Abstract
Rubinosporus, a new bolete genus from tropical forests of Thailand is introduced with R. auriporus as the type species. The genus is unique among Xerocomoideae in producing dark ruby spore deposits. It can be differentiated from all other Boletaceae genera by the following combination of characters: pileus surface evenly covered with matted tomentum; stipe surface with evenly scattered minute squamules; golden yellow tubular hymenophore, which is relatively thin especially when young; unchanging surfaces and context when bruised or cut; smooth, broadly ellipsoid basidiospores; and dark ruby spore deposits. The Boletaceae-wide and Xerocomoideae-wide phylogenetic analyses based on four-gene data sets (atp6, cox3, rpb2, and tef1) support Rubinosporus as monophyletic and places it in Boletaceae subfamily Xerocomoideae. Full descriptions and illustrations of the new genus and species are presented.
Keywords: fungal diversity, multigene phylogeny, new genus, taxonomy
1. Introduction
The family Boletaceae has been widely studied for over one hundred years. The former traditional taxonomy was based only on morphological characters. However, since molecular techniques and phylogenetic analyses have been developed and used as an advanced tool for the modern concepts in systematics and taxonomy, many genera, and species in Boletaceae have been recognized and described as new, e.g., [1,2,3]. In the last five years only, nine new Boletaceae genera have been described worldwide, namely Afrocastellanoa M.E. Smith & Orihara, Cacaoporus Raspé & Vadthanarat, Carolinigaster M.E. Sm. & S. Cruz, Erythrophylloporus Ming Zhang & T.H. Li, Indoporus A. Parihar, K. Das, Hembrom & Vizzini, Ionosporus O. Khmelnitsky, Phylloporopsis Angelini, A. Farid, Gelardi, M.E. Smith, Costanzo, & Vizzini, Spongispora G. Wu, S.M.L. Lee, E. Horak & Zhu L. Yang, and Longistriata Sulzbacher, Orihara, Grebenc, M.P. Martín & Baseia [4,5,6,7,8,9,10,11,12]. Five of those genera were described from tropical to subtropical Asia, where high fungal diversity has been reported, but yet, remains poorly known to science, e.g., [13,14].
Based on the current multiple gene phylogenies, the Boletaceae are classified into six sub-families and one phylogenetically unsupported group [2,3]. Xerocomoideae is one of the six sub-families, which consists of nine genera namely Alessioporus Gelardi, Vizzini & Simonini, Aureoboletus Pouzar, Boletellus Murrill, Heimioporus E. Horak, Hemileccinum Šutara, Hourangia Xue T. Zhu & Zhu L. Yang, Phylloporus Quél., Pulchroboletus Gelardi, Vizzini & Simonini, and Xerocomus Quél [2,3]. Two additional genera, Corneroboletus and Sinoboletus, were also described in the subfamily but were later synonymized with Hemileccinum and Aureoboletus, respectively [3]. Typical characters of this subfamily are boletoid or phylloporoid basidiomata; dry or viscid pileus with smooth or subtomentose to tomentose pellis; absent or rarely present veil; yellowish to yellow context; often bluing or sometimes redding or unchanging; smooth or ornamented stipe surface; basidiospores with bacillate, reticulate, longitudinally striate, or pitted ornamentations, or occasionally smooth; spore deposit with more or less olive-brown tint [2,3,15].
We have carried out surveys of the diversity of boletes in Thailand since 2010. Some collections with striking morphological characters were made and carefully studied. The collections combined typical characters of two genera that are widely distributed in tropical to subtropical regions, Aureoboletus with a golden yellow hymenium, and Baorangia G. Wu & Zhu L. Yang, which has a thin hymenophoral layer [3,16,17]. However, the collections showed a surprising dark ruby spore deposit, which is clearly distinct from the two genera and other genera in Boletaceae. Therefore, family-wide and subfamily-wide phylogenies were performed and showed that the collections belong in a generic lineage different from other genera in Boletaceae. Consequently, a new genus and a new species are introduced, with full descriptions and illustrations.
2. Materials and Methods
2.1. Specimens Collecting
The specimens were collected during the rainy season, from May to June, between 2015 and 2017, in Chiang Mai Province, northern Thailand. The specimens were wrapped in aluminum foil and taken to the laboratory for morphological description. After the description of macroscopic characters, the specimens were dried in an electric drier at 45–50 °C for 24 h or until dried properly. Then, they were deposited in the following herbaria Chiang Mai University (CMUB), and Meise Botanic Garden (BR) [18].
2.2. Morphological Study
The macroscopic descriptions were made based on detailed field notes and photos of fresh basidiomata taken in the habitat and the laboratory. Color codes were given based on a Methuen Handbook of Colour [19]. Chemical solutions including 10% potassium hydroxide (KOH) and 28–30% ammonium hydroxide (NH4OH), were used to determine the chemical reactions (color reactions) of the pileus, pileus context, stipe, stipe context, and hymenophore. For the microscopical study, the dried specimens were observed using 5% KOH, NH4OH, Melzer’s reagent, or stained with 1% ammoniacal Congo red. A minimum of 50 basidiospores, 20 basidia, and 20 cystidia were randomly measured under a Nikon Eclipse Ni microscope using the NIS-Elements D software. The notation ‘[m/n/p]’ represents the number of basidiospores “m” measured from “n” basidiomata of “p” collections. Dimensions of microscopic structures are presented in the following format: (a–)b–c–d(–e), in which “c” represents the average, “b” the 5th percentile, “d” the 95th percentile, “a” the minimum, and “e” the maximum. Q, the length/width ratio, is presented in the same format. Section of the pileus surface was radially and perpendicularly cut to the surface at a point halfway between the center and margin of the pileus. Sections of stipitipellis were taken from halfway up the stipe and longitudinally cut perpendicularly to the surface. All microscopic features were drawn by freehand using an Olympus Camera Lucida model U−DA fitted to Olympus CX31 compound microscope. For scanning electron microscopy (SEM), a spore print was mounted onto an SEM stub with double-sided carbon tape. The sample was coated with gold, then examined and photographed with a JEOL JSM–5910 LV SEM (JEOL, Tokyo, Japan).
2.3. DNA Extraction, PCR Amplification and DNA Sequencing
Genomic DNA was extracted from about 10–15 mg of dried specimen or fresh tissue preserved in cetyltrimethylammonium bromide (CTAB), using a CTAB isolation procedure adapted from Doyle and Doyle [20]. Portions of the genes atp6, cox3, rpb2, and tef1 were amplified by polymerase chain reaction (PCR) and sequenced by Sanger sequencing. The primer pairs ATP6-1M40F/ATP6-2M [21], COX3M1-F/ COX3M1-R [11], bRPB2-6F/bRPB2-7.1R [22], and EF1-983F/EF1-2218R [23] were used to amplify atp6, cox3, rpb2, and tef1, respectively. PCR products were purified by adding 1 U of exonuclease I and 0.5 U FastAP alkaline phosphatase (Thermo Scientific, St. Leon-Rot, Germany) and incubated at 37 °C for 1 h, followed by inactivation at 80 °C for 15 min. Standard Sanger sequencing was performed in both directions by Macrogen with PCR primers, except for atp6, for which universal primers M13F-pUC(−40) and M13F(−20) were used; for tef1, additional sequencing was performed with two internal primers, EF1-1577F and EF1-1567R [23].
2.4. Alignment and Phylogeny Inference
The two reads of newly generated sequences were assembled in GENEIOUS Pro v. 6.0.6 (Biomatters). A Boletaceae-wide sequence dataset, including selected sequences representative of the whole family, downloaded from GenBank, was aligned using MAFFT [24] on the server accessed at http://mafft.cbrc.jp/alignment/server/ (accessed on 19 December 2021). For this dataset, the introns in rpb2 and tef1 were removed based on the amino acid sequence of previously published sequences. Maximum likelihood (ML) phylogenetic inference was performed using RAxML on the CIPRES web portal (RAxML-HPC2 on XSEDE) [25,26]. The phylogenetic tree was inferred by a single analysis with four partitions (one for each gene), using the general time reversible computerized adaptive testing (GTRCAT) model with 25 categories. The outgroup consisted of two Buchwaldoboletus and seven Chalciporus species from sub-family Chalciporoideae, based on previous phylogenies e.g., [1,2,3,11]. Statistical support of clades was obtained with 1000 rapid bootstrap replicates. For Bayesian Inference (BI), the best-fit model of substitution among those implementable in MrBayes was estimated separately for each region using jModeltest [27] on the CIPRES portal, based on the Bayesian Information Criterion (BIC). The selected models were HKY + I + G for atp6, GTR+I+G for cox3 and tef1 exons, and K80 + I + G for rpb2 exons. Partitioned Bayesian analysis was performed with MrBayes 3.2.6 software for Windows [28]. Two runs of five chains were run for 11,000,000 generations and sampled every 1000 generations. The chain temperature was decreased to 0.02 to improve convergence. At the end of the run, the average deviation of split frequencies was 0.008614. A total of 8252 trees were used to construct a 50% majority rule consensus tree and calculate the Bayesian posterior probabilities (BPP).
For a subfamily Xerocomoideae-wide tree, all selected taxa in Xerocomoideae were aligned using the MAFFT online software (introns included). ML phylogenetic tree was inferred by a single analysis with five partitions (atp6, cox3, rpb2 exons, tef1 exons, and intron of rpb2 + introns of tef1) (one for each gene), outgroup were four Butyriboletus species in Pulveroboletus group, using the same analytical software and model used for family Boletaceae-wide tree. For BI, the same analytical software for family Boletaceae-wide tree was used. However, the selected models were GTR+I+G for atp6, cox3, and intron of rpb2 + introns of tef1, K80 + I + G for rpb2 exons, and SYM+I+G for tef1 exons. Two runs of five chains were sampled every 200 generations and stopped after 800,000 generations. At the end of the run, the average deviation of split frequencies was 0.007928. A total of 2709 trees were used to construct a 50% majority rule consensus tree and calculate the BPPs.
3. Results
3.1. Phylogenetic Analyses
A total of fourteen sequences were newly generated in this study and deposited in GenBank (Table 1). For the Boletaceae-wide tree, the alignment contained 776 sequences comprising four genes (162 for atp6, 133 for cox3, 231 for rpb2, 250 for tef1) from 257 voucher specimens corresponding to 252 taxa, and was 2946 characters long (TreeBase number: 28,349). The sequences of Rubinosporus voucher SV0934 (atp6 and cox3) were not added to the analyses because they were identical to the holotype (SV0090). Maximum likelihood and BI trees of the combined four-gene dataset were similar in topology, without any supported conflict (BS ≥ 70% and PP ≥ 0.90). The phylogram of RAxML bipartition (Figure 1) retrieved the six subfamily clades, namely Austroboletoideae (BS = 99% and PP = 1), Boletoideae (BS = 54% and PP = 0.93), Chalciporoideae (BS = 100% and PP = 1), Leccinoideae (BS = 99% and PP = 1), Xerocomoideae (BS = 99% and PP = 1), and Zangioideae (BS = 100% and PP = 1). The Pulveroboletus group of Wu et al. [2,3] was not monophyletic, like in previously published phylogenies. However, the monophyly of each genus in this group was highly supported. The selected Rubinosporus auriporus specimens were monophyletic (BS = 100% and PP = 1) and clustered in the highly supported Xerocomoideae clade.
