedures have shown great promise in prevention of chronic pain and neuroma in both mixed motor/sensory and pure sensory nerves.13,15–21 Regenerative peripheral nerve interface (RPNI) and targeted muscle reinnervation (TMR) techniques direct axonal growth into target muscles to prevent unorganized axonal sprouting and neuroma formation. RPNI is a procedure in which the transected proximal nerve stump is inserted into a denervated muscle cuff, which allows for targeted axonal ingrowth (Fig. 1B).20,22 Similarly, TMR is a surgical technique that connects the proximal nerve stump to a muscle motor nerve branch allowing for directed axonal growth (Fig. 1C).18,23,24 A combination of TMR with vascularized RPNI has also been described.15 Another employed technique is relocation nerve grafting (Fig. 1D).25 After nerve transection, a long autograft or allograft is used for coaptation of the proximal nerve stump to a muscle remote from the zone of injury. In principle, this allows axons to regenerate along a long graft, with only few axons reaching the end target (muscle). In addition, reset neurectomy has been discussed in the context of diffuse nerve injury when no discrete zone of injury can be identified (Fig. 2).26 The nerve is transected proximal to the area of pain with immediate coaptation to the distal nerve end with or without nerve graft. This eliminates afferent nerve signals and allows the nerve to regenerate along the native nerve, which has become a “graft.” To our knowledge, the concept of reset neurectomy has not been combined with relocation nerve grafting.
Fig. 2.
Reset neurectomy.
In the context of headache surgery, the most common method to address the proximal nerve stump after GON/LON transection remains burial in muscle. No other techniques have been described in detail in this context. The aim of this article is to describe and prove the feasibility of RPNI/TMR following GON/LON transection in headache surgery. Further, we describe a novel technique combining reset neurectomy with GON autograft relocation to address the transected proximal GON stump.
SURGICAL TECHNIQUES TO ADDRESS THE PROXIMAL NERVE STUMP AFTER NERVE TRANSECTION OR EXCISION OF NEUROMAS
Case Report One: RPNI
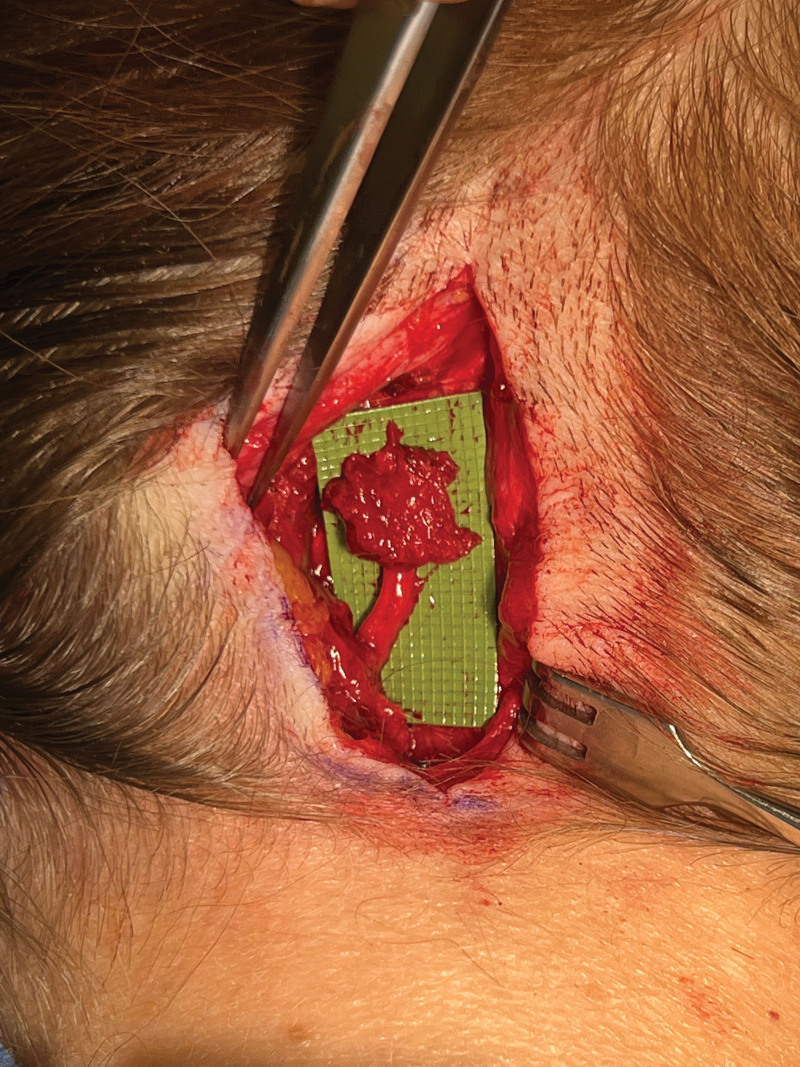
