Abstract
Background: Computer-based programs have been implemented from a psychosocial approach for the care of people with dementia (PwD). However, several factors may determine adherence of older PwD to this type of treatment. The aim of this paper was to identify the sociodemographic, cognitive, psychological, and physical-health determinants that helped predict adherence or not to a “GRADIOR” computerized cognitive training (CCT) program in people with mild cognitive impairment (MCI) and mild dementia. Method: This study was part of a randomized clinical trial (RCT) (ISRCTN: 15742788). However, this study will only focus on the experimental group (n = 43) included in the RCT. This group was divided into adherent people (compliance: ≥60% of the sessions and persistence in treatment up to 4 months) and non-adherent. The participants were 60–90 age and diagnosed with MCI and mild dementia. We selected from the evaluation protocol for the RCT, tests that evaluated cognitive aspects (memory and executive functioning), psychological and physical health. The CCT with GRADIOR consisted of attending 2–3 weekly sessions for 4 months with a duration of 30 min Data analysis: Phi and Biserial-point correlations, a multiple logical regression analysis was obtained to find the adherence model and U Mann–Whitney was used. Results: The adherence model was made up of the Digit Symbol and Arithmetic of Wechsler Adult Intelligence Scale (WAIS-III) and Lexical Verbal Fluency (LVF) -R tests. This model had 90% sensitivity, 50% specificity and 75% precision. The goodness-of-fit p-value of the model was 0.02. Conclusions: good executive functioning in attention, working memory (WM), phonological verbal fluency and cognitive flexibility predicted a greater probability that a person would be adherent.
Keywords: dementia, rehabilitation, software, computer-based, cognition, psychology
1. Introduction
The World Alzheimer Report 2019 estimated that there are more than 50 million people with dementia (PwD) in the world, a number that will increase to 152 million by 2050 [1]. In recent years, various investigations have been developed on the effectiveness of computer-based cognitive training (CCT) programs to help delay decline and maintain cognitive status in people with mild cognitive impairment (MCI) and mild dementia [2,3]. However, it is not only important to study the effectiveness, but it is also necessary to take into consideration the rate and therefore, the possible characteristics that determine adherence to CCT program.
Adherence has been mostly studied in association with the use of drugs [4], there being few studies about adherence to CCT programs in people with MCI and dementia [5]. The WHO defines adherence as the degree to which a person’s behavior with respect to a treatment corresponds to the recommendations provided by a healthcare professional [6]. Therapeutic compliance has been used as a synonym for adherence, this refers to the degree to which a patient acts according to a therapeutic regimen [7]. That is, the frequency, duration, and latency of a specific treatment. Recently, the literature emphasized persistence to help complement the definition of adherence, terms often used interchangeably, but differing. Persistence refers to the time of treatment from its beginning to its end; in which, there could be a “grace period”. That is, a permitted time interval in which the person temporarily suspends the treatment, but retakes it until its end [8]. Spanish Society of Pharmacy, Clinic, Family and Community (SEFAC) proposes that for a patient to be completely adherent, he/she must be compliant and persistent [9]. However, there will be cases where a person may be compliant, but not necessarily persistent, vice versa or neither [10].
The WHO pointed out the lack of adherence as a public health problem and proposed some factors that could determine it, such as: socioeconomic level, the person, the therapy, different conditions, the health system, and care team [9]. Our research will particularly focus on investigating the determinants of adherence associated with the person.
So, adherence can be predicted and explained from a cognitive, psychological, social, neurocognitive, and even technological point of view. Scase et al. [11] pointed out the mild level of deterioration, social interaction, and the availability of technological support to explain the adherence of a group of people with MCI to computer-based gamified environments. Evers et al. [12] mentioned that poor baseline performance in memory, attention, and semantic verbal fluency (SVF) tests helped to predict greater adherence in older women.
Park et al. [13] tried to associate adherence to virtual reality (VR) training program with the improvement of cognitive functioning. Turunen et al. [14] mentioned good memory performance as one of the variables that helped to predict the adherence to a CCT program in adults at risk of dementia. Han et al. [15] found a correlation between the improvement of memory skills and adherence to The Ubiquitous Spaced Retrieval-based Memory Advancement and Rehabilitation Training (USMART) in people with MCI. On the other hand, when older adults are aware of their cognitive decline, specifically when it affects their executive functioning, there was an increase in their adherence [16]. Similarly, de Wit et al. [17] mentioned that the compensatory strategies offered by a memory support system training in people with MCI could influence adherence. Certain psychological variables such as positive expectations at the beginning of a CCT program were found among the main factors that help predict adherence [14]. On the other hand, it seems that people with cognitive impairment spent more time on web-based CT sessions than people with depressive symptoms or other psychiatric disorders [18].
Moreover, earlier studies have already remarked the difficulties in finding features linked to low adherence to the intervention based on individual Cognitive Stimulation Therapy (iCST) [19]. Consequently, the objective of this study was to identify the sociodemographic, cognitive, psychological, and physical-health determinants that helped predict adherence (therapeutic compliance and persistence) or not to a “GRADIOR” CCT program in people with MCI and mild dementia.
2. Materials and Methods
2.1. Study Design
This study was part of a multicenter simple-blind, randomized clinical trial (RCT) on the effectiveness of the GRADIOR cognitive rehabilitation program in people with MCI and mild dementia [20,21]. This trial was registered at isrctn.com (ISRCTN: 15742788) [22] and was approved by the Drug Research Ethics Committee of the Zamora Health Area (Number: 387-E.C). The recruitment period began in June 2018 and lasted until December 2019. The protocol of this RCT had variations, the main one being related to the design because the ehcoBUTLER platform was excluded. This was not completed on time when the RCT started, which led to modifications in the design with respect to the number of parallel groups, the sample size, and the type of randomization.
The participants included in this RCT were randomly assigned (1:2) to the control or experimental group. Those in the control group (CG) maintained their daily activities, remaining on the waiting list, and those in the experimental group (EG) attended CCT sessions using the GRADIOR program for 4 months. However, the present study focused on the EG. From which, two groups were formed: adherent (compliance-persistent) and non-adherent (those who did not meet any or none of the conditions).
2.2. Participants
The study sample included 43 participants aged 60 to 86, and 72.1% had a basic primary educational level. Participants were selected from day centers, memory clinics and hospitals in the Spanish regions of Castile and León and Galicia. The inclusion criteria were as follows: (1) clinical diagnosis of MCI according to Petersen’s criteria [23] and mild dementia according to the diagnostic criteria of the Diagnostic and Statistical Manual of Mental Disorders (DSM-V) [24] (this diagnostic was carried out by a psychogeriatrician and neurologist); the types of MCI included were amnestic, and for mild dementia were Alzheimer’s disease, vascular dementia, mixed dementia, and frontotemporal dementia; (2) score on the Yesavage Geriatric Depression Scale (GDS) ≤ 5; (3) voluntary participation of each people; (4) participation of a reference caregiver; 5) speaking and understanding Spanish. An additional criterion was the Mini-Mental State Exam (MMSE) scores, the cut-off point for MCI was ≤27 and for mild dementia was 20 ≥ X ≤ 25. MMSE scores were adjusted according to age and educational level of each people [25].
The exclusion criteria were: (1) severe physical comorbidity; (2) severe sensory alterations (auditory or visual) indicated after clinical evaluation by the psychogeriatrician or neurologist; (3) clinically proven psychopathological disorders (depression, anxiety, bipolar disorder, psychosis); (4) neurological disorders (Huntington’s disease, stroke, Parkinson’s disease, dementia with Lewy bodies); (5) history of substance use (e.g., alcohol, tobacco).
To make up the group of adherents, the conditions of compliance and persistence were considered. Regarding therapeutic compliance, people who attended at least 66% of the sessions of the maximum number of sessions, for 30–40 min per session. Additionally, to consider a person persistent or not, the person had to attend weekly for 16 weeks (4 months) of intervention, and therefore they should not exceed the only “grace period” or allowed interval of absence from the CCT of two continuous weeks. If the person met only one or neither of the two conditions, they were considered non-adherent.
2.3. Neuropsychological Assessment
Possible predictor variables of adherence were associated with the baseline of RCT. We constructed an evaluation protocol for the RCT, which included several scales that evaluated different aspects. However, we selected the variables for our study, considering the literature and our objectives. Sociodemographic aspects such as age, sex, educational level and years of education were assessed. Appendix A describes each of the test used to specifically measure global cognitive performance, memory and EF: MMSE [26], Memory of words, Word recognition and Total of Alzheimer’s Disease Assessment Scale—Cognitive Sub-scale (ADASCog) [27], Trail Making Test (TMT) forms A–B [28], Digits, Arithmetic and Digit Symbol of Wechsler Adult Intelligence Scale (WAIS-III) [29], Visual Recognition of the Rivermead Behavior Memory Test (RBMT) [30], Visual Reasoning of the Cambridge Cognition Examination (CAMCOG) [31] and Verbal Fluency (Semantic and Lexical) [32].
