Abstract
We studied the evolution of resistance to quinolones in Escherichia coli from 1992 to 1997 in Barcelona, Spain. An increasing proportion of quinolone-resistant E. coli (QREC) infections was observed. QREC strains were more common in patients with nosocomial infections but also increased in patients with community-acquired infections (9% in 1992 to 17% in 1996). Seventy (12%) of 572 episodes of E. coli bacteremia were due to QREC. Factors significantly associated with QREC bacteremia were the presence of underlying disease, recent exposure to antibiotics, and bacteremia of unknown origin. In the multivariate analysis, only prior exposure to antimicrobial agents (P < 0.001; odds ratio [OR] = 2), specifically, to quinolones (P < 0.001; OR = 14), and the presence of a urinary catheter (P < 0.001; OR = 2) were significantly associated with QREC bacteremia. Among 16 QREC isolates from cultures of blood of community origin selected at random, 13 different pulsed-field gel electrophoresis patterns were recognized, showing the genetic diversity of these isolates and in turn indicating the independent emergence of QREC in the community. The prevalence of QREC in the feces of healthy people was unexpectedly high (24% in adults and 26% in children). A survey of the prevalence of QREC of avian and porcine origin revealed a very high proportion of QREC in animal feces (up to 90% of chickens harbored QREC). The high prevalence of QREC in the stools of healthy humans in our area could be linked to the high prevalence of resistant isolates in poultry and pork.
The resistance of Escherichia coli to quinolones has remained rare until recently, when the widespread use of quinolones as therapy for urinary tract infections (1, 15, 16) and prophylaxis for patients with granulocytopenia (6–8) and cirrhosis (5) has been associated with the emergence of resistant strains. The resistant mutants are usually cross-resistant to other quinolones (19).
Recently, we have observed a considerable increase in the prevalence of resistance to ciprofloxacin among E. coli strains isolated from blood cultures in our hospital. In this study we describe the evolution of the resistance of E. coli to ciprofloxacin, the risk factors for quinolone-resistant E. coli (QREC) bacteremia, and the relationship between human consumption of quinolones in our area and the emergence of QREC. We also sought to establish a correlation between the resistance to quinolones of E. coli from animal sources and the emergence and dissemination of this resistance in the community.
MATERIALS AND METHODS
Population studied.
The Hospital Mútua de Terrassa is an acute-care center in Terrassa, a city in the province of Barcelona, Spain, that forms part of a health district of 230,000 inhabitants. It is a teaching institution with 500 beds; approximately 20,000 patients are admitted to the hospital each year. It serves as a referral center for certain pathologies for a population of circa 1 million, and it has a hematooncology unit with 22 inpatient rooms and an active peripheral bone marrow transplantation program. Fluoroquinolones were introduced in the country in 1986, and four drugs of this class were available at the time of the study. Prophylaxis with ciprofloxacin (500 mg twice daily) was given to cancer patients who were neutropenic (neutrophil count, <500 cells/mm3). This practice was introduced in early 1991 but was discontinued in December 1996. A prospective surveillance program of all patients with bacteremia has been active since 1988. Data for all patients are recorded in a computer-assisted protocol that includes patient demographics, chronic underlying disease, prior surgery, origin of bacteremia, presence of a urinary catheter, and prior receipt of antibiotics.
To study risk factors for the acquisition of QREC bacteremia, a comparison of patients with QREC bacteremia and patients with quinolone-susceptible E. coli bacteremia was carried out. For these purposes, we retrospectively examined all episodes of documented E. coli bacteremia from January 1992 to December 1997. Data on quinolone consumption in our area were provided by the National Center of Microbiology and by the local Section of Epidemiology of the Health Service of Catalonia (22). Consumption was expressed as defined daily doses (DDDs).
Definitions.
Bacteremia was considered to have been nosocomially acquired if it appeared 48 h after admission and no evidence of infection was present on admission; all other episodes of bacteremia were considered to have been community acquired. The source of infection was designated as one of the following: lower respiratory tract, urinary tract, surgical wound, intra-abdominal, intravenous catheter, or unknown origin. Complicated urinary tract infection (UTI) was defined as the infection of the urinary tract in the presence of anatomical (calculus stricture, prostatic enlargement) or functional (pregnancy, neurogenic bladder, vesicourethral reflux) abnormalities of the urinary tract. Prior antibiotic use was defined as administration of a fluoroquinolone or other antibiotic for more than 48 h during the previous 3 months. Overall mortality was defined as death during hospitalization.
