Abstract
The increasing resistance of Candida species to fluconazole is cause for concern. To determine the molecular mechanisms involved in resistance to fluconazole, I used a scheme of transposon mutagenesis in Saccharomyces cerevisiae, a genetically tractable yeast that is closely related to Candida albicans. This technique, which permits the generation and analysis of multiple random Tn3::LEU2::lacZ fusions, can be used as a disruption mutagen (N. B. Burns et al., Genes Dev. 8:1087–1105, 1994). By using the Tn3::LEU2::lacZ library as a disruption mutagen, I found recessive mutations in genes that were previously found to be involved in azole resistance, e.g., PDR5 and CPR1, and in genes previously found to be involved in azole sensitivity, e.g., ERG3. This approach also enabled me to identify recessive mutations in three genes not previously known to be involved in azole sensitivity. Two of the genes, ADA3 and SPT7, are general transcriptional regulators; the third, YMR034c, is a putative sterol transporter. Finally, by screening the Tn3::LEU2::lacZ library for lacZ fusions induced by a low concentration of fluconazole, I identified genes known to be induced by azoles as well as a variety of other genes not previously known to be induced by the drug. In conclusion, transposon mutagenesis is a promising screening tool for use in identifying novel drug targets and in uncovering the mechanisms involved in the response of S. cerevisiae to antifungal drugs.
Fluconazole, a widely used azole, selectively inhibits the cytochrome P-450-dependent C14 lanosterol demethylase (P-450 14-DM), a key enzyme involved in ergosterol biosynthesis in fungi (19). The emerging resistance of Candida species to fluconazole is a matter of concern (20). A better understanding of the molecular responses of Candida species to fluconazole could enable physicians to make more effective use of this agent.
Saccharomyces cerevisiae, a genetically tractable fungus, is an attractive experimental system for the study of azole resistance. S. cerevisiae is closely related to the genetically intractable Candida albicans, and it has long served as a model system for studies of sterol biosynthesis (15). All of the reported mechanisms of fluconazole resistance in S. cerevisiae have also been described for C. albicans and involve the same gene products (11, 20). One well-characterized mechanism of azole resistance in Saccharomyces is conferred by loss-of-function mutations in sterol Δ5,6-desaturase, which is the product of the ERG3 gene (13, 20). Another mechanism of azole resistance is mediated by the pleiotropic drug resistance 5 gene (PDR5), an ATP-binding cassette efflux transporter, through decreased accumulation of fluconazole (2, 4). A complex regulatory network controls the PDR phenotype (4). Finally, loss of function of NADPH-dependent cytochrome P-450–oxidoreductase, which is encoded by the CPR1 gene, results in azole hypersensitivity (18). On the other hand, the importance of target-site (P-450 14-DM) alterations is unclear (11, 13, 20).
Despite advances in understanding the mechanisms of azole resistance in Saccharomyces, the components of the response pathways are not fully known. Recent methods such as DNA microarray technology (5) are precise and powerful tools for real-time analysis of the dynamic change of the host in response to signals, including drugs. However, these technologies will always rely on classical genetics and on the effects of gene disruption to explain gene function.
In the current work, I used an insertional Tn3::LEU2::lacZ transposon mutagenesis scheme to study the mechanisms of azole resistance in Saccharomyces. This strategy permits the generation and analysis of a large number of independent lacZ gene fusions (3). This approach offers several advantages over the traditional methods and oligonucleotide-based probe assays: (i) it frequently creates loss-of-function mutations by insertional mutagenesis, (ii) it facilitates easy identification of expression candidates by a simple colorimetric assay, and (iii) it allows for rapid sequencing of candidate genes (3). Using the transposon mutagenesis scheme, I found recessive mutations in genes that were previously found to be involved in azole resistance (e.g., PDR5 and CPR1) and sensitivity (e.g., ERG3). This approach also enabled me to identify recessive mutations in three genes previously not known to be involved in azole sensitivity. Two of the genes, ADA3 and SPT7, are general transcriptional activators or repressors; the third, YMR034c, is a putative sterol transporter. My results implicate the role of transposon mutagenesis as a promising tool for identifying novel drug targets and in identifying the molecular mechanisms involved in the resistance of S. cerevisiae to azoles.
MATERIALS AND METHODS
Strains.
I used standard methods for making the yeast growth media and standard techniques for yeast manipulation (12). All work was done in the Σ1278b genetic background. Fluconazole was a gift from Pfizer, Inc. (New York, N.Y.).