This 62-year-old woman underwent left GON and LON decompression 1 year before. At the time of primary GON decompression, her nerve was found to be compressed by trapezius fascia with absent vasa vasorum and yellow discoloration of the nerve. Her pain initially improved, but then returned at around 7 months postoperatively. She had a total improvement in symptoms of 40% from baseline. Given her persistent symptoms with exquisite pain over the GON exit point from the semispinalis muscle 3 cm distal to the occipital protuberance and 1.5 cm lateral to the midline that improved completely for 48 hours after nerve block, the decision was made to proceed with GON exploration and possible transection. The patient was informed about the side effects (permanent numbness of the occiput) and risks (importantly paresthesia and formation of painful neuroma) prior to surgery. Preoperatively, the area of maximum pain was marked. A 5 cm incision was made distal to the occipital protuberance at the site of primary decompression. The incision was carried down through the dermis to the midline raphe where the nerve was encased in a fat pad that was elevated and wrapped around the nerve at the time of primary decompression. The nerve was found distal to the fat pad and traced proximally. The GON was evaluated to determine whether there were specific regions of the nerve that showed macroscopic signs of damage. The GON was transected proximal to the maximum point of pain and proximal to macroscopic nerve damage. A small rectangular muscle cuff of semispinalis capitis muscle was harvested and wrapped around the GON as an RPNI (Fig. 3). The incision was closed in a standard fashion.6
Fig. 3.
RPNI. A small rectangular segment of semispinalis capitis muscle was harvested and wrapped around the GON stump. The muscle envelope was closed at the distal end with 3.0 Monocryl suture. Another 4.0 Monocryl suture was passed through the muscle and epineurium at the proximal RPNI end to keep the RPNI in place.
Case Report Two: TMR
This 65-year-old woman suffered from right-sided occipital pain for 40 years. She initially developed pain after resection of a malignant tumor of the scalp. She underwent multiple reconstructive procedures after tumor resection, including skin grafting, tissue expander placement, and scalp flap advancement. Subsequent to these procedures, she developed severe pain in the occipital region on the right. The pain was constant. Her pain sketch showed a typical GON radiation pattern on the right.27 On examination, she had severe pain and a positive Tinel sign on palpation of the GON and LON. After nerve block, her pain improved from 8 to 2 on a visual analogue scale. The decision was made to offer her nerve exploration with possible nerve decompression, possible nerve transaction, and possible removal of neuroma.
