Abstract
Background: Total hip replacement (THR) is a common and cost-effective procedure for end-stage osteoarthritis, but inappropriate utilization may be devaluing its true impact. The purpose of this study was to develop and test the internal validity of a prognostic algorithm for predicting the probability of non-response to THR surgery at 1 year. Methods: Analysis of outcome data extracted from an institutional registry of individuals (N = 2177) following elective THR performed between January 2012 and December 2019. OMERACT-OARSI responder criteria were applied to Western Ontario and McMaster Universities Arthritis Index (WOMAC) pain and function scores at pre- and 1 year post-THR, to determine non-response to surgery. Independent prognostic correlates of post-operative non-response observed in adjusted modelling were then used to develop a nomogram. Results: A total of 194 (8.9%) cases were deemed non-responders to THR. The degree of contribution (OR, 95% CI) of each explanatory factor to non-response on the nomogram was, morbid obesity (1.88, 1.16, 3.05), Kellgren–Lawrence grade <4 (1.89, 1.39, 2.56), WOMAC Global rating per 10 units (0.86, 0.79, 0.94) and the following co-morbidities: cerebrovascular disease (2.39, 1.33, 4.30), chronic pulmonary disease (1.64; 1.00, 2.71), connective tissue disease (1.99, 1.17, 3.39), diabetes (1.86, 1.26, 2.75) and liver disease (2.28, 0.99, 5.27). The concordance index for the nomogram was 0.70. Conclusion: We have developed a prognostic nomogram to calculate the probability of non-response to THR surgery. In doing so, we determined that both the probability of and predictive prognostic factors for non-response to THR differed from a previously developed nomogram for total knee replacement (TKR), confirming the benefit of designing decision support tools that are both condition and surgery site specific. Future external validation of the nomogram is required to confirm its generalisability.
Keywords: hip replacement, outcome, risk prediction, nomogram
1. Introduction
Total hip joint replacement (THR) continues to be one of the most common and cost-effective surgeries performed in the history of orthopaedics and medicine [1] and its popularity for treating end-stage osteoarthritis is reflected in over 50,000 procedures that are performed each year in Australia [2]. Estimates for Australia now predict a rise of 276% for total hip and 208% for total knee replacements by 2030, with current (direct and indirect) costs of over $5 billion/year expected to also rise [3,4]. With a dissatisfaction rate of between 20 and 30% after total hip and knee replacement (TJR) surgery [5], a rate of inappropriate surgery of 20–25% [6] and the lack of uniform capture of validated and relevant outcome measures, the question is not whether these procedures are cost-effective, which they are [7], but whether how and/or when TJR is utilized may be devaluing its true impact.
Decision aids are known to improve the efficiency of surgical decisions [8,9], but are more commonly constructed to predict immediate-term outcomes such as length of stay, need for rehabilitation and post-surgery complications [10]. This is likely due to the availability of these types of data. We have previously explored surgeons’ perceptions of utilizing decision aids to identify patients at risk of poor pain and functional outcomes (non-responders) following total knee replacement (TKR) [11]. We found that surgeons prioritise clinical acumen but do believe that decision aids can improve communication and patient-informed consent. Consequently, with data from our institutional registry, we constructed a prediction tool to help surgeons identify potential non-responders to total knee replacement (TKR) so that those who are deemed responders may be more efficiently processed for surgery [12], while those who are not would either be referred for non-surgical care or have their modifiable factors (obesity, mental health, co-and morbidities) optimised and further consideration given for surgery afterwards.
It is well established that total knee and total hip replacement recipients differ in their trajectory of recovery after surgery [13], with a tendency for people undergoing THR to reach their peak improvement in pain and function earlier than those undergoing TKR. However, maximum improvement is achieved for both within 1 year, albeit a higher proportion of THR recipients report greater pain and function improvements than those who undergo TKR [14]. The degree to which baseline patient characteristics predict pain and function outcomes varies across the literature [13,14,15], although factors such as baseline symptom and radiographic OA severity, obesity, mental health, and co-morbidities (variously measured) are consistently reported.
Given the variation in patterns of recovery following hip and knee TJR, we aimed to determine whether pre-operative prognostic variables that predict non-response to THR aligned with those that informed our previously constructed TKR nomogram [12] and therefore determine whether use of the existing tool could be interchangeable across procedures. Our end goal was to produce an internally validated nomogram that accurately predicted the likelihood of non-response to THR surgery at 1 year. We envisaged that whether existing or in the form of a new tool, a prognostic nomogram would be useful in informing targeted interventions for improving outcomes in people at risk of non-response to THR.
2. Patients and Methods
2.1. Study Setting and Participants
This study was undertaken at St. Vincent’s Hospital (SVH), in Melbourne Australia. All primary elective THR performed at SVH between January 2012 and December 2019, were assessed for study inclusion. A consecutive cohort of unilateral, elective, primary THR with baseline and 12 month follow-up data was included.
2.2. Data Collection
Data of individuals who had undergone THR between 2012 and 2019 were extracted from our institutional registry. The St. Vincent’s Melbourne Arthroplasty and Outcomes (SMART) registry is a clinical registry of all elective hip and knee joint replacements undertaken at the hospital from 1998 and was the same data source for our previously constructed TKR nomogram [12]. The SMART registry and data dictionary [16] have been previously described in detail. The registry holds an extensive array of prospectively collected demographic, surgical and clinical data, as well as patient-reported outcome measures. An osteoarthritis questionnaire (Western Ontario and McMaster Universities Arthritis Index (WOMAC)) and a general health questionnaire Veteran’s Rand Survey (VR12) are routinely collected within 12 weeks of surgery and at regular time points post-surgery, including at 1 year. Data are loaded onto the SMART registry by dedicated registry staff. Data quality audits are conducted annually, with missing and implausible data investigated by the research team and rectified where possible.