Transposon mutagenesis screening for fluconazole sensitivity and resistance.
The S. cerevisiae haploid strain 10512-3C (MATa leu2::hisG his3::hisG; Fink laboratory, Whitehead Institute for Biomedical Research, Cambridge, Mass.) was transformed with the Tn3::LEU2::lacZ transposon genomic DNA library (Snyder laboratory, Yale University, New Haven, Conn.) (3). Approximately 50,000 Leu+ transformant colonies were pooled and replated at a density of ∼600 colonies/plate on synthetic complete (SC)-leucine medium. To screen for sensitive mutants, colonies were replica plated on SC-leucine–8-μg/ml fluconazole plates. To screen for resistant mutants, the pooled transformants of 10512-3C were spread at approximately 108 cells on SC-leucine–128-μg/ml fluconazole plates and incubated for 7 days at 30°C.
Transposon mutagenesis screening for fluconazole-responsive lacZ fusions.
The S. cerevisiae diploid strain L5803 (MATa/α ura3-52/ura3-52 leu2::hisG/leu2::hisG; Fink laboratory) was transformed with the Tn3::LEU2::lacZ transposon-mutagenized yeast genomic DNA library. Approximately 280,000 transformants were obtained by selection on SC-leucine medium. The transformants were pooled and replated on SC medium at a density of ∼600 colonies/plate. These colonies were printed to the surface of 8.26-cm filter circles (model no. 576; Schleicher & Schuell) on SC medium and on SC medium containing subinhibitory concentrations of fluconazole (8 μg/ml) and grown at 30°C for 18 to 24 h. The wild-type L5803 strain failed to grow at a concentration of 64 μg/ml. Filters were then processed with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) as follows. The filters were first dipped into liquid nitrogen for 1 min and then thawed and placed on an 8.26-cm circular blotter (model no. 593; Schleicher & Schuell) saturated with 0.03% X-Gal in 2.5 ml of Z buffer. The mutant ypl110c::Tn3::LEU2::lacZ (which I found not to respond to fluconazole) was used as a blue-color control. The β-galactosidase reaction was stopped by Na2CO3 after 4 h. Mutant colonies, which were more blue on the fluconazole plate filters, were patched onto SC medium, retested, purified, and retested again. Of the 18,000 blue colonies screened from a total plating of 180,000 colonies (only a subset of insertions express lacZ [3]), 62 mutants were found to be reproducibly induced. The pattern of β-galactosidase induction for each mutant was studied by comparing the intensity of fluconazole-induced blue-color development with the color response to different classes of antifungals (amphotericin B, 10 mg/ml; nystatin, 25,000 U/ml; and 5-fluorouracil, 10 mg/ml) or other growth inhibitors (canavanine, 20 mg/ml; cycloheximide, 100 μg/ml; and 100% alcohol). To determine haploid phenotypes, the diploid expression mutants were sporulated and dissected on yeast extract-peptone-dextrose medium. Tetrads (10 per diploid) of ascospore colonies were replica plated to SC-leucine and filters on SC plates. The fluconazole growth phenotypes of haploid expression mutants were assayed by replica plating to SC plates containing different concentrations of fluconazole. The growth of reconstructed diploids formed by crossing mutants with wild-type sister spores was similarly tested to check for recessivity.
Molecular biology and biochemical methods.
Genomic DNA immediately adjacent to Tn3::LEU2::lacZ in the mutants of interest was cloned as described earlier (3). DNA and protein homology searches were performed with the BLAST network (1).
RESULTS
Identification of genes known to be involved in azole resistance and sensitivity.
I found mutants with a Tn3::LEU2::lacZ disruption of PDR5 to be hypersensitive to fluconazole (2 μg/ml) (Fig. 1). The disruption phenotype of pdr5 mutants also included pleiotropic hypersensitivity to a variety of agents. I also found mutants with a Tn3::LEU2::lacZ disruption of CPR1 to be exquisitively sensitive specifically to fluconazole (0.1 μg/ml) (Fig. 1). Finally, I found several fluconazole-resistant mutants with a Tn3::LEU2::lacZ disruption of ERG3. The erg3 mutant alleles, despite their resistance to azoles, had a pleiotropic sensitivity to a variety of agents.