Microbiological studies.
Blood cultures were performed by using an automated system (VITAL; bioMérieux, Marcy l’Etoile, France). E. coli strains recovered from blood were identified and tested for antimicrobial susceptibility by using Vitek commercial panels (bioMérieux Vitek, Inc., Hazelwood, Mo.). The MIC breakpoints for ciprofloxacin were as follows: susceptible, ≤1 μg/ml; resistant, ≥4 μg/ml. The breakpoints are recommended by the National Committee for Clinical Laboratory Standards (17).
Selected strains were further characterized by biotyping (21), serogrouping, and pulsed-field gel electrophoresis (PFGE). O grouping was done by bacterial agglutination with specific antisera (Rijksinstituut voor Volkgezondheid en Milieuhygiëne, Bilthoven, The Netherlands, and Difco Laboratories, Detroit, Mich.). Strains not agglutinated by any of the antisera were defined as nontypeable. Subtyping was performed by PFGE (10). DNA for PFGE analysis was prepared by a modification of the method of Smith et al. (23). Digestion of the agarose blocks was carried out with XbaI (Pharmacia P-L Biochemicals, Uppsala, Sweden) in accordance with the manufacturer’s instructions. PFGE was performed by orthogonal-field alternation electrophoresis (Gene Navigator; Pharmacia LKB Biotechnology, Uppsala, Sweden).
Sampling of stools was performed from 1996 to 1998. Stool samples from adults and children were obtained either with a rectal swab or from freshly passed feces and were transferred to the laboratory in Ames transport medium (Eurotubo, Barcelona, Spain). Sampling from animal sources was performed in 1997. Samples were obtained through a sterile incision of the large bowel under aseptic conditions and were transferred in Ames transport medium. Stool samples were routinely plated on MacConkey agar (bioMérieux) and MacConkey agar (Oxoid) supplemented with 2 μg of ciprofloxacin per ml. Biochemical identification was performed by standard methods. Antimicrobial susceptibility tests were performed by the agar diffusion method with Mueller-Hinton agar (bioMérieux), and the MICs were determined by the E test (AB Biodisc, Solna, Sweden).
Statistical methods.
All data were analyzed by using a statistical software package (SPSS). Student’s unpaired t test, Fisher’s exact test, and the chi-square test were used for univariate analysis of the significance of associations. The annual percentage of QREC was compared by an analysis for linear trend in proportions by the Mantel-Haenszel test. The independent importance of potential risk factors significantly associated with bacteremic patients in univariate analysis was evaluated by stepwise logistic regression analysis. Confounding effects were assessed by comparing odds ratios (ORs) in the original and extended models. All possible two-way interactions were analyzed by a stepwise addition procedure. Differences were considered statistically significant at a P value of ≤0.05 (two-tailed) for all tests.
RESULTS
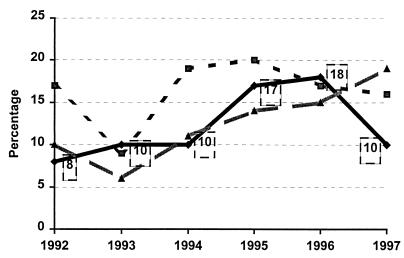
During the study period a total of 572 E. coli-positive blood cultures corresponding to 572 episodes of sepsis were obtained. Seventy (12.2%) of these episodes were due to QREC isolates. The prevalence of QREC strains among all patients with E. coli bacteremia steadily increased from 1992 to 1996: 8.3% in 1992, 9.8% in 1993, 10.3% in 1994, 17% in 1995, and 18% in 1996. In 1997, coincident with the discontinuation of the ciprofloxacin prophylaxis program for neutropenic patients, the annual incidence decreased to 10.4%, a rate similar to that found in the early years of the study (Fig. 1). The number of episodes due to community-acquired QREC infections increased significantly from 1992 to 1997 (P = 0.01; OR = 2.33), while the number of nosocomially acquired cases of QREC infections remained steady until 1997, when a sudden reduction at the expense of QREC isolates in neutropenic patients took place. Figure 1 shows the global trends of QREC bacteremia compared to the global prevalence of QREC among all community-acquired and nosocomial isolates.
FIG. 1.
Ciprofloxacin-resistant E. coli evolution (1992 to 1997). ⧫, isolates from blood (n = 564); ░⃞, nosocomially acquired isolates (n = 3,349); ▴, community-acquired isolates (n = 10,690). Numbers in squares indicate percentages of resistant isolates recovered each year.