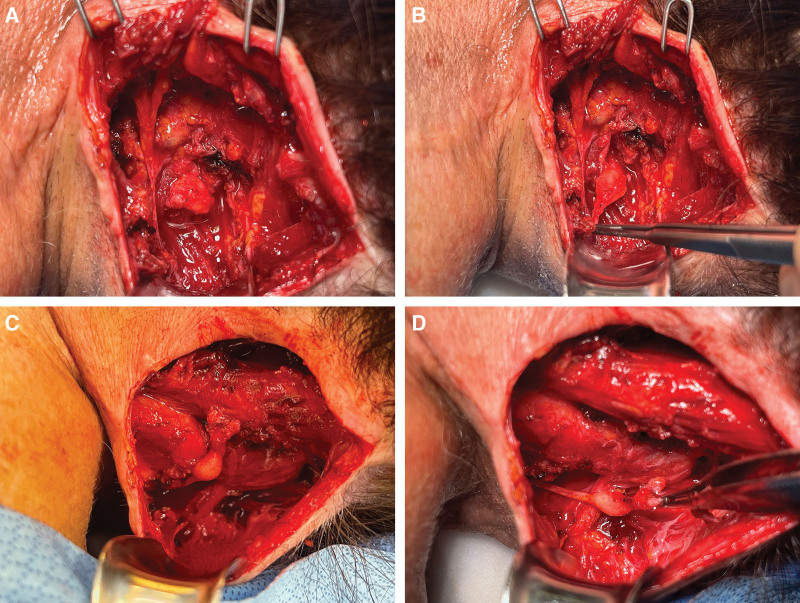
Preoperatively, the area of maximum pain was marked. After re-opening of a previous oblique incision along her scalp flap, careful dissection down to the trapezius muscle and midline raphe was performed. The GON was identified and found to be transected on the right. A large (~1 cm) stump neuroma was present (Fig. 4A, B). The dissection was then carried laterally, where the LON was identified and dissected free. Similarly, the nerve appeared to be transected with a large stump neuroma (Fig. 4C, D). The neuromas of the GON and LON were resected back to healthy nerve. Two motor branches of the semispinalis capitis muscle were identified using nerve stimulation, and the proximal GON and LON stumps were sutured to the semispinalis capitis motor nerve branches in an end-to-end fashion using 9.0 Nylon suture. (See Video [online], which displays greater and lesser occipital nerve TMR with microsurgical coaptation to small motor branches of the semispinalis capitis muscle.) The incision was closed in a standard fashion.6
Fig. 4.
Neuromas of the greater occipital nerve and the lesser occipital nerve. A, B, The greater occipital nerve (GON) neuroma was found at the exit point of the nerve from the semispinalis capitis muscle. C, D, The lesser occipital nerve neuroma was found lateral to the GON neuroma. Both neuromas were at the maximum point of pain perceived by the patient.
Video 1. This video displays greater and lesser occipital nerve targeted muscle reinnervation with microsurgical coaptation to small motor branches of the semispinalis capitis muscle.
Case Report Three: Reset Neurectomy with GON Autograft Relocation
This 67-year-old man underwent bilateral GON decompression 3 years before his presentation. He reported that he was pain free for 2 years before his symptoms slowly returned only on the left side. Given his recurrent symptoms with pain over the left GON with good response to nerve block, the decision was made to proceed with GON exploration and possible transection.
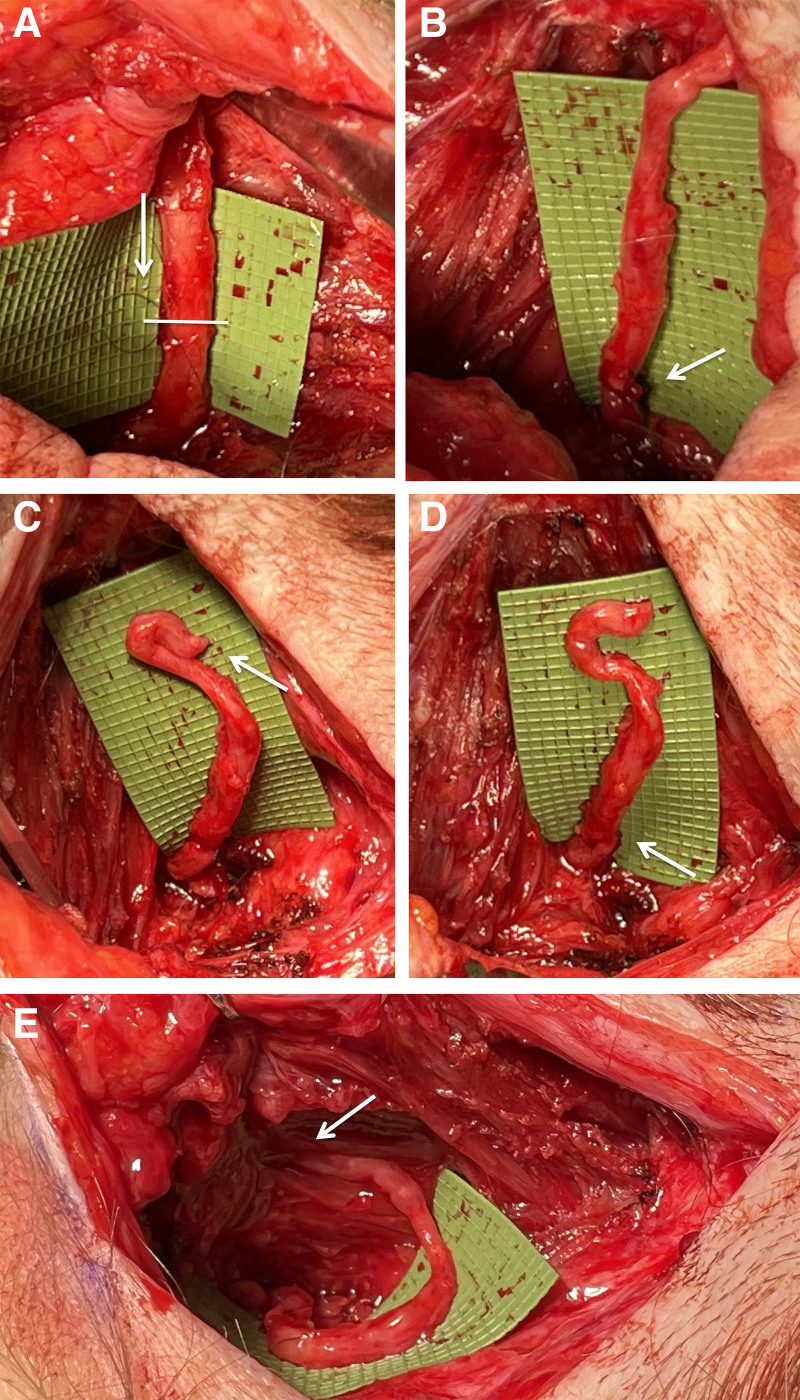