Table 1.
List of collections used for phylogenetic analyses, with origin, GenBank accession numbers, and reference(s).
| Species | Voucher | Origin | atp6 | cox3 | rpb2 | tef1 | Reference(s) |
|---|---|---|---|---|---|---|---|
| Afroboletus aff. multijugus | JD671 | Burundi | MH614651 | MH614794 | MH614747 | MH614700 | [11] |
| Afroboletus costatisporus | ADK4644 | Togo | KT823958 | MH614795 * | KT823991 | KT824024 | [21], [11] * |
| Afroboletus luteolus | ADK4844 | Togo | MH614652 | MH614796 | MH614748 | MH614701 | [11] |
| Aureoboletus auriflammeus | CFMR:BOS-699 | USA | – | – | MK766269 | MK721060 | [29] |
| Aureoboletus catenarius | HKAS54467 | China | – | KT990349 | KT990711 | [3] | |
| Aureoboletus duplicatoporus | HKAS50498 | China | – | – | KF112754 | KF112230 | [2] |
| Aureoboletus formosus | GDGM44441 | China | – | – | KT291751 | KT291744 | [30] |
| Aureoboletus gentilis | ADK4865 | Belgium | KT823961 | MH614797 * | KT823994 | KT824027 | [21], [11] * |
| Aureoboletus glutinosus | GDGM44477 | China | – | – | MH700229 | MH700205 | [31] |
| Aureoboletus innixus | CFMR:BOS-544 | USA | – | – | MK766270 | MK721061 | [29] |
| Aureoboletus moravicus | VDKO1120 | Belgium | MG212528 | MH614798 * | MG212615 | MG212573 | [32], [11] * |
| Aureoboletus nephrosporus | HKAS74929 | China | – | – | KT990358 | KT990721 | [3] |
| Aureoboletus projectellus | AFTOL-ID-713 | USA | DQ534604 * | – | AY787218 | AY879116 | [33] *, Unpublished |
| Aureoboletus raphanaceus | GDGM 53127 | China | – | – | MN549706 | MN549676 | [31] |
| Aureoboletus singeri | CFMR:BOS-468 | Belize | – | – | MK766274 | MK721065 | [29] |
| Aureoboletus sp. | OR0245 | China | MH614653 | MH614799 | MH614749 | MH614702 | [11] |
| Aureoboletus sp. | OR0369 | Thailand | MH614654 | MH614800 | MH614750 | MH614703 | [11] |
| Aureoboletus tenuis | GDGM42601 | China | – | – | KT291754 | KT291745 | [30] |
| Aureoboletus thibetanus | AFTOL-ID-450 | China | DQ534600 * | – | DQ366279 | DQ029199 | [33] *, Unpublished |
| Aureoboletus tomentosus | HKAS90216 | China | – | – | KT990355 | KT990717 | [3] |
| Aureoboletus viscidipes | HKAS77103 | China | – | – | KT990360 | KT990723 | [3] |
| Aureoboletus viscosus | OR0361 | Thailand | MH614655 | MH614801 | MH614751 | MH614704 | [11] |
| Australopilus palumanus | REH-9433 | Australia | – | – | MK766276 | MK721067 | [29] |
| Austroboletus cf. dictyotus | OR0045 | Thailand | KT823966 | MH614802 * | KT823999 | KT824032 | [21], [11] * |
| Austroboletus cf. subvirens | OR0573 | Thailand | MH614656 | MH614803 | MH614752 | MH614705 | [11] |
| Austroboletus olivaceoglutinosus | HKAS57756 | China | – | – | KF112764 | KF112212 | [2] |
| Austroboletus sp. | OR0891 | Thailand | MH614657 | MH614804 | MH614753 | MH614706 | [11] |
| Boletellus aff. ananas | NY815459 | Costa Rica | – | – | KF112760 | KF112308 | [2] |
| Boletellus aff. emodensis | OR0061 | Thailand | KT823970 | MH614806 * | KT824003 | KT824036 | [21], [11] * |
| Boletellus ananas | K(M)123769 | Belize | MH614658 | MH614807 | MH614754 | MH614707 | [11] |
| Boletellus areolatus | TNS-F-61444 | Japan | – | AB989025 | AB999754 | – | [34] |
| Boletellus aurocontextus | TNS-F-61501 | Japan | – | AB989037 | AB999770 | – | [34] |
| Boletellus emodensis | TNS-F-61564 | Japan | – | AB989053 | AB999782 | – | [34] |
| Boletellus sp. | OR0621 | Thailand | MG212529 | MH614808 * | MG212616 | MG212574 | [32], [11] * |
| Boletus aereus | VDKO1055 | Belgium | MG212530 | MH614809 * | MG212617 | MG212575 | [32], [11] * |
| Boletus albobrunnescens | OR0131 | Thailand | KT823973 | MH614810 * | KT824006 | KT824039 | [21], [11] * |
| Boletus edulis | VDKO0869 | Belgium | MG212531 | MH614811 * | MG212618 | MG212576 | [32], [11] * |
| Boletus rubriceps | MICH:KUO-08150719 | USA | – | – | MK766284 | MK721076 | [29] |
| Boletus s.s. sp. | OR0446 | China | MG212532 | MH614813 * | KF112703 | MG212577 | [32], [11] * |
| Borofutus dhakanus | OR0345 | Thailand | MH614660 | MH614814 | MH614755 | MH614709 | [11] |
| Buchwaldoboletus lignicola | HKAS76674 | China | – | – | KF112819 | KF112277 | [2] |
| Buchwaldoboletus lignicola | VDKO1140 | Belgium | MH614661 | MH614815 | MH614756 | MH614710 | [11] |
| Butyriboletus appendiculatus | VDKO0193b | Belgium | MG212537 | MH614816 * | MG212624 | MG212582 | [32], [11] * |
| Butyriboletus cf. roseoflavus | OR0230 | China | KT823974 | MH614819 * | KT824007 | KT824040 | [21], [11] * |
| Butyriboletus floridanus | BOS-617 | Belize | – | – | MK766287 | MK721079 | [29] |
| Butyriboletus frostii | NY815462 | USA | – | – | KF112675 | KF112164 | [2] |
| Butyriboletus pseudoregius | VDKO0925 | Belgium | MG212538 | MH614817 * | MG212625 | MG212583 | [32], [11] * |
| Butyriboletus roseopurpureus | BOTH4497 | USA | MG897418 | MH614818 * | MG897438 | MG897428 | [35], [11] * |
| Butyriboletus subsplendidus | HKAS50444 | China | – | – | KT990379 | KT990742 | [3] |
| Butyriboletus yicibus | HKAS55413 | China | – | – | KF112674 | KF112157 | [2] |
| Cacaoporus pallidicarneus | SV0221 | Thailand | MK372262 | MK372299 | MK372286 | MK372273 | [11] |
| Cacaoporus tenebrosus | SV0223 | Thailand | MK372266 | MK372303 | MK372290 | MK372277 | [11] |
| Caloboletus calopus | ADK4087 | Belgium | MG212539 | MH614820 | KP055030 | KJ184566 | [32], [36], [37], [11] |
| Caloboletus firmus | BOS-372 | Belize | – | – | MK766288 | MK721080 | [29] |
| Caloboletus inedulis | BOTH3963 | USA | MG897414 | MH614821 * | MG897434 | MG897424 | [35], [11] * |
| Caloboletus radicans | VDKO1187 | Belgium | MG212540 | MH614822 * | MG212626 | MG212584 | [32], [11] * |
| Caloboletus sp. | OR0068 | Thailand | MH614662 | MH614823 | MH614757 | MH614711 | [11] |
| Caloboletus yunnanensis | HKAS69214 | China | – | – | KT990396 | KJ184568 | [36], [3] |
| Chalciporus aff. piperatus | OR0586 | Thailand | KT823976 | MH614824 * | KT824009 | KT824042 | [21], [11] * |
| Chalciporus aff. rubinus | OR0139 | China | MH614663 | – | MH614758 | MH614712 | [11] |
| Chalciporus africanus | JD517 | Cameroon | KT823963 | MH614825 * | KT823996 | KT824029 | [21], [11] * |
| Chalciporus piperatus | VDKO1063 | Belgium | MH614664 | MH614826 | MH614759 | MH614713 | [11] |
| Chalciporus rubinus | AF2835 | Belgium | KT823962 | – | KT823995 | KT824028 | [21] |
| Chalciporus sp. | OR0363 | Thailand | MH645586 | MH645607 | MH645602 | MH645594 | [11] |
| Chalciporus sp. | OR0373 | Thailand | MH645587 | MH645608 | MH645603 | MH645595 | [11] |
| Chamonixia brevicolumna | DBG_F28707 | USA | – | – | MK766291 | MK721083 | [29] |
| Chamonixia caespitosa | OSC117571 | USA | – | – | MK766293 | MK721085 | [29] |
| Chiua sp. | OR0141 | China | MH614665 | MH614827 | MH614760 | MH614714 | [11] |
| Chiua virens | OR0266 | China | MG212541 | MH614828 * | MG212627 | MG212585 | [32], [11] * |
| Chiua viridula | HKAS74928 | China | – | – | KF112794 | KF112273 | [2] |
| Crocinoboletus cf. laetissimus | OR0576 | Thailand | KT823975 | MH614833 * | KT824008 | KT824041 | [21], [11] * |
| Crocinoboletus rufoaureus | HKAS53424 | China | – | – | KF112710 | KF112206 | [2] |
| Cupreoboletus poikilochromus | GS10070 | Italy | – | – | KT157068 | KT157072 | [38] |
| Cyanoboletus brunneoruber | OR0233 | China | MG212542 | MH614834 * | MG212628 | MG212586 | [32], [11] * |
| Cyanoboletus pulverulentus | RW109 | Belgium | KT823980 | MH614835 * | KT824013 | KT824046 | [21], [11] * |
| Cyanoboletus sinopulverulentus | HKAS59609 | China | – | – | KF112700 | KF112193 | [2] |
| Cyanoboletus sp. | OR0257 | China | MG212543 | MH614836 * | MG212629 | MG212587 | [32], [11] * |
| Cyanoboletus sp. | OR0322 | Thailand | MH614673 | MH614837 | MH614768 | MH614722 | [11] |
| Cyanoboletus sp. | OR0961 | Thailand | MH614675 | MH614839 | MH614770 | MH614724 | [11] |
| Erythrophylloporus aurantiacus | REH7271 | Costa Rica | MH614666 | MH614829 | MH614761 | MH614715 | [39] |
| Erythrophylloporus fagicola | Garay215 | Mexico | MH614667 | MH614830 | MH614762 | MH614716 | [39] |
| Erythrophylloporus paucicarpus | OR1151 | Thailand | MH614670 | MH614831 | MH614765 | MH614719 | [39] |
| Erythrophylloporus suthepense | SV0236 | Thailand | MH614672 | MH614832 | MH614767 | MH614721 | [39] |