Affective state, motivation and expectations were also evaluated as part of the psychological dimension (Appendix A). The affective component was evaluated using the GDS [33]. Motivation was valued using the following question: “Do you need someone to encourage you to attend the workshop?” The other questions included in the questionnaire were associated with the following expectations: (1) memory improvement; (2) improvement in quality of life; (3) spending free time in a pleasant way; (4) meeting new people at the workshop. Data on the use of technologies were collected based on the question “Do you usually use any technology?”
Finally, we used the EuroQol (EQ-5D-5L) test [34], which assesses the patients’ perception about the physical-health dimension: mobility, self-care, daily activities, pain-discomfort and anxiety-depression, and patients’ perception regarding their current health condition (Appendix A).
2.4. Computer-Based Cognitive Training (CCT) and Adherence
GRADIOR is a computer-based cognitive rehabilitation program and allows CCT of different cognitive functions that present a deficit or deterioration based on different etiologies, including dementia [35]. It includes a series of exercises associated with orientation, memory, attention, perception, executive functioning, reasoning, and calculation (Figure 1). Each of these cognitive modalities (cognitive functions) includes various sub-modalities (cognitive processes) to customize CCT to the user’s cognitive profile [36]. The RCT was based on a cognitive training plan designed for people with MCI and mild dementia, independently (Table 1). This meant that the type of exercises was a function of diagnosis. However, the plan was personalized for each patient according to his/her cognitive level in each exercise. Participants had to attend two or three weekly sessions (this was determined for each center), each lasting 30–40 min. The interface of GRADIOR uses an intuitive touchscreen system and meets usability standards [37,38], providing good user experience [39] to adapt to the needs of older people.
Figure 1.
Puzzle exercise. Cognitive modality: executive function. II; software pause.
Table 1.
“GRADIOR” computer-based cognitive training (CCT) plan according to modalities and sub-modalities for participants with mild cognitive impairment (MCI) and mild dementia.
| Cognitive Function | Mild Dementia | Both | Mild Cognitive Impairment (MCI) |
|---|---|---|---|
| Orientation | Orientation | ||
| Attention | Selective sequential visual | ||
| Selective visual-simultaneous | |||
| Vigilance color | |||
| Memory | Span numbers direct | Hearing short term | Word-Word Associative |
| Immediate graphic | Associative image-word | ||
| Span numbers inverse | Span direct lyrics | ||
| Location | |||
| Verbal compound short term | |||
| Associative face-name | |||
| Span direct objects | |||
| Executive Function | Puzzles | Numbers and letters | |
| Keys | Change rules | ||
| Visual inhibition | Ordination stories | ||
| Interference | |||
| Perception | Visual sizes | Graphic colors | Visual figures |
| Visual faces | Text colors | ||
| Calculation | Number identification | ||
| Arithmetic problems | |||
| Reasoning | Sort charts |
To calculate the adherence rate, the number of sessions attended by each person at the CCT during the 4 months was divided by the maximum number of sessions attended, the result was multiplied by one hundred, except for participants who died or dropped out because of medical reasons, for whom the adherence rate was calculated only until the time of drop-out. We consider the cut-off point of 66% for the rate of adherence or therapeutic compliance according to the literature [5,40,41]. Like compliance, persistence or not was considered as a dichotomous variable [42] and was measured taking into account whether or not the person completed the 4 months of intervention, considering the grace period.
2.5. Statistical Analyses
The statistical analysis was performed with the Software Statistical Package for the Social Sciences (SPSS) [43]. We used the punctual biserial correlation to find the degree of association between the dichotomous and dependent variable (adherent and non-adherent) and the quantitative and independent variables (socio-demographic, cognitive, psychological and physical-health). Additionally, the Phi correlation to find the association between dichotomous variables.
The independent variables that were significantly correlated with the dependent variable were taken into consideration as possible predictor variables of adherence and, therefore, were introduced in the analysis with Multiple Logistic Regression. This was used to identify the IVs that helped to predict adherence or not to CCT. We used this analysis because the dependent variable was dichotomous and we had several independent variables, some metric and some qualitative.
Then, we have used a non-parametric analysis for two independent samples (Mann–Whitney) due to the size of the sample and because not all variables followed a normal distribution (Shapiro–Wilk). This analysis allowed us (1) to compare the performance between adherent and non-adherent group, (2) to investigate if there were significant differences between people with MCI and mild dementia in relation to each group (adherent and non-adherent) and, (3) to evaluate if there were significant differences between MCI-adherent vs. MCI-non-adherent and mild dementia-adherent vs. mild dementia-non-adherent with respect to the variables (Digit Symbol and Arithmetic of WAIS-III and LVF-R) that made up the adherence model.
3. Results
3.1. Participant Characteristics
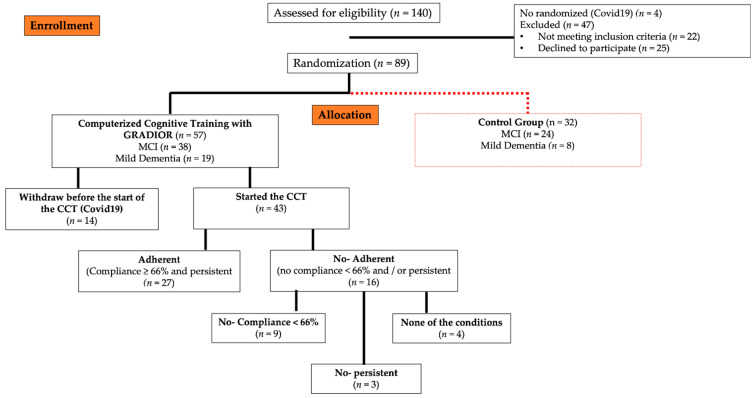
In total, 140 people were contacted to enter the study, and 47 (33.6%) were excluded due to the following reasons: they did not meet the criteria (n = 22) and did not want to participate (n = 25). Additionally, four (2.9%) were not randomized due to the onset of COVID-19. A total of 89 (63.6%) people were randomized. Of these, 57 were assigned to the EG. However, only 75.4% (n = 43) participants managed to initiate the intervention and 24.6% (n = 14) did not start any session due to the start of COVID-19 (Figure 2).
Figure 2.
Sample randomization process of randomized clinical trial (RCT). Additionally, conformation of the adherent and non-adherent group in the experimental group (EG). CCT, Computerized cognitive training.
The EG participants were classified into two groups (adherent and non-adherent). Twenty-seven (62.8%) made up the group of adherents. The mean age of this group was 73.6 ± 6.0, and 40.7% were men. The mean years of education were 9.6 ± 2.8. Sixteen (59.3%) participants were diagnosed with MCI and 40.7% (n = 11) with mild dementia. The mean adherence rate of this group was 83.3 ± 8.6. The non-adherent group (n = 16) had a mean of 76.1 ± 7.5 for age and 8.4 ± 1.1 for years of education. Of this group, 37.5% (n = 6) were men, 68.8% (n = 11) were diagnosed with MCI and 31.2% (n = 5) with mild dementia. Additionally, the mean adherence rate was 59.2 ± 16.1. However, there were no significant differences between the two groups (adherent and non-adherent) with respect to age, sex and years of education (Table 2). Of the non-adherent participants, 56% (n = 9) did not comply with 66% of the CCT sessions, 19% (n = 3) were not persistent or dropped-out during the training period (4 months), and 25% (n = 4) did not meet any of the above conditions (Figure 2).
Table 2.
Baseline characteristics for the adherent and non-adherent group.