Preoperatively, the area of maximum pain was marked. An incision was made at the prior midline incision and carried down to the midline raphe. Similar to case report 1, the GON was found and dissected out lateral to the raphe. Before transection of the GON, an 8.0 Nylon suture was passed through the proximal and distal nerve end epineurium (Fig. 5A). Nerve transection was performed in between the proximal and distal sutures. The nerve was evaluated for bleeding and presence of healthy fascicles. The suture was sewn down, allowing for nerve coaptation after transection (reset neurectomy) without retraction of the nerve and loss of nerve orientation (Fig. 5B). If the nerve end appears unhealthy (no bleeding, abnormal fascicles), the nerve must be trimmed back to healthy tissue. The terminal GON branches were then transected distally, resulting in a 7 cm autograft (Fig. 5C, D) that was buried in the semispinalis capitis muscle (Fig. 5E).
Fig. 5.
Reset neurectomy of the greater occipital nerve. A, Prior to transection of the GON, a suture is placed proximal and distal to the planned site of division. This will prevent the proximal nerve stump from retracting deep into the cervical musculature. B, After nerve transection, the suture is tied down. C, Then, the distal nerve stump is cut and another suture (D) is placed to ensure correct alignment of the nerve under no tension. E, The distal nerve end is inserted into the semispinalis capitis muscle.
DISCUSSION
Advanced techniques to address the proximal nerve stump after nerve transection such as RPNI, TMR, relocation nerve grafting, and resect neurectomy have been shown to improve chronic pain and neuroma formation after transection of peripheral nerves.16,17,19,20,26,28 These techniques have not been described in the head and neck region. In the context of headache surgery, GON transection is performed when the nerve appears severely damaged, if symptoms are recurrent or persistent, and when neuromas are excised.9 LON excision is commonly performed during primary surgery. This article describes the feasibility of using RPNI and TMR to prevent chronic pain and neuroma formation after GON/LON transection. Further, we introduce reset neurectomy with GON autograft relocation as another method to address the nerve stump in the setting of diffuse nerve injury.