2.3. Surgery
All patients underwent an elective primary THR, performed or supervised by one of 19 surgeons. The use of either cemented or uncemented implants was determined by surgeon preferences and implants were used consistently throughout the study period.
2.4. Outcome of Interest
Our primary outcome was the likelihood of non-response to THR 1 year after surgery. We used the same process for deriving the primary outcome as we did when constructing our prior knee nomogram [12]. Changes in pain, function and global scores post-THR, relative to baseline, were derived from the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), a self-administered measure of outcome of OA interventions, validated for use in THR [17]. We then used the OMERACT-OARSI responder criteria [18] to classify patients as either a responder or non-responder to THR based on the change in WOMAC scores. To ascertain whether a patient has achieved a meaningful response to THR, a normalised score (0 to 100) for each subscale of pain, function and global is created. Following OMERACT-OARSI criteria, a patient is considered a responder to THR if they report an improvement from baseline scores in either pain or function subscales of at least 50% and 20 points. If this threshold is not reached, then improvement in two of the three following criteria is also considered clinically meaningful; a minimum of 20% and 10 points in pain score; a minimum of 20% and 10 points in function score; and a minimum of 20% and 10 points in the global score. Patients who do not meet these thresholds are deemed non-responders and, according to OMERACT-OARSI criteria, have not achieved a clinically meaningful improvement from THR surgery.
2.5. Candidate Predictors
Pre-operative prognostic variables were chosen a priori as candidate predictors based on clinical reasoning and prior research. Aside from age, sex and aetiology, candidate predictors were limited to those that were considered potentially modifiable. Potential covariates therefore included smoking status and body mass index, which was assessed as both a continuous variable and according to World Health Organisation criteria as non-obese (BMI < 30 kg/m2), obese class I (30 ≤ BMI < 35 kg/m2), obese class II (35 ≤ BMI < 40) and obese class III (BMI ≥ 40 kg/m2). The American Anaesthesiologist’ (ASA) Physical Status Classification (1–4) [19] and the Charlson Co-morbidity Index (CCI) [20] were used as co-morbidity measures, with the latter assessed as a continuous variable and by each individual Charlson co-morbidity. The pre-operative anteroposterior radiographs taken within three months of surgery were used to assess radiographic osteoarthritis severity and assign a Kellgren–Lawrence (K-L) grade (0–4) [21]. Quality of life was derived from the routinely collected VR12 baseline physical (PCS) and mental (MCS) component scores [22].
Patient postcodes were used to assign a Socio-Economic Index for Areas (SEIFA) score (1–10) [23] and a geographic accessibility index (ARIA+) [24], which serve as a proxy for socio-economic status and remoteness. The use of interpreter services as a measure of English language proficiency was also recorded, but these were not included as candidate predictors. As the purpose of this study was to develop a predictive nomogram for use as a decision support tool prior to undergoing surgery, procedural characteristics such as prosthesis type, fixation and surgical approach as well as post-operative length of stay (days), discharge disposition (home vs. rehabilitation) and adverse events (according to Clavien–Dindo criteria) [25] are presented for descriptive purposes only.
2.6. Statistical Analyses
Categorical variables were presented as the frequency and percentage. Continuous variables were presented as the mean and standard deviation (SD) or the median and inter-quartile range (IQR). Aligning with the development of our knee nomogram [12], the statistical approach was a follows:
Unadjusted and then adjusted logistic regression models clustered on the individual patient were run, with the candidate predictors described above analysed for associations with non-response to THR surgery at 1 year.
Quadratic transformations were incorporated into the models to test for the linearity of association between candidate explanatory variables and the non-response endpoints.
Multivariable models were then assessed for collinearity and potential interactions between pairs of candidate predictors were further tested.
Overall fit of the model was tested using a Hosmer and Lemeshow goodness-of-fit test.
The Akaike and Bayesian Information criteria were used to assess relative goodness of fit between multiple, competing multivariable model solutions prior to the selection of the final model for the development of the final prognostic nomogram.
2.6.1. Nomogram Construction
Following the same method as previously described by Katten et al. [26], independent prognostic correlates of post-operative non-response that we observed in our adjusted modelling were used to derive a prognostic nomogram for non-response to THR.
2.6.2. Nomogram Validation
As per our knee nomogram [12], internal validation was derived via the concordance index and evaluation of nomogram calibration. The concordance index captures the likelihood that a study case drawn at random from the analysis dataset that was classified as a non-responder before another case drawn at random records a higher likelihood of non-response on the prognostic nomogram. The index was ascertained from the original 2177 THR used to derive the multivariable model described above, with 500 bootstrapped random samples. A separate round of 500 bootstrapped resamples was then used for calibration of the nomogram. Cases were categorized in accordance with their non-response probabilities as predicted by the nomogram and the mean scores of these probability groups were compared to the empirically observed non-response estimates on a calibration curve. The calibration curve is representative of the degree of agreement between predicted and observed values across a spectrum of predicted probabilities of non-response.
All analyses were performed using R version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria).
3. Results
3.1. Study Population
A total of 2248 primary elective THR were performed in 1950 patients during the study timeframe, of whom all had complete baseline data. Eight cases (16 hips) of simultaneous bilateral THR and 20 cases who underwent revision surgery within 1 year were excluded. Thirty-five cases were missing a 12 month WOMAC questionnaire and therefore also excluded due to death (n = 25), declining to complete the survey (n = 6) or having relocated overseas (n = 4). This left 2177 of 2212 (98.4%) eligible cases with complete data for analysis. In total, 194,2177 (8.9%) cases failed to reach the threshold for achieving a clinically meaningful response to surgery as per OMERACT-OARSI definitions and were considered non-responders to THR.
Demographic and clinical characteristics of the cohort are outlined in Table 1. The mean (SD) age was 66.5 (11.7) years and 1218 (58.9%) were female. Just over eight percent of the cohort required the use of an interpreter and 41.9% resided in a geographically disadvantaged area (SEIFA score ≤ 5) according to postcode. The probability of non-response to THR was higher amongst those who required an interpreter or were from a relatively disadvantaged postcode (Table 1).