Patients with QREC bacteremia (n = 70) were compared with patients with ciprofloxacin-susceptible E. coli bacteremia (n = 502), and the results are summarized in Table 1. Factors significantly associated with QREC bacteremia were the presence of chronic underlying disease (87 versus 33%; P < 0.001), recent exposure to antibiotics (51 versus 8%; P < 0.001), recent quinolone use (36 versus 1%; P < 0.0001), and an episode of unknown origin (27 versus 8%; P = 0.002). The overall mortality rate was 23% among patients with QREC bacteremia, whereas it was 11% among those infected with ciprofloxacin-susceptible strains (P < 0.0002).
TABLE 1.
Risk factors for ciprofloxacin-resistant E. coli bacteremia (univariate analysis)
| Characteristic | Patients infected with ciprofloxacin-susceptible strains (n = 502) | Patients infected with ciprofloxacin-resistant strains (n = 70) | OR (95% CIa) | P |
|---|---|---|---|---|
| Age (yr [mean ± SDb]) | 65.8 ± 18.4 | 70.2 ± 13 | 0.015 | |
| Sex (no. of males/no. of females) | 264/238 | 44/26 | 0.10 | |
| No. (%) of patients with: | ||||
| underlying disease | 164 (33) | 61 (87) | 3.2 (2–7) | 0.001 |
| Source of acquisition | ||||
| Community | 407 (81) | 39 (56) | ||
| Nosocomial | 95 (19) | 31 (44) | 3.4 (2–6) | <0.001 |
| Source of infection | ||||
| Urinary tract | 312 (62) | 32 (46) | 0.5 (0.3–0.8) | 0.009 |
| Intra-abdominal | 135 (26) | 17 (24) | NSc | |
| Unknown | 42 (8) | 19 (27) | 4 (2–8) | <0.001 |
| Urinary catheter | 68 (14) | 28 (40) | 4.3 (2.5–7.7) | <0.001 |
| Prior antibiotic use | 41 (8) | 36 (51) | 11.9 (7–25) | <0.001 |
| Prior quinolone use | 6 (1) | 25 (36) | 46 (17–117) | <0.001 |
| Mortality | 53 (11) | 20 (23) | 3.3 (2–6) | <0.001 |
CI, confidence interval.
SD, standard deviation.
NS, not significant.
In a logistic regression model in which QREC bacteremia was the dependent variable and which was adjusted for variables significant in the univariate analysis, prior exposure to antimicrobial agents (P < 0.001; OR = 2), specifically, to quinolones (P < 0.001; OR = 14), and the presence of a urinary catheter (P < 0.001; OR = 2) showed the strongest association with QREC bacteremia. When adjusted for severity of disease and comorbidity, the increased mortality in the QREC group was not significant.
The subset of patients with UTIs and febrile granulocytopenia was analyzed further. In patients with UTIs (Table 2) factors significantly associated with bacteremia were complicated UTI (47 versus 25%; P = 0.014), the presence of a urinary catheter (56 versus 17%; P < 0.0001), and prior use of quinolones (22 versus 2%; P < 0.0001; OR = 17). Fourteen of 18 granulocytopenic febrile patients with QREC bacteremia had been given prophylaxis with ciprofloxacin, whereas none of the 15 patients with ciprofloxacin-susceptible E. coli bacteremia had been given quinolone prophylaxis (P < 0.0001) (Table 3). In 1997, the discontinuation of the ciprofloxacin prophylaxis policy for granulocytopenic patients was associated with a drop in the rate of QREC bacteremia of nosocomial origin, and the global incidence reflects the increasing importance of community-acquired QREC bacteremia. No cases of cross-infection were documented.
TABLE 2.
Risk factors for ciprofloxacin-resistant E. coli bacteremia of urinary origin (univariate analysis)
| Characteristic | Patients infected with ciproflaxacin-susceptible strains (n = 312) | Patients infected with ciprofloxacin-resistant strains (n = 32) | P (OR) |
|---|---|---|---|
| Age (yr [mean ± SDa]) | 65 ± 18.2 | 72 ± 13.7 | 0.06 |
| Sex (no. of males/no. of females) | 125/187 | 21/11 | 0.005 |
| No. (%) of patients with: | |||
| Complicated UTI | 78 (25) | 15 (47) | 0.014 (2.6) |
| Urinary catheter | 53 (17) | 18 (56) | <0.001 (6.2) |
| Prior antibiotic use | 16 (5) | 10 (31) | <0.001 (8.4) |
| Prior quinolone use | 5 (2) | 7 (22) | <0.001 (17) |
SD, standard deviation.
