Abstract
Levofloxacin is among the more active fluoroquinolones against streptococci and staphylococci. It is effective against moderately severe infections caused by these organisms, but its efficacy in the treatment of bacteremia and serious infections such as endocarditis is not well defined. We compared the efficacy of levofloxacin to those of standard agents in the rabbit model of aortic-valve endocarditis caused by fluoroquinolone-susceptible strains including a penicillin-susceptible strain of Streptococcus sanguis, a penicillin-resistant strain of Streptococcus mitis, a methicillin-resistant strain of Staphylococcus aureus, and a methicillin-susceptible strain of S. aureus. Levofloxacin administered intramuscularly at dosages of 20 to 40 mg/kg of body weight twice daily (b.i.d.) was completely ineffective against the penicillin-susceptible strain, with mean vegetation titers after 3 days of therapy not statistically significantly different from those for controls. Levofloxacin was no more effective than penicillin against the penicillin-resistant strain. Levofloxacin administered for 4 days at a dosage of 20 mg/kg b.i.d. was at least as effective as vancomycin administered intravenously at a dosage of 25 mg/kg b.i.d. against the methicillin-resistant S. aureus strain and was as effective as nafcillin administered intramuscularly at 100 mg three times daily against the methicillin-susceptible strain. Emergence of resistance to levofloxacin in vitro was less likely to occur than resistance to ciprofloxacin, and resistance to levofloxacin was not observed in vivo. Levofloxacin-rifampin combinations were antagonistic in vitro and in vivo. Levofloxacin was highly effective as a single agent against experimental staphylococcal endocarditis but was surprisingly ineffective against streptococcal endocarditis, suggesting that it has a potential role as treatment for serious S. aureus but not viridans group streptococcal infections in humans.
Levofloxacin, a fluoroquinolone with somewhat enhanced activity against gram-positive cocci, is the l-stereoisomer of ofloxacin, which is a racemic mixture that contains equal parts of the l- and d-stereoisomers. Since the d-stereoisomer has no antibacterial activity, levofloxacin is twice as potent by weight as ofloxacin, although their antibacterial spectra are otherwise identical (10, 32). Levofloxacin is more active in vitro than ciprofloxacin against gram-positive organisms, including streptococci and staphylococci (3, 25, 29, 31). The purpose of these studies was to investigate whether the in vitro activity of levofloxacin against gram-positive cocci is predictive of in vivo efficacy. The rabbit model of aortic-valve endocarditis caused by viridans group streptococci and methicillin-susceptible and methicillin-resistant strains of Staphylococcus aureus was used to compare the activity of levofloxacin to those of the first-line agents used to treat these infections in humans. Emergence of resistance during therapy has been a concern with fluoroquinolones when these are used as single agents (13, 14, 19, 26, 28). Accordingly, selection for levofloxacin-resistant mutants was examined in vitro and in vivo. Since ciprofloxacin and rifampin in combination prevent the emergence of resistance and are used clinically (9, 14, 34), the effect of the combination of rifampin and levofloxacin on the antibacterial activity of rifampin was also examined.
MATERIALS AND METHODS
Bacterial strains.
Strain M99 is a penicillin-susceptible (MIC, <0.1 μg/ml) strain of Streptococcus sanguis. Strain 543 is a penicillin-resistant (MIC, 2 μg/ml) strain of Streptococcus mitis. Strain 76 is a beta-lactamase-producing, high-level methicillin-resistant strain of S. aureus. Strain 1-63 is a methicillin-susceptible beta-lactamase-producing strain of S. aureus. Both S. aureus strains were ciprofloxacin susceptible.
Thirty-six S. aureus clinical isolates, 26 ciprofloxacin-susceptible isolates and 10 ciprofloxacin-resistant isolates (kindly provided by David Hooper, Harvard Medical School), were used for in vitro experiments designed to assess susceptibility, cross-resistance, and frequency of occurrence of mutants with resistance to ciprofloxacin and levofloxacin.
Susceptibility studies.
MICs were determined by the standard broth dilution method in 1-ml volumes of cation-supplemented Mueller-Hinton broth (MHB) for S. aureus strains and in Todd-Hewitt broth (THB) for viridans group streptococci at an inoculum of approximately 3 × 105 CFU/ml. MICs were read after a 24-h incubation at 35°C. Minimal bactericidal concentrations (MBCs), defined as a 99.9% reduction in the original inoculum, were determined by subculturing 10 μl from each clear tube onto blood agar and incubation for 24 h.