| Variable | Sub-Categories | T.Student/Mann–Whitney/Χ2 | p | d-cohen/rb | Adherent | No-Adherent | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| ± SD | Number | % | ± SD | Number | % | |||||
| Age | −1.185 | 0.243 | −0.374 | 73.6 ± 6.0 | 76.1 ± 7.5 | |||||
| Sex | ||||||||||
| Female | 0.044 | 0.834 | 0.032 | 16 | 61,50% | 10 | 38,50% | |||
| Male | 11 | 64,70% | 6 | 35,30% | ||||||
| Years of education | 264.000 | 0.120 | 0.222 | 9.6 ± 2.8 | 8.4 ± 1.1 | |||||
| Clinical Group | ||||||||||
| MCI | 16 | 59,30% | 11 | 40,70% | ||||||
| Mild dementia | 11 | 68,80% | 5 | 31,30% | ||||||
| Adherence Rate | 83.3 ± 8.6 | 59.2 ± 16.1 | ||||||||
| MMSE | 24.4 ± 2.4 | 22.6 ± 3.9 | ||||||||
| ADAS-Cog: Memory of words | 6.1 ± 1.3 | 6.4 ± 1.5 | ||||||||
| ADAS-Cog: word recognition | 3.4 ± 1.9 | 4.6 ± 3.6 | ||||||||
| ADAS-Cog: Total | 13.7 ± 5.0 | 17.2 ± 6.7 | ||||||||
| TMTA_Mistakes | 0.4 ± 0.8 | 0.4 ± 0.7 | ||||||||
| TMTA_Time | 12.8 ± 12.0 | 6.3 ± 2.4 | ||||||||
| WAIS-III: Total Digit | 10.8 ± 2.6 | 9.3 ± 2.5 | ||||||||
| CAMCOG: Visual Reasoning | 2.4 ± 1.4 | 1.9 ± 1.5 | ||||||||
| RBMT: Drawing recognition | 7.7 ± 2.2 | 8.0 ± 2.7 | ||||||||
| WAIS-III: Digit Symbol | 312.000 | 0.016 * | 0.444 | 10.2 ± 2.7 | 8.3 ± 2.8 | |||||
| WAIS-III: Arithmetic | 306.500 | 0.022 * | 0.419 | 10.3 ± 2.9 | 7.7 ± 3.0 | |||||
| SVF | 7.2 ± 3.0 | 5.6 ± 2.5 | ||||||||
| LVF-P | 7.7 ± 3.1 | 6.3 ± 3.2 | ||||||||
| LVF-M | 8.1 ± 3.8 | 6.3 ± 3.5 | ||||||||
| LVF-R | 2.575 | 0.014 * | 0.812 | 8.8 ± 2.5 | 6.6 ± 3.0 | |||||
| GDS | 4.1 ± 4.0 | 4.4 ± 2.9 | ||||||||
| Health condition | 67.0 ± 21.4 | 76.3 ± 19.6 | ||||||||
| Motivation:Attend | Nothing | 20 | 62,50% | 12 | 37,50% | |||||
| Somethings | 4 | 66,70% | 2 | 33,30% | ||||||
| I’m not sure | 1 | 100,00% | 0 | 0,00% | ||||||
| Quite a lot | 2 | 50,00% | 2 | 50,00% | ||||||
| Expectations: Memory | Nothing | 3 | 60,00% | 2 | 40,00% | |||||
| I’m not sure | 3 | 75,00% | 1 | 25,00% | ||||||
| Quite a lot | 13 | 56,50% | 10 | 43,50% | ||||||
| A lot | 8 | 72,70% | 3 | 27,30% | ||||||
| Expectations: Quality of life | Nothing | 1 | 33,30% | 2 | 66,70% | |||||
| I’m not sure | 4 | 100,00% | 0 | 0,00% | ||||||
| Quite a lot | 14 | 56,00% | 11 | 44,00% | ||||||
| A lot | 8 | 72,70% | 3 | 27,30% | ||||||
| Expectations: Free time | I’m not sure | 1 | 100,00% | 0 | 0,00% | |||||
| Quite a lot | 10 | 66,70% | 5 | 33,30% | ||||||
| A lot | 16 | 59,30% | 11 | 40,70% | ||||||
| Expectations: Relating | Nothing | 2 | 100,00% | 0 | 0,00% | |||||
| Somethings | 2 | 50,00% | 2 | 50,00% | ||||||
| I’m not sure | 3 | 100,00% | 0 | 0,00% | ||||||
| Quite a lot | 10 | 47,60% | 11 | 52,40% | ||||||
| A lot | 10 | 76,90% | 3 | 23,10% | ||||||
| EQ-5D-5L: Mobility | I have no problem | 21 | 70,00% | 9 | 30,00% | |||||
| Minor problems | 3 | 60,00% | 2 | 40,00% | ||||||
| Moderate problems | 2 | 40,00% | 3 | 60,00% | ||||||
| serious problems | 1 | 33,30% | 2 | 66,70% | ||||||
| EQ-5D-5L: Self-care | I have no problem | 23 | 63,90% | 13 | 36,10% | |||||
| Minor problems | 1 | 33,30% | 2 | 66,70% | ||||||
| Moderate problems | 3 | 100,00% | 0 | 0,00% | ||||||
| serious problems | 0 | 0,00% | 1 | 100,00% | ||||||
| EQ-5D-5L: Everyday activities | I have no problem | 20 | 60,60% | 13 | 39,40% | |||||
| Minor problems | 1 | 33,30% | 2 | 66,70% | ||||||
| Moderate problems | 5 | 83,30% | 1 | 16,70% | ||||||
| serious problems | 1 | 100,00% | 0 | 0,00% | ||||||
| EQ-5D-5L: Pain/discomfort | I have no problem | 15 | 75,00% | 5 | 25,00% | |||||
| Minor problems | 5 | 41,70% | 7 | 58,30% | ||||||
| Moderate problems | 3 | 60,00% | 2 | 40,00% | ||||||
| serious problems | 4 | 80,00% | 1 | 20,00% | ||||||
| I can’t | 0 | 0,00% | 1 | 100,00% | ||||||
| EQ-5D-5L: Anxiety/depression | I have no problem | 18 | 72,00% | 7 | 28,00% | |||||
| Minor problems | 2 | 33,30% | 4 | 66,70% | ||||||
| Moderate problems | 4 | 57,10% | 3 | 42,90% | ||||||
| serious problems | 2 | 50,00% | 2 | 50,00% | ||||||
| I can’t | 1 | 100,00% | 0 | 0,00% | ||||||
| Prior use of technology | No | 4 | 66,70% | 2 | 33,30% | |||||
| Yes | 23 | 62,20% | 14 | 37,80% | ||||||
Note: , mean; * p-value ≤ 0.05; CAMCOG, Cambridge Cognition Examination; EQ-5D-5L, EuroQol; GDS, Geriatric Depression Scale; LVF, Lexical Verbal Fluency (forms P, M, R); MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; RBMT, The Rivermead Behavioural Memory Test; SD, standard deviation; SVF, Semantic Verbal Fluency; TMT, Trail Making Test; WAIS-III, Wechsler Adult Intelligence Scale. Bold Data; significance effect size.
3.2. Adherence Prediction Model to CCT Program
The variables that were directly correlated with the adherent group to the CCT were: Digit Symbol of WAIS-III (p = 0.02), Arithmetic of WAIS-III (p = 0.02) and lexical verbal fluency (LVF)-R (p = 0.03).
These variables were significantly correlated with the dependent variable and were included in a multiple logistic regression model. The final model proposed that the predictors of adherence were associated with performance in Digit Symbol of WAIS-III (OR: 1.06; 95 CI: 0.77–1.47), Arithmetic of WAIS-III (OR: 1.22; 95 CI: 0.90–1.65) and LVF-R (OR: 1.22; 95 CI: 0.91–1.62) (Table 3). Therefore, an increase in performance in these tests predicts an increase in the probability that a person is adherent (compliant and persistent). The adherent group obtained a better performance with moderate-high effect sizes than the non-adherent group in Digit Symbol of WAIS-III (p = 0.016; rb = 0–444), Arithmetic of WAIS-III (p = 0.022; rb = 0.419) and LVF-R (p = 0.014; d-cohen = 0.812) (Table 2). The goodness-of-fit p-value of the model was 0.02, with a McFadden R-squared value of 0.174 (Table 3). This model has 90% sensitivity, 50% specificity and 75% precision to correctly identify the adherence group (compliance-persistence). Of the 16 people who were not adherent, the model would only be able to correctly predict 8 subjects.
Table 3.
Model of adherence to a CCT program “GRADIOR”.
| Predictor Variable | McFadden R2 | p-Value | Estimate | Standard Error | OR | z | 95% CI |
|---|---|---|---|---|---|---|---|
| WAIS-III: Digit Symbol | 0.174 | 0.019 | 0.064 | 0.164 | 1.066 | 0.387 | 0.773–1.470 |
| WAIS III: Arithmetic | 0.203 | 0.153 | 1.225 | 1.329 | 0.908–1.653 | ||
| LVF-R | 0.200 | 0.146 | 1.222 | 1.368 | 0.917–1.627 |
Note: CCT, computer-based cognitive training; CI, confidence interval; LVF-R, Lexical Verbal Fluency; OR, odds ratios; WAIS-III, Wechsler Adult Intelligence Scale.
Regarding the adherent group, there were significant differences with moderate effect sizes between the group of people with MCI and mild dementia with respect to performance in Digit Symbol of WAIS-III (p = 0.010; rb = −0–591), Arithmetic of WAIS-III (p = 0.005; rb = −0.642) and LVF-R (p = 0.030; rb = −0.500). Better performance was seen in people with MCI compared to people with mild dementia (Table 4).
Table 4.