TABLE 3.
Risk factors for ciprofloxacin-resistant E. coli bacteremia in febrile granulocytopenic patients (univariate analysis)
| Characteristic | Patients infected with ciprofloxacin-susceptible strains (n = 15) | Patients infected with ciprofloxacin-resistant strains (n = 18) | P (OR) |
|---|---|---|---|
| Age (yr [mean ± SDa]) | 63 ± 13 | 62 ± 11.3 | 0.26 |
| Sex (no. of males/no. of females) | 8/7 | 8/10 | NSb |
| No. (%) of patients with: | |||
| Urinary catheter | 0 | 3 (17) | 0.04 |
| Prior antibiotic use | 2 (13) | 17 (94) | <0.001 (110) |
| Prior quinolone use | 0 | 14 (78) | <0.001 |
SD, standard deviation.
NS, not significant.
The ciprofloxacin MICs for QREC strains ranged between 4 and 128 μg/ml, with a mean of 32 μg/ml. Of the 56 ciprofloxacin-resistant strains studied, all were cross-resistant to the other available quinolones, and 37.5% were resistant to three or more other antibiotics. The E. coli resistance patterns for ciprofloxacin-resistant and ciprofloxacin-susceptible strains are shown in Table 4. Ciprofloxacin-resistant strains were frequently associated with ampicillin, co-trimoxazole, and, more strikingly, gentamicin resistance (46% versus 4% for ciprofloxacin-susceptible strains; P < 0.001).
TABLE 4.
E. coli resistance patterns in ciprofloxacin-resistant and ciprofloxacin-susceptible strains
| Drug | % Strains resistant
|
P | |
|---|---|---|---|
| Ciprofloxacin-susceptible strains (n = 180) | Ciprofloxacin-resistant strains (n = 56) | ||
| Gentamicin | 4 | 46 | <0.001 |
| Co-trimoxazole | 23 | 67 | <0.001 |
| Cefotaxime | 1 | 7 | 0.009 |
| Amoxicillin-clavulanic acid | 8 | 16 | 0.06 |
| Ampicillin | 50 | 80 | <0.001 |
Among QREC isolates from patients with bacteremia of community origin, 16 isolates were selected at random and were further characterized by serotyping and PFGE. Thirteen different PFGE patterns were recognized among strains that belonged to 7 different serogroups (five nontypeable strains) and 13 different biotypes (Table 5), showing the genetic diversity of these QREC isolates and, in turn, indicating the independent emergence of resistant mutants in the community.
TABLE 5.
PFGE patterns, serogroups, and biotypes of 16 selected quinolone-resistant E. coli isolates from blood of patients with community-acquired infection
| Strain | PFGE pattern | Serogroup | Biotype |
|---|---|---|---|
| 92/80545H | 1 | 088 | K |
| 92/77239H | Lysed | AAa | M |
| 92/77650H | 2 | 025 | L |
| 92/77300H | 3 | 025 | L |
| 92/78119H | Lysed | 077 | J |
| 93/78326H0 | 4 | 0101 | H |
| 93/78167H | 5 | NTb | I |
| 94/T1304H | 6 | AA | D |
| 94/T1304H1 | 6 | AA | F |
| 94/00311H | 7 | NT | A |
| 94/T1107H1 | 8 | AA | E |
| 94/T1107H2 | 9 | AA | E |
| 94/78611H | 10 | NT | G |
| 95/60506H | 11 | 086 | A |
| 95/D1204H | 12 | 0160 | C |
| 95/M0306H | 13 | NT | B |
AA, autoagglutinable.
NT, nontypeable.
Prevalence of QREC in human fecal samples.
A total of 104 samples were collected from 104 adults, (42 males and 62 females; mean age, 69.8 years; age range, 17 to 94 years) who came to the hospital emergency room with a noninfectious diseases condition and who specifically denied having received antibiotics in the preceding 3 months. QREC strains were isolated from 25 (24%) patients. Twenty-nine strains of QREC were isolated from these 25 people; ciprofloxacin MICs ranged from 4 to ≥32 μg/ml, and 6 of the 29 strains (20.7%) were resistant to gentamicin.