| Fistulinella prunicolor | REH9880 | Australia | MH614676 | MH614840 | MH614771 | MH614725 | [11] |
| Harrya chromapes | HKAS50527 | China | – | – | KF112792 | KF112270 | [2] |
| Harrya moniliformis | HKAS49627 | China | – | – | KT990500 | KT990881 | [3] |
| Heimioporus conicus | HKAS53451 | China | – | – | KF112805 | KF112226 | [3] |
| Heimioporus australis | REH9288 | Australia | – | – | – | KP327703 | [40] |
| Heimioporus cooloolae | REH9817 | Australia | – | – | – | KP327710 | [40] |
| Heimioporus fruticicola | REH8962 | Australia | – | – | – | KP327696 | [40] |
| Heimioporus gaojiaocong | HKAS80582 | China | – | – | KT990409 | KT990770 | [3] |
| Heimioporus ivoryi | REH8620 | Costa Rica | – | – | – | KP327683 | [40] |
| Heimioporus japonicus | OR0114 | Thailand | KT823971 | – | KT824004 | KT824037 | [21] |
| Heimioporus japonicus | SV0016 | Thailand | MT136776 | – | MT136766 | MT136771 | [41] |
| Heimioporus mandarinus | OR0218 | Thailand | MG212546 | – | MG212632 | MG212590 | [32] |
| Heimioporus subcostatus | SV0235 | Thailand | MT136780 | – | MT136770 | MT136775 | [41] |
| Hemileccinum depilatum | AF2845 | Belgium | MG212547 | MH614843 * | MG212633 | MG212591 | [32], [11] * |
| Hemileccinum hortonii | MICH:KUO-07050706 | USA | – | – | MK766377 | MK721175 | [29] |
| Hemileccinum impolitum | ADK4078 | Belgium | MG212548 | MH614844 * | MG212634 | MG212592 | [32], [11] * |
| Hemileccinum indecorum | OR0863 | Thailand | MH614677 | MH614845 | MH614772 | MH614726 | [11] |
| Hemileccinum rubropunctum | REH-8501 | USA | – | – | MK766327 | MK721122 | [29] |
| Hemileccinum rugosum | HKAS84355 | China | – | – | KT990413 | KT990774 | [3] |
| Hemileccinum sp. | HKAS59445 | China | – | – | KT990414 | KT990775 | [3] |
| Hemileccinum sp. | HKAS53421 | China | – | – | KF112751 | KF112235 | [2] |
| Hemileccinum subglabripes | MICH:KUO-07230802 | USA | – | – | MK766300 | MK721092 | [29] |
| Hortiboletus amygdalinus | HKAS54166 | China | – | – | KT990416 | KT990777 | [3] |
| Hortiboletus campestris | MICH:KUO-08240502 | USA | – | – | MK766302 | MK721094 | [29] |
| Hortiboletus rubellus | VDKO0403 | Belgium | MH614679 | MH614847 | MH614774 | – | [11] |
| Hortiboletus subpaludosus | HKAS59608 | China | – | – | KF112696 | KF112185 | [2] |
| Hourangia cf. pumila | OR0762 | Thailand | MH614680 | MH614848 | MH614775 | MH614728 | [11] |
| Hourangia cheoi | HKAS52269 | China | – | – | KF112773 | KF112286 | [15] |
| Hourangia microcarpa | HKAS53378 | China | – | – | KF112775 | KF112300 | [2] |
| Hourangia nigropunctata | HKAS 57427 | China | – | – | KP136978 | KP136927 | [15] |
| Hourangia sp. | HKAS68178 | China | – | – | KF112776 | KF112301 | [2] |
| Hymenoboletus luteopurpureus | HKAS46334 | China | – | – | KF112795 | KF112271 | [2] |
| Imleria badia | VDKO0709 | Belgium | KT823983 | MH614849 * | KT824016 | KT824049 | [21], [11] * |
| Imleria obscurebrunnea | OR0263 | China | MH614681 | MH614850 | MH614776 | MH614729 | [11] |
| Imleria pallidus | BOTH4356 | USA | MH614659 | MH614812 | – | MH614708 | [11] |
| Lanmaoa angustispora | HKAS74752 | China | – | – | KM605177 | KM605154 | [17] |
| Lanmaoa asiatica | OR0228 | China | MH614682 | MH614851 | MH614777 | MH614730 | [11] |
| Lanmaoa carminipes | BOTH4591 | USA | MG897419 | MH614852 * | MG897439 | MG897429 | [35], [11] * |
| Lanmaoa pallidorosea | BOTH4432 | USA | MG897417 | MH614853 * | MG897437 | MG897427 | [35], [11] * |
| Lanmaoa sp. | OR0130 | Thailand | MH614683 | MH614854 | MH614778 | MH614731 | [11] |
| Lanmaoa sp. | OR0370 | Thailand | MH614684 | MH614855 | MH614779 | MH614732 | [11] |
| Leccinellum aff. crocipodium | HKAS76658 | China | – | – | KF112728 | KF112252 | [2] |
| Leccinellum aff. griseum | KPM-NC-0017832 | Japan | KC552164 | – | – | JN378450* | unpublished, [42] * |
| Leccinellum cremeum | HKAS90639 | China | – | – | KT990420 | KT990781 | [3] |
| Leccinum scabrum | VDKO0938 | Belgium | MG212549 | MH614858 * | MG212635 | MG212593 | [32], [11] * |
| Leccinum schistophilum | VDKO1128 | Belgium | KT823989 | MH614859 * | KT824022 | KT824055 | [21], [11] * |
| Leccinum variicolor | VDKO0844 | Belgium | MG212550 | MH614860 * | MG212636 | MG212594 | [32], [11] * |
| Mucilopilus castaneiceps | HKAS75045 | China | – | – | KF112735 | KF112211 | [2] |
| Mycoamaranthus cambodgensis | SV0197 | Thailand | MZ355900 | MZ355909 | – | – | This study |
| Neoboletus brunneissimus | OR0249 | China | MG212551 | MH614861 * | MG212637 | MG212595 | [32], [11] * |
| Neoboletus ferrugineus | HKAS77718 | China | – | – | KT990431 | KT990789 | [3] |
| Neoboletus flavidus | HKAS59443 | China | – | – | KU974144 | KU974136 | [3] |
| Neoboletus hainanensis | HKAS59469 | China | – | – | KF112669 | KF112175 | [2] |
| Neoboletus junquilleus | AF2922 | France | MG212552 | MH614862 * | MG212638 | MG212596 | [32], [11] * |
| Neoboletus magnificus | HKAS74939 | China | – | – | KF112653 | KF112148 | [2] |
| Neoboletus obscureumbrinus | OR0553 | Thailand | MK372271 | – | MK372294 | MK372282 | [11] |
| Neoboletus sp. | OR0128 | Thailand | MH614686 | MH614863 | MH614781 | MH614734 | [11] |
| Neoboletus tomentulosus | HKAS53369 | China | – | – | KF112659 | KF112154 | [2] |
| Neoboletus erythropus | VDKO0690 | Belgium | KT823982 | MH614864 * | KT824015 | KT824048 | [21], [11] * |
| Octaviania asterosperma | AQUI3899 | Italy | KC552159 | – | – | KC552093 | [43] |
| Octaviania cyanescens | PNW-FUNGI-5603 | USA | KC552160 | – | – | JN378438 | [43], [42] |
| Octaviania decimae | KPM-NC17763 | Japan | KC552145 | – | – | JN378409 | [43], [42] |
| Octaviania tasmanica | MEL2128484 | Australia | KC552157 | – | – | JN378437 | [43], [42] |
| Octaviania zelleri | MES270 | USA | KC552161 | – | – | JN378440 | [43], [42] |
| Phylloporus bellus | OR0473 | China | MH580778 | MH614866 * | MH580818 | MH580798 | [44], [11] * |
| Phylloporus brunneiceps | OR0050 | Thailand | KT823968 | MH614867 * | KT824001 | KT824034 | [21], [11] * |
| Phylloporus castanopsidis | OR0052 | Thailand | KT823969 | MH614868 * | KT824002 | KT824035 | [21], [11] * |
| Phylloporus maculatus | OR0285 | China | MH580780 | – | MH580820 | MH580800 | [44] |
| Phylloporus pachycystidiatus | HKAS53422 | China | – | – | KF112777 | KF112288 | [2] |
| Phylloporus pelletieri | WU18746 | Austria | MH580781 | MH614869 * | MH580821 | MH580801 | [44], [11] * |
| Phylloporus pusillus | OR1158 | Thailand | MH580783 | MH614870 * | MH580823 | MH580803 | [44], [11] * |
| Phylloporus rhodoxanthus | WU17978 | Austria | MH580785 | MH614871 * | MH580824 | MH580805 | [44], [11] * |
| Phylloporus rubeolus | OR0251 | China | MH580786 | MH614872 * | MH580825 | MH580806 | [44], [11] * |
| Phylloporus rubiginosus | OR0169 | China | MH580788 | MH614873 * | MH580827 | MH580808 | [44], [11] * |
| Phylloporus rubrosquamosus | HKAS52552 | China | – | – | KF112780 | KF112289 | [2] |
| Phylloporus scabripes | CFMR:BOS-621 | Belize | – | – | MK766359 | MK721156 | [29] |
| Phylloporus sp. | OR0896 | Thailand | MH580790 | MH614874 * | MH580829 | MH580810 | [44], [11] * |
| Phylloporus subbacillisporus | OR0436 | China | MH580792 | MH614875 * | MH580831 | MH580812 | [44], [11] * |
| Phylloporus subrubeolus | BC022 | Thailand | MH580793 | MH614876 * | MH580832 | MH580813 | [44], [11] * |
| Phylloporus yunnanensis | OR0448 | China | MG212554 | MH614877 * | MG212640 | MG212598 | [32], [11] * |
| Porphyrellus castaneus | OR0241 | China | MG212555 | MH614878 * | MG212641 | MG212599 | [32], [11] * |
| Porphyrellus aff. nigropurpureus | ADK3733 | Benin | MH614687 | MH614879 | MH614782 | MH614735 | [11] |
| Porphyrellus nigropurpureus | HKAS74938 | China | – | – | KF112763 | KF112246 | [2] |
| Porphyrellus porphyrosporus | MB97 023 | Germany | DQ534609 | – | GU187800 | GU187734 | [33], [45] |
| Porphyrellus sp. | JD659 | Burundi | MH614688 | MH614880 | MH614783 | MH614736 | [11] |
| Porphyrellus sp. | OR0222 | Thailand | MH614689 | MH614881 | MH614784 | MH614737 | [11] |
| Pulchroboletus sclerotiorum | FLAS F 60333 | USA | – | – | MF614169 | MF614167 | [46] |
| Pulchroboletus sclerotiorum | FLAS F 60334 | USA | – | – | MF614164 | MF614165 | [46] |
| Pulveroboletus aff. ravenelii | ADK4360 | Togo | KT823957 | MH614882 * | KT823990 | KT824023 | [21], [11] * |