Mann–Whitney U test. Comparison between people with MCI and mild dementia in relation to each group (adherent and non-adherent).
| Variable | Group | Adherent | No-Adherent | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mann–Whitney | p-Value | rb | N | ± SD | Mann–Whitney | p-Value | rb | N | ± SD | ||
| WAIS-III: Digit Symbol | Mild Dementia | 36.000 | 0.010 ** | −0.591 | 11 | 8.7 ± 0.7 | 11.000 | 0.065 | −0.600 | 5 | 6.3 ± 1.5 |
| MCI | 16 | 11.3 ± 0.6 | 11 | 9.2 ± 0.6 | |||||||
| WAIS III: Arithmetic | Mild Dementia | 31.500 | 0.005 ** | −0.642 | 11 | 8.2 ± 0.7 | 16.000 | 0.205 | −0.418 | 5 | 6.4 ± 1.4 |
| MCI | 16 | 11.7 ± 0.6 | 11 | 8.3 ± 0.9 | |||||||
| LVF-R | Mild Dementia | 44.000 | 0.030 * | −0.500 | 11 | 7.5 ± 0.8 | 10.000 | 0.052 * | −0.636 | 5 | 4.2 ± 1.6 |
| MCI | 16 | 9.7 ± 0.5 | 11 | 7.7 ± 0.6 | |||||||
Note: * p-value ≤ 0.05; ** p-value ≤ 0.01; , mean; LVF, Lexical Verbal Fluency; rb, Rank biserial correlation; WAIS-III, Wechsler Adult Intelligence Scale. Bold Data; significance effect size.
On the other hand, we wanted to compare people with MCI-adherents with MCI-non-adherents and the same for people with dementia. Regarding people with MCI, there were low effect sizes between adherents and non-adherents in relation to performance in Digit Symbol and Arithmetic (rb = −0.358). We observed a better performance of the MCI-adherent group compared to the MCI-non-adherence group. While in the dementia group, there were low–medium effect sizes (rb = −0.417) for Arithmetic and LVF-R. We found that the mild dementia-adherent group performed better than the mild dementia-non-adherent group (Table 5).
Table 5.
Mann–Whitney U test. Comparison between MCI-adherent vs. MCI-non-adherent and mild dementia-adherent vs. dementia-non-adherent.
| Variable | Group | Dementia | MCI | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mann–Whitney | p | rb | N | ± SD | Mann–Whitney | p | rb | N | ± SD | ||
| WAIS-III: Digit Symbol | No-Adherent | 17.500 | 0.464 | −0.271 | 4 | 6.6 ± 1.9 | 52.000 | 0.137 | −0.358 | 9 | 9.9 ± 0.5 |
| Adherent | 12 | 8.4 ± 0.7 | 18 | 10.7 ± 0.7 | |||||||
| WAIS III: Arithmetic | No-Adherent | 14.000 | 0.232 | −0.417 | 4 | 6.0 ± 1.8 | 52.000 | 0.139 | −0.358 | 9 | 9.0 ± 0.9 |
| Adherent | 12 | 8.2 ± 0.6 | 18 | 10.9 ± 0.7 | |||||||
| LVF-R | No-Adherent | 14.000 | 0.244 | −0.417 | 4 | 4.8 ± 1.9 | 61.500 | 0.324 | −0.241 | 9 | 8.2 ± 0.7 |
| Adherent | 12 | 7.1 ± 0.9 | 18 | 9.2 ± 0.6 | |||||||
Note: , mean; LVF, Lexical Verbal Fluency; rb, Rank biserial correlation; WAIS-III, Wechsler Adult Intelligence Scale. Bold Data; significance effect size.
4. Discussion
It is well known that the recruitment and involvement phase of CCT is the most difficult, due to therapeutic nihilism among families and often general practitioners (GPs), and because of patients’ fear of dealing with something new [44]. However, difficulties do not end at the beginning, as patients require motivation to continue the CCT program. In all these stages, we can identify several features that are intrinsic to the person and could intervene and predict adherence to a CCT program. Our purpose was to identify the sociodemographic, cognitive, psychological, and physical-health determinants that helped predict adherence or not to a “GRADIOR” CCT program in people with MCI and mild dementia. It can also be used as guidance to personalize the intervention so that patients might gain more benefits and to improve the sustainability of the care system and take care about the risks of drop-out in specific patients.
4.1. Socio-Demographic Variables and Adherence
We did not find age to be a predictor of adherence to CCT, contrary to some studies such as Maseda et al. [45]. Even so, it is difficult to support that age is the only factor that influences adherence itself, but it is probably associated with other problems or capacities that affect adherence. Indeed, age may also be associated with greater cognitive impairment.
Another sociodemographic factor considered was years of education, and we formally hypothesized that it was going to influence on adherence. However, we did not find any relationship between these variables [14]. In contrast, some studies reported educational level as a predictor of adherence [46,47]. We cannot establish any formal recommendations in this regard since different research conclusions are reached. We consider that future studies should assess this aspect more thoroughly to define its relevance. Moreover, it will probably be necessary to combine educational level with other intellectual activities as reading, work performance, hobbies, etc., to identify the main feature involved in adherence.
As for the variables sex and diagnosis, these were not predictors of adherence. However, it is likely that our results were influenced by the greater number of women and people with MCI that made up our sample. Therefore, it would be interesting for future studies to include more balanced groups in terms of sex and diagnosis to determine their level of influence on adherence.
4.2. Cognitive Profile and Adherence
Different studies support the presence of executive dysfunction associated with alterations in response inhibition and cognitive flexibility in people with MCI [48] and deficits in working memory (WM) [49], inhibition process, sensitivity to interference [50], flexibility and reasoning [51] in people with Alzheimer’s. In this order of ideas, our findings indicated how some executive functions (EF) such as attention, WM, numerical reasoning, and verbal fluency, evaluated by Arithmetic, Digit Symbol and LVF-R test are involved in adherence to a CCT program such as GRADIOR. Additionally, our previous study on the effectiveness of GRADIOR indicated a trend of improvement in these cognitive processes after 4 months of intervention in the EG [21].
Anderson-Hanley, Arciero, Barcelos, Nimon, Rocha, Thurin and Maloney [16] pointed out EF as a motivator of adherence. In turn, poor executive functioning has been identified as an important factor that helps negatively predict the prognosis of dementia [52].
In particular, the role of WM as an adherence factor has been pointed out in studies on the improvement of this cognitive function (WM) after CCT sessions [53,54], while other studies have associated adherence with memory, the latter from a more general level [14,15]. Other studies have pointed out the association between adherence and delayed recall [55]. The functioning of the WM depends on the process of attention for the performance of the tasks associated with the Arithmetic and Digit Symbol scales. In our study, the WM sub-processes involved in performing these tasks were particularly associated with (1) maintaining information for a short time, which allowed for the (2) using, manipulating, or processing of this information. In some cases, this allowed for the activation of more complex cognitive functions such as numerical reasoning (Arithmetic).
It is probable that the attention, maintenance and manipulating of information associated with WM and the phonological verbal fluency are more directly linked to adherence. In fact, there was a significantly better performance at the beginning of the CCT by the adherent group compared to the non-adherent group in these functions. Likewise, when we simplify the comparison by clinical groups, we find that the MCI/dementia adherent group performed better for these functions compared to the MCI/dementia non-adherent group. In accordance with our findings, we suggest paying more attention to those people with a lower cognitive performance in these tests and, therefore, a greater deterioration in these EF, because they may not be as adherent to a CCT program. So, the development of strategies to link and maintain these people in a CCT program will take more effort, from conducting an adequate neuropsychological evaluation to determining the presence and severity of deficits to planning a personalized CCT intervention plan.
4.3. Physical-Health Variables and Adherence
Our findings did not mention any physical-health variable that was able of predicting a person’s adherence to a CCT program such as GRADIOR. Most participants agreed that they did not have mobility problems, self-care, ADL, pain and discomfort, anxiety, and depression. Possibly, if we had found greater problems in these dimensions, this could have somehow influenced adherence to the CCT with GRADIOR. However, this did not happen, which could be explained because the sample was made up of people with MCI and mild dementia, and not people in advanced stages of the disease.
In this regard, Tolea et al. [56] pointed out that cognitive deterioration is linked with physical deterioration. In this way, people who progress in their cognitive deterioration also progress in their physical deterioration. These changes can also vary according to the etiology, and per example, people with vascular dementia progress more quickly to physical deterioration than people with Alzheimer’s dementia.
Despite our findings, it’s necessary that technologies, including CCT programs, should be available and accessible not only in a clinical center, but also at home in order to improve the access people suffering physical limitations to the treatment [57,58].
4.4. Psychological Profile and Adherence
None of the psychological variables (expectations, motivation, and mood) were associated with the CCT adherence. Regarding the initial expectations, the participants agreed that the CCT with GRADIOR would help to improve their memory, quality of life, free time and relating to others. However, our findings did not point to any of these expectations as predictors of adherence. Probably, GRADIOR could help to satisfy these expectations and consequently 62.8% of the participants were adherents (compliant and persistent).
In fact, the users of the “GRADIOR” CCT program improved their social network throughout the sessions. They found it easier to meet and interact with new people because they shared the same needs and interests “now, I have friends who understand me, and I can have a coffee with them after working with GRADIOR”. According to the systematic review of Heins et al. [59], interventions with technology have shown to improve the social support network and therefore alleviate feelings of loneliness. Similarly, CCT probably encourages PwD to become active and participate in the group, improving their mood and motivation [38,60,61]. In our study, mood did not help to predict adherent.