Time-kill studies were conducted with the S. aureus strains to determine the effect of rifampin on the bactericidal activity of levofloxacin. Levofloxacin at a concentration of 4 μg/ml and rifampin at a concentration of 1 μg/ml were tested alone and in combination in 10-ml volumes of Trypticase soy broth (TSB) for S. aureus at 37°C. Samples of 100 μl taken after 0, 4, and 24 h of antibiotic exposure were serially diluted 10-fold and were cultured onto blood agar. Colonies were counted after incubation for 24 h at 37°C.
The frequency of occurrence of resistant mutants among the S. aureus strains was determined by quantitatively inoculating an overnight culture onto Trypticase soy agar (TSA) containing ciprofloxacin or levofloxacin at concentrations of 0, 2×, 4×, 8×, 16×, and 32× the MIC. The inoculum was prepared by suspending the pellet obtained by centrifugation of a 10-ml culture in TSB into 1 ml of 0.85% saline. Tenfold serial dilutions were prepared. Ten-microliter volumes were taken from the suspension and spotted onto the agar to give a final inoculum of approximately 107 CFU, a value which approximates the total bacterial burden present in aortic-value vegetations of infected rabbits at the start of antimicrobial therapy. Cultures were incubated for 48 h at 37°C, and the numbers of colonies that grew at each concentration were counted. The frequency of occurrence of resistant mutants was expressed as the ratio of the number of CFU on antibiotic-containing agar to the number of CFU on drug-free agar.
Rabbit endocarditis model.
To establish endocarditis, a catheter was positioned across the aortic valve and was secured in place for the duration of the experiment. After the catheter had been in place for an hour, 1 ml of approximately 107 CFU in 0.9% saline was injected intravenously. Twenty-four hours later, antimicrobial therapy was begun. Levofloxacin was administered intramuscularly at a dosage of 20 or 40 mg/kg of body weight twice daily (b.i.d.) to approximate the peak concentrations achievable in the sera of humans (8, 18). The mean concentration in serum, which was determined by the agar diffusion bioassay method with S. aureus 209P, was 5.8 ± 1.0 μg/ml (n = 3) at 1 h after administration of a 20-mg/kg dose and 0.2 ± 0.1 μg/ml at 8 h. The corresponding values for the 40-mg/kg dose were 11.0 ± 1.5 (n = 3) and 0.5 ± 0.3 μg/ml. The half-life was 1.5 h for both doses.
Comparator drugs were administered at doses that have previously been shown in the rabbit model to approximate concentrations achievable in the sera of humans. Penicillin was administered at a dosage of 150,000 U of penicillin G plus 150,000 U of procaine penicillin intramuscularly three times daily (t.i.d.) which achieves concentrations in serum of 38 ± 8 μg/ml 1 h after dosing, with a half-life of 3.2 h (6). Vancomycin was administered intravenously at a dosage of 25 mg/kg b.i.d. which produces mean concentrations in serum of 43 ± 7 μg/ml 1 h after dosing, with a half-life of 1.3 h (5). Nafcillin was given at a dosage of 100 mg/kg t.i.d., which achieves concentrations in serum of 28 ± 11 μg/ml 1 h after dosing, with a half-life of 1.7 h (5). Rifampin was administered intramuscularly at a dosage of 5 mg/kg b.i.d., which achieves mean concentrations in serum of 3.6 ± 0.6 μg/ml 1 h after dosing, with a half-life of 7.7 h (7).
Untreated control rabbits were killed 18 to 24 h after infection, and aortic-valve vegetations were removed for culture. The remaining rabbits were given antimicrobial therapy for 3 days (for viridans group streptococcal endocarditis) or 4 days (S. aureus endocarditis). The rabbits were killed and aortic-valve vegetations were harvested 12 h following administration of the last dose of drug. The vegetations were homogenized and were quantitatively subcultured onto blood agar to determine the number of organisms remaining in the vegetation. Vegetations from rabbits in the experiments with S. aureus in which levofloxacin alone was compared to levofloxacin plus rifampin were also cultured onto TSA containing 5 μg of either levofloxacin or rifampin per ml to screen for the emergence of resistance. The number of organisms remaining in the vegetation of each rabbit was expressed as the vegetation titer, defined as log10 CFU per gram of vegetation.
Statistical analysis.
Differences in frequencies of the occurrence of resistant mutants was determined by paired Student’s t test. Differences in mean vegetation titers for treated and untreated rabbits were analyzed for statistical significance (defined as P < 0.05) by analysis of variance. An unpaired Student’s t test with the Bonferoni correction was used post hoc to determine statistical significance.