It is known that some peripheral nerves are more likely to develop painful neuromas after transection than others.29,30 Although the incidence and prevalence of neuromas affecting peri-cranial nerves is unknown, our case example proves that the GON and LON can form painful neuromas after injury. Given the risk of neuroma formation, careful consideration should be given to the expected side effects and potential risks of GON division prior to offering this procedure to patients. It is important to discuss known side effects such as permanent numbness of the occiput and transient tingling. Further, the potential risks such as paresthesia, and formation of painful neuromas at the nerve stump should be discussed. Further, fat grafting of nerves with recurrent pain should be considered prior to division of the GON.31
To minimize the risk of painful neuroma formation after nerve transection, the free proximal nerve end must be addressed. Different methods of nerve stump reconstruction have not been analyzed in the context of GON/LON excision. However, there is no reason to suggest that the techniques that have been shown to improve/ prevent pain in sensory nerves of the extremities would be less efficacious in the region of the head and neck.16,17,19,20,26,28
RPNI and TMR are both feasible options to address the GON/LON proximal nerve stump after transection. The semispinalis muscle is in close proximity to the nerve. A small cuff of muscle can easily be harvested (RPNI) and wrapped around the GON. Importantly, the RPNI has to be small and buried deep in order not to cause irritation due to a superficial location. Further, with a nerve stimulator, it is easy to identify the semispinalis capitis motor nerve branches adjacent to the transected nerve to perform TMR. This method requires microsurgical techniques but allows for deep burial of the GON, minimizing the risk for focal irritation. If the GON requires very proximal division due to diffuse injury/low focal point of pain, reset neurectomy with relocation nerve grafting prevents retraction of the GON after division. Further, given that the GON is used as a long autograft, harvest of autograft or use of allograft is not required.
Our patient numbers and follow-up time does currently not allow for comparison of outcomes between these techniques. With the treatment of additional patients, we plan on illustrating an algorithm for selection of the best candidates for the presented techniques.
At this time, we think that both TMR and RPNI are good options for focal nerve damage at or distal to the exit point of the GON from the semispinalis capitis muscle. Importantly, for TMR, nerve excision must be planned in close proximity to good motor branch targets to allow for coaptation. If RPNI is chosen to address the proximal nerve stump, it is imperative to make a small and thin RPNI that allows for deep burial of the proximal nerve stump in a small space. However, several authors have demonstrated that RPNI is a good option in tight spaces such as in the digits after neuroma excision.32
If diffuse injury is present and nerve transection has to be performed at a very proximal level, reset neurectomy is the better technique to avoid retraction of the proximal nerve stump deep into the soft tissues of the neck. Animal studies have shown that nerve regeneration across a long (5 cm) allograft is permanently prevented, and dorsal root ganglia gene expression is altered to arrest growth over a period of 5 months.33 Similarly, senescence increases and nerve regeneration decreases as nerve autograft lengths increase.34 Therefore, regenerating axons will likely not reach the end of a GON autograft that is ~7 cm in length. However, senescence has not been studied in the context of GON autografts. Therefore, the distal GON end was buried in muscle to prevent axonal ingrowth into the dermis, which may cause recurrent pain.
For the LON, both TMR and RPNI are good options to address the nerve stump. In our practice, we do not dissect out the LON in its entire course precluding the use of reset neurectomy with LON autograft.
Although our short-term results using RPNI, TMR, and reset neurectomy with GON autograft relocation are promising, we will carefully follow these patients and present objective long-term data. In the future, a multi-institutional study to compare surgical techniques after GON/LON transection is needed to shed light on the best method to address proximal nerve endings after nerve transection in headache surgery.
CONCLUSIONS
Newer techniques to address the proximal nerve stump after transection aimed at prevention of chronic neuropathic pain and neuroma formation such as RPNI and TMR are feasible in patients undergoing GON/LON transection. Further, relocation nerve grafting with GON autograft is a method that can be considered in patients with diffuse nerve injury requiring proximal nerve division.