Regarding the level of motivation, it did not help to predict adherent. However, we observed that participants did not need external motivation to participate in the RCT. It can be explained by the consent granted by each participant, since this could be a bias of their participation and voluntary commitment.
The questionnaire used to measure expectations, motivation and mood could have been influenced in part by the cognitive impairment in language comprehension commonly associated with people with dementia [62]. Impairment of this cognitive process is usually more evident and significant as dementia progresses, and based on our initial assessment, most people understood the information. Likewise, and with the aim of reducing this problem, the evaluator explained and repeated each of the questions to the participants as many times as necessary. Due to the above, we consider that we tried to reduce biases regarding the understanding of the questions that made up the questionnaire.
4.5. Previous Use of Technology and Adherence
The previous use of computers was not associated with higher probability of initiating CCT, contrary to what was mentioned by Turunen, Hokkanen, Bäckman, Stigsdotter-Neely, Hänninen, Paajanen, Soininen, Kivipelto and Ngandu [14]. Currently, the use of technology is probably more frequent, and it is supposed to rise in the next years. Consequently, no previous experience can be a challenge for the patient, and the excitement of using technology for the first time can balance prior experience with it.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
4.6. Strengths and Limitations
Regarding the limitations of the study, we are aware that the sample was medium. Nevertheless, this problem is representative of most of the studies carried out with CCT [63]. The main reason was the impact of the onset of a COVID-19 pandemic, which led to the end of the selection process and, in general, the RCT, due to wide mobility restrictions and strict confinement for this vulnerable population. However, this RCT methodologically provides the inclusion of older people with cognitive impairment and their participation for 4 months in a CCT, which is important because most of the published studies on CCT in older people contemplate short periods of CCT [64,65,66]. It makes this study as a good contribution to the clinic field.
Carrying out this study as part of an RCT presented a series of drawbacks in (1) the process of selecting the sample of older people and (2) its maintenance throughout the 4-month CCT. The above is due to different characteristics, which could negatively influence these two scenarios, such as the following: high mortality rate of the sample, mobility alterations, emotional alterations, the stigma of the disease, the start of a pandemic, little financing, and the high cost of resources [21].
On the other hand, we consider that 83.3% to be a high adherence rate for an RCT with the characteristics described and contributes to a little-explored field “adherence to CCT programs”. Even so, the results are more likely to be generalized to people with MCI, as only 37.2% of our sample were people with mild dementia. We consider that adherence to psychosocial approaches will be higher in the early stages of dementia and, consequently, the timely diagnosis of MCI and dementia is strategic for improving the prognosis, quality of life and social health of these people. Indeed, the progression of cognitive impairment leads to fewer resources and less motivation to attend a complex CCT program, even when improvement is predicted [67].
Our study conducted a comprehensive compilation of possible predictors at the person level. Perhaps a variable at the personal level that was not considered in our study was genetics; for example, we know that the APOE-e4 gene is associated with a higher risk of Alzheimer dementia. These types of variables probably exert some influence on the adherence of these people to various treatments, including CCT. Therefore, it would be interesting to develop this type of study.
Orrell, Yates, Leung, Kang, Hoare, Whitaker, Burns, Knapp, Leroi, Moniz-Cook, Pearson, Simpson, Spector, Roberts, Russell, de Waal, Woods and Orgeta [19] suggested contextual variables and person features modify adherence. However, it was not found in our study, but undoubtedly the contexts in which the interventions were carried out could be different and people supporting the intervention could also have different levels of motivation, experience, and skills. All of them could have influenced in the adherence of the participants to the CCT with “GRADIOR”. In any case, it should be noted that all the professionals involved received training and support during the entire RCT to maximize adherence.
Our study did not consider usability and user-experience variables associated with the interface of the “GRADIOR” program in adherence because we had previously performed several usability studies [37] and user-experience. Irazoki, Sánchez-Gómez, Contreras-Somoza, Toribio-Guzmán, Martín-Cilleros, Verdugo-Castro, Jenaro-Río and Franco-Martín [39] and Santos Golino and Flores-Mendoza [68] found that the improvement of instructions helped to rise adherence rates in a CCT program for the elderly. Therefore, it would be interesting for future studies to investigate the association between variables such as usability, user-experience and adherence to CCT programs.
4.7. Recommendations and Implications
Our study has a scientific value because it provides a predictive model of adherence based on evidence, indicating several predictive cognitive processes for being considered in the implementation of CCT programs in people with MCI and mild dementia. Adherence to CCT programs is a subject little studied, and most scientific efforts are focused on effectiveness studies [69,70,71,72]. In turn, adherence is a factor that helps determine the effectiveness of a CCT program. Consequently, adherence studies are of great interest, and therefore, scientific efforts should also focus on the development of future research that investigates, corroborates and improves the adherent profiles [73].
The lack of and poor adherence to different psychosocial interventions, including CCT programs such as GRADIOR has clinical implications for people with MCI and mild dementia because it could worsen their cognitive impairment and even social and emotional level. Therefore, this study has clinical value because it helps to propose some strategies to increase adherence to treatment:
Make an adequate neuropsychological evaluation, focused on processes such as the following: attention, WM, numerical reasoning, phonological verbal fluency, and cognitive flexibility. To identify those people with the greatest commitment of these processes and, therefore, carry out a more personalized accompaniment and increase the probability of adherence to the CCT.
Design personalized CCT plans focused on tasks that involve executive functioning training, specifically in attention, WM, number reasoning, phonological verbal fluency, and cognitive flexibility. Additionally, in turn, modify the level of difficulty of each of the tasks associated with each of the cognitive processes, considering the level and cognitive profile of each of the patients. This will prevent the person from getting bored by the ease of the task or frustrated by its difficulty [74]. In this way, it will be possible to increase the degree of adherence.
It would be interesting for future studies to investigate the influence of involving people with cognitive impairment in decision making on their voluntary participation in the GRADIOR CCT and adherence. Decision making is a process that requires functions such as attention and WM, linked to the adherent rate. We know that decision making could be altered, so the inclusion and support of a reference family member in the process was crucial. However, this point deserves to be studied.
Finally, our study has a practical value because it suggests the importance of developing studies that applying user-centered methodological designs for the development of CCT programs, including end users from the initial stages of their development [74]. It will permit to develop of programs that to meet the physical, cognitive, psychological, and social needs and allow a greater adherence of users.
5. Conclusions
Finally, 62.8% of the participants were adherents to the “GRADIOR” CCT program. Likewise, this group had a high adherence rate of 83.3%. Regarding the predictors, the adherence model consisted of three tests (Digit Symbol of WAIS-III, Arithmetic of WAIS-III and LVF-R). This means that good executive functioning associated with attention, WM, numerical reasoning, phonological verbal fluency, and cognitive flexibility helped predict adherence. Thus, people with MCI and mild dementia with worse scores in these cognitive functions should be considered with higher priority to intervene for preventing the drop out from a CCT program. The adherent group performed better than the non-adherent group in these functions.
Acknowledgments
The research presented in this paper was carried out as part of the Marie Curie Initial Training Network (ITN) action, H2020-MSCA-ITN-2015, under grant agreement number 676265. INDUCT: Interdisciplinary Network for Dementia Using Current Technology. H2020 Marie Skłodowska Curie Actions—Innovative Training Networks, 2015. Department of Research and Development, INTRAS Foundation, Zamora, Spain.
Abbreviations
| ADASCog | Alzheimer’s Disease Assessment Scale—Cognitive Sub-scale |
| CAMCOG | Cambridge Cognition Examination. |
| CCT | Computer-based cognitive training. |
| EQ-5D-5L | EuroQol. |
| EF | Executive function. |
| GDS | Yesavage Geriatric Depression Scale. |
| GPs | General Practitioners. |
| iCST | Cognitive Stimulation Therapy. |
| LVF | Lexical Verbal Fluency. |
| MCI | Mild Cognitive Impairment. |
| MMSE | Mini-Mental State Exam. |
| PwD | People with Dementia. |
| RBMT | Rivermead Behavior Memory Test. |
| RCT | Randomized clinical trial. |
| SVF | Semantic Verbal Fluency. |
| TMT | Trail Making Test form A–B. |
| WAIS-III | Wechsler Adult Intelligence Scale. |
| WM | Working Memory |
Appendix A
Table A1.