Table 1.
Baseline patient demographics and characteristics.
| Variable | Overall (N = 2177) |
Responder (N = 1983) | Non-Responder (N = 194) | p-Value |
|---|---|---|---|---|
| Sex [N (%)] | 0.825 | |||
| Female | 1218 (55.9) | 1108 (91.0) | 110 (9.0) | |
| Male | 959 (44.1) | 875 (91.2) | 84 (8.8) | |
| Age [mean (SD)] | 66.5 (11.7) | 66.3 (11.7) | 68.0 (11.6) | 0.051 |
| $ BMI [mean (SD)] | 30.5 (6.2) | 30.4 (6.1) | 31.3 (6.5) | |
| Obesity Class [N (%)] | <0.043 | |||
| BMI < 30 kg/m2 | 1116 (51.3) | 1026 (91.9) | 90 (8.1) | |
| 30 ≤ BMI < 35 kg/m2 | 612 (28.1) | 560 (91.5) | 52 (8.5) | |
| 35 ≤ BMI < 40 kg/m2 | 286 (13.1) | 258 (90.2) | 28 (9.8) | |
| BMI ≥ 40 kg/m2 | 163 (7.5) | 139 (72.9) | 24 (14.7) | |
| & ASA Score [N (%)] | 0.013 | |||
| ≤2 | 1283 (58.9) | 1185 (92.4) | 98 (7.6) | |
| ≥3 | 894 (41.1) | 798 (89.3) | 96 (10.7) | |
| Charlson Comorbidity [N (%)] | ||||
| 0 | 1273 (58.8) | 1187 (93.2) | 86 (6.8) | <0.001 |
| 1 | 521 (23.9) | 469 (90.0) | 12 (10.0) | |
| ≥2 | 383 (17.6) | 327 (85.4) | 56 (14.6) | |
| Smoker [N (%)] | 0.307 | |||
| Current | 309 (14.2) | 275 (89.0) | 34 (11.0) | |
| Ex | 616 (28.3) | 567 (92.0) | 49 (8.0) | |
| Never | 1252 (57.5) | 1141 (91.1) | 111 (8.9) | |
| + K-L Grade [N (%)] | <0.001 | |||
| ≤3 | 616 (28.3) | 534 (86.7) | 82 (13.3) | |
| 4 | 1561 (71.7) | 1449 (92.8) | 112 (7.2) | |
| Aetiology [N (%)] | 0.328 | |||
| Osteoarthritis | 1869 (85.9) | 1711 (91.5) | 158 (8.5) | |
| Inflammatory Arthritis | 91 (4.2) | 80 (87.9) | 11 (12.1) | |
| Avascular Necrosis | 146 (6.7) | 129 (88.4) | 17 (11.6) | |
| Dysplasia | 71 (3.3) | 63 (88.7) | 8 (11.3) | |
| Pre ^ WOMAC-Pain [mean (SD)] | 65.6 (18.2) | 66.0 (18.0) | 61.6 (19.7) | 0.001 |
| Pre WOMAC-Motion [mean (SD)] | 70.0 (20.4) | 70.3 (20.3) | 66.9 (20.8) | 0.028 |
| Pre WOMAC-Function [mean (SD)] | 68.2 (17.5) | 68.6 (17.4) | 64.6 (17.7) | 0.002 |
| Pre WOMAC-Global [mean (SD)] | 67.8 (16.7) | 68.2 (17.2) | 64.1 (17.2) | 0.001 |
| Pre-surgery VR12 * MCS [mean (SD)] | 41.1 (14.9) | 41.2 (15.0) | 39.9 (14.1) | 0.226 |
| Pre-surgery VR12 # PCS [mean (SD)] | 24.3 (7.4) | 24.4 (7.4) | 23.9 (6.9) | 0.387 |
| Interpreter Required | 0.025 | |||
| Yes | 178 (8.2) | 170 (87.6) | 24 (12.4) | |
| No | 1999 (91.8) | 1829 (91.6) | 154 (8.4) | |
| Socio-Economic Index | 0.011 | |||
| ≤5 | 913 (41.9) | 815 (89.3) | 98 (10.7) | |
| ≥6 | 1264 (58.1) | 1168 (92.8) | 96 (7.2) | |
| Rurality [N (%)] | 0.428 | |||
| Metropolitan | 1731 (79.5) | 1581 (91.3) | 150 (8.7) | |
| Regional | 446 (20.5) | 402 (90.1) | 44 (9.9) |
$ BMI = body mass index; & ASA = American Society of Anaesthesiologists; + K-L = Kellgren–Lawrence; ^ WOMAC = Western Ontario and McMaster Universities Arthritis Index; * MCS = Mental Component Scale; # PCS = Physical Component Scale.
The average length of stay was 4.5 (2.6) days and 1723 (79.1%) of cases were discharged home, whereas 454 (20.9%) cases required inpatient rehabilitation. Most adverse events were minor; either Clavien–Dindo grade 1 (9.0%) or grade 2 (7.3%). In those who experienced a grade 3 complication (n = 90; 4.1%), a higher proportion (n = 16) were non-responders; however, these numbers were relatively low. There was no difference in responder rates between those who did or did not incur an unplanned readmission (Supplementary Table S1).