The prevalence of QREC in stools from healthy children was also high. Of a total of 65 children studied (41 males and 24 females; mean age, 2.7 years; age range, 6 months to 9 years), the stools of 17 (26%) had E. coli isolates that were resistant to ciprofloxacin (MIC range, 4 to ≥32 μg/ml), and 5 of these strains (29.4%) were also resistant to gentamicin.
In 1998, 380 stool samples collected from children (212 males and 168 females; mean age, 2.4 years; age range, 6 months to 10 years) with gastroenteritis in the emergency room prior to any antibiotic exposure were also studied for the prevalence of QREC. One hundred fifty-two isolates (40%) were resistant to ciprofloxacin, with MICs ranging from 4 to ≥32 μg/ml. Of 75 strains selected at random, 18 (24.0%) were resistant to gentamicin.
Prevalence of QREC in animal fecal samples.
The prevalence of QREC (ciprofloxacin MIC range, 4 to ≥32 μg/ml) isolates from cattle, pigs, and poultry (chickens) was obtained for animals from three different slaughterhouses in the area around Barcelona. QREC isolates were not found in any of the 51 stool samples obtained from cattle, although an isolate from 1 sample was found to be resistant to nalidixic acid. Among the 56 samples from 56 different pigs, ciprofloxacin resistance was found for isolates from 25 (44.6%), almost half of the samples tested. Among the 105 isolates from poultry, 102 of 105 (97%) and 95 of 105 (90.4%) of the E. coli isolates were resistant to nalidixic acid and ciprofloxacin, respectively.
Quinolone consumption in the area.
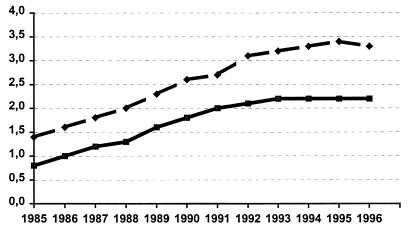
Information concerning fluoroquinolone consumption in the Barcelona area during the period of study was available from two sources. The first was from the National Health Care System Database. The number of metric tons of fluoroquinolones sold per year in the province of Barcelona and their conversion to DDDs/1,000 inhabitants are depicted in Fig. 2. The DDDs/1,000 inhabitants increased steadily from 1985 to 1992 (from 0.8 to 2.2) and then reached a plateau that lasted until 1996. Another independent source of data was the Prescriptions of Primary Health Service Database for the central region in Catalonia for the years 1991 to 1995 (22). DDDs/1,000 inhabitants were calculated for the most important antibiotic groups. Fluoroquinolone consumption was 1.8 DDDs/1,000 inhabitants in 1991 and increased steadily until 1993 (2.2 DDDs/1,000 inhabitants), when it reached a plateau that lasted until 1995, the last year of observation.
FIG. 2.
Consumption of quinolones in the province of Barcelona. ⧫, metric tons; ■, DDDs/1,000 inhabitants.
DISCUSSION
In this study we have shown that the incidence of QREC among isolates from the blood of patients with both community- and hospital-acquired infections was already high early in the 1990s (8.3% in 1992) and that it continued to increase, particularly at the community level (18% in 1996). Prior exposure of the patient to a fluoroquinolone was the single most significant risk factor for the isolation of resistant organisms; the urinary tract was the most frequent source of QREC bacteremia, in keeping with previous reports (1, 9, 18). In addition, patients with QREC bacteremia appeared to be sicker than control patients, as indicated by the number of underlying diseases, the number of cases of bacteremia of unknown origin, and the increased mortality rates. The latter, however, were not a significant finding in our regression model; in all likelihood, these higher mortality rates reflect the older age and the increased number and severity of underlying disorders among patients in the group infected with QREC. The high level of ciprofloxacin resistance in many of these isolates for which MICs are >64 μg/ml indicates the presence of multiple mutations. It is therefore very unlikely that these strains were selected in an individual patient with limited or no quinolone exposure. This constitutes a strong argument in favor of the preexistence of strains with at least some initial resistance mutations. Continued exposure to quinolones would explain the occurrence and relative frequency of occurrence of strains with high-level resistance to quinolones in the community.
A complicated UTI and the presence of a urinary catheter were also found to be risk factors associated with QREC infection in patients with UTIs, confirming the findings of others (9). The use of quinolones in patients harboring E. coli strains with single-step mutations would select isolates with high levels of resistance to ciprofloxacin. QREC may have emerged in direct response to the selective pressure exerted by antibiotic use. The lack of patient-to-patient spread of resistant organisms clearly indicates the preexistence of QREC strains that were colonizing these patients.