Instrument description.
| Determinants | Test | Sub-Scale | Measure | Measurement Scale |
|---|---|---|---|---|
| Cognition | MMSE | GCS | Score: 0–30 | |
| ADAS-Cog | GCS | Score: 0–70. | ||
| 70 = Worse or lower cognitive performance | ||||
| Memory of words | Memory: Verbal free recall | 10 = maximum number of words not remembered | ||
| Word recognition | Memory: verbal recognition | 12 = maximum number of words not remembered | ||
| TMT-A | Time | Processing speed | Time (Percentile): 5–95 | |
| Mistakes | EF: Selective-sustained attention. Cognitive flexibility. | Mistakes = 0–4. 4 = Maximum number of mistakes |
||
| WAIS-III | Total Digits | EF: WM. Auditory immediate memory and attention | Scalar score: 1–19 | |
| Digit Symbol | EF: WM and attention | |||
| Arithmetic | EF: attention. WM. Numerical reasoning | |||
| CAMCOG | Visual Reasoning | FE: Visual abstract reasoning | Score: 0–6. | |
| 6 = Maximum number of hits | ||||
| RBMT | Drawing recognition | Memory: Visual Recognition | Score: 0–10. 10 = Maximum number of hits |
|
| SVF | EF: Fluency, cognitive flexibility, categorization, and monitoring of performance | Scalar score: 2–18 | ||
| LVF-P | ||||
| LVF-M | ||||
| LVF-R | ||||
| Psychological | GDS | Depression level | Score: 0–15 points. | |
| 15 = maximum symptoms of depression | ||||
| Motivation | Attend | Do you need someone to encourage you to attend GRADIOR? | Score: 1–5. 1 = Nothing. 2 = Something. 3 = I am not sure. 4 = Quite a lot. 5 = A lot |
|
| Expectations | Memory | I think GRADIOR will help my memory? | ||
| Quality of Life | Do I think my quality of life will improve after GRADIOR? | |||
| Free time | Do I think that the workshop with GRADIOR will occupy my time in a pleasant way? | |||
| Relating | I would like to meet new people in the workshop with GRADIOR? | |||
| physical health | EQ-5D-5L | Mobility | Subjective perception of mobility problems | Score: 1–5. 1 = I have no problems. 2 = minor problems. 3 = moderate problems. 4 = serious problems. 5 = I cannot |
| Self-Care | Subjective perception of problems bathing and dressing | |||
| Everyday Activities | Subjective perception of problems to perform DLA | |||
| Pain/Discomfort | Subjective perception of pain or discomfort | |||
| Anxiety/Depression | Subjective perception of depression or anxiety | |||
| Health Condition | Subjective perception of general health status | Score: 0–100. 100 = Excellent health |
||
| Technology | Prior Use of Technology | 1 = yes. 2 = No | ||
Note: ADAS-Cog, Alzheimer’s Disease Assessment Scale—Cognitive Sub-scale; CAMCOG, Cambridge Cognition Examination; DLA, daily life activities; EF, Executive Function; EQ-5D-5L, EuroQol; GCS, Global cognitive state; GDS, Geriatric Depression Scale; LVF, Lexical Verbal Fluency (form P, M R); MMSE, Mini-Mental State Examination; RBMT, The Rivermead Behavioural Memory Test; SVF, Semantic Verbal Fluency; TMT, Trail Making Test; WAIS-III, Wechsler Adult Intelligence Scale; WM, Working memory.
Author Contributions
Conceptualization, A.A.D.B., M.A.F.-M., M.V.P.B., and H.G.v.d.R.; database, A.A.D.B., and J.M.T.-G.; formal Analysis, A.A.D.B., and F.M.-A.; investigation, A.A.D.B., M.A.F.-M., and H.G.v.d.R.; methodology, A.A.D.B., J.M.T.-G., and H.G.v.d.R.; project administration, A.A.D.B., M.A.F.-M., H.G.v.d.R., and E.P.V.; software, M.A.F.-M., and Y.B.A.; writing—original draft preparation, A.A.D.B., writing—review and editing, A.A.D.B., M.A.F.-M., M.V.P.B., and H.G.v.d.R.; supervision, M.A.F.-M., H.G.v.d.R., and M.V.P.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research presented in this paper was carried out as part of the Marie Curie Initial Training Network (ITN) action, H2020-MSCA-ITN-2015, under grant agreement number 676265. INDUCT: Interdisciplinary Network for Dementia Using Current Technology. H2020 Marie Skłodowska Curie Actions—Innovative Training Networks, 2015.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Drug Research. Zamora Health Area (Registry number: 387-E.C).
Informed Consent Statement
All subjects gave their informed consent for inclusion before they participated in the study.
Data Availability Statement
The raw data will be provided by the main author to whom it is requested.
Conflicts of Interest
We declare the following interests: A.A.D.B is a paid member of INTRAS Foundation responsible for the development and distribution of the GRADIOR software. M.A.F.M was the initial designer of GRADIOR. J.M.T.G, Y.B.A, E.P.V are workers of INTRAS. M.V.P.B, H.G.v.d.R and F.M.A did not report conflicts of interests.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alzheimer’s Disease International World Alzheimer Report 2019: Attitudes to dementia. Alzheimer’s Dis. Int. Lond. 2019 [Google Scholar]
- 2.Nousia A., Siokas V., Aretouli E., Messinis L., Aloizou A.-M., Martzoukou M., Karala M., Koumpoulis C., Nasios G., Dardiotis E. Beneficial Effect of Multidomain Cognitive Training on the Neuropsychological Performance of Patients with Early-Stage Alzheimer’s Disease. Neural Plast. 2018;2018:2845176. doi: 10.1155/2018/2845176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sukontapol C., Kemsen S., Chansirikarn S., Nakawiro D., Kuha O., Taemeeyapradit U. The effectiveness of a cognitive training program in people with mild cognitive impairment: A study in urban community. Asian J. Psychiatr. 2018;35:18–23. doi: 10.1016/j.ajp.2018.04.028. [DOI] [PubMed] [Google Scholar]
- 4.Arlt S., Lindner R., Rösler A., von Renteln-Kruse W. Adherence to medication in patients with dementia: Predictors and strategies for improvement. Drugs Aging. 2008;25:1033–1047. doi: 10.2165/0002512-200825120-00005. [DOI] [PubMed] [Google Scholar]
- 5.Coley N., Ngandu T., Lehtisalo J., Soininen H., Vellas B., Richard E., Kivipelto M., Andrieu S. Adherence to multidomain interventions for dementia prevention: Data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15:729–741. doi: 10.1016/j.jalz.2019.03.005. [DOI] [PubMed] [Google Scholar]
- 6.Sabaté E. In: Adherence to Long-Term Therapies: Evidence for Action. World Health Organization, editor. World Health Organization; Ginebra, Switzerland: 2003. [PubMed] [Google Scholar]
- 7.Cramer J.A., Roy A., Burrell A., Fairchild C.J., Fuldeor M.J., Ollendorf D.A., Wong P.K. Medication compliance and persistence: Terminology and definitions. Value Health. 2008;11:44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 8.Dilla T., Valladares A., Lizán L., Sacristán J.A. Treatment adherence and persistence: Causes, consequences and improvement strategies. Aten Primaria. 2009;41:342–348. doi: 10.1016/j.aprim.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.SEFAC Plan. de Adherencia al Tratamiento. Uso Responsable del Medicamento. 2010. [(accessed on 18 February 2022)]. Available online: https://www.sefac.org/sites/default/files/sefac2010/private/documentos_sefac/documentos/farmaindustria-plan-de-adherencia.pdf.