3.2. Predictors of Non-Response
On unadjusted modelling (Table 2), obese class III (BMI ≥ 40 kg/m2) was associated with 1.87-fold the odds of non-response (uOR 1.87; 95% CI 1.18, 2.98), relative to non-obese (BMI < 30 kg/m2). ASA Class ≥ 3 was associated with 1.45-fold the odds of non-response (uOR 1.45; 95% CI 1.08, 1.96) and CCI scores of 1 (uOR 1.53; 95% CI 1.06, 2.20) and ≥2 (uOR2.36; 95% CI 1.64, 3.40) were associated with an increased odds of non-response to THR. To assess whether the association between CCI ≥ 2 and non-response to THR was due to multiple Charlson co-morbidities or severity of the Charlson co-morbidity, each individual Charlson comorbidity as defined by Charlson et al., 1987 [27] was assessed for probability of non-response (Supplementary Table S2). This revealed that cerebrovascular disease (uOR 2.29; 95% CI 1.29, 4.07), chronic pulmonary disease (uOR 1.72; 95% CI 1.05, 2.82), connective tissue disease (uOR 1.90; 95% CI 1.13, 3.21), diabetes (uOR 1.83; 95% CI 1.26, 2.66) and mild liver disease (uOR 2.44; 95% CI 1.06, 5.61) correlated with non-response to THR (Table 2). Kellgren–Lawrence (K-L) grade < 4 also correlated with increased odds of non-response to THR relative to K-L 4 (uOR 1.99; 95% CI 1.47, 2.69) and each 10-point increase in baseline WOMAC Global score correlated with a reduction in post-operative non-response (uOR 0.87; 95% CI 0.80, 0.94).
Table 2.
Predictors of non-response to THR.
| Factor | Level | uOR (95% CI) | p-Value | aOR (95% CI) | p-Value * |
|---|---|---|---|---|---|
| Obesity Class III | <40 kg/m2 | Reference | Reference | ||
| >40 kg/m2 | 1.87 (1.18, 2.98) | 0.008 | 1.89 (1.16, 3.07) | 0.010 | |
| ^ Diabetes | 1.83 (1.26, 2.66) | 0.002 | 1.86 (1.26, 2.75) | 0.002 | |
| ^ Chronic pulmonary disease | 1.72 (1.05, 2.82) | 0.030 | NS | ||
| ^ Connective tissue disease | 1.90 (1.13, 3.21) | 0.016 | 1.95 (1.14, 3.33) | 0.014 | |
| ^ Cerebrovascular disease | 2.29 (1.29, 4.07) | 0.005 | 2.39 (1.33, 4.30) | 0.004 | |
| ^ Mild liver disease | 2.44 (1.06, 5.91) | 0.036 | NS | ||
| K-L grade | 4 | Reference | Reference | ||
| ≤3 | 1.99 (1.47, 2.69) | <0.001 | 1.91 (1.41, 2.61) | <0.001 | |
| Pre-op WOMAC Global ^ | 0.87 (0.80, 0.94) | 0.001 | 0.86 (0.79, 0.94) | <0.001 |
* Hosmer and Lemeshow: p > 0.05; ^ per 10 units; ^ comorbidities derived from the Charlson Comorbidity Index.
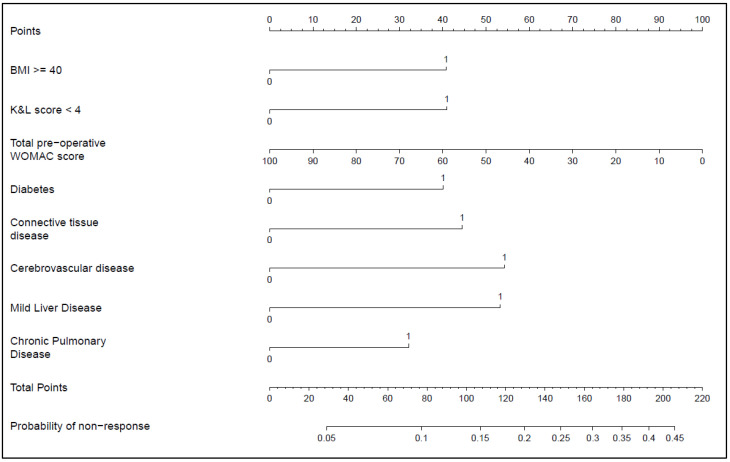
Two multivariable models were run (Supplementary Table S3), model one using the CCI score and model two using individual Charlson comorbidities, with the latter demonstrating a stronger association with probability of non-response to THR and therefore selected as the model of choice to construct our nomogram (Figure 1). The final candidate variables associated with an increased odds of non-response and selected for our nomogram (Table 2) were therefore obese class III (adjusted OR 1.88; 95% CI 1.16, 3.05), cerebrovascular disease (aOR 2.39; 95% CI 1.33, 4.30), chronic pulmonary disease (aOR 1.64; 1.00, 2.71), connective tissue disease (aOR 1.99; 95% CI 1.17, 3.39), diabetes (aOR 1.86; 95% CI 1.26, 2.75), liver disease (aOR 2.28: 0.99, 5.27) and K-L grade < 4 (aOR 1.89; 95% CI 1.39, 2.56). In contrast, each 10-point increase in pre-operative WOMAC Global score was associated with a 14% reduction in the odds of non-response (aOR 0.86; 95% CI 0.79, 0.94), when other predictors were controlled for. A worked example is provided as a Supplementary File (see Supplementary Figure S1).
Figure 1.
Nomogram for non-response to THR.
To estimate an individual’s likelihood of non-response to THR, match each response or level of predictor for each explanatory variable to the corresponding points (top line) and then sum to produce a total score. Match the total points to the corresponding probability of non-response scale (bottom line) to derive the final estimate.