RESULTS
Susceptibility studies.
The levofloxacin MICs for the streptococcal strains were determined on two separate occasions in THB and were 2 and 4 μg/ml, respectively (Table 1), with corresponding MBCs of 2 to 4 and 4 to 8 μg/ml. These MICs were somewhat higher than the published MICs of ≤2 μg/ml for streptococcal strains (3, 25, 31). To determine whether higher MICs could be due to the use of THB, MIC determinations were repeated with cation-adjusted MHB plus 2 to 5% lysed horse blood, according to National Committee for Clinical Laboratory Standard methods (20). The MICs obtained by this method were 0.5 and 2 μg/ml, respectively.
TABLE 1.
MICs for the strains that caused experimental endocarditis
| Strain | MIC (μg/ml)
|
||||
|---|---|---|---|---|---|
| Levofloxacin | Nafcillin | Penicillin | Rifampin | Vancomycin | |
| S. sanguis M99 | 2a | <0.1 | |||
| S. mitis 543 | 4a | 2 | |||
| S. aureus 1-63 | 0.5 | 0.25 | <0.5 | ||
| S. aureus 76 | 1 | >64 | <0.5 | 1 | |
Results for the S. sanguis and S. mitis strains were determined in THB. The MICs determined in cation-adjusted MHB plus 2 to 5% lysed horse blood were 0.5 μg/ml for strain M99 and 2 μg/ml for strain 543.
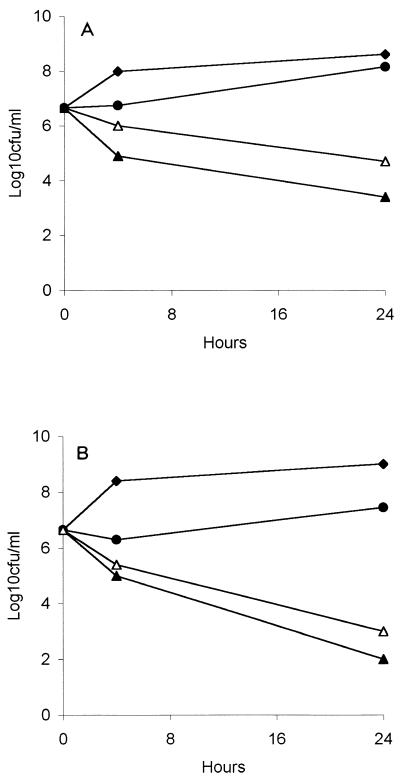
The levofloxacin MICs for the S. aureus strains were 0.5 and 1 μg/ml with MBCs of 1 and 2 μg/ml. In time-kill studies levofloxacin alone produced a 3- to 4-log10 CFU/ml reduction in the initial inoculum of each strain after 24 h (Fig. 1). The addition of rifampin to levofloxacin resulted in approximately 1-log10 CFU/ml increase in the number of surviving organisms at 24 h compared to the number after treatment with levofloxacin alone.
FIG. 1.
Time-kill curves for methicillin-resistant strain S. aureus 76 (A) and methicillin-susceptible strain S. aureus 1-63 (B). Black diamond, no drug; black circle, rifampin at 1 μg/ml; white triangle, levofloxacin at 4 μg/ml; black triangle, levofloxacin plus rifampin.
The MICs for the 36 clinical isolates ranged from 0.12 to >64 μg/ml for ciprofloxacin and 0.12 to 32 μg/ml for levofloxacin. The geometric mean MIC of ciprofloxacin for the 10 ciprofloxacin-resistant (defined as an MIC of ≥4 μg/ml) isolates was 18 μg/ml (range, 4 to 128 μg/ml), whereas the mean for levofloxacin was 4 μg/ml (range, 1 to 32 μg/ml). Three ciprofloxacin-resistant strains and nine ciprofloxacin-susceptible strains were chosen for studies that compared the frequencies of occurrence of resistant mutants upon exposure to ciprofloxacin or levofloxacin. Approximately 1 in 4.58 ± 1.68 log10 CFU grew on agar containing ciprofloxacin at 4× the MIC, whereas 1 in 5.74 ± 1.45 log10 CFU grew on agar containing levofloxacin at 4× the MIC (P < 0.017). Similar differences were observed at 2× and 8× the MIC, although insufficient data were available to make a direct statistical comparison due to overgrowth on some plates with 2× the MIC of ciprofloxacin and no growth on plates with a 8× the MIC of levofloxacin.