Fluoroquinolones have been shown to reduce the incidence of infections caused by gram-negative bacteria in patients with neutropenia (8), and their use as prophylactic agents in this population has been widespread among many hematology wards (7). All strains of E. coli isolated from cultures of blood from neutropenic patients with fever were susceptible to ciprofloxacin until 1992. However, 14 of 18 (78%) strains of E. coli isolated from blood cultures since then and until 1996, a period when all neutropenic patients received ciprofloxacin as prophylaxis, were resistant to ciprofloxacin. Our findings are similar to those reported by others (6) and confirm that the patients who are receiving quinolone prophylaxis are at risk of developing bacteremia caused by QREC strains. The fact that Carratalá et al. (6) isolated QREC from the stools of 40% of a group of neutropenic patients who were receiving norfloxacin prophylaxis, as well as the high prevalence of QREC that we have shown to occur in the stools of healthy adults, strongly suggests the rapid selection of preexisting resistant commensal organisms; it is not surprising that this phenomenon surfaces in a highly select group of individuals predisposed to QREC infection (2). A recently published study of QREC as a cause of bacteremia in neutropenic patients in Korea revealed little evidence of clonal spread, disproving the initial assumption of an outbreak that originated from a single clone (24).
The genetic diversity of the QREC isolates obtained from the blood of patients with community-acquired infection, as well as their prevalence in the feces of healthy subjects, indicates the widespread presence of resistant clones colonizing the population at large. Fluoroquinolones have been extensively used in Spain since 1988. If their extensive use in the community for many years were the exclusive explanation for such prevalence, it would be difficult to explain their high incidence in healthy adults and even more so in children never previously exposed to quinolones; it is also difficult to reconcile the high prevalence in our area with the low prevalence of these resistant mutants in other locations where the use of fluoroquinolones has been equally extensive. There must be, therefore, other factors that explain the present situation of the high prevalence of QREC in our community.
The high prevalence of QREC in the feces of healthy people from the area was a surprise. The healthy members of a community represent the largest reservoir for bacteria resistant to antimicrobial agents (13). This excess resistance could be due to environmental conditions, such as poor sanitation or contamination of food, that tend to disseminate resistant strains and/or to practices related to the use of antimicrobial agents that select for the overgrowth of resistant strains (12). In our study, without minimizing the importance of the high level of consumption of antimicrobial agents, including quinolones, in our area, environmental effects are strongly suggested by the observation that the isolates from children who had never received quinolones had no lower levels of resistance than isolates from adults who had possibly been exposed to them. Also, the transmission of resistant isolates might occur between adults and children in families or day care and school settings. The higher prevalence (40%) of QREC in the feces of sick children with gastroenteritis was unexpected and remains unexplained.
In this regard, it was of great importance to know that the prevalence of QREC of avian origin is very high in our area. The presence of QREC in poultry has been documented previously. A recent study from Spain (4) showed a prevalence of QREC of up to 17% among sick chickens and 7% in the feces of healthy chickens. The occurrence of resistance in E. coli from animal sources, specifically, chickens, has been linked to the use of enrofloxacin in this animal population since 1990. In our study, 45% of pigs and 90% of chickens harbored QREC. To our knowledge, this is the highest prevalence of resistance to quinolones in E. coli that has ever been reported. The different prevalences of QREC between pigs and chickens could be related to the different intensities and durations of their exposure to quinolones. In Spain, the addition of antimicrobial agents to feed or water for therapeutic or prophylactic purposes remains uncontrolled, and the volume of drugs used is high (3). It may be, as pointed out by Blanco et al. (4), that the abusive and anarchic use of antibiotics is probably the leading factor for the high percentages of resistance detected among Spanish avian E. coli strains. Intensively farmed animals are often treated as a group and are given massive amounts of medication, and antibiotics may select for resistant E. coli strains in the fecal flora. The animals are in constant contact with feces and are therefore also continuously exposed to contamination with fecal bacteria.
More than one-third of QREC isolates from humans were resistant to three or more other antimicrobial agents. Strikingly, the rate of the coresistance to gentamicin among these strains was very high (46%); the reason for this has not been determined. In any event, the real problem of such multiple-drug-resistant strains is that the use of any one of these antibiotics may lead to selection and maintenance of resistance to other agents as well.