| Pulveroboletus aff. ravenelii | ADK4650 | Togo | KT823959 | MH614883 * | KT823992 | KT824025 | [21], [11] * |
| Pulveroboletus brunneopunctatus | HKAS55369 | China | – | – | KT990455 | KT990814 | [3] |
| Pulveroboletus fragrans | OR0673 | Thailand | KT823977 | MH614884 * | KT824010 | KT824043 | [21], [11] * |
| Pulveroboletus ravenelii | REH2565 | USA | KU665635 | MH614885 * | KU665637 | KU665636 | [21], [11] * |
| Retiboletus aff. nigerrimus | OR0049 | Thailand | KT823967 | MH614886 * | KT824000 | KT824033 | [21], [11] * |
| Retiboletus brunneolus | HKAS52680 | China | – | – | KF112690 | KF112179 | [2] |
| Retiboletus fuscus | OR0231 | China | MG212556 | MH614887 * | MG212642 | MG212600 | [32], [11] * |
| Retiboletus griseus | MB03 079 | USA | KT823964 | MH614888 * | KT823997 | KT824030 | [21], [11] * |
| Retiboletus kauffmanii | OR0278 | China | MG212557 | MH614889 * | MG212643 | MG212601 | [32], [11] * |
| Retiboletus nigerrimus | HKAS53418 | China | – | – | KT990462 | KT990824 | [3] |
| Rhodactina himalayensis | CMU25117 | Thailand | MG212558 | – | – | MG212602, MG212603 | [32] |
| Rhodactina rostratispora | SV0170 | Thailand | MG212560 | – | MG212645 | MG212605 | [32] |
| Rossbeevera cryptocyanea | KPM-NC17843 | Japan | KT581441 | – | – | KC552072 | [43] |
| Rossbeevera griseovelutina | TNS-F-36989 | Japan | KC552124 | – | – | KC552076 | [43] |
| Rossbeevera pachydermis | KPM-NC23336 | New Zealand | KJ001064 | – | – | KP222912 | [43] |
| Royoungia rubina | HKAS53379 | China | – | – | KF112796 | KF112274 | [2] |
| Rubinosporus auriporus | SV0090 | Thailand | MZ355896 | MZ355905 | MZ355901 | MZ355903 | This study |
| Rubinosporus auriporus | SV0101 | Thailand | MZ355897 | MZ355906 | MZ355902 | MZ355904 | This study |
| Rubinosporus auriporus | SV0394 | Thailand | MZ355898 | MZ355907 | – | – | This study |
| Rubinosporus auriporus | SV0396 | Thailand | MZ355899 | MZ355908 | – | – | This study |
| Rubroboletus legaliae | VDKO0936 | Belgium | KT823985 | MH614890 * | KT824018 | KT824051 | [21], [11] * |
| Rubroboletus rhodosanguineus | BOTH4263 | USA | MG897416 | MH614891 * | MG897436 | MG897426 | [35], [11] * |
| Rubroboletus rhodoxanthus | HKAS84879 | China | – | – | KT990468 | KT990831 | [3] |
| Rubroboletus satanas | VDKO0968 | Belgium | KT823986 | MH614892 * | KT824019 | KT824052 | [21], [11] * |
| Rugiboletus andinus | REH-7705 | Costa rica | – | – | MK766316 | MK721111 | [29] |
| Rugiboletus brunneiporus | HKAS83209 | China | – | – | KM605168 | KM605144 | [17] |
| Rugiboletus extremiorientalis | OR0406 | Thailand | MG212562 | MH614893 * | MG212647 | MG212607 | [32], [11] * |
| Singerocomus inundabilis | TWH9199 | Guyana | MH645588 | MH645609 | LC043089* | MH645596 | [47] *, [11] |
| Singerocomus rubriflavus | TWH9585 | Guyana | MH645589 | MH645610 | – | MH645597 | [11] |
| Spongiforma thailandica | DED7873 | Thailand | MG212563 | MH614894 ** | MG212648 | KF030436* | [1] *, [32], [11] ** |
| Strobilomyces echinocephalus | OR0243 | China | MG212564 | – | MG212649 | MG212608 | [32] |
| Strobilomyces floccopus | RW103 | Belgium | KT823978 | MH614895 * | KT824011 | KT824044 | [21], [11] * |
| Strobilomyces mirandus | OR0115 | Thailand | KT823972 | MH614896 * | KT824005 | KT824038 | [21], [11] * |
| Strobilomyces sp. | OR0259 | China | MG212565 | MH614897 * | MG212650 | MG212609 | [32], [11] * |
| Strobilomyces sp. | OR0319 | Thailand | MH614690 | MH614898 | MH614785 | MH614738 | [11] |
| Strobilomyces sp. | OR0778 | Thailand | MG212566 | MH614899 * | MG212651 | MG212610 | [32], [11] * |
| Strobilomyces sp. | OR1092 | Thailand | MH614691 | MH614900 | MH614786 | MH614739 | [11] |
| Strobilomyces verruculosus | HKAS55389 | China | – | – | KF112813 | KF112259 | [2] |
| Suillellus luridus | VDKO0241b | Belgium | KT823981 | MH614901 * | KT824014 | KT824047 | [21], [11] * |
| Suillellus queletii | VDKO1185 | Belgium | MH645590 | MH645611 | MH645604 | MH645598 | [11] |
| Suillellus subamygdalinus | HKAS57262 | China | – | – | KF112660 | KF112174 | [2] |
| Sutorius australiensis | REH9441 | Australia | MG212567 | MK386576 ** | MG212652 | JQ327032* | [48] *, [32], [11] ** |
| Sutorius eximius | REH9400 | USA | MG212568 | MH614902 ** | MG212653 | JQ327029* | [48] *, [32], [11] ** |
| Sutorius pachypus | OR0411 | Thailand | MN067465 | – | MN067500 | MN067484 | [49] |
| Sutorius pseudotylopilus | OR0378B | Thailand | MH614692 | MH614903 | MH614787 | MH614740 | [11] |
| Sutorius rubinus | OR0379 | Thailand | MH614693 | MH614904 | MH614788 | MH614741 | [11] |
| Sutorius ubonensis | SV0032 | Thailand | MN067472 | – | MN067507 | MN067491 | [49] |
| Tengioboletus glutinosus | HKAS53425 | China | – | – | KF112800 | KF112204 | [2] |
| Tengioboletus reticulatus | HKAS53426 | China | – | – | KF112828 | KF112313 | [2] |
| Turmalinea persicina | KPM-NC18001 | Japan | KC552130 | – | – | KC552082 | [43] |
| Turmalinea yuwanensis | KPM-NC18011 | Japan | KC552138 | – | – | KC552089 | [43] |
| Tylopilus balloui s.l. | OR0039 | Thailand | KT823965 | MH614905 * | KT823998 | KT824031 | [21], [11] * |
| Tylopilus felleus | VDKO0992 | Belgium | KT823987 | MH614906 * | KT824020 | KT824053 | [21], [11] * |
| Tylopilus ferrugineus | BOTH3639 | USA | MH614694 | MH614907 | MH614789 | MH614742 | [11] |
| Tylopilus otsuensis | HKAS53401 | China | – | – | KF112797 | KF112224 | [2] |
| Tylopilus sp. | JD598 | Gabon | MH614695 | MH614908 | MH614790 | MH614743 | [11] |
| Tylopilus sp. | OR0252 | China | MG212569 | MH614909 * | MG212654 | MG212611 | [32], [11] * |
| Tylopilus sp. | OR0542 | Thailand | MG212570 | MH614910 * | MG212655 | MG212612 | [32], [11] * |
| Tylopilus sp. | OR1009 | Thailand | MH614697 | MH614911 | MH614791 | – | [11] |
| Tylopilus vinaceipallidus | OR0137 | China | MG212571 | MH614912 * | MG212656 | MG212613 | [32], [11] * |
| Tylopilus violaceobrunneus | HKAS89443 | China | – | – | KT990504 | KT990886 | [3] |
| Veloporphyrellus conicus | REH8510 | Belize | MH614698 | MH614913 | MH614792 | MH614745 | [11] |
| Veloporphyrellus gracilioides | HKAS53590 | China | – | – | KF112734 | KF112210 | [2] |
| Veloporphyrellus pseudovelatus | HKAS59444 | China | JX984519 | – | – | JX984553 | [50] |
| Veloporphyrellus velatus | HKAS63668 | China | JX984523 | – | – | JX984554 | [50] |
| Xanthoconium affine | NY00815399 | USA | – | – | KT990486 | KT990850 | [3] |
| Xanthoconium purpureum | MICH:KUO-07061405 | USA | – | – | MK766372 | MK721170 | [29] |
| Xanthoconium sinense | HKAS77651 | China | – | – | KT990488 | KT990853 | [3] |
| Xerocomellus chrysenteron | VDKO0821 | Belgium | KT823984 | MH614914 * | KT824017 | KT824050 | [21], [11] * |
| Xerocomellus cisalpinus | ADK4864 | Belgium | KT823960 | MH614915 * | KT823993 | KT824026 | [21], [11] * |
| Xerocomellus communis | HKAS50467 | China | – | – | KT990494 | KT990858 | [3] |
| Xerocomellus ripariellus | VDKO0404 | Belgium | MH614699 | MH614916 | MH614793 | MH614746 | [11] |
| Xerocomus ferrugineus | CFMR:BOS-545 | USA | – | – | MK766375 | MK721173 | [29] |
| Xerocomus fulvipes | HKAS76666 | China | – | – | KF112789 | KF112292 | [2] |
| Xerocomus magniporus | HKAS58000 | China | – | – | KF112781 | KF112293 | [2] |
| Xerocomus puniceiporus | HKAS80683 | China | – | – | KU974146 | KU974138 | [3] |
| Xerocomus rugosellus | HKAS58865 | China | – | – | KF112784 | KF112294 | [2] |
| Xerocomus s.s. sp. | OR0237 | China | MH580796 | – | MH580835 | MH580816 | [44] |
| Xerocomus s.s. sp. | OR0443 | China | MH580797 | MH614917 * | MH580836 | MH580817 | [44], [11] * |
| Xerocomus s.s. sp. | OR0053 | Thailand | MH580795 | MH614918 * | MH580834 | MH580815 | [44], [11] * |
| Xerocomus spadiceus var. gracilis | MICH:KUO-07080702 | USA | – | – | MK766378 | MK721176 | [29] |
| Xerocomus subtomentosus | VDKO0987 | Belgium | MG212572 | MH614919 * | MG212657 | MG212614 | [32], [11] * |
| Xerocomus tenax | MICH:KUO-08241404 | USA | – | – | MK766379 | MK721177 | [29] |
| Zangia citrina | HKAS52684 | China | HQ326850 | – | – | HQ326872 | [51] |
| Zangia olivaceobrunnea | HKAS52272 | China | HQ326857 | – | – | HQ326876 | [51] |
| Zangia roseola | HKAS51137 | China | HQ326858 | – | – | HQ326877 | [51] |
Figure 1.