The lowest concentrations of ciprofloxacin that permitted no growth of CFU from a 107 inoculum for the three ciprofloxacin-resistant strains for which MICs were 4, 16, and 16 μg/ml were 32, 64, and 128 μg/ml, respectively. The corresponding concentrations for levofloxacin were MICs of 1, 2, and 8 μg/ml and no growth at 4, 8, and 32 μg/ml. The geometric mean ciprofloxacin MIC for the nine susceptible strains was 0.34 μg/ml (range, 0.12 to 0.5 μg/ml), whereas the mean MIC of levofloxacin was 0.17 μg/ml (range, 0.12 to 0.25 μg/ml). The geometric mean of the lowest concentration of ciprofloxacin that permitted no growth in susceptible strains was 10 μg/ml (range, 4 to 16 μg/ml), whereas it was 2 μg/ml for levofloxacin (range, 1 to 8 μg/ml). The MICs for resistant mutants obtained from four representative susceptible strains after a single passage on agar containing either ciprofloxacin at 2× the MIC or higher demonstrated that regardless of which fluoroquinolone was used to select for resistance, the MICs of ciprofloxacin were approximately 2 to 2.5 times the MICs of levofloxacin (Table 2).
TABLE 2.
MICs for parents and first-step fluoroquinolone-resistant mutants of four ciprofloxacin-susceptible S. aureus clinical isolates
| Strain | Geometric mean (range) MIC (μg/ml)
|
|
|---|---|---|
| Ciprofloxacin | Levofloxacin | |
| Parent strains (n = 4) | 0.35 (0.125–0.5) | 0.21 (0.125–0.5) |
| First-step mutants selected with ciprofloxacin (n = 7) | 8.8 (2–32) | 4.4 (1–16) |
| First-step mutants selected with levofloxacin (n = 6) | 14 (8–32) | 5.0 (2–8) |
Endocarditis experiments.
Levofloxacin was relatively ineffective against both the penicillin-susceptible and the penicillin-resistant strains of viridans group streptococci. The densities of the penicillin-susceptible organisms in the vegetations of rabbits treated with either 20- or 40-mg/kg doses of levofloxacin were not statistically significantly different from those in the vegetations of control rabbits and were inferior to those in the vegetations of rabbits treated with penicillin (Table 3). The 40-mg/kg regimen was more active than the 20-mg/kg regimen, indicating a dose response. Production of this difference, however, required peak concentrations in serum at the upper limit of those achievable in humans (8). The efficacy of the 40-mg/kg b.i.d. dosage regimen of levofloxacin was no better and was perhaps inferior to that of penicillin in rabbits infected with a penicillin-resistant strain, although the results did not achieve statistical significance. No further experiments were conducted with the penicillin-resistant strain because power calculations indicated that more than 60 rabbits would have to be treated to have an 80% probability of observing a statistically significant result, which the trend indicated would likely favor penicillin over levofloxacin. The possibility that levofloxacin would be better than penicillin even for rabbits infected with the penicillin-resistant strain seemed remote, particularly given the results obtained with the penicillin-susceptible strain.
TABLE 3.
Titers in vegetations of rabbits treated with levofloxacin or penicillin for penicillin-susceptible and -resistant strains of viridans group streptococci
| Strain | Treatment (dosage) | Vegetation titer (log10 CFU/g [no. of rabbits]) |
|---|---|---|
| S. sanguis M99, penicillin susceptible | No treatment | 8.1 ± 0.3 (7) |
| Penicillin (300,000 U t.i.d.) | 4.4 ± 2.1 (13)a | |
| Levofloxacin (20 mg/kg b.i.d.) | 8.3 ± 0.6 (13)bc | |
| Levofloxacin (40 mg/kg b.i.d.) | 6.9 ± 1.6 (10)bc | |
| S. mitis 543, penicillin resistant | No treatment | 7.7 ± 0.58 (4) |
| Penicillin (300,000 U t.i.d.) | 4.1 ± 3.8 (5)b | |
| Levofloxacin (40 mg/kg b.i.d.) | 5.5 ± 2.7 (5)b |
P < 0.001 versus no treatment.
P > 0.05 versus no treatment.
P < 0.05 versus penicillin and P < 0.05 for levofloxacin at 20 versus levofloxacin at 40 mg/kg.