Our study has several limitations. The first is that the total number of slaughterhouses in the area is 29, but we studied samples from only 3 of them. The number of animals killed daily is in the thousands, but our study included samples from only several hundred obtained at random during an 8-day period. A second limitation is that we have not proved that this extraordinarily high prevalence of resistance to quinolones in E. coli was due to the use or misuse of enrofloxacin. It is known, however, that the level of consumption of enrofloxacin in veterinary medicine is high and that in Spain several generic forms of enrofloxacin are marketed. Another limitation of our study is that we have not demonstrated the link between QREC strains from animal sources and QREC strains that produce infections in humans. Beyond the formidable methodological difficulties that determination of such a link would entail, it was conclusively shown some time ago by Linton (14) that antibiotic-resistant E. coli could be transferred from poultry to a food-handler’s hands during food preparation and, finally, to the foodstuff. The transmission of enteric bacteria to consumers via this route has been established, and prevention of food poisoning is the basis for food hygiene and public health regulations in many countries (20). Consequently, we believe that this offers the best available explanation for the high prevalence of these resistant strains in the guts of healthy children in our area.
In summary, we have shown an increasing prevalence of QREC in our community during this decade. It has now reached a frequency of 22%. We have also shown that prior use of quinolones, the presence of a urinary catheter, and the use of these agents as prophylaxis for neutropenic patients are risk factors for blood-borne QREC infections. We believe that the high prevalence of QREC in the feces of healthy people, including children, in our area should be linked to the high prevalence of these resistant strains in poultry and pork. These findings should reinforce the message that control of the spread of antibiotic resistance requires the prudent use of antibiotics not only in humans but also in veterinary medicine (11).
ACKNOWLEDGMENT
This study received financial support from El Fondo de Medicina, Hospital Mútua de Terrassa, Barcelona, Spain.
REFERENCES
- 1.Aguiar J M, Chacon J, Canton R, Baquero F. The emergence of highly fluoroquinolone-resistant E. coliin community-acquired urinary tract infections. J Antimicrob Chemother. 1992;29:349–350. doi: 10.1093/jac/29.3.349. [DOI] [PubMed] [Google Scholar]
- 2.Ball P. Is resistant Escherichia coli bacteremia an inevitable outcome for neutropenic patients receiving a fluoroquinolone as prophylaxis? Clin Infect Dis. 1995;20:561–563. . (Editorial response.) [Google Scholar]
- 3.Baquero F the Task Force of the General Direction for Health Planning of the Spanish Ministry of Health. Antibiotic resistance in Spain: what can be done? Clin Infect Dis. 1996;23:819–823. doi: 10.1093/clinids/23.4.819. [DOI] [PubMed] [Google Scholar]
- 4.Blanco J E, Blanco M, Mora A, Blanco J. Prevalence of bacterial resistance to quinolones and other antimicrobials among avian Escherichia colistrains isolated from septicemic and healthy chickens in Spain. J Clin Microbiol. 1997;35:2184–2185. doi: 10.1128/jcm.35.8.2184-2185.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campillo B, Dupeyron C, Richardet J P, Mangeney N, Leluan G. Epidemiology of severe hospital-acquired infections in patients with liver cirrhosis: effect of long-term administration of norfloxacin. Clin Infect Dis. 1998;26:1066–1070. doi: 10.1086/520273. [DOI] [PubMed] [Google Scholar]
- 6.Carratalá J, Fernandez-Sevilla A, Tubau F, Callis M, Gudiol F. Emergence of quinolone-resistant Escherichia colibacteremia in neutropenic patients with cancer who have received prophylactic norfloxacin. Clin Infect Dis. 1995;20:557–560. doi: 10.1093/clinids/20.3.557. [DOI] [PubMed] [Google Scholar]
- 7.Cometta A, Calandra T, Bille J, Glauser M P. Escherichia coliresistant to fluoroquinolones in patients with cancer and neutropenia. N Engl J Med. 1994;330:1240–1241. doi: 10.1056/NEJM199404283301717. [DOI] [PubMed] [Google Scholar]