Boletaceae-wide Maximum Likelihood phylogenetic tree inferred from the four-gene dataset (atp6, cox3, rpb2, and tef1) (introns excluded), showing position of the new genus Rubinosporus in Xerocomoideae. Bootstrap support values (BS ≥ 70%) and the corresponding Bayesian posterior probabilities (PP ≥ 0.90) are shown above the supported branches. The two Buchwaldoboletus and seven Chalciporus species (subfamily Chalciporoideae) were used as the outgroup. All taxa belonging to subfamilies Austroboletoideae, Boletoideae, Chalciporoideae, Leccinoideae, and Zangioideae were collapsed into subfamily clades. All generic clades in subfamily Xerocomoideae and Pulveroboletus group that were highly supported were also collapsed.
The Xerocomoideae-wide alignment contained 243 sequences comprising four genes (42 for atp6, 38 for cox3, 81 for rpb2, 82 for tef1) from 86 voucher specimens corresponding to 82 taxa and was 3176 characters long (TreeBase number: 28350). ML and BI trees showed similar topologies without any supported conflict. The phylogram of RAxML bipartition (Figure 2) retrieved nine highly supported generic clades, for which BS = 100% and PP = 1 for six clades, Aureoboletus, Pulchroboletus, Heimioporus, Hemileccinum, Hourangia, and the new genus Rubinosporus, while the others had only slightly less support, Boletellus (BS = 85% and PP = 1), Phylloporus (BS = 99% and PP = 1), and Xerocomus (BS = 75% and PP = 1).
Figure 2.
Xerocomoideae-wide phylogenetic tree inferred from the four-gene dataset (atp6, cox3, rpb2, and tef1) (introns included), including new genus Rubinosporus and selected Xerocomoideae using Maximum Likelihood and Bayesian Inference methods (ML tree is presented). The four Butyriboletus species in Pulveroboletus group were used as the outgroup. Bootstrap support values (BS ≥ 70%) and posterior probabilities (PP ≥ 0.90) are shown above the supported branches.
3.2. Taxonomy
Rubinosporus Vadthanarat, Raspé & Lumyong, gen. nov.
Typus generis—Rubinosporus auriporus Vadthanarat, Raspé & Lumyong
MycoBank—MB840262
Etymology—from Latin “rubineus” and “sporus” referring to its production of dark ruby spore deposits.
Diagnosis—Distinguished from the other genera in Boletaceae by the following combination of characters: pileus surface even, with matted, cracked tomentum; stipe surface even, scattered with minute squamules, golden yellow tubular hymenophore which is relatively thin, especially when young; unchanging surfaces and context when touched or cut; smooth, broadly ellipsoid basidiospores; dark ruby spore deposit.
Description—Basidiomata stipitate-pileate with tubular hymenophore, medium-sized. Pileus hemispherical at first then convex to plano-convex or applanate in age; margin inflexed to deflexed, exact to slightly exceeding; surface even to subrugulose at places, dull, greyish red to pastel red to reddish brown, with greyish yellow, greyish orange to brownish orange to brown matted, cracked tomentum; context firm, off-white to yellowish white, unchanging when cut. Stipe central, terete, or sometimes slightly compressed, cylindrical or subcylindrical with slightly wider base; surface topography even, yellowish white to pinkish white at places, with scattered yellowish white to orange to light brown minute squamules, to bright yellow near the top; basal mycelium yellowish white; context solid, off-white to yellowish white, unchanging when cut. Hymenophore tubulate, narrowly adnate, mostly segmentiform to subventricose. Tubes relatively thin, especially when young, golden yellow becoming orange-yellow, separable from the pileus context, unchanging when bruised. Pores topography subirregular, irregularly arranged, roundish to slightly angular composite pores; golden yellow at first, golden yellow to greyish yellow with irregularly reddish brown at places in age, unchanging when touched. Odor mild fungoid. Taste mild to slightly sweet. Spore print dark ruby in mass. Basidiospores broadly ellipsoid, thin-walled, smooth, yellowish to brownish hyaline in water, yellowish hyaline in KOH or NH4OH, yellowish to reddish in Melzer’s reagent (weakly dextrinoid). Basidia 4-spored, clavate without basal clamp connection. Cheilocystidia clavate with rounded apex or fusiform to broadly fusiform or utriform, thin-walled, hyaline to yellowish hyaline in KOH or NH4OH. Pleurocystidia fusiform with narrower apex, thin-walled, hyaline to yellowish hyaline in KOH or NH4OH. Pileipellis a tomentum to intricate trichoderm, composed of moderately interwoven thin-walled hyphae; terminal cells cylindrical with obtuse apex, hyaline to yellowish at places in KOH. Stipitipellis a tomentum composed of loosely to moderately interwoven cylindrical hyphae, anastomosing at places, scattered with groups of rising cells to clusters of basidiole-like cells mixed with caulocystidia, and rarely with caulobasidia, hyaline to yellowish hyaline in KOH or NH4OH. Clamp connections were not seen in any tissue.
Known distribution—Currently known only from Thailand.
Notes—The morphologically closely resembling genera are Aureoboletus and Baorangia, the former sharing the bright yellow to golden yellow hymenium, and the latter sharing the thin hymenophore [3,17,31]. However, Rubinosporus is easily distinguished from those two genera by the dark ruby spore deposit, which has an olive brown tint in Aureoboletus and Baorangia.
Rubinosporus auriporus Vadthanarat, Raspé & Lumyong, sp. nov. Figure 3, Figure 4 and Figure 5
Figure 3.
Fresh basidiomata of Rubinosporus auriporus: (A,B) SV0090 (Holotype); (C,D) SV0394, spores deposit on the cap showing dark ruby color (white arrow); (E) SV0396; (F) the golden yellow pores, irregularly reddish brown at places in (SV0394)—Bars (A–E) = 1, (F) = 5 mm.
Figure 4.
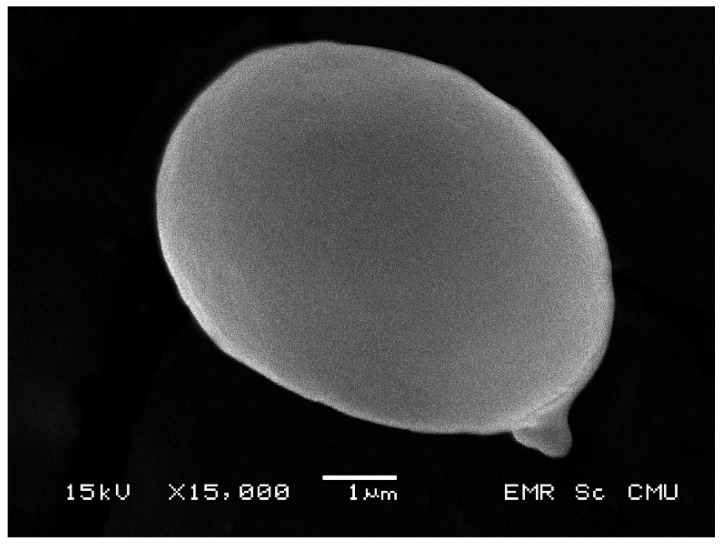
Scanning electron micrographs of Rubinosporus auriporus basidiospores from the holotype—Bar = 1 µm.
Figure 5.
Microscopic features of Rubinosporus auriporus: (A) Basidiospores; (B) Basidia; (C) Two shapes of cheilocystidia; (D) Caulocystidia; (E) Pileipellis; (F) Stipitipellis.—Bars A–D = 10 µm, E,F = 50 µm. All drawings were made from the type (SV0090).
MycoBank—MB840263
Holotype—THAILAND, Chiang Mai Province, Mae Taeng District, 19°06’37.5” N–98°44’40.0” E, elev. 1,090 m, 2 June 2015, Santhiti Vadthanarat, SV0090 (CMUB; isotype BR).
Etymology—from Latin referring to the golden yellow hymenophore.
Description—Basidiomata medium-sized. Pileus (14–)33–92(–174) mm in diameter, hemispherical at first then convex to plano-convex or applanate in age; margin inflexed to deflexed, exact to slightly exceeding somewhere (0.5 mm); surface even to subrugulose at places, dull, greyish red to pastel red to reddish brown (8E5–6, 9B/C/D5–7, 9E6), at first densely to moderately covered with greyish yellow, greyish orange to brownish orange to brown (4B3–5, 5B3–5, 5C3–6, 5D3–4, 6E7–8) matted, cracked tomentum becoming less in age; context 4–15 mm thick half-way to the margin, firm, off-white to yellowish white (1A1–2), occasionally pale yellow (1A2–3) above the hymenium or under pileipellis, unchanging when cut. Stipe 37–109 × 11–45 mm, central, terete or slightly compressed sometimes, mostly cylindrical to cylindrical with slightly wider base; surface topography even, slightly shiny, yellowish white (2A2–3, 3A2) with pinkish white (8A2) at places, scattered with yellowish white (2A2–3, 3A2) to orange to light brown (5A/B7, 7D7–8) minute squamules, to bright yellow (2–3A7) near the top; basal mycelium little developed, yellowish white (2A2); context solid, off-white to yellowish white (1A1–2), even to virgate at places, unchanging when cut. Hymenophore tubulate, narrowly adnate, mostly segmentiform to subventricose. Tubes (0.8)2–4.5(7) mm long half-way to the margin, relatively thin when young 1/4 to 1/5 times then 1/2 to 1/3 times that of the pileus context when mature, golden yellow (3A7) becoming orange yellow (4B7), separable from the pileus context, unchanging when bruised. Pores 0.4–0.8(1) mm wide at mid-radius, topography subirregular, irregularly arranged, composite pores composed of roundish to slightly angular pores in age, golden yellow (3–4A8) at first, golden yellow to greyish yellow (4A/B/C7) with irregularly reddish brown (8E/F8) at places in age, unchanging when touched. Odor mild fungoid. Taste mild to slightly sweet. Spore print dark ruby (12F7) in mass.