In contrast to the results for streptococcal endocarditis, levofloxacin was highly effective against S. aureus infection. Levofloxacin produced a mean reduction in bacterial density of 6 to 7.5 log10 CFU/g compared to that for control rabbits infected with methicillin-resistant strain 76. The vegetation titers in control rabbits and rabbits treated with vancomycin at 25 mg/kg b.i.d., levofloxacin at 20 mg/kg b.i.d., and levofloxacin at 40 mg/kg b.i.d. were 9.2 ± 0.5 (n = 7), 5.8 ± 3.1 (n = 8), 3.3 ± 2.1 (n = 7), and 1.6 ± 1.4 (n = 9) log10 CFU/ml, respectively. For vancomycin and levofloxacin at 20 and 40 mg/kg, P was <0.05 versus no treatment. Levofloxacin at the 20-mg/kg dose produced a 2.5 log10 greater reduction in CFU than vancomycin, although the difference did not achieve statistical significance. Levofloxacin at the 40-mg/kg dose, however, was significantly more effective than vancomycin (P < 0.01). The addition of rifampin to levofloxacin was antagonistic in vivo. Treatment with levofloxacin alone in a separate experiment at the 20-mg/kg b.i.d. dosage resulted in a mean vegetation titer of 2.3 ± 2.5 log10 CFU/g (n = 7), whereas the mean vegetation titer was 5.5 + 0.9 log10 CFU/g (n = 7) for levofloxacin plus rifampin at 5 mg/kg b.i.d. (P < 0.01). Emergence of resistance, indicated by growth of CFU on antibiotic-containing agar, was not observed for either regimen.
The results for the methicillin-susceptible strain were similar to those for the resistant strain. The vegetation titers in control rabbits and rabbits treated with nafcillin at 100 mg/kg t.i.d., levofloxacin at 20 mg/kg b.i.d., and levofloxacin plus rifampin at 5 mg/kg b.i.d. were 9.0 ± 0.3 (n = 6), 0.0 ± 0.0 (n = 8), 1.3 ± 1.6 (n = 8), and 3.3 ± 1.3 (n = 9) log10 CFU/g, respectively. For nafcillin and levofloxacin alone, P was < 0.001 versus the control. Levofloxacin was about as effective as nafcillin in reducing vegetation titers (P > 0.05 for levofloxacin alone versus nafcillin), although all vegetations from the nafcillin-treated group were sterile, whereas four of the eight levofloxacin-treated group were sterile. Vegetation titers in rabbits given the levofloxacin-rifampin combination were significantly higher than vegetation titers in rabbits given levofloxacin alone (P < 0.05). Emergence of resistance, indicated by growth of CFU on antibiotic-containing agar, was not observed for either levofloxacin or rifampin.
DISCUSSION
Lack of coverage of gram-positive cocci is considered an important weakness in the antibacterial spectrum of fluoroquinolones. The MICs of many fluoroquinolones for streptococci and staphylococci are just below the breakpoint for resistance and are just below the concentrations that are achievable in serum and tissues. Although ciprofloxacin has been used successfully to treat a variety of respiratory tract and skin and soft-tissue infections caused by gram-positive cocci (21, 23), the emergence of resistance and reports of treatment failures (particularly in serious and often high-inoculum infections, such as endocarditis, osteomyelitis, and meningitis) (2, 13, 16, 17, 24, 30, 33) have led to avoidance of fluoroquinolones for the treatment of infections caused by gram-positive cocci.
Levofloxacin is slightly more active than ciprofloxacin against streptococci and staphylococci, and higher peak concentrations in serum can be achieved with the recommended doses. On the basis of this profile, levofloxacin was developed and is approved by the U.S. Food and Drug Administration for use in the treatment of community-acquired pneumonia, acute maxillary sinusitis, and uncomplicated skin and skin-structure infections caused by Streptococcus pyogenes, Streptococcus pneumoniae, and S. aureus. Although it is active in vitro against viridans group streptococci, the clinical significance of this activity is unknown. Our results with experimental streptococcal endocarditis indicate that this improved activity in vitro may not translate to in vivo conditions, at least for serious infections. The reason for this lack of efficacy is not clear. Emergence of resistance, although not specifically sought in the experiments with streptococci, is possible but seems unlikely given the short period of exposure and the complete lack of response at the 20-mg/kg dose with no reduction in vegetation titers compared to the titers in untreated controls. Notably, the MICs in THB were higher than those in cation-supplemented MHB with lysed horse blood, suggesting that viridans group streptococci may be intrinsically more resistant to fluoroquinolones than other streptococcal species and that standard susceptibility tests overestimate activity. Further studies with other isolates are required to determine if this is a general phenomenon or peculiar to the strains that we used. A lack of efficacy of trovafloxacin against experimental viridans group streptococcal endocarditis (27) suggests that the phenomenon could be a general one and that fluoroquinolones, despite MICs in the susceptible range, may not be efficacious as treatment for serious infections caused by strains of viridans group streptococci.