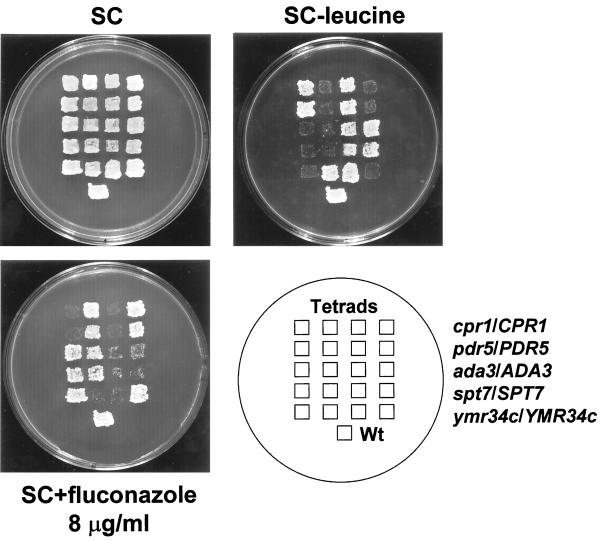
FIG. 1.
The Tn3::LEU2::lacZ transposon mutagenesis scheme identifies known genes and genes previously not known to be involved in azole resistance. One representative four-spore tetrad of ascospores of each of the diploids cpr1::Tn3::LEU2::lacZ/CPR1 leu2::hisG/leu2::hisG ura3-52/ura3-52, pdr5::Tn3::LEU2::lacZ/PDR5 leu2::hisG/leu2::hisG ura3-52/ura3-52, ada3::Tn3::LEU2::lacZ/ADA3 leu2::hisG/leu2::hisG ura3-52/ura3-52, spt7::Tn3::LEU2::lacZ::/SPT7 leu2::hisG/leu2::hisG ura3-52/ura3-52, and ymr034c::Tn3::LEU2::lacZ/YMR034c leu2::hisG/leu2::hisG ura3-52/ura3-52 shows that the cpr1::LEU2::lacZ, pdr5::Tn3::LEU2::lacZ, ada3::Tn3::LEU2::lacZ, spt7::Tn3::LEU2::lacZ, and ymr034c::Tn3::LEU2::lacZ disruptants segregate 2:2 (Leu+ and fluconazole hypersensitive). The spores were patched to SC plates and then printed to SC-leucine and SC–8-μg/ml fluconazole plates. Shown is the growth after 2 days at 30°C. The 10512-3C strain transformed with a cen LEU2 plasmid was also put in the plate as a haploid wild-type (Wt) control.
Identification of genes previously not known to be involved in azole resistance.
Tn3::LEU2::lacZ insertions into three genes previously not known to be involved in sensitivity to azoles resulted in sensitivity to fluconazole.
Disruption of ADA3 resulted in hypersensitivity to 8 μg of fluconazole per ml (Fig. 1). ADA genes encode transcriptional activators-corepressors (9). The ada3 mutant was sensitive specifically to fluconazole. Other ada mutants (ada1, ada2, ada3, ada5, and gcn5; Guarente laboratory, Massachusetts Institute of Technology, Cambridge) were also tested for fluconazole sensitivity. All but the gcn5 mutant were sensitive specifically to fluconazole compared with the isogenic wild-type control. The ada1 and ada5 mutants exhibited the most severe sensitivity, whereas ada2 and ada3 mutants were only moderately sensitive to the drug.
Disruption of SPT7 conferred hypersensitivity to 4 μg of fluconazole per ml (Fig. 1). The spt7 allele mutant grew slowly and was an inositol auxotroph, as previously described (6). This mutant, in contrast to the ada3 mutant, had pleiotropic sensitivity to many other agents. SPT genes, like ADA genes, encode proteins thought to be part of the in vivo transcription activation machinery (6, 9). Other spt mutants (spt3, spt6, spt7, spt8, spt15, spt20, and gcn5; Winston laboratory, Harvard Medical School, Boston, Mass.) were also tested for fluconazole sensitivity. Among those, only spt7 and spt20 mutants were hypersensitive.
Tn3::LEU2::lacZ disruption in the gene YMR034c, a putative sterol transporter (1), resulted in sensitivity to 8 μg of fluconazole per ml (Fig. 1).
All of the mutations in the aforementioned genes, whether previously known or unknown, that conferred resistance or sensitivity to fluconazole due to the Tn3::LEU2::lacZ disruption were recessive.
Identification of genes known to be involved in response mechanisms to azoles.
I found multiple pdr5::LEU2::lacZ fusions to be specifically induced by fluconazole (Fig. 2; Table 1). In addition, an erg3::LEU2::lacZ fusion was found to be induced specifically by fluconazole. Finally, a variety of previously known genes were identified for the first time to be induced by fluconazole (Table 1).