3.3. Prognostic Nomogram
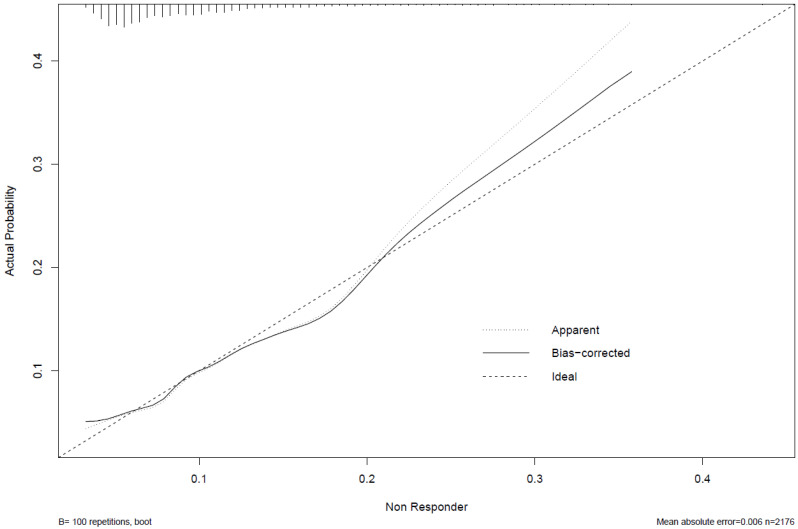
Independent correlates of non-response taken from adjusted logistic model two described above, were used to derive a prognostic nomogram that estimated the likelihood of non-response based on this suite of explanatory variables (Figure 1). The degree of contribution of each explanatory factor to non-response nomogram points in descending order was cerebrovascular disease, connective tissue disease, K-L grade, BMI ≥ 40 kg/m2, diabetes, and baseline WOMAC Global. The model’s concordance index was 0.70. The calibration curve (Figure 2) is an illustration of how accurately the nomogram predicted a probability of non-response when compared with the actual dataset-observed outcomes.
Figure 2.
Calibration curve. The x-axis represents the nomogram-predicted probability, the y-axis represents the empirically observed probability and the dashed diagonal line depicts perfect agreement between the predicted and observed probability of non-response to THR.
4. Discussion
In this large cohort study of 2177 consecutive cases of THR, we identified a non-responder rate of 9%, which is lower than for our TKR cohort (15%) and this is in keeping with the literature, whereby improvements in pain and function and satisfaction with THR are generally higher than for TKR [28,29]. Whilst our non-responder rate for THR was comparatively lower than for TKR, at a cost of $26 k per procedure [30], selection of people who do not respond to TJR could be costing Australia up to $119 million per year in direct hospital cost alone, on what can be regarded as low-value and wasteful care.
Both the THR cohort in the current study and TKR cohort [12] in our prior study shared some prognostic factors for non-response to surgery, including morbid obesity, and milder radiographic and clinical symptom severity; however, there were also some notable differences. Unique to our THR cohort, a patient’s comorbidity profile appeared to play an important role in predicting risk of non-response to surgery. We also found no association between pre-operative mental well-being and response to THR, which in contrast had featured strongly as a predictor of poor pain and function outcome in our TKR nomogram cohort [12]. These differences have important implications for tailoring non-operative therapy and pre-surgery optimisation programmes for people with advanced hip and knee OA who may be considering TJR.
Current Royal Australian College of General Practitioner (RACGP) guidelines [31] indicate that people who receive the best outcomes following TJR include those with well-controlled comorbidities, a BMI between 20 and 30, “good mental health status” and radiographic osteoarthritis severity of K-L grade 3 or 4. Yet our current study and the broader literature are not entirely congruent with these recommendations. For example, both our hip and knee nomograms suggest that a K-L grade < 4 is prognostic for poor response to surgery, and that BMI is only prognostic for poor pain and function outcomes in those who are morbidly obese [6,7,8,9,10,11,12].
Twenty eight percent of our THR cohort had a K-L grade 3 OA at the time of surgery, and eight percent were morbidly obese, indicating possible missed opportunities for guideline-endorsed [32] non-surgical treatment in a substantial proportion of this cohort. Although it is unclear from the current study what, if any, non-operative interventions were attempted by patients prior to presenting for surgery, it has been reported that 57% of Australians who present for TJR have not received appropriate first-line treatments such as exercise therapy and weight loss [33]. A substantial body of evidence does suggest that implementation of standardized evidence-based non-surgical therapies, even in those with established OA, can result in meaningful improvement in both pain and quality of life for many, as well as cost savings based on deferral or avoidance of TJR [34].
Morbid obesity poses a challenge to treat patients with advanced OA, with diet and education proving most effective in people with lower levels of obesity [35]. A recent randomised controlled trial of a new pharmacologic option appears safe and effective in those with severe obesity, although not specifically trialled in the OA population [36]. Our randomised clinical trial of bariatric surgery in patients with advanced knee OA demonstrated that one-third of severely obese patients (BMI ≥ 35 kg/m2) declined to proceed with planned TKR up to 5 years post-original consent for surgery, due to symptom improvement [37]. This suggests a role for surgical weight loss interventions in definitively managing advanced lower-limb OA in patients who are morbidly obese [38]. It is also worth noting, however, that more than 40% of our THR cohort had a BMI within the range of 30 and 39 kg/m2 and while this group may benefit from diet and exercise, delay in referral to an orthopaedic surgeon in this group may not be warranted based on our findings.
Several Charlson comorbidities predicted poor response to THR in the current study. Prior literature has shown that comorbidities, whether assessed individually or by using validated indices [39,40], are associated with poor functional outcome after THR. In people with OA, comorbidities such as respiratory and cardiovascular disease have been linked with lower physical activity levels, compared to those without significant comorbidities [41] and current evidence demonstrates that activity levels do not improve after THR [42,43]. This suggests a role for increasing physical activity in patients with significant comorbidities prior to undergoing THR to maximise post-operative outcomes. However, there is inadequate knowledge and motivation for adhering to physical activity recommendations among people with OA and concomitant comorbidities who undergo joint replacement surgery, which poses a barrier to change [44].