Levofloxacin was at least as effective as comparator drugs and was perhaps more effective that vancomycin against experimental S. aureus endocarditis. Unlike the experience with ciprofloxacin in vitro and in animal models, in which emergence of resistance readily occurs (11, 14, 15), no emergence of resistance occurred with levofloxacin. These results are consistent with the findings of other investigators who found no emergence of resistance during levofloxacin exposure (11, 15, 22). In vitro levofloxacin selected for resistant mutants at a 10-fold lower frequency than ciprofloxacin, as others have reported (12). Levofloxacin MICs for first-step mutants averaged 4 to 5 μg/ml, whereas they were 9 to 14 μg/ml for ciprofloxacin. Levofloxacin MICs for first-step mutants tended to be below the peak concentrations in serum produced in the experimental model and achievable in humans, whereas ciprofloxacin MICs were well above the maximum achievable concentration in serum of approximately 4 μg/ml. Thus, levofloxacin is slightly more active than ciprofloxacin, selects for resistance at a lower frequency, and achieves concentrations in vivo that require two mutations instead of one mutation to produce resistance at that level. These properties probably account for the failure to observe resistance in these experiments, which produced an infection with a pretreatment inoculum of approximately 107 CFU. Resistance might occur, however, with higher bacterial burdens, particularly if these persist in the presence of drug after long periods of exposure.
The results of these experiments are applicable only to fully fluoroquinolone-susceptible strains of S. aureus. Many methicillin-resistant strains are also ciprofloxacin resistant (1). Cross-resistance to other fluoroquinolones is the rule, and it is therefore unlikely that levofloxacin would be effective either due to outright failure or due to the emergence of resistance.
Whether or not rifampin should be used in combination with levofloxacin for the treatment of serious S. aureus infections is unclear. The rationale for a rifampin-fluoroquinolone combination is twofold: to take advantage of the excellent antistaphylococcal activity of rifampin and to prevent the emergence of resistance, which can occur with either antibiotic when it is used as a single agent. However, in vitro and during the relatively short period of drug exposure for these studies, the levofloxacin-rifampin combination was antagonistic, as has been observed for cephalosporin-rifampin combinations against experimental staphylococcal endocarditis (4). The levofloxacin-rifampin combination was still bactericidal in vivo, and it may be that over longer courses of treatment these differences would disappear and be of no clinical significance. If the emergence of resistance proves to be a problem, as it has been with other fluoroquinolones in high-inoculum infections, osteomyelitis, or foreign-body infections, there could very well be benefit to the addition of rifampin to the regimen (2, 9, 34). Further studies with other animal models of infection and clinical trials and experience are required to resolve this issue and to define indications for combination therapy.
ACKNOWLEDGMENT
This work was supported by a grant from Ortho-McNeil Pharmaceutical Company.