Macrochemical reactions: KOH, yellow to orange on cap, stipe, and hymenium; none or yellowish on pileus context and stipe context; NH4OH, yellow to orange to brown on cap, stipe and hymenophore; none or yellowish on pileus context, stipe context and hymenium.
Spores [293/5/2] (6.5–)7.1–7.9–8.7(–9.3) × (4.4–)5.2–5.8–6.4(–6.9) µm Q = (1.19–)1.25–1.36–1.52(–1.68). From the type (7–)7.1–7.7–8.6(–9) × (4.4–)4.8–5.7–6.5(–6.8) µm, Q = (1.19–)1.23–1.36–1.53(–1.66), N = 60, broadly ellipsoid, thin-walled, smooth, yellowish to brownish hyaline in water, yellowish hyaline in KOH or NH4OH, yellowish to reddish in Melzer’s reagent (inamyloid to weakly dextrinoid). Basidia 4-spored, (18–)19–24–27(–28) × (9–)9–11–12(–12) µm, clavate without basal clamp connection, hyaline to yellowish hyaline in KOH or NH4OH; sterigmata up to 4 µm long. Cheilocystidia of two types, (1) clavate with rounded apex, frequent, (14–)15–25–36(–38) × (9–)10–12–16(–16) µm, thin-walled, hyaline to yellowish hyaline in KOH or NH4OH, and (2) fusiform to broadly fusiform or utriform, frequent, (21–)22–34–41(–41) × (10–)10–12–15(–16) µm, thin-walled, hyaline to yellowish hyaline in KOH or NH4OH. Pleurocystidia (29–)30–47–58(–61) × (9–)9–12–16(–18) µm, frequent and more near the pores, fusiform with narrower apex, thin-walled, hyaline to yellowish hyaline in KOH or NH4OH. Hymenophoral trama divergent, 57–106 µm wide, with 16–32 µm wide of subregular mediostratum, composed of cylindrical, 4–12 µm wide hyphae, slightly yellowish to hyaline in KOH or NH4OH. Pileipellis a tomentum to intricate trichoderm, 125–230 µm thick, composed of moderately interwoven thin-walled hyphae; terminal cells 21–68 × 3.5–9 µm, cylindrical with obtuse apex, hyaline to yellowish at places in KOH. Pileus context made of strongly interwoven, thin-walled, hyaline hyphae, 7–23 µm wide, hyaline in KOH. Stipitipellis a tomentum composed of loosely to moderately interwoven cylindrical hyphae (3–9 µm wide), anastomosing at places, scattered with groups of rising cells to clusters of basidiole-like cells ((14–)15–23–38(–39) × (5–)6–8–10(–11) µm) mixed with two types of caulocystidia, and rarely with caulobasidia, 120–170 µm thick (including the height of rising cells), hyaline to yellowish hyaline in KOH or NH4OH; terminal cells 24–81 × 5–9 µm, more or less parallel to the surface of the stipe, thin-walled, elongated cylindrical with obtuse to slightly swollen apex. Caulocystidia of two types, 1) fusiform, not frequent, (25–)26–45–72(–76) × (9–)9–14–18(–18) µm, thin-walled, hyaline to yellowish hyaline in KOH, and 2) broadly clavate, not frequent, (14–)14–23–34(–34) × (10–)10–15–21(–21) µm, thin-walled, hyaline to yellowish hyaline in KOH. Stipe context composed of parallel, 6–18(23) µm wide hyphae, hyaline to yellowish hyaline in KOH or NH4OH. Clamp connections were not seen in any tissue.
Habitat and distribution—Gregarious (up to 6 basidiomata) to fasciculate of 2–4 basidiomata, on soil in hill evergreen forest dominated by Fagaceae mixed with Dipterocarpaceae: Dipterocarpus obtusifolius, D. costatus, Shorea siamensis, Hopea sp. Currently known only from the type locality in Chiang Mai Province, northern Thailand.
Specimens examined—THAILAND, Chiang Mai Province, Mae Taeng District, 19°06’32.7” N–98°44’33.6” E, elev. 1,070 m, 4 Jun 2015, Santhiti Vadthanarat, SV0101 (CMUB, BR); ibid. 19°06’33.8” N–98°44’20.9” E, elev. 1110 m, 23 May 2017, Santhiti Vadthanarat, SV0394 (CMUB, BR); ibid. 19°06’36.2” N–98°44’41.1” E, elev. 1080 m, 23 May 2017, Santhiti Vadthanarat, SV0396 (CMUB, BR).
Notes—In the new species, the hymenophoral cystidia contained greenish yellow (1A8) pigments when fresh specimens were observed in water under a compound microscope. However, the pigment was discolored when the cystidia were observed in KOH or NH4OH, or after treatment of the specimen with heat (drying at 45–50 °C).
A macro-morphologically similar species, Butyriboletus roseoflavus (Hai B. Li & Hai L. Wei) D. Arora & J.L. Frank originally described from China, has a similar color tone of basidiomata with a light pink, light purplish red to rose-red pileus; and lemon-yellow, olive-yellow or honey-yellow hymenophore. However, it can be differentiated from R. auriporus by having a yellower and reticulated stipe which is lemon-yellow or light yellow with almost entirely reticulate stipe or at least in lower part; yellower context which is lemon-yellow and also variable staining reaction in response to bruising, bruising blue slowly or unchanging; bruising blue promptly hymenophore; subfusiform basidiospores; olive brown spore deposit; and the habitat in Pinus or mixed forests dominated by Pinus [52,53].
The chemical reaction of basidiospores with Melzer’s reagent which was negative to weakly dextrinoid in R. auriporus is also present in two Xerocomoideae species, Alessioporus ichnusanus (Alessio, Galli & Littini) Gelardi, Vizzini & Simonini, and Pulchroboletus roseoalbidus (Alessio & Littini) Gelardi, Vizzini & Simonini. However, the two species are different from R. auriporus by their basidiospore shapes, which are sub-cylindrical or ellipsoid or ellipsoid-fusoid, the strong discoloration (bluing or darkening) in parts of basidiomata, and olive-brown spore deposit [54].
4. Discussion
The new genus Rubinosporus is distinguished from other Boletaceae by a combination of striking characters, i.e., a golden yellow tubular hymenophore that is relatively thin especially when young, and dark ruby spore deposits. The character of golden yellow tubular hymenophore is also found in Aureoboletus, Alessioporus, and Pulchroboletus, which also belong to the subfamily Xerocomoideae. However, Aureoboletus species differ from Rubinosporus in usually having a viscid pileus surface especially when moist, olive brown spore deposit, and subfusiform or oblong ovoid to subglobose basidiospores [3,16,31]. Alessioporus is clearly different by the reticulated stipe occasionally with a granular ring-like zone in the middle or lower half of the stipe; rapidly bluing hymenophore, stipe surface, and context when bruised or exposed; sub-ellipsoid to fusiform, ellipsoid to subcylindrical basidiospores; olive brown spore deposit; and distribution in Mediterranean Italy and subtropical USA [54,55]. Pulchroboletus differs by the stipe surface with scattered red to reddish brown punctae, occasionally with reticulum or longitudinal striations, and with a pseudo-annulus; hymenophore and context usually intensively staining blue when bruised or cut; ellipsoidal to ellipsoid-fusoid basidiospores; olive brown colored spore deposit; so far found in Mediterranean Europe and tropical to subtropical America [46,54,56].
The thin hymenophore is also present in Baorangia and Lanmaoa G. Wu & Zhu L. Yang, which both belong to the Pulveroboletus group. They have a very thin hymenophore, with a tube length 1/3 to 1/5 times the thickness of the pileus context. However, Baorangia and Lanmaoa differ from Rubinosporus by having yellow hymenium (not golden or bright yellow) that immediately turns light blue to greenish blue when touched; olive-brown spore deposit; and subfusiform to elongated subfusiform basidiospores [5,17,35].
Although the color of spore deposit in Rubinosporus is somewhat similar to the color tone in Austroboletus (Corner) Wolfe and Ionosporus Khmeln., Austroboletus has a rufous madder to chocolate to purplish vinaceous spore deposit which is browner than in Rubinosporus. Also, Austroboletus species produce basidiomata with pileipellis markedly exceeding pileus margin, embracing the stipe in young basidiomata, whitish to pinkish hymenium, and ornamented basidiospores [3,57,58]. Ionosporus has pale violet to reddish brown spore deposit. However, their basidiospores have an obvious reaction in potassium hydroxide solution, turning deep purple violet. The basidiospores also have granulose pitted surface under SEM. Moreover, Austroboletus and Ionosporus phylogenetically belong to different subfamilies, the Austroboletoideae and Leccinoideae, respectively [2,10]. The color of spore deposit is one of the important character used to differentiate mushroom genera, both in the Agaricales and Boletales. Several previous studies used this character to differentiate new genera. For example, Tylopilus eximius (Peck) Singer, which has a reddish-brown spore deposit, was separated from Tylopilus (having a pinkish spore deposit), and placed in a new genus, Sutorius [48]. Cacaoporus is distinguished from the most similar genus Sutorius by its dark brown spore deposit while the genus Sutorius has a reddish-brown spore deposit [11]. Moreover, they were all supported by the phylogenies.
Most morphological characters of Rubinosporus fit the typical characters of Xerocomoideae genera, as described in Wu et. al. [2,3] and Zhu et al. [15]. However, the color of the spore deposit, which in all Xerocomoideae so far described has an olive-brown tint, is dark ruby in Rubinosporus. The differences in spore deposit color between genera within the same subfamily in Boletaceae, are also found in Boletoideae which varies from olive green (Boletus), blackish brown (Strobilomyces), light yellow or yellow golden (Xanthoconium), pinkish (Tylopilus) e.g., [2,3,59,60]. Spore print color, however, is mostly conserved at genus level, with only slight variations. Most Xerocomoideae genera, i.e., Boletellus, Hemileccinum, Heimioporus, Hourangia, Phylloporus, Xerocomus and some species in Aureoboletus produce ornamented basidiospores [3,15,40,61,62,63,64]. Exceptions exist, however, e.g., in Phylloporus and Xerocomus [44]. Smooth basidiospores are found in Alessioporus, Pulchroboletus, most species in Aureoboletus [3,31,46,54,55], and the new genus Rubinosporus. The basidiospores of the single Rubinosporus species, R. auriporus showed a weakly dextrinoid reaction in Melzer’s reagent, similar to two species in Alessioporus and Pulchroboletus, namely A. ichnusanus and P. roseoalbidus whereas the other species in the two latter genera are not dextrinoid [46,54,55,56]. Therefore, the character cannot be considered typical for Rubinosporus.