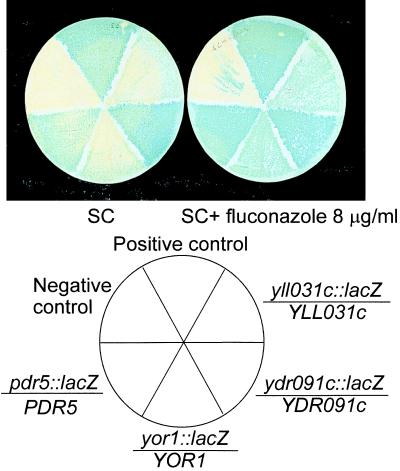
FIG. 2.
Fluconazole induces expression of transporters in yeast. Diploid strains of indicated genotype (PDR5/pdr5::Tn3::LEU2::lacZ, YOR1/yor1::Tn3::LEU2::lacZ, YDR091c/ydr091c::Tn3::LEU2::lacZ, and YLL031c/yll031c::Tn3::LEU2::lacZ are shown. The YDR091c/ydr091c::Tn3::LEU2::lacZ diploid appears to be induced the least. The YPL110c/ypl110c::Tn3::LEU2::lacZ mutant and strain L5803 were used as blue- and white-color controls, respectively. Strains were grown on filters in SC and SC-fluconazole (8 μg/ml) at 30°C for 24 h. Filters were then processed with X-Gal. The reaction was stopped after 6 h.
TABLE 1.
Fluconazole-responsive lacZ fusions
| Gene | na | Expressionb | Insertionc | Functiond |
|---|---|---|---|---|
| Transporters | ||||
| PDR5 | 7H | U, S | +602, +1016e | ABC transporter |
| YOR1 | 4 | U, S | +154, +1005, +1253, +1253 | ABC transporter |
| YDR091c | 2L | U, S | +947, +947 | Similar to ABC transporters |
| OSR | 1 | U, NS | ND | Cation efflux transporter |
| YBR241c | 2 | U, S | +522, +522 | Glucose transporter, MFS superfamily |
| YLL031c | 2L | U, NS | +329, +731 | Member of MFS superfamily |
| Ergosterol pathway-acetyl coenzyme A regeneration | ||||
| ERG3 | 1R | U, S | +2 | C5 desaturase |
| CIT2 | 1 | U, S | ND | Citrate synthetase |
| YDR213w | 2 | U, S | +233 | Sterol uptake control |
| YBL098w | 1L | U, NS | +609 | Similar to kynurenin-3-monooxygenase |
| ACR1 | 2 | U, ND | +363, +286 | Acetyl coenzyme A synthetase regulator |
| Gluconeogenesis-glycolysis | ||||
| FBP1 | 1 | U, NS | +406 | Fructose-1,6-biphosphatase |
| GDH2 | 1 | U, S | +3225 | NADPH generation |
| SUC2 | 1 | U, S | +107 | Glucose repressible, invertase |
| ADH2 | 1 | U, S | +863 | Glucose repressible, alcohol dehydrogenase II |
| YOR095c | 1L | U, NS | +79 | Ribose-5-isomerase |
| Genes expressed in mitochondria-oxidoreductases | ||||
| YMC1 | 2 | U, NS | +682, +698 | Mitochondrial carrier protein |
| NCA2 | 2 | U, S | +717, +717 | Control of mitochondrial synthesis of ATPp |
| NDI1 | 1 | U, NS | +465 | NAD-ubiquinone oxidoreductase |
| POS5 | 1 | U, NS | +206 | Similar to FRE2 (ferric reductase) |
| RNR2 | 1 | U, S | +597 | Ribonucleoside reductase, DNA damage repair |
| Cell wall biosynthesis | ||||
| ALG9 | 1 | U, NS | +1163 | Mannosyltransferase |
| ECM10 | 2 | U, S | +1140, +1140 | Involved in cell wall structure |
| AMS1 | 1 | U, S | +1407 | Vacuolar mannosidase (?), mannan turnover |
| YPL087w | 1 | U, NS | +890 | Probable membrane protein |
| YGR131w | 2 | U, S | +461, +504 | Probable membrane protein |
| YAL053w | 1 | U, S | +1107 | Probable membrane protein |
| YPR030w | 1 | U, S | +2421 | Similar to Ecm21p |
| Other | ||||
| ALK1 | 1 | U, NS | +258 | DNA damage repair |
| YDL244w | 1 | U, S | +595 | Similar to Thi5p (pyrimidine biosynthetic enzyme) |
| DBF3 | 1L | U, NS | +4763 | U5 snRNA-associated splicing factor |
| RPRS1 | 1 | U, S | +1199 | De novo synthesis of purines-pyrimidines |
| YGR090w | 1L | U, NS | +833 | Similar to Ribp, a flavin biosynthetic enzyme |
| CDC95 | 1L | U, NS | +484 | Probable translation initiation factor 6 |
| TIN1 | 1 | U, NS | +2136 | TOR inhibitory protein |
| MOT3 | 1L | U, NS | +912 | High-copy suppressor of MOT1-SPT3 lethality |
| YHR209w | 2 | U, S | +242, +285 | |
| YLR425w | 1 | U, S | +212, +212 | |
| YPL280w | 1 | U, S | +695 | |
| YJL150w | 1L | U, ND | +822 | |
| YGR239c | 1 | U, S | +500 | |
| YGR110w | 1 | U, NS | +875 | |
| YOR391c | 1 | U, NS | +653 | |
| YJL105w | 1 | U, NS | +202 |
n, number of isolates; H, hypersensitive; L, lethal; R, resistant.