REFERENCES
- 1.Acar J F, Goldstein F W. Trends in bacterial resistance to fluoroquinolones. Clin Infect Dis. 1997;24(Suppl. 1):S67–S73. doi: 10.1093/clinids/24.supplement_1.s67. [DOI] [PubMed] [Google Scholar]
- 2.Ball P. Emergent resistance to ciprofloxacin amongst Pseudomonas aeruginosa and Staphylococcus aureus: clinical significance and therapeutic approaches. J Antimicrob Chemother. 1990;26(Suppl. F):165–179. doi: 10.1093/jac/26.suppl_f.165. [DOI] [PubMed] [Google Scholar]
- 3.Biedenbach D J, Jones R N. The comparative antimicrobial activity of levofloxacin tested against 350 clinical isolates of streptococci. Diagn Microbiol Infect Dis. 1996;25:47–51. doi: 10.1016/0732-8893(96)00066-1. [DOI] [PubMed] [Google Scholar]
- 4.Brandt C M, Rouse M S, Tallan B M, Wilson W R, Steckelberg J M. Failure of time-kill synergy studies using subinhibitory antimicrobial concentrations to predict in vivo antagonism of cephalosporin-rifampin combinations against Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:2191–2193. doi: 10.1128/aac.38.9.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers H F, Sachdeva M, Kennedy S. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of beta-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;162:705–710. doi: 10.1093/infdis/162.3.705. [DOI] [PubMed] [Google Scholar]
- 6.Chambers H F. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for penicillin-binding protein 2a. Antimicrob Agents Chemother. 1995;39:462–466. doi: 10.1128/aac.39.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chambers H F, Kartalija M, Sande M A. Ampicillin, sulbactam and rifampin combination therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J Infect Dis. 1995;171:897–902. doi: 10.1093/infdis/171.4.897. [DOI] [PubMed] [Google Scholar]
- 8.Chien S C, Wong F A, Fowler C L, Callery-D’Amico S V, Williams R R, Nayak R, Chow A T. Double-blind evaluation of the safety and pharmacokinetics of multiple oral once-daily 750-milligram and 1-gram doses of levofloxacin in healthy volunteers. Antimicrob Agents Chemother. 1998;42:885–888. doi: 10.1128/aac.42.4.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dworkin R J, Lee B L, Sande M A, Chambers H F. Ciprofloxacin with rifampin: a predominantly oral regimen for right-sided Staphylococcus aureus endocarditis in intravenous drug abusers. Lancet. 1989;ii:1071–1073. doi: 10.1016/s0140-6736(89)91083-0. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos G M, Wennersten C B, Moellering R C., Jr Comparative in vitro activity of levofloxacin and ofloxacin against gram-positive bacteria. Diagn Microbiol Infect Dis. 1996;25:35–41. doi: 10.1016/0732-8893(96)00069-7. [DOI] [PubMed] [Google Scholar]
- 11.Entenza J M, Vouillamoz J, Glauser M P, Moreillon P. Levofloxacin versus ciprofloxacin, flucloxacillin, or vancomycin for treatment of experimental endocarditis due to methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1997;41:1662–1667. doi: 10.1128/aac.41.8.1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans M E, Titlow W B. Levofloxacin selects fluoroquinolone-resistant methicillin-resistant Staphylococcus aureus less frequently than ciprofloxacin. J Antimicrob Chemother. 1998;41:285–288. doi: 10.1093/jac/41.2.285. [DOI] [PubMed] [Google Scholar]
- 13.Greenberg R N, Kennedy D J, Reilly P M, Luppen K L, Weinandt W J, Bollinger M R, Aguirre F, Kodesch F, Saeed A M. Treatment of bone, joint, and soft-tissue infections with oral ciprofloxacin. Antimicrob Agents Chemother. 1987;31:151–155. doi: 10.1128/aac.31.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaatz G W, Seo S M, Barriere S L, Albrecht L M, Rybak M J. Ciprofloxacin and rifampin, alone and in combination, for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1989;33:1184–1187. doi: 10.1128/aac.33.8.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang S L, Rybak M J, McGrath B J, Kaatz G W, Seo S M. Pharmacodynamics of levofloxacin, ofloxacin, and ciprofloxacin, alone and in combination with rifampin, against methicillin-susceptible and -resistant Staphylococcus aureus in an in vitro infection model. Antimicrob Agents Chemother. 1994;38:2702–2709. doi: 10.1128/aac.38.12.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kimbrough R C, III, Gerecht W B, Husted F C, Wolfe J E. The failure of ciprofloxacin to prevent the progression of Streptococcus pneumoniae infections to meningitis. Missouri Med. 1991;88:635–637. [PubMed] [Google Scholar]
- 17.Lee B L, Padula A M, Kimbrough R C, Jones S R, Chaisson R E, Mills J, Sande M A. Infectious complications with respiratory pathogens despite ciprofloxacin therapy. N Engl J Med. 1991;325:520–521. doi: 10.1056/nejm199108153250719. [DOI] [PubMed] [Google Scholar]