In the Xerocomoideae-wide phylogeny obtained in this study, the monophyly of all genera was highly supported. However, no sequences of Alessioporus were added in the phylogeny because among the genes that were used to infer our phylogeny, only a partial tef1 sequence of A. ichnusanus was available in GenBank. However, in ITS and combined ITS+ LSU+tef1 phylogenies of previous studies, Alessioporus was sister to Pulchroboletus [46,54,56]. In this study, Pulchroboletus was sister to Aureoboletus with high support, and distant from Rubinosporus. Moreover, Alessioporus is morphologically clearly different from Rubinosporus as discussed above. The relationship of Rubinosporus to the other genera within Xerocomoideae remains unclear. It formed a clade close to Hemileccinum with poor support. More species, genes, phylogenies are needed to reveal the sister relationship of Rubinosporus. In addition, the three genera Phylloporus, Hourangia, and Xerocomus formed a highly supported clade. Phylloporus formed a clade sister to Xerocomus, and both genera are sisters to Hourangia. The result is slightly different from Wu et al. [3] phylogeny (based on 28S, tef1, rpb1, and rpb2), in which the three genera also formed a highly supported clade but Phylloporus was sister to Hourangia, not Xerocomus like in this study.
Most of the Boletaceae genera have been recognized as important ectomycorrhizal fungi in forest ecosystems [65,66]. Rubinosporus also presumably forms ectomycorrhizal relationships with either Dipterocarpaceae or Fagaceae, or both. These two tree families were dominant around the area where the genus was found. However, further research is needed to confirm the ectomycorrhizal host species of Rubinosporus.
Rubinosporus is the third novel bolete genus described from Thailand, after Spongiforma Desjardin, Manfr. Binder, Roekring & Flegel, and Cacaoporus Raspé & Vadthanarat were described in 2009 and 2019, respectively [11,67]. Prior to this study, Boletaceae subfamily Xerocomoideae consisted of nine genera [2,3]. Based on the morphological and phylogenetic results in the present study, the tenth genus, Rubinosporus is introduced in the subfamily Xerocomoideae.
Author Contributions
Conceived and designed study, O.R., S.V. and S.L.; analyzed data, S.V. and O.R.; collected specimens, S.V.; performed experiments, S.V. and O.R.; wrote the manuscript, S.V. and O.R.; reviewed and edited the manuscript, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research work was partially supported by Chiang Mai University, and also supported by a Postdoctoral Fellowship from Mae Fah Luang University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: (https://www.ncbi.nlm.nih.gov/; http://purl.org/phylo/treebase, submission ID 28349 and 28350; accessed on 1 December 2021).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nuhn M.E., Binder M., Taylor A.F.S., Halling R.E., Hibbett D.S. Phylogenetic overview of the Boletineae. Fungal Biol. 2013;117:479–511. doi: 10.1016/j.funbio.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 2.Wu G., Feng B., Xu J., Zhu X.T., Li Y.C., Zeng N.K., Hosen I., Yang Z.L. Molecular phylogenetic analyses redefine seven major clades and reveal 22 new generic clades in the fungal family Boletaceae. Fungal Divers. 2014;69:93–115. doi: 10.1007/s13225-014-0283-8. [DOI] [Google Scholar]
- 3.Wu G., Li Y.C., Zhu X.T., Zhao K., Han L.H., Cui Y.Y., Li F., Xu J.P., Yang Z.L. One hundred noteworthy boletes from China. Fungal Divers. 2016;81:25–188. doi: 10.1007/s13225-016-0375-8. [DOI] [Google Scholar]
- 4.Orihara T., Smith M.E. Unique phylogenetic position of the African truffle-like fungus, Octaviania ivoryana (Boletaceae, Boletales), and the proposal of a new genus, Afrocastellanoa. Mycologia. 2017;109:323–332. doi: 10.1080/00275514.2017.1301750. [DOI] [PubMed] [Google Scholar]
- 5.Crous P.W., Luangsa-ard J.J., Wingfield M.J., Carnegie A.J., Hernández-Restrepo M., Lombard L., Groenewald J.Z. Fungal Planet description sheets: 785–867. Persoonia. 2018;41:238–417. doi: 10.3767/persoonia.2018.41.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Farid A., Gelardi M., Angelina C., Franck A.R., Costanzo F., Kaminsky L., Ercole E., Baroni T.J., White A.L., Garey J.R., et al. Phylloporus and Phylloboletellus are no longer alone: Phylloporopsis gen. nov. (Boletaceae), a new smooth-spored lamellate genus to accommodate the American species Phylloporus boletinoides. Fungal Syst. Evol. 2018;2:341–359. doi: 10.3114/fuse.2018.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ming Z., Hui T.L. Erythrophylloporus (Boletaceae, Boletales), a new genus inferred from morphological and molecular data from subtropical and tropical China. Mycosystema. 2018;37:1111–1126. [Google Scholar]
- 8.Parihar A., Hembrom M.E., Vizzini A., Das K. Indoporus shoreae gen. et sp. nov. (Boletaceae) from tropical India. Cryptogam. Mycol. 2018;39:447–466. doi: 10.7872/crym/v39.iss4.2018.447. [DOI] [Google Scholar]
- 9.Wu G., Lee S.M.L., Horak E., Yang Z.L. Spongispora temakensis, a new boletoid genus and species from Singapore. Mycologia. 2018;110:919–929. doi: 10.1080/00275514.2018.1496387. [DOI] [PubMed] [Google Scholar]
- 10.Khmelnitsky O., Davoodian N., Singh P., Raspé O., Lee S.M., Fechner N., Bonito G., Lebel T., Halling R.E. Ionosporus: A new genus for Boletus longipes (Boletaceae), with a new species, I. australis, from Australia. Mycol. Prog. 2019;18:439–451. doi: 10.1007/s11557-018-01463-1. [DOI] [Google Scholar]
- 11.Vadthanarat S., Lumyong S., Raspé O. Cacaoporus, a new Boletaceae genus, with two new species from Thailand. MycoKeys. 2019;54:1–29. doi: 10.3897/mycokeys.54.35018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sulzbacher M.A., Orihara T., Grebenc T., Wartchow F., Smith M.E., Martín M.P., Giachini A.J., Baseia I.G. Longistriata flava (Boletaceae, Basidiomycota)—A new monotypic sequestrate genus and species from Brazilian Atlantic Forest. MycoKeys. 2020;62:53–73. doi: 10.3897/mycokeys.62.39699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hyde K.D., Norphanphoun C., Chen J., Dissanayake A.J., Doilom M., Hongsanan S., Jayawardena R.S., Jeewon R., Perera R.H., Thongbai B., et al. Thailand’s amazing diversity: Up to 96% of fungi in northern Thailand may be novel. Fungal Divers. 2018;93:215–239. doi: 10.1007/s13225-018-0415-7. [DOI] [Google Scholar]
- 14.Thongbai B., Hyde K.D., Lumyong S., Raspé O. High undescribed diversity of Amanita section Vaginatae in northern Thailand. Mycosphere. 2018;9:462–494. doi: 10.5943/mycosphere/9/3/3. [DOI] [Google Scholar]
- 15.Zhu X.T., Wu G., Zhao K., Halling R.E., Yang Z.L. Hourangia, a new genus of Boletaceae to accommodate Xerocomus cheoi and its allied species. Mycol. Prog. 2015;14:37. doi: 10.1007/s11557-015-1060-0. [DOI] [Google Scholar]
- 16.Pouzar Z. Nova genera macromycetum I. Ceská Mykol. 1957;11:48–50. [Google Scholar]
- 17.Wu G., Zhao K., Li Y.C., Zeng N.K., Feng B., Halling R.E., Yang Z.L. Four new genera of the fungal family Boletaceae. Fungal Divers. 2015;81:1–24. doi: 10.1007/s13225-015-0322-0. [DOI] [Google Scholar]
- 18.Thiers B. Continuously Updated—Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. [(accessed on 8 March 2022)]. Available online: http://sweetgum.nybg.org/science/ih/
- 19.Kornerup A., Wanscher J.H. Methuen Handbook of Colour. 3rd ed. Eyre Methuen Ltd.; London, UK: 1978. pp. 1–252. [Google Scholar]
- 20.Doyle J.J., Doyle J.L. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- 21.Raspé O., Vadthanarat S., De Kesel A., Degreef J., Hyde K.D., Lumyong S. Pulveroboletus fragrans, a new Boletaceae species from Northern Thailand, with a remarkable aromatic odor. Mycol. Prog. 2016;15:38. doi: 10.1007/s11557-016-1179-7. [DOI] [Google Scholar]
- 22.Matheny P.B. Improving phylogenetic inference of mushrooms with RPB1 and RPB2 nucleotide sequences (Inocybe; Agaricales) Mol. Phylogenet. Evol. 2005;35:1–20. doi: 10.1016/j.ympev.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Rehner S.A., Buckley E. A Beauveria phylogeny inferred from nuclear ITS and EF1-α sequences: Evidence for cryptic diversification and links to Cordyceps teleomorphs. Mycologia. 2005;97:84–98. doi: 10.3852/mycologia.97.1.84. [DOI] [PubMed] [Google Scholar]
- 24.Katoh K., Standley D.M. MAFFT Multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stamatakis A. RAxML-vi-hpc: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 26.Miller M.A., Holder M.T., Vos R., Midford P.E., Liebowitz T., Chan L., Hoover P., Warnow T. The CIPRES portals. CIPRES. 2009. [(accessed on 19 December 2021)]. Available online: http://www.phylo.org/portal2/home.