- 8.Dekker A W, Rozemberg-Arska M, Verhoef J. Infection prophylaxis in acute leukemia: a comparison of ciprofloxacin with trimethroprim-sulfamethoxazole and colistin. Ann Intern Med. 1987;106:7–12. doi: 10.7326/0003-4819-106-1-7. [DOI] [PubMed] [Google Scholar]
- 9.Ena J, Amador C, Martínez C, Ortiz de la Tabla V. Risk factors for acquisition of urinary tract infections caused by ciprofloxacin resistant Escherichia coli. J Urol. 1995;193:117–120. doi: 10.1097/00005392-199501000-00040. [DOI] [PubMed] [Google Scholar]
- 10.Gautom R K. Rapid pulsed-field gel electrophoresis protocol for typing of Escherichia coliO157:H7 and other gram-negative organisms in 1 day. J Clin Microbiol. 1997;35:2977–2980. doi: 10.1128/jcm.35.11.2977-2980.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson A P. Veterinary use of antimicrobial agents and problems of resistance in human bacterial infections. J Antimicrob Chemother. 1997;39:285–286. doi: 10.1093/jac/39.2.285. [DOI] [PubMed] [Google Scholar]
- 12.Kunin C M, Lipton H L, Tupasi T, Sacks T, Scheckler W E, Jivani A, et al. Social, behavioural, and practical factors affecting antibiotic use worldwide: report of Task Force 4. Rev Infect Dis. 1987;9(Suppl. 3):S270–S285. doi: 10.1093/clinids/9.supplement_3.s270. [DOI] [PubMed] [Google Scholar]
- 13.Lester S C, Pla M P, Wang F, Perez Schael I, Jiang H, O’Brien T F. The carriage of Escherichia coliresistant to antimicrobial agents by healthy children in Boston, in Caracas, Venezuela, and in Quin Pu, China. N Engl J Med. 1990;323:285–289. doi: 10.1056/NEJM199008023230501. [DOI] [PubMed] [Google Scholar]
- 14.Linton A H. Animal to man transmission of Enterobacteriaceae. R Soc Health J. 1977;97:115–118. doi: 10.1177/146642407709700308. [DOI] [PubMed] [Google Scholar]
- 15.Llordés M, Xercavins M, Quintana S, Garau J. Program and abstracts of the 6th European Congress on Clinical Microbiology and Infectious Diseases. Seville, Spain: European Society of Clinical Microbiology and Infectious Diseases; 1993. Emergence of ciprofloxacin resistance in E. coli, abstr. 1041. [Google Scholar]
- 16.Muder R R, Brennen C, Goetz A M, Wagener M M, Rihs J D. Association with prior fluoroquinolone therapy of widespread ciprofloxacin resistance among gram-negative isolates in a Veterans Affairs medical center. Antimicrob Agents Chemother. 1991;35:256–258. doi: 10.1128/aac.35.2.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard M7-A3. 3rd ed. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1993. [Google Scholar]
- 18.Peña C, Pallarés R, Pujol M, Tubau F, Ariza J. Relationship between quinolone use and emergence of ciprofloxacin-resistant Escherichia coli. Antimicrob Agents Chemother. 1995;39:520–524. doi: 10.1128/aac.39.2.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piddock L J V, Wise R. Mechanisms of resistance to quinolones and clinical perspectives. J Antimicrob Chemother. 1989;23:475–483. doi: 10.1093/jac/23.4.475. [DOI] [PubMed] [Google Scholar]
- 20.Piddock L J V. Does the use of antimicrobial agents in veterinary medicine and animal husbandry select antibiotic resistant bacteria that infect man and compromise antimicrobial therapy? J Antimicrob Chemother. 1996;38:1–3. doi: 10.1093/jac/38.1.1. [DOI] [PubMed] [Google Scholar]
- 21.Richard C. Une methode simple de marquage épidemiologique: la biotypie, application a Enterobacter cloacae & Escherichia coli. Bull Assoc Anc Elèves Inst Pasteur. 1981;87:14–21. [Google Scholar]
- 22.Sale M R, Campanera M T. Program and abstracts of the 7th International Congress for Infectious Diseases. Hong Kong: International Society for Infectious Diseases; 1993. Trends in antibiotic prescription in primary health care in Catalonia, abstr. 60.015; p. 138. [Google Scholar]
- 23.Smith C L, Klco S R, Cantor C R. Pulsed-field gel electrophoresis and the technology of large DNA molecules. In: Davies K, editor. Genome analysis: a practical approach. Oxford, United Kingdom: IRLC Press; 1997. pp. 41–72. [Google Scholar]
- 24.Yoo I-H, Huh D-H, Choi J-H, Shim W-S, Kang M-W, Kim C-C, Kim D-J. Molecular epidemiological analysis of quinolone-resistant Escherichia colicausing bacteremia in neutropenic patients with leukemia in Korea. Clin Infect Dis. 1997;25:1385–1391. doi: 10.1086/516132. [DOI] [PubMed] [Google Scholar]