- 18.Medical Economics Co., Inc. Physicians’ desk reference. 52nd ed. Montvale, N.J: Medical Economics, Co., Inc.; 1998. pp. 2001–2011. [Google Scholar]
- 19.Mulligan M E, Ruane P J, Johnston L, Wong P, Wheelock J P, MacDonald K, Reinhardt J F, Johnson C C, Statner B, Blomquist I, et al. Ciprofloxacin for eradication of methicillin-resistant Staphylococcus aureus colonization. Am J Med. 1987;82(Suppl. 2):215–219. [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 3rd ed. Approved standard. NCCLS Document M7-A3. Vol. 13 1993. , no. 25, National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 21.Nicodemo A C, Robledo J A, Jasovich A, Neto W. A multicentre, double-blind, randomised study comparing the efficacy and safety of oral levofloxacin versus ciprofloxacin in the treatment of uncomplicated skin and skin structure infections. Int J Clin Pract. 1998;52:69–74. [PubMed] [Google Scholar]
- 22.Palmer S M, Rybak M J. Pharmacodynamics of once- or twice-daily levofloxacin versus vancomycin, with or without rifampin, against Staphylococcus aureus in an in vitro model with infected platelet-fibrin clots. Antimicrob Agents Chemother. 1966;40:701–705. doi: 10.1128/aac.40.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pankey G A. Multicenter, phase IV evaluation of intravenous ciprofloxacin as initial therapy in patients with lower respiratory tract, urinary tract, and skin/skin structure infections. Clin Ther. 1995;7:353–365. doi: 10.1016/0149-2918(95)80101-4. [DOI] [PubMed] [Google Scholar]
- 24.Pérez-Trallero E, Garcia-Arenzana J M, Jimenez J A, Peris A. Therapeutic failure and selection of resistance to quinolones in a case of pneumococcal pneumonia treated with ciprofloxacin. Eur J Clin Microbiol Infect Dis. 1990;9:905–906. doi: 10.1007/BF01967510. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 25.Pfaller M A, Jones R N. Comparative antistreptococcal activity of two newer fluoroquinolones, levofloxacin and sparfloxacin. Diagn Microbiol Infect Dis. 1997;29:199–201. doi: 10.1016/s0732-8893(97)81810-x. [DOI] [PubMed] [Google Scholar]
- 26.Piercy E A, Barbaro D, Luby J P, Mackowiack P A. Ciprofloxacin for methicillin-resistant Staphylococcus aureus infection. Antimicrob Agents Chemother. 1989;33:128–130. doi: 10.1128/aac.33.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piper K E, Rouse M S, Patel R, Wilson W R, Steckelberg J M. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1997. Trovafloxacin treatment of viridans streptococcal experimental endocarditis, abstr. B20; p. 30. [Google Scholar]
- 28.Schmitz F J, Hofmann B, Hansen B, Scheuring S, Lückefahr M, Klootwijk M, Verhoef J, Fluit A, Heinz H P, Köhrer K, Jones M E. Relationship between ciprofloxacin, ofloxacin, levofloxacin, sparfloxacin and moxifloxacin (BAY 12-8039) MICs and mutations in grlA, grlB, gyrA and gyrB in 116 unrelated clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1998;41:481–484. doi: 10.1093/jac/41.4.481. [DOI] [PubMed] [Google Scholar]
- 29.Smith S M, Eng H K, Tecson-Tumang F. Ciprofloxacin therapy for methicillin-resistant Staphylococcus aureus infections or colonizations. Antimicrob Agents Chemother. 1989;33:181–184. doi: 10.1128/aac.33.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tebas P, Martinez-Ruiz R, Roman F, Mendaza P, Rodriguez-Diaz J C, Daza R, de Letona J M. Early resistance to rifampin and ciprofloxacin in the treatment of right-sided Staphylococcus aureus endocarditis. J Infect Dis. 1991;163:204–205. doi: 10.1093/infdis/163.1.204-a. . (Letter.) [DOI] [PubMed] [Google Scholar]
- 31.Tuohy M, Washington J A. Antimicrobial susceptibility of viridans group streptococci. Diagn Microbiol Infect Dis. 1997;29:277–280. doi: 10.1016/s0732-8893(97)00140-5. [DOI] [PubMed] [Google Scholar]
- 32.von Eiff C, Peters G. In-vitro activity of ofloxacin, levofloxacin and d-ofloxacin against staphylococci. J Antimicrob Chemother. 1996;38:259–263. doi: 10.1093/jac/38.2.259. [DOI] [PubMed] [Google Scholar]
- 33.Yamaguti A, Trevisanello C, Lobo I M, Carvalho M C, Bortoletto M L, Silva M L, Brasil-Filho R, Levi G C, Mendonça J S. Oral ciprofloxacin for treatment of chronic osteomyelitis. Int J Clin Pharmacol Res. 1993;13:75–79. [PubMed] [Google Scholar]
- 34.Zimmerli W, Widmer A F, Blatter M, Frei R, Ochsner P E. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: a randomized controlled trial. JAMA. 1998;279:1537–1541. doi: 10.1001/jama.279.19.1537. [DOI] [PubMed] [Google Scholar]