- 27.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ronquist F., Teslenko M., van der Mark P., Ayres D., Darling A., Höhna S., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo M., Ortiz-Santana B. Revision of leccinoid fungi, with emphasis on North American taxa, based on molecular and morphological data. Mycologia. 2020;112:197–211. doi: 10.1080/00275514.2019.1685351. [DOI] [PubMed] [Google Scholar]
- 30.Zhang M., Li T.H., Wang C.Q., Song B., Xu J. Aureoboletus formosus, a new bolete species from Hunan Province of China. Mycol. Prog. 2015;14:118. doi: 10.1007/s11557-015-1142-z. [DOI] [Google Scholar]
- 31.Zhang M., Li T.H., Wang C.Q., Zeng N.K., Deng W.Q. Phylogenetic overview of Aureoboletus (Boletaceae, Boletales), with descriptions of six new species from China. MycoKeys. 2019;61:111–145. doi: 10.3897/mycokeys.61.47520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vadthanarat S., Raspé O., Lumyong S. Phylogenetic affinities of the sequestrate genus Rhodactina (Boletaceae), with a new species, R. rostratispora from Thailand. MycoKeys. 2018;29:63–80. doi: 10.3897/mycokeys.29.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Binder M., Hibbett D.S. Molecular systematics and biological diversification of Boletales. Mycologia. 2006;98:971–981. doi: 10.1080/15572536.2006.11832626. [DOI] [PubMed] [Google Scholar]
- 34.Sato H., Hattori T. New species of Boletellus section Boletellus (Boletaceae, Boletales) from Japan, B. aurocontextus sp. nov. and B. areolatus sp. nov. PLoS ONE. 2015;10:e0128184. doi: 10.1371/journal.pone.0128184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phookamsak R., Hyde K.D., Jeewon R., Bhat D.J., Jones E.B.G., Maharachchikumbura S.S.N., Raspé O., Karunarathna S.C., Wanasinghe D.N., Hongsanan S., et al. Fungal diversity notes 929–1035: Taxonomic and phylogenetic contributions on genera and species of fungi. Fungal Divers. 2019;95:1–273. doi: 10.1007/s13225-019-00421-w. [DOI] [Google Scholar]
- 36.Zhao K., Wu G., Feng B., Yang Z.L. Molecular phylogeny of Caloboletus (Boletaceae) and a new species in East Asia. Mycol. Prog. 2014;13:1127–1136. doi: 10.1007/s11557-014-1001-3. [DOI] [Google Scholar]
- 37.Zhao K., Wu G., Yang Z.L. A new genus, Rubroboletus, to accommodate Boletus sinicus and its allies. Phytotaxa. 2014;188:61–77. doi: 10.11646/phytotaxa.188.2.1. [DOI] [Google Scholar]
- 38.Gelardi M., Simonini G., Ercole E., Davoli P., Vizzini A. Cupreoboletus (Boletaceae, Boletineae), a new monotypic genus segregated from Boletus sect Luridi to reassign the Mediterranean species B. poikilochromus. Mycologia. 2015;107:1254–1269. doi: 10.3852/15-070. [DOI] [PubMed] [Google Scholar]
- 39.Vadthanarat S., Amalfi M., Halling R.E., Bandala V., Lumyong S., Raspé O. Two new Erythrophylloporus species (Boletaceae) from Thailand, with two new combinations of American species. MycoKeys. 2019;55:29–57. doi: 10.3897/mycokeys.55.34570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halling R.E., Fechner N., Nuhn M., Osmundson T., Soytong K., Arora D., Binder M., Hibbett D. Evolutionary relationships of Heimioporus and Boletellus (Boletales), with an emphasis on Australian taxa including new species and new combinations in Aureoboletus, Hemileccinum and Xerocomus. Aust. Syst. Bot. 2015;28:1–22. doi: 10.1071/SB14049. [DOI] [Google Scholar]
- 41.Vadthanarat S., Lumyong S., Raspé O. Heimioporus subcostatus, a new Boletaceae species from northern and northeastern Thailand. Phytotaxa. 2020;475:018–028. doi: 10.11646/phytotaxa.475.1.2. [DOI] [Google Scholar]
- 42.Orihara T., Smith M.E., Shimomura N., Iwase K., Maekawa N. Diversity and systematics of the sequestrate genus Octaviania in Japan: Two new subgenera and eleven new species. Persoonia. 2012;28:85–112. doi: 10.3767/003158512X650121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orihara T., Lebel T., Ge Z.W., Smith M.E., Maekawa N. Evolutionary history of the sequestrate genus Rossbeevera (Boletaceae) reveals a new genus Turmalinea and highlights the utility of ITS minisatellite-like insertions for molecular identification. Persoonia. 2016;37:173–198. doi: 10.3767/003158516X691212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chuankid B., Vadthanarat S., Hyde K.D., Thongklang N., Zhao R., Lumyong S., Raspé O. Three new Phylloporus species from tropical China and Thailand. Mycol. Prog. 2019;18:603–614. doi: 10.1007/s11557-019-01474-6. [DOI] [Google Scholar]
- 45.Binder M., Larsson K.H., Matheny B.P., Hibbett D.S. Amylocorticiales ord. nov. and Jaapiales ord. nov.: Early diverging clades of Agaricomycetidae dominated by corticioid forms. Mycologia. 2010;102:865–880. doi: 10.3852/09-288. [DOI] [PubMed] [Google Scholar]
- 46.Crous P.W., Wingfield M.J., Lombard L., Roets F., Swart W.J., Alvarado P., Groenewald J.Z. Fungal Planet description sheets: 951–1041. Persoonia. 2019;43:223–425. doi: 10.3767/persoonia.2019.43.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henkel T.W., Obase K., Husbands D., Uehling J.K., Bonito G., Aime M.C., Smith M.E. New Boletaceae taxa from Guyana: Binderoboletus segoi gen. and sp. nov., Guyanaporus albipodus gen. and sp. nov., Singerocomus rubriflavus gen. and sp. nov., and a new combination for Xerocomus inundabilis. Mycologia. 2016;108:157–173. doi: 10.3852/15-075. [DOI] [PubMed] [Google Scholar]
- 48.Halling R.E., Nuhn M., Fechner N.A., Osmandson T.W., Soytong K., Arora D., Hibbett D.S., Binder M. Sutorius: A new genus for Boletus eximius. Mycologia. 2012;104:951–961. doi: 10.3852/11-376. [DOI] [PubMed] [Google Scholar]
- 49.Vadthanarat S., Halling R.E., Amalfi M., Lumyong S., Raspé O. An unexpectedly high number of new Sutorius (Boletaceae) species from northern and northeastern Thailand. Front. Microbiol. 2021;12:1–27. doi: 10.3389/fmicb.2021.643505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y.C., Ortiz-Santana B., Zeng N.K., Feng B. Molecular phylogeny and taxonomy of the genus Veloporphyrellus. Mycologia. 2014;106:291–306. doi: 10.3852/106.2.291. [DOI] [PubMed] [Google Scholar]
- 51.Li Y.C., Feng B., Yang Z.L. Zangia, a new genus of Boletaceae supported by molecular and morphological evidence. Fungal Divers. 2011;49:125–143. doi: 10.1007/s13225-011-0096-y. [DOI] [Google Scholar]
- 52.Li H., Wei H., Peng H., Ding H., Wang L., He L., Fu L. Boletus roseoflavus, a new species of Boletus in section Appendiculati from China. Mycol. Prog. 2013;13:21–31. doi: 10.1007/s11557-013-0888-4. [DOI] [Google Scholar]
- 53.Arora D., Frank J.L. Clarifying the butter Boletes: A new genus, Butyriboletus, is established to accommodate Boletus sect. Appendiculati, and six new species are described. Mycologia. 2014;106:464–480. doi: 10.3852/13-052. [DOI] [PubMed] [Google Scholar]
- 54.Gelardi M., Simonini G., Ercole E., Vizzini A. Alessioporus and Pulchroboletus gen. nov. (Boletaceae, Boletineae), two novel genera to accommodate Xerocomus ichnusanus and X. roseoalbidus from European Mediterranean basin: Molecular and morphological evidence. Mycologia. 2014;106:1168–1187. doi: 10.3852/14-042. [DOI] [PubMed] [Google Scholar]
- 55.Frank J.L., Bessette A.R., Bessette A.E. Alessioporus rubriflavus (Boletaceae), a new species from the eastern United States. N. Am. Fungi. 2017;12:1–8. [Google Scholar]
- 56.Farid A., Franck A.R., Garey J.R. Boletus rubricitrinus belongs in Pulchroboletus (Boletaceae) Czech Mycol. 2017;69:143–162. doi: 10.33585/cmy.69204. [DOI] [Google Scholar]
- 57.Horak E. Revision of Malaysian Species of Boletales s.l. (Basidiomycota) Described by Corner EJH (1972, 1974) Forest Research Institute and Ministry of Natural Resources and Environment; Selangor, Malaysia: 2011. pp. 1–283. [Google Scholar]
- 58.Fechner N., Bonito G., Bougher N.L., Lebel T., Halling R.E. New species of Austroboletus (Boletaceae) in Australia. Mycol. Prog. 2017;16:769–775. doi: 10.1007/s11557-017-1314-0. [DOI] [Google Scholar]
- 59.Halling R.E., Desjardin D.E., Fechner N., Arora D., Soytong K., Dentinger B.T.M. New porcini (Boletus sect. Boletus) from Australia and Thailand. Mycologia. 2014;106:830–834. doi: 10.3852/13-340. [DOI] [PubMed] [Google Scholar]
- 60.Han L.H., Hao Y.J., Liu C., Dai D.Q., Zhao K., Tang L.Z. Strobilomyces rubrobrunneus (Boletaceae), a new species with reddish brown scales from eastern China. Phytotaxa. 2018;376:167–176. doi: 10.11646/phytotaxa.376.4.2. [DOI] [Google Scholar]
- 61.Horak E. Heimioporus E. Horak gen. nov.—Replacing Heimiella Boedijn (1951, syn. post., Boletales, Basidiomycota) Sydowia. 2004;56:237–240. [Google Scholar]
- 62.Šutara J. Xerocomus s. l. in the light of the present state of knowledge. Czech Mycol. 2008;60:29–62. doi: 10.33585/cmy.60104. [DOI] [Google Scholar]
- 63.Neves M.A., Halling R.E. Study on species of Phylloporus I: Neotropics and North America. Mycologia. 2010;102:923–943. doi: 10.3852/09-215. [DOI] [PubMed] [Google Scholar]
- 64.Neves M.A., Binder M., Halling R., Hibbett D., Soytong K. The phylogeny of selected Phylloporus species inferred from NUC-LSU and ITS sequences, and descriptions of new species from the Old World. Fungal Divers. 2012;55:109–123. doi: 10.1007/s13225-012-0154-0. [DOI] [Google Scholar]
- 65.Tedersoo L., Smith M.E. Lineages of ectomycorrhizal fungi revisited: Foraging strategies and novel lineages revealed by sequences from belowground. Fungal Biol. Rev. 2013;27:83–99. doi: 10.1016/j.fbr.2013.09.001. [DOI] [Google Scholar]
- 66.Husbands D.R., Henkel T.W., Bonito G., Vilgalys R., Smith M.E. New species of Xerocomus (Boletales) from the Guiana Shield, with notes on their mycorrhizal status and fruiting occurrence. Mycologia. 2013;105:422–435. doi: 10.3852/12-146. [DOI] [PubMed] [Google Scholar]
- 67.Desjardin D.E., Binder M., Roekring S., Flegel T. Spongiforma, a new genus of gasteroid boletes from Thailand. Fungal Divers. 2009;37:1–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: (https://www.ncbi.nlm.nih.gov/; http://purl.org/phylo/treebase, submission ID 28349 and 28350; accessed on 1 December 2021).