Aside from our final candidate predictors, there were several patient demographic variables that were associated with non-response to THR, but these were not included in our final model. Our decision not to include socio-economic status and English language proficiency despite a clear association with non-response to THR was based on our a priori decision to only include candidate predictors that were deemed modifiable. Our use of SIEFA as a marker of socio-economic status has limited applicability for individual patient-level risk prediction as it is a relative index of socio-economic advantage/disadvantage according to geographic location. Nonetheless, patients who present from geographically disadvantaged locations and/or poor English language proficiency may require assessment for additional community supports both before and after surgery.
There are several strengths to this study including the large sample size, the near-complete follow-up of the cohort, as well as a broad range of candidate predictors available for assessment. The methods used for assessing the internal validity of our tool were robust and the internal validation itself suggested good nomogram performance. However, best practice requires external validation to confirm the generalisability of prediction tools [45], which poses a challenge, given the lack of available and comparable datasets for TJR [46]. In that regard, external validity of our prognostic nomogram for knee replacement has been independently assessed and only partially supported [47], albeit in a dataset with notable differences in sample characteristics. While the external validation study [47] substantiated the importance of baseline radiographic and clinical symptom severity in predicting non-response to TKR, the roles of BMI and mental well-being (also predictors in our knee nomogram study) [12] were not supported. However, there were very few morbidly obese patients (n = 17) in the external validation cohort and the timeline for capturing mental well-being prior to surgery was variable, highlighting the limited scope when using study cohorts that do not mimic the source dataset.
It is worthwhile noting that while the registry used in this study contains a comprehensive array of variables, it is by no means exhaustive. It is therefore entirely possible that risk factors for poor response to THR exist that were not captured in the registry and therefore were not included in our model, although a recent systematic review of pre-operative predictors of THR outcomes does align with our findings [48]. We also excluded non-modifiable factors such as socio-economic status and the use of an interpreter from our model despite a higher rate of non-response to THR among these groups. We felt these factors are better addressed as part of surgery and discharge planning, rather than influencing the decision to refer to or proceed with surgery.
5. Conclusions
In this study, we observed several pre-operative patient characteristics that were associated with poor patient outcomes which informed the development of a prognostic calculator for estimating the likelihood of non-response to THR. We believe this is the first tool to predict non-response to THR based on patient-reported outcomes. Notability, we found that both the probability of and predictive prognostic factors for non-response to surgery differ between total hip and total knee replacement (TKR), confirming the benefit of designing decision support tools that are both condition and surgery site specific. Like our knee nomogram, with external validation, we envisage our nomogram for THR may serve as a useful tool that supports the shared decision-making process between patients and their allied health or general practitioners, when considering specialist referral or for use by surgeons to highlight with their patients opportunities for pre-surgery optimisation.
Acknowledgments
M.M.D. is the recipient of an NHMRC Career Development Fellowship (GNT1122526) and University of Melbourne Dame Kate Campbell Fellowship. P.F.M.C. is the recipient of an NHMRC Practitioner Fellowship (GNT1154203).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/jcm11061649/s1, Figure S1: Worked example of how to use nomogram. Table S1: Clinical characteristics. Table S2: Charlson Comorbidities. Table S3: Predictors of non-response to THR.
Author Contributions
All authors (M.M.D., T.S. and P.F.M.C.) made substantial contributions to conception and design, analysis, data interpretation and drafting this article. Authors who made a substantial contribution to acquisition of data: M.M.D. and P.F.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The SMART registry has been approved by the Human Research Ethics Committee of St. Vincent’s Hospital Melbourne, (HREC-A 100/14).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Consent for data sharing was not obtained and ethics approval would be required from the study institutions for future use of these data.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Elmallah R.K., Chughtai M., Khlopas A., Bhowmik-Stoker M., Bozic K.J., Kurtz S.M., Mont M.A. Determining Cost-Effectiveness of Total Hip and Knee Arthroplasty Using the Short Form-6D Utility Measure. J. Arthroplast. 2017;32:351–354. doi: 10.1016/j.arth.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 2.Australian Orthopedic Association National Joint Replacement Registry . Hip, Knee & Shoulder Arthroplasty: 2021 Annual Report. Australian Orthopedic Association National Joint Replacement Registry; Adelaide, Australia: 2021. [Google Scholar]