Footnotes
Published online 25 March 2022.
Disclosure: I.V. and K.E. are consultants for AxoGen, Integra, and Checkpoint. All the other authors have no financial interest to declare in relation to the content of this article.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Mosser SW, Guyuron B, Janis JE, et al. The anatomy of the greater occipital nerve: implications for the etiology of migraine headaches. Plast Reconstr Surg. 2004;113:693–697; discussion 698. [DOI] [PubMed] [Google Scholar]
- 2.Janis JE, Hatef DA, Ducic I, et al. The anatomy of the greater occipital nerve: part II. Compression point topography. Plast Reconstr Surg. 2010;126:1563–1572. [DOI] [PubMed] [Google Scholar]
- 3.Janis JE, Hatef DA, Reece EM, et al. Neurovascular compression of the greater occipital nerve: implications for migraine headaches. Plast Reconstr Surg. 2010;126:1996–2001. [DOI] [PubMed] [Google Scholar]
- 4.Ducic I, Moriarty M, Al-Attar A. Anatomical variations of the occipital nerves: implications for the treatment of chronic headaches. Plast Reconstr Surg 2009;123:859–863; discussion 864. [DOI] [PubMed] [Google Scholar]
- 5.Israel JS, Kempton SJ, Afifi AM. Prospective analysis of the greater occipital nerve location in patients undergoing occipital nerve decompression. Ann Plast Surg. 2018;81:71–74. [DOI] [PubMed] [Google Scholar]
- 6.Gfrerer L, Dayan E, Austen WG, Jr. Trigger-site deactivation surgery for nerve compression headaches. Plast Reconstr Surg. 2021;147:1004e–1021e. [DOI] [PubMed] [Google Scholar]
- 7.Lee M, Brown M, Chepla K, et al. An anatomical study of the lesser occipital nerve and its potential compression points: implications for surgical treatment of migraine headaches. Plast Reconstr Surg. 2013;132:1551–1556. [DOI] [PubMed] [Google Scholar]
- 8.Peled ZM, Pietramaggiori G, Scherer S. Anatomic and compression topography of the lesser occipital nerve. Plast Reconstr Surg Glob Open. 2016;4:e639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ducic I, Felder JM, III, Khan N, et al. Greater occipital nerve excision for occipital neuralgia refractory to nerve decompression. Ann Plast Surg. 2014;72:184–187. [DOI] [PubMed] [Google Scholar]
- 10.Gfrerer L, Hansdorfer MA, Ortiz R, et al. Muscle fascia changes in patients with occipital neuralgia, headache, or migraine. Plast Reconstr Surg. 2021;147:176–180. [DOI] [PubMed] [Google Scholar]
- 11.McNutt S, Hallan DR, Rizk E. Evaluating the evidence: is neurolysis or neurectomy a better treatment for occipital neuralgia? Cureus. 2020;12:e11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zabaglo M, Dreyer MA. Neuroma. Treasure Island, Fla.: StatPearls; 2021. [Google Scholar]
- 13.Eberlin KR, Ducic I. Surgical algorithm for neuroma management: a changing treatment paradigm. Plast Reconstr Surg Glob Open. 2018;6:e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guse DM, Moran SL. Outcomes of the surgical treatment of peripheral neuromas of the hand and forearm: a 25-year comparative outcome study. Ann Plast Surg. 2013;71:654–658. [DOI] [PubMed] [Google Scholar]
- 15.Valerio I, Schulz SA, West J, et al. Targeted muscle reinnervation combined with a vascularized pedicled regenerative peripheral nerve interface. Plast Reconstr Surg Glob Open. 2020;8:e2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frantz TL, Everhart JS, West JM, et al. Targeted muscle reinnervation at the time of major limb amputation in traumatic amputees: early experience of an effective treatment strategy to improve pain. JB JS Open Access. 2020;5:e0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Brien AL, Jordan SW, West JM, et al. Targeted muscle reinnervation at the time of upper-extremity amputation for the treatment of pain severity and symptoms. J Hand Surg Am. 2021;46:72.e1–72.e10. [DOI] [PubMed] [Google Scholar]