- 10.Alfian S.D., Denig P., Coelho A., Hak E. Pharmacy-based predictors of non-adherence, non-persistence and reinitiation of antihypertensive drugs among patients on oral diabetes drugs in the Netherlands. PLoS ONE. 2019;14:e0225390. doi: 10.1371/journal.pone.0225390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scase M., Marandure B., Hancox J., Kreiner K., Hanke S., Kropf J. Development of and Adherence to a Computer-Based Gamified Environment Designed to Promote Health and Wellbeing in Older People with Mild Cognitive Impairment. Stud. Health Technol. Inform. 2017;236:348–355. [PubMed] [Google Scholar]
- 12.Evers A., Klusmann V., Schwarzer R., Heuser I. Improving cognition by adherence to physical or mental exercise: A moderated mediation analysis. Aging Ment. Health. 2011;15:446–455. doi: 10.1080/13607863.2010.543657. [DOI] [PubMed] [Google Scholar]
- 13.Park J.H., Liao Y., Kim D.R., Song S., Lim J.H., Park H., Lee Y., Park K.W. Feasibility and Tolerability of a Culture-Based Virtual Reality (VR) Training Program in Patients with Mild Cognitive Impairment: A Randomized Controlled Pilot Study. Int. J. Environ. Res. Public Health. 2020;17:3030. doi: 10.3390/ijerph17093030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turunen M., Hokkanen L., Bäckman L., Stigsdotter-Neely A., Hänninen T., Paajanen T., Soininen H., Kivipelto M., Ngandu T. Computer-based cognitive training for older adults: Determinants of adherence. PLoS ONE. 2019;14:e0219541. doi: 10.1371/journal.pone.0219541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han J.W., Oh K., Yoo S., Kim E., Ahn K.-H., Son Y.-J., Kim T.H., Chi Y.K., Kim K.W. Development of the ubiquitous spaced retrieval-based memory advancement and rehabilitation training program. Psychiatry Investig. 2014;11:52–58. doi: 10.4306/pi.2014.11.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson-Hanley C., Arciero P.J., Barcelos N., Nimon J., Rocha T., Thurin M., Maloney M. Executive function and self-regulated exergaming adherence among older adults. Front. Hum. Neurosci. 2014;8:989. doi: 10.3389/fnhum.2014.00989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Wit L., Chandler M., Amofa P., DeFeis B., Mejia A., O’Shea D., Locke D., Fields J., Smith G. Memory Support System training in mild cognitive impairment: Predictors of learning and adherence. Neuropsychol. Rehabil. 2019;31:210–220. doi: 10.1080/09602011.2019.1667833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tedim Cruz V., Pais J., Alves I., Ruano L., Mateus C., Barreto R., Bento V., Colunas M., Rocha N., Coutinho P. Web-based cognitive training: Patient adherence and intensity of treatment in an outpatient memory clinic. J. Med. Internet Res. 2014;16:e122. doi: 10.2196/jmir.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orrell M., Yates L., Leung P., Kang S., Hoare Z., Whitaker C., Burns A., Knapp M., Leroi I., Moniz-Cook E., et al. The impact of individual Cognitive Stimulation Therapy (iCST) on cognition, quality of life, caregiver health, and family relationships in dementia: A randomised controlled trial. PLoS Med. 2017;14:e1002269. doi: 10.1371/journal.pmed.1002269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vanova M., Irazoki E., García-Casal J.A., Martínez-Abad F., Botella C., Shiells K.R., Franco-Martín M.A. The effectiveness of ICT-based neurocognitive and psychosocial rehabilitation programmes in people with mild dementia and mild cognitive impairment using GRADIOR and ehcoBUTLER: Study protocol for a randomised controlled trial. Trials. 2018;19:100. doi: 10.1186/s13063-017-2371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diaz Baquero A.A., Franco-Martín M.A., Parra Vidales E., Toribio-Guzmán J.M., Bueno-Aguado Y., Martínez Abad F., Perea Bartolomé M.V., Asl A.M., van der Roest H.G. The Effectiveness of GRADIOR: A Neuropsychological Rehabilitation Program for People with Mild Cognitive Impairment and Mild Dementia. Results of a Randomized Controlled Trial After 4 and 12 Months of Treatment. J. Alzheimer’s Dis. 2022;86:1–17. doi: 10.3233/JAD-215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franco-Martín M.A. Evaluation of GRADIOR, a neuropsychological rehabilitation programme for people with mild dementia and mild cognitive impairment. ISRCTN Registry. 2017 doi: 10.1186/ISRCTN15742788. [DOI] [Google Scholar]
- 23.Petersen R.C. Clinical practice. Mild cognitive impairment. N. Engl. J. Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 24.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Arlington, VA, USA: 2013. [Google Scholar]
- 25.Blesa R., Pujol M., Aguilar M. Clinical Validity of the“Mini-Mental State” for Spanish speaking communities. Neuropsychologia. 2001;39:1150–1157. doi: 10.1016/S0028-3932(01)00055-0. [DOI] [PubMed] [Google Scholar]
- 26.Folstein M.F., Folstein S.E., McHugh P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 27.Rosen W.G., Mohs R.C., Davis K.L. A new rating scale for Alzheimer’s disease. Am. J. Psychiatry. 1984;141:1356–1364. doi: 10.1176/ajp.141.11.1356. [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2004;19:203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 29.Wechsler D. WAIS-III administration and scoring manual. Psychol. Corp. 1997 [Google Scholar]
- 30.Wilson B., Cockburn J., Baddeley A., Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. J. Clin. Exp. Neuropsychol. 1989;11:855–870. doi: 10.1080/01688638908400940. [DOI] [PubMed] [Google Scholar]
- 31.Roth M., Tym E., Mountjoy C.Q., Huppert F.A., Hendrie H., Verma S., Goddard R. CAMDEX. A standardised instrument for the diagnosis of mental disorder in the elderly with special reference to the early detection of dementia. Br. J. Psychiatry J. Ment. Sci. 1986;149:698–709. doi: 10.1192/bjp.149.6.698. [DOI] [PubMed] [Google Scholar]
- 32.Benton A.L. Differential behavioral effects in frontal lobe disease. Psychology. 1968;6:53–60. doi: 10.1016/0028-3932(68)90038-9. [DOI] [Google Scholar]
- 33.Sheikh J.I., Yesavage J.A. Geriatric Depression Scale (GDS): Recent evidence and development of a shorter version. Clin. Gerontol. 1986;5:165–173. doi: 10.1300/J018v05n01. [DOI] [Google Scholar]
- 34.Herdman M., Gudex C., Lloyd A., Janssen M., Kind P., Parkin D., Badia X. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L) Qual. Life Res. 2011;20:1727–1736. doi: 10.1007/s11136-011-9903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toribio-Guzmán J.M., Vidales E.P., Rodríguez M.J.V., Aguado Y.B., Bartolomé M.T.C., Franco-Martin M. Rehabilitación cognitiva por ordenador en personas mayores: Programa gradior. Ed. Univ. De Salamanca. 2018;24:61–75. doi: 10.14201/aula2018246175. [DOI] [Google Scholar]
- 36.Franco-Martin M.A., Diaz-Baquero A.A., Bueno-Aguado Y., Cid-Bartolomé M.T., Parra Vidales E., Perea Bartolomé M.V., de la Torre Díez I., van der Roest H.G. Computer-based cognitive rehabilitation program GRADIOR for mild dementia and mild cognitive impairment: New features. BMC J. BMC Med. Inform. Decis. Mak. 2020;20:1–15. doi: 10.1186/s12911-020-01293-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Franco-Martín M., Palau F.G., Ruiz Y., Vargas E., Solis A., Mellado J.G., Bartolomé L. Usability of a cognitive (gradior) and physical training program based in new software technologies in patients with mild dementia, mild cognitive impairment and healthy elderly people: Long Lasting Memories preliminary findings. Neurosci. Lett. 2011;500:e6. doi: 10.1016/j.neulet.2011.05.079. [DOI] [Google Scholar]
- 38.Góngora Alonso S., Toribio Guzmán J.M., Sainz de Abajo B., Muñoz Sánchez J.L., Martín M.F., de la Torre Díez I. Usability evaluation of the eHealth Long Lasting Memories program in Spanish elderly people. Health Inform. J. 2019;26:1728–1741. doi: 10.1177/1460458219889501. [DOI] [PubMed] [Google Scholar]
- 39.Irazoki E., Sánchez-Gómez M.C., Contreras-Somoza L.M., Toribio-Guzmán J.M., Martín-Cilleros M.V., Verdugo-Castro S., Jenaro-Río C., Franco-Martín M.A. A Qualitative Study of the Cognitive Rehabilitation Program GRADIOR for People with Cognitive Impairment: Outcomes of the Focus Group Methodology. J. Clin. Med. 2021;10:859. doi: 10.3390/jcm10040859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heesch K.C., Mâsse L.C., Dunn A.l., Frankowski R.F., Mullen P.D. Does adherence to a lifestyle physical activity intervention predict changes in physical activity? J. Behav Med. 2003;26:333–348. doi: 10.1023/A:1024205011001. [DOI] [PubMed] [Google Scholar]
- 41.Sjösten N.M., Salonoja M., Piirtola M., Vahlberg T.J., Isoaho R., Hyttinen H.K., Aarnio P.T., Kivelä S.-L. A multifactorial fall prevention programme in the community-dwelling aged: Predictors of adherence. Eur. J. Public Health. 2007;17:464–470. doi: 10.1093/eurpub/ckl272. [DOI] [PubMed] [Google Scholar]
- 42.Leslie S.R., Gwadry-Sridhar F., Thiebaud P., Patel B.V. Calculating medication compliance, adherence and persistence in administrative pharmacy claims databases. Pharm. Program. 2008;1:13–19. doi: 10.1179/175709208X334614. [DOI] [Google Scholar]
- 43.IBM-Corp . IBM SPSS Statistics for Windows 25.0. IBM-Corp; Armonk, NY, USA: 2017. [Google Scholar]