Determined by filter X-Gal assay in SC and SC–8-μg/ml fluconazole plates. U, upregulation; S, β-galactosidase expression in the filters only in the presence of fluconazole (8 μg/ml); NS, β-galactosidase expression in the filters in the presence of both fluconazole and other drugs; ND, not determined.
Approximate position (within 25 bp) of Tn3::LEU2 insertions relative to the translational start site ATG at position +1. ND, not determined.
See reference 7. ABC, ATP-binding cassette; MFS, major facilitator superfamily.
Two of seven pdr5::LEU2::lacZ hypersensitive mutants were sequenced.
DISCUSSION
Using transposon mutagenesis, I found genes known to be involved in fluconazole resistance (PDR5 and CPR1) and fluconazole sensitivity (ERG3). In addition, this approach led to the identification of novel genes. Hence, I found that general transcriptional repressors-activators affect azole sensitivity. Of note, Spt7p, Spt13p, and Spt20p as well as Ada2p, Ada3p, and Gcn5p are located in the transcriptional complex called SAGA (Spt-Ada-Gcn5-acetyltransferase) (8). Our data suggest a GCN5-independent role of the SAGA complex in regulating gene expression, because, whereas spt7 and spt20 mutants were hypersensitive to fluconazole, gcn5 mutants were not. The mechanism of action of the aforementioned transcription factors is not known. It may involve regulation of the PDR network and thus, indirectly, the efficiency of drug efflux. Evidence of the interaction between PDR1, a positive regulator of PDR5 (2, 4), and ADA3 has been reported with a two-hybrid system (14). Further work is needed to define the role of the general transcriptional machinery in the various drug-specific responses. Finally, I found that a disruption in the YMR034c gene caused sensitivity to azoles. YMR034c is homologous with bacterial (arsenic resistance protein), plant, and mammalian (sodium-dependent bile acid cotransporter) transporters (1). Loss of function of YMR034c could affect efflux of fluconazole either directly through decreased transport of the drug or through alterations of lipid fluidity of the fungal membrane and thus indirectly through impaired extrusion of fluconazole by Pdr5p.
I also screened for fluconazole-responsive lacZ fusions in a defined medium (SC), which lacks the lipid extracts, in order to avoid the influence of exogenous lipids on the expression of genes such as ERG3 whose transcription is affected by feedback mechanisms by sterol levels (17). I found genes known to be involved in azole response mechanisms as well as genes that were previously unknown to be upregulated by azoles. Transporters appeared to constitute an important element of the response to fluconazole. PDR5, in particular, was found to be specifically induced by fluconazole in my study. Induction of CDR1, the PDR5 homologue in C. albicans, by fluconazole has been reported for both laboratory and fluconazole-resistant clinical isolates of C. albicans (10, 16, 20).
My work has multiple implications. First, transposon mutagenesis holds promise for enabling us to uncover novel molecular targets involved in azole sensitivity and could be applicable to other classes of antifungals. Second, elucidation of the role of transcription factors or sterol transporters in azole resistance could lead to the discovery of new, potentially therapeutic targets. Finally, combining mutants with fluconazole-responsive lacZ fusions to form double mutants could reveal additional unknown drug phenotypes and shed light on the regulatory mechanisms involved in azole response.