- 3.Ackerman I.N., Bohensky M.A., Zomer E., Tacey M., Gorelik A., Brand C.A., De Steiger R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019;20:90. doi: 10.1186/s12891-019-2411-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inacio M.C.S., Graves S., Pratt N., Roughead L., Nemes S. Increase in Total Joint Arthroplasty Projected from 2014 to 2046 in Australia: A Conservative Local Model with International Implications. Clin. Orthop. Relat. Res. 2017;475:2130–2137. doi: 10.1007/s11999-017-5377-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klem N.-R., Smith A., O’Sullivan P., Dowsey M.M., Schütze R., Kent P., Choong P.F., Bunzli S. What Influences Patient Satisfaction after TKA? A Qualitative Investigation. Clin. Orthop. Relat. Res. 2020;478:1850–1866. doi: 10.1097/CORR.0000000000001284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dowsey M.M., Gunn J., Choong P.F. Selecting those to refer for joint replacement: Who will likely benefit and who will not? Best Pr. Res. Clin. Rheumatol. 2014;28:157–171. doi: 10.1016/j.berh.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Chen A.T., Bronsther C.I., Stanley E.E., Paltiel A.D., Sullivan J.K., Collins J.E., Neogi T., Katz J.N., Losina E. The Value of Total Knee Replacement in Patients with Knee Osteoarthritis and a Body Mass Index of 40 kg/m2 or Greater: A Cost-Effectiveness Analysis. Ann. Intern. Med. 2021;174:747–757. doi: 10.7326/M20-4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mangla M., Bedair H., Dwyer M., Freiberg A., Sepucha K. Pilot Study Examining Feasibility and Comparing the Effectiveness of Decision Aids for Hip and Knee Osteoarthritis: A Randomized Trial. MDM Policy Pract. 2019;4:2381468319827278. doi: 10.1177/2381468319827278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sepucha K., Bedair H., Yu L., Dorrwachter J.M., Dwyer M., Talmo C.T., Vo H., Freiberg A.A. Decision Support Strategies for Hip and Knee Osteoarthritis: Less Is More: A Randomized Comparative Effectiveness Trial (DECIDE-OA Study) J. Bone Jt. Surg. Am. 2019;101:1645–1653. doi: 10.2106/JBJS.19.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konopka J.F., Hansen V.J., Rubash H.E., Freiberg A.A. Risk Assessment Tools Used to Predict Outcomes of Total Hip and Total Knee Arthroplasty. Orthop. Clin. N. Am. 2015;46:351–362. doi: 10.1016/j.ocl.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Bunzli S., Nelson E., Scott A., French S., Choong P., Dowsey M. Barriers and facilitators to orthopaedic surgeons’ uptake of decision aids for total knee arthroplasty: A qualitative study. BMJ Open. 2017;7:e018614. doi: 10.1136/bmjopen-2017-018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dowsey M.M., Spelman T., Choong P.F. Development of a Prognostic Nomogram for Predicting the Probability of Nonresponse to Total Knee Arthroplasty 1 Year After Surgery. J. Arthroplast. 2016;31:1654–1660. doi: 10.1016/j.arth.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 13.Dailiana Z.H., Papakostidou I., Varitimidis S., Liaropoulos L., Zintzaras E., Karachalios T., Michelinakis E., Malizos K.N. Patient-reported quality of life after primary major joint arthroplasty: A prospective comparison of hip and knee arthroplasty. BMC Musculoskelet. Disord. 2015;16:366. doi: 10.1186/s12891-015-0814-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dainty J.R., Smith T.O., Clark E.M., Whitehouse M.R., Price A.J., MacGregor A.J. Trajectories of pain and function in the first five years after total hip and knee arthroplasty: An analysis of patient reported outcome data from the National Joint Registry. Bone Jt. J. 2021;103:1111–1118. doi: 10.1302/0301-620X.103B6.BJJ-2020-1437.R1. [DOI] [PubMed] [Google Scholar]
- 15.Dowsey M.M., Choong P.F.M. Predictors of Pain and Function Following Total Joint Replacement. In: Kinov P., editor. Arthroplsaty—Update. Intech; Rijeka, Croatia: 2013. pp. 67–98. [DOI] [Google Scholar]
- 16.Gould D., Thuraisingam S., Shadbolt C., Knight J., Young J., Schilling C., Choong P.F., Dowsey M.M. Cohort profile: The St Vincent’s Melbourne Arthroplasty Outcomes (SMART) Registry, a pragmatic prospective database defining outcomes in total hip and knee replacement patients. BMJ Open. 2021;11:e040408. doi: 10.1136/bmjopen-2020-040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bellamy N.B.W., Goldsmith C. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J. Orthop. Rheumatol. 1988;1:95–108. [PubMed] [Google Scholar]
- 18.Escobar A., Gonzalez M., Quintana J.M., Vrotsou K., Bilbao A., Herrera-Espiñeira C., García-Pérez L., Aizpuru F., Sarasqueta C. Patient acceptable symptom state and OMERACT-OARSI set of responder criteria in joint replacement. Identification of cut-off values. Osteoarthr. Cartil. 2012;20:87–92. doi: 10.1016/j.joca.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 19.Owens W.D., Felts J.A., Spitznagel E.L., Jr. ASA physical status classifications: A study of consistency of ratings. Anesthesiology. 1978;49:239–243. doi: 10.1097/00000542-197810000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Hall W.H., Ramachandran R., Narayan S., Jani A.B., Vijayakumar S. An electronic application for rapidly calculating Charlson comorbidity score. BMC Cancer. 2004;4:94. doi: 10.1186/1471-2407-4-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kellgren J.H., Lawrence J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sanderson K., Andrews G. The SF-12 in the Australian population: Cross-validation of item selection. Aust. N. Z. J. Public Health. 2002;26:343–345. doi: 10.1111/j.1467-842X.2002.tb00182.x. [DOI] [PubMed] [Google Scholar]
- 23.Dowsey M.M., Nikpour M., Choong P.F. Outcomes following large joint arthroplasty: Does socio-economic status matter? BMC Musculoskelet. Disord. 2014;15:148. doi: 10.1186/1471-2474-15-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dowsey M.M., Petterwood J., Lisik J.P., Gunn J., Choong P.F. Prospective analysis of rural-urban differences in demographic patterns and outcomes following total joint replacement. Aust. J. Rural Health. 2014;22:241–248. doi: 10.1111/ajr.12100. [DOI] [PubMed] [Google Scholar]
- 25.Clavien P.A., Barkun J., de Oliveira M.L., Vauthey J.N., Dindo D., Schulick R.D., de Santibañes E., Pekolj J., Slankamenac K., Bassi C., et al. The Clavien-Dindo Classification of Surgical Complications: Five-year experience. Ann. Surg. 2009;250:187–196. doi: 10.1097/SLA.0b013e3181b13ca2. [DOI] [PubMed] [Google Scholar]