- 44.Moore V., Cahill S. Diagnosis and disclosure of dementia--a comparative qualitative study of Irish and Swedish General Practitioners. Aging Ment. Health. 2013;17:77–84. doi: 10.1080/13607863.2012.692763. [DOI] [PubMed] [Google Scholar]
- 45.Maseda A., Millán-Calenti J.C., Lorenzo-López L., Núñez-Naveira L. Efficacy of a computerized cognitive training application for older adults with and without memory impairments. Aging Clin. Exp. Res. 2013;25:411–419. doi: 10.1007/s40520-013-0070-5. [DOI] [PubMed] [Google Scholar]
- 46.Shatil E. Does combined cognitive training and physical activity training enhance cognitive abilities more than either alone? A four-condition randomized controlled trial among healthy older adults. Front. Aging Neurosci. 2013;5:8. doi: 10.3389/fnagi.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bagwell D., West R. Assessing compliance: Active versus inactive trainees in a memory intervention. Clin. Interv. Aging. 2008;3:371–382. doi: 10.2147/CIA.S1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Traykov L., Raoux N., Latour F., Gallo L., Hanon O., Baudic S., Bayle C., Wenisch E., Remy P., Rigaud A.S. Executive functions deficit in mild cognitive impairment. Cogn. Behav. Neurol. 2007;20:219–224. doi: 10.1097/WNN.0b013e31815e6254. [DOI] [PubMed] [Google Scholar]
- 49.Rainville C., Amieva H., Lafont S., Dartigues J.F., Orgogozo J.M., Fabrigoule C. Executive function deficits in patients with dementia of the Alzheimer’s type: A study with a Tower of London task. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2002;17:513–530. doi: 10.1093/arclin/17.6.513. [DOI] [PubMed] [Google Scholar]
- 50.D’Onofrio G., Panza F., Sancarlo D., Addante F., Solfrizzi V., Cantarini C., Mangiacotti A., Lauriola M., Cascavilla L., Paris F., et al. Executive Dysfunction Detected with the Frontal Assessment Battery in Alzheimer’s Disease Versus Vascular Dementia. J. Alzheimer’s Dis. 2018;62:699–711. doi: 10.3233/JAD-170365. [DOI] [PubMed] [Google Scholar]
- 51.Baudic S., Barba G.D., Thibaudet M.C., Smagghe A., Remy P., Traykov L. Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2006;21:15–21. doi: 10.1016/j.acn.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Mansbach W.E., Mace R.A. Predicting Functional Dependence in Mild Cognitive Impairment: Differential Contributions of Memory and Executive Functions. Gerontologist. 2019;59:925–935. doi: 10.1093/geront/gny097. [DOI] [PubMed] [Google Scholar]
- 53.Hyer L., Scott C., Atkinson M.M., Mullen C.M., Lee A., Johnson A., McKenzie L.C. Cognitive Training Program to Improve Working Memory in Older Adults with MCI. Clin. Gerontol. 2016;39:410–427. doi: 10.1080/07317115.2015.1120257. [DOI] [PubMed] [Google Scholar]
- 54.Vermeij A., Claassen J.A., Dautzenberg P.L., Kessels R.P. Transfer and maintenance effects of online working-memory training in normal ageing and mild cognitive impairment. Neuropsychol. Rehabil. 2016;26:783–809. doi: 10.1080/09602011.2015.1048694. [DOI] [PubMed] [Google Scholar]
- 55.Lam L.C., Chan W.C., Leung T., Fung A.W., Leung E.M. Would older adults with mild cognitive impairment adhere to and benefit from a structured lifestyle activity intervention to enhance cognition?: A cluster randomized controlled trial. PLoS ONE. 2015;10:e0118173. doi: 10.1371/journal.pone.0118173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tolea M.I., Morris J.C., Galvin J.E. Trajectory of Mobility Decline by Type of Dementia. Alzheimer Dis. Assoc. Disord. 2016;30:60–66. doi: 10.1097/WAD.0000000000000091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gigler K.L., Blomeke K., Shatil E., Weintraub S., Reber P.J. Preliminary evidence for the feasibility of at-home online cognitive training with older adults. Gerontechnology. 2013;12:26–35. doi: 10.4017/gt.2013.12.1.007.00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blackwood J., Shubert T., Fogarty K., Chase C. The Impact of a Home-Based Computerized Cognitive Training Intervention on Fall Risk Measure Performance in Community Dwelling Older Adults, a Pilot Study. J. Nutr. Health Aging. 2016;20:138–145. doi: 10.1007/s12603-015-0598-5. [DOI] [PubMed] [Google Scholar]
- 59.Heins P., Boots L.M.M., Koh W.Q., Neven A., Verhey F.R.J., de Vugt M.E. The Effects of Technological Interventions on Social Participation of Community-Dwelling Older Adults with and without Dementia: A Systematic Review. J. Clin. Med. 2021;10:2308. doi: 10.3390/jcm10112308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pinto-Bruno Á.C., García-Casal J.A., Csipke E., Jenaro-Río C., Franco-Martín M. ICT-based applications to improve social health and social participation in older adults with dementia. A systematic literature review. Aging Ment. Health. 2017;21:58–65. doi: 10.1080/13607863.2016.1262818. [DOI] [PubMed] [Google Scholar]
- 61.Franco Martín M.A., Criado C.H., Orihuela-Villameriel T., Bueno-Aguado Y. Intervención Psicoterapéutica en Afectados de Enfermedad de Alzheimer con Deterioro Leve. IMSERSO; Madrid, Spain: 2002. [Google Scholar]
- 62.Szatloczki G., Hoffmann I., Vincze V., Kalman J., Pakaski M. Speaking in Alzheimer’s Disease, is That an Early Sign? Importance of Changes in Language Abilities in Alzheimer’s Disease. Front. Aging Neurosci. 2015;7:195. doi: 10.3389/fnagi.2015.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klimova B., Maresova P. Computer-Based Training Programs for Older People with Mild Cognitive Impairment and/or Dementia. Front. Hum. Neurosci. 2017;11:262. doi: 10.3389/fnhum.2017.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee G.J., Bang H.J., Lee K.M., Kong H.H., Seo H.S., Oh M., Bang M. A comparison of the effects between 2 computerized cognitive training programs, Bettercog and COMCOG, on elderly patients with MCI and mild dementia: A single-blind randomized controlled study. Medicine. 2018;97:e13007. doi: 10.1097/MD.0000000000013007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bahar-Fuchs A., Webb S., Bartsch L., Clare L., Rebok G., Cherbuin N., Anstey K.J. Tailored and Adaptive Computerized Cognitive Training in Older Adults at Risk for Dementia: A Randomized Controlled Trial. J. Alzheimers Dis. 2017;60:889–911. doi: 10.3233/JAD-170404. [DOI] [PubMed] [Google Scholar]
- 66.Gates N.J., Rutjes A.W.S., Di Nisio M., Karim S., Chong L.Y., March E., Martínez G., Vernooij R.W.M. Computerised cognitive training for 12 or more weeks for maintaining cognitive function in cognitively healthy people in late life. Cochrane Database Syst. Rev. 2020;2020:CD012277. doi: 10.1002/14651858.CD012277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Choi J., Twamley E.W. Cognitive rehabilitation therapies for Alzheimer’s disease: A review of methods to improve treatment engagement and self-efficacy. Neuropsychol. Rev. 2013;23:48–62. doi: 10.1007/s11065-013-9227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Santos Golino M.T., Flores-Mendoza C.E. Development of a cognitive training program for the elderly. Rev. Bras. Geriatr. Gerontol. Rio De JaneiRo. 2016;19:769–785. doi: 10.1590/1809-98232016019.150144. [DOI] [Google Scholar]
- 69.Gaitán A., Garolera M., Cerulla N., Chico G., Rodriguez-Querol M., Canela-Soler J. Efficacy of an adjunctive computer-based cognitive training program in amnestic mild cognitive impairment and Alzheimer’s disease: A single-blind, randomized clinical trial. Int J. Geriatr. Psychiatry. 2013;28:91–99. doi: 10.1002/gps.3794. [DOI] [PubMed] [Google Scholar]
- 70.González-Palau F., Franco M., Bamidis P., Losada R., Parra E., Papageorgiou S.G., Vivas A.B. The effects of a computer-based cognitive and physical training program in a healthy and mildly cognitive impaired aging sample. Aging Ment. Health. 2014;18:838–846. doi: 10.1080/13607863.2014.899972. [DOI] [PubMed] [Google Scholar]
- 71.Hwang J.H., Cha H.G., Cho Y.S., Kim T.S., Cho H.S. The effects of computer-assisted cognitive rehabilitation on Alzheimer’s dementia patients memories. J. Phys. Ther. Sci. 2015;27:2921–2923. doi: 10.1589/jpts.27.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mendoza Laiz N., Del Valle Díaz S., Rioja Collado N., Gomez-Pilar J., Hornero R. Potential benefits of a cognitive training program in mild cognitive impairment (MCI) Restor. Neurol. Neurosci. 2018;36:207–213. doi: 10.3233/RNN-170754. [DOI] [PubMed] [Google Scholar]
- 73.Vermeire E., Hearnshaw H., Van Royen P., Denekens J. Patient adherence to treatment: Three decades of research. A comprehensive review. J. Clin. Pharm. Ther. 2001;26:331–342. doi: 10.1046/j.1365-2710.2001.00363.x. [DOI] [PubMed] [Google Scholar]
- 74.Diaz Baquero A.A., Dröes R.-M., Perea Bartolomé M.V., Irazoki E., Toribio-Guzmán J.M., Franco-Martín M.A., van der Roest H. Methodological Designs Applied in the Development of Computer-Based Training Programs for the Cognitive Rehabilitation in People with Mild Cognitive Impairment (MCI) and Mild Dementia. Systematic Review. J. Clin. Med. 2021;10:1222. doi: 10.3390/jcm10061222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data will be provided by the main author to whom it is requested.