ACKNOWLEDGMENTS
Part of this work was performed at the Whitehead Institute for Biomedical Research (Fink laboratory) in Cambridge, Mass., when D.P.K. was a fellow in the Clinical Investigator Training Program (supported by Pfizer, Inc.) at the Harvard Massachusetts Institute of Technology Division of Health Sciences and Technology and a fellow in Infectious Diseases at Massachusetts General Hospital, Harvard Medical School, in Boston, Mass. This work was also supported by the Cancer Center (Core) Grant (CA16672) from The University of Texas M. D. Anderson Cancer Center.
I thank the Winston and Guarente laboratories for providing strains; T. Milne and K. Hirschi for their critical review of the manuscript; and C. A. Styles, R. H. Rubin, G. R. Fink, and other members of the Fink laboratory for helpful advice.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Balzi E, Wang M, Leterme S, Dyck L, Goffeau A. PDR5, a novel yeast multidrug resistance conferring transporter controlled by the transcription regulator PDR1. J Biol Chem. 1994;269:2206–2214. [PubMed] [Google Scholar]
- 3.Burns N B, Grimwade P B, Ross-Macdonald E Y, Choi K, Finbery K, Roeder G S, Synder M. Large-scale analysis of gene expression, protein localization, and gene disruption in Saccharomyces cerevisiae. Genes Dev. 1994;8:1087–1105. doi: 10.1101/gad.8.9.1087. [DOI] [PubMed] [Google Scholar]
- 4.Decottignies A, Goffeau A. Complete inventory of the yeast ABC proteins. Nat Genet. 1997;15:137–145. doi: 10.1038/ng0297-137. [DOI] [PubMed] [Google Scholar]
- 5.DeRisi J L, Iyer V R, Brown P O. Exploring the metabolic and genetic control of gene expression on a genomic scale. Science. 1997;278:680–686. doi: 10.1126/science.278.5338.680. [DOI] [PubMed] [Google Scholar]
- 6.Gansheroff L J, Dollard C, Tan P, Winston F. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo. Genetics. 1995;139:523–536. doi: 10.1093/genetics/139.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garrels J I. YPD—a database for the proteins of Saccharomyces cerevisiae. Nucleic Acids Res. 1994;24:46–49. doi: 10.1093/nar/24.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 9.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernaez M L, Gil C, Pla J, Nombela C. Induced expression of Candida albicans multidrug resistance gene in response to fluconazole and other antifungals. Yeast. 1998;14:517–526. doi: 10.1002/(SICI)1097-0061(19980430)14:6<517::AID-YEA250>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 11.Joseph-Horne T, Hollomon D W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149:141–149. doi: 10.1111/j.1574-6968.1997.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 12.Kaiser C, Michaelis S, Mitchell A. Methods in yeast genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1994. [Google Scholar]
- 13.Kelly S L, Arnoldi A, Kelly D. Molecular genetic analysis of azole antifungal mode of action. Biochem Soc Trans. 1993;21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 14.Martens J A, Genereaux J, Saleh A, Brandl C J. Transcriptional activation by yeast PDR1p is inhibited by its association with NGG1p/ADA3p. J Biol Chem. 1996;271:15884–15890. doi: 10.1074/jbc.271.27.15884. [DOI] [PubMed] [Google Scholar]
- 15.Paultauf F, Kohlwein S D, Henry S A. Regulation and compartmentalization of lipid synthesis in yeast. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 415–500. [Google Scholar]
- 16.Sanglard D, Kuchler K, Ischer F, Pagani J L, Monod M, Billie J. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob Agents Chemother. 1995;39:2378–2386. doi: 10.1128/aac.39.11.2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith S J, Crowley J H, Parks L W. Transcriptional regulation by ergosterol in the yeast Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:5427–5432. doi: 10.1128/mcb.16.10.5427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutter T R, Loper J C. Disruption of the Saccharomyces cerevisiae gene for NADPH-cytochrome P450 reductase causes increased sensitivity to ketoconazole. Biochem Biophys Res Commun. 1989;160:1257–1266. doi: 10.1016/s0006-291x(89)80139-1. [DOI] [PubMed] [Google Scholar]
- 19.Vanden Bossche H, Koymans L, Moereels H. P450 inhibitors of use in medical treatment: focus on mechanisms of action. Pharmacol Ther. 1995;67:79–100. doi: 10.1016/0163-7258(95)00011-5. [DOI] [PubMed] [Google Scholar]
- 20.White T, Marr K, Bowden R. Clinical, cellular and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]