- 18.Souza JM, Cheesborough JE, Ko JH, et al. Targeted muscle reinnervation: a novel approach to postamputation neuroma pain. Clin Orthop Relat Res. 2014;472:2984–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kubiak CA, Adidharma W, Kung TA, et al. Decreasing postamputation pain with the regenerative peripheral nerve interface (RPNI). Ann Vasc Surg. 2022;79:421–426. [DOI] [PubMed] [Google Scholar]
- 20.Kubiak CA, Kemp SWP, Cederna PS. Regenerative peripheral nerve interface for management of postamputation neuroma. JAMA Surg. 2018;153:681–682. [DOI] [PubMed] [Google Scholar]
- 21.Woo SL, Kung TA, Brown DL, et al. Regenerative peripheral nerve interfaces for the treatment of postamputation neuroma pain: a pilot study. Plast Reconstr Surg Glob Open. 2016;4:e1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kung TA, Langhals NB, Martin DC, et al. Regenerative peripheral nerve interface viability and signal transduction with an implanted electrode. Plast Reconstr Surg. 2014;133:1380–1394. [DOI] [PubMed] [Google Scholar]
- 23.Kim PS, Ko JH, O’Shaughnessy KK, et al. The effects of targeted muscle reinnervation on neuromas in a rabbit rectus abdominis flap model. J Hand Surg Am. 2012;37:1609–1616. [DOI] [PubMed] [Google Scholar]
- 24.Fracol ME, Dumanian GA, Janes LE, et al. Management of sural nerve neuromas with targeted muscle reinnervation. Plast Reconstr Surg Glob Open. 2020;8:e2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackinnon SE. Wandering nerve graft technique for management of the recalcitrant painful neuroma in the hand: a case report. Microsurgery. 1988;9:95–102. [DOI] [PubMed] [Google Scholar]
- 26.Eberlin KR, Pickrell BB, Hamaguchi R, et al. Reset neurectomy for cutaneous nerve injuries. Plast Reconstr Surg Glob Open. 2021;9:e3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gfrerer L, Hansdorfer MA, Ortiz R, et al. Patient pain sketches can predict surgical outcomes in trigger-site deactivation surgery for headaches. Plast Reconstr Surg. 2020;146:863–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dumanian GA, Potter BK, Mioton LM, et al. Targeted muscle reinnervation treats neuroma and phantom pain in major limb amputees: a randomized clinical trial. Ann Surg. 2019;270:238–246. [DOI] [PubMed] [Google Scholar]
- 29.Wolvetang NHA, Lans J, Verhiel SHWL, et al. Surgery for symptomatic neuroma: anatomic distribution and predictors of secondary surgery. Plast Reconstr Surg. 2019;143:1762–1771. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy AM, Elliot D. Surgery for symptomatic neuroma: anatomic distribution and predictors of secondary surgery. Plast Reconstr Surg. 2020;145:879e–880e. [DOI] [PubMed] [Google Scholar]
- 31.Guyuron B, Pourtaheri N. Therapeutic role of fat injection in the treatment of recalcitrant migraine headaches. Plast Reconstr Surg. 2019;143:877–885. [DOI] [PubMed] [Google Scholar]
- 32.Hooper RC, Cederna PS, Brown DL, et al. Regenerative peripheral nerve interfaces for the management of symptomatic hand and digital neuromas. Plast Reconstr Surg Glob Open. 2020;8:e2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pan D, Bichanich M, Wood IS, et al. Long acellular nerve allografts cap transected nerve to arrest axon regeneration and alter upstream gene expression in a rat neuroma model. Plast Reconstr Surg. 2021;148:32e–41e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoben GM, Ee X, Schellhardt L, et al. Increasing nerve autograft length increases senescence and reduces regeneration. Plast Reconstr Surg. 2018;142:952–961. [DOI] [PMC free article] [PubMed] [Google Scholar]