- 26.Kattan M.W., Leung D.H., Brennan M.F. Postoperative nomogram for 12-year sarcoma-specific death. J. Clin. Oncol. 2002;20:791–796. doi: 10.1200/JCO.2002.20.3.791. [DOI] [PubMed] [Google Scholar]
- 27.Charlson M.E., Pompei P., Ales K.L., MacKenzie C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Alzahrani K., Gandhi R., Debeer J., Petruccelli D., Mahomed N. Prevalence of Clinically Significant Improvement Following Total Knee Replacement. J. Rheumatol. 2011;38:753–759. doi: 10.3899/jrheum.100233. [DOI] [PubMed] [Google Scholar]
- 29.Quintana J.M., Escobar A., Arostegui I., Bilbao A., Azkarate J., Goenaga J.I., Arenaza J.C. Health-Related Quality of Life and Appropriateness of Knee or Hip Joint Replacement. Arch. Intern. Med. 2006;166:220–226. doi: 10.1001/archinte.166.2.220. [DOI] [PubMed] [Google Scholar]
- 30.Royal Australian College of Surgeons and Medibank . Surgical Variance Report 2017: Orthopaedic Surgery. RACGP; Melbourne, Australia: 2017. pp. 1–28. [Google Scholar]
- 31.Royal Australian College of Surgeons . Guidelines for the Management of Knee and Hip Osteoarthritis. 2nd ed. RACGP; Melbourne, Australia: 2018. [Google Scholar]
- 32.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., Callahan L., Copenhaver C., Dodge C., Felson D., et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020;72:149–162. doi: 10.1002/acr.24131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hinman R.S., Nicolson P.J.A., Dobson F.L., Bennell K.L. Use of Nondrug, Nonoperative Interventions by Community-Dwelling People With Hip and Knee Osteoarthritis. Arthritis Care Res. 2015;67:305–309. doi: 10.1002/acr.22395. [DOI] [PubMed] [Google Scholar]
- 34.Ackerman I.N., Skou S.T., Roos E.M., Barton C.J., Kemp J.L., Crossley K.M., Liew D., Ademi Z. Implementing a national first-line management program for moderate-severe knee osteoarthritis in Australia: A budget impact analysis focusing on knee replacement avoidance. Osteoarthr. Cartil. Open. 2020;2:100070. doi: 10.1016/j.ocarto.2020.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Losina E., Smith K.C., Paltiel A.D., Collins J.E., Suter L.G., Hunter D.J., Katz J.N., Messier S.P. Cost-Effectiveness of Diet and Exercise for Overweight and Obese Patients with Knee Osteoarthritis. Arthritis Care Res. 2019;71:855–864. doi: 10.1002/acr.23716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilding J.P.H., Batterham R.L., Calanna S., Davies M., Van Gaal L.F., Lingvay I., McGowan B.M., Rosenstock J., Tran M.T., Wadden T.A., et al. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N. Engl. J. Med. 2021;384:989–1002. doi: 10.1056/NEJMoa2032183. [DOI] [PubMed] [Google Scholar]
- 37.Dowsey M.M.B.W., Cochrane A., Burton P.R., Liew D., Choong P.F. Effect of Bariatric Surgery Preceding Total Knee Arthroplasty. A randomized Clinical Trial. JAMA Netw. Open. :2022. doi: 10.1001/jamanetworkopen.2022.6722. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rishi L., Bhandari M., Kumar R. Can bariatric surgery delay the need for knee replacement in morbidly obese osteoarthritis patients. J. Minimal Access Surg. 2018;14:13–17. doi: 10.4103/jmas.JMAS_129_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh J.A., Lewallen D.G. Predictors of Activity Limitation and Dependence on Walking Aids After Primary Total Hip Arthroplasty. J. Am. Geriatr. Soc. 2010;58:2387–2393. doi: 10.1111/j.1532-5415.2010.03182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buirs L.D., Beers L.W.A.H.V., Scholtes V.A.B., Pastoors T., Sprague S., Poolman R. Predictors of physical functioning after total hip arthroplasty: A systematic review. BMJ Open. 2016;6:e010725. doi: 10.1136/bmjopen-2015-010725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McKevitt S., Healey E., Jinks C., Rathod-Mistry T., Quicke J. The association between comorbidity and physical activity levels in people with osteoarthritis: Secondary analysis from two randomised controlled trials. Osteoarthr. Cartil. Open. 2020;2:100057. doi: 10.1016/j.ocarto.2020.100057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harding P., Holland A., Delany C., Hinman R.S. Do Activity Levels Increase After Total Hip and Knee Arthroplasty? Clin. Orthop. Relat. Res. 2014;472:1502–1511. doi: 10.1007/s11999-013-3427-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hawke L.J., Shields N., Dowsey M.M., Choong P.F.M., Taylor N.F. Effectiveness of behavioural interventions on physical activity levels after hip or knee joint replacement: A systematic review. Disabil. Rehabil. 2020;42:3573–3580. doi: 10.1080/09638288.2019.1603328. [DOI] [PubMed] [Google Scholar]
- 44.Hawke L.J., Taylor N.F., Dowsey M.M., Choong P.F.M., Shields N. In the dark about physical activity—Exploring patient perceptions of physical activity after elective total knee joint replacement: A qualitative study. Arthritis Care Res. 2021 doi: 10.1002/acr.24718. [DOI] [PubMed] [Google Scholar]
- 45.Manning D.W., Edelstein A., Alvi H.M. Risk Prediction Tools for Hip and Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2016;24:19–27. doi: 10.5435/JAAOS-D-15-00072. [DOI] [PubMed] [Google Scholar]
- 46.Rolfson O., Rothwell A., Sedrakyan A., Chenok K.E., Bohm E., Bozic K.J., Garellick G. Use of Patient-Reported Outcomes in the Context of Different Levels of Data. J. Bone Jt. Surg. Am. 2011;93((Suppl. S3)):66–71. doi: 10.2106/JBJS.K.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Riddle D.L., Golladay G.J., Jiranek W.A., Perera R.A. External Validation of a Prognostic Model for Predicting Nonresponse Following Knee Arthroplasty. J. Arthroplast. 2017;32:1153–1158.e1. doi: 10.1016/j.arth.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hofstede S.N., Gademan M.G.J., Vlieland T.V., Nelissen R., De Mheen P.J.M.-V. Preoperative predictors for outcomes after total hip replacement in patients with osteoarthritis: A systematic review. BMC Musculoskelet. Disord. 2016;17:212. doi: 10.1186/s12891-016-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Consent for data sharing was not obtained and ethics approval would be required from the study institutions for future use of these data.