Abstract
Striga hermonthica, a member of the Orobanchaceae family, is an obligate root parasite of staple cereal crops, which poses a tremendous threat to food security, contributing to malnutrition and poverty in many African countries. Depleting Striga seed reservoirs from infested soils is one of the crucial approaches to minimize subterranean damage to crops. The dependency of Striga germination on the host-released strigolactones (SLs) has prompted the development of the “Suicidal Germination” strategy to reduce the accumulated seed bank of Striga. The success of aforementioned strategy depends not only on the activity of the applied SL analogs, but also requires suitable application protocol with simple, efficient, and handy formulation for rain-fed African agriculture. Here, we developed a new formulation “Emulsifiable Concentration (EC)” for the two previously field-assessed SL analogs Methyl phenlactonoate 3 (MP3) and Nijmegen-1. The new EC formulation was evaluated for biological activities under lab, greenhouse, mini-field, and field conditions in comparison to the previously used Atlas G-1086 formulation. The EC formulation of SL analogs showed better activities on Striga germination with lower EC50 and high stability under Lab conditions. Moreover, EC formulated SL analogs at 1.0 µM concentrations reduced 89–99% Striga emergence in greenhouse. The two EC formulated SL analogs showed also a considerable reduction in Striga emergence in mini-field and field experiments. In conclusion, we have successfully developed a desired formulation for applying SL analogs as suicidal agents for large-scale field application. The encouraging results presented in this study pave the way for integrating the suicidal germination approach in sustainable Striga management strategies for African agriculture.
Keywords: Striga hermonthica, seedbank, suicidal germination, strigolactone analogs, witch weeds, methyl phenlactonoate
1. Introduction
Striga hermonthica, an obligate root-parasitic weed, is one of the major biotic constraints to cereal production in sub-Saharan Africa [1,2,3,4]. After spreading in about 32 African countries, Striga has infested about 50 million hectares of arable land in Africa [5,6]. The crop yield losses due to Striga infestation can vary from 40 to 100%, causing annual losses of around US $10 billion and threatening the life and food security of 300 million African people [7,8,9,10,11]. Developing suitable Striga control strategies is crucial for not only reducing the extent of damage but also retaining further spread into the non-contaminant fields [12]. However, the management of Striga is challenging due to the production of ~0.1 million seeds per plant [13], up to 20 years of seed longevity [14], complex life cycle [3], underground damage [15], and host dependency of Striga seed germination [16].
The germination of Striga seed requires favorable hot and humid conditions [17] and, most importantly, the perception of host-released chemical signals, such as strigolactones (SLs) [18,19,20]. The germinated Striga seeds should establish a connection to the root system of the host to survive due to very limited food reserve in tiny seeds for a short period of time [3,21]. This essential step of Striga life cycle leads to a basis for a promising control strategy, known as “Suicidal Germination” [22,23]. The germination of Striga seeds can be triggered in the bare soil by direct application of synthetic germination stimulants [24,25]. This germination in the host’s absence is lethal for Striga, which can be exploited as a tool to eliminate the accumulated Striga seed bank [22,23,26]. Although this concept has been proposed in a number of previous studies [22,23,26,27], but the availability of simple, easy-to-synthesize, and affordable synthetic SL analogs with suitable formulation and application under natural, hot, and rain-fed field conditions is still arguable [28]. Indeed, the selection of suitable germination stimulants, application protocol, and appropriate formulation for field application remain as challenging barriers to the success of this technology.
Several SL analogs and mimics were developed over the last three decades, but the problem of their efficacy, stability, and synthesis remains unsolved [29,30,31]. To identify suitable suicidal germination stimulants, a few SL analogs have been screened during the past 10 years based on the bioactivity of Striga germination under different conditions [25,32,33,34]. Indeed, two potent SL analogs, namely, MP3 and Nijmegen-1, were selected to be tested for suicidal germination activity under field conditions [23,32]. Another crucial step was to devise an appropriate protocol for successful field application. Keeping in mind that scarcity of water, type of soil, frequency and concentration of application, a rain-fed based suicidal application protocol has been proposed recently [23]. In addition, the development of active and viable formulation, suitable for harsh African agricultural conditions, is very crucial for the success of suicidal technology. Although the use of SL analogs formulated in Atlas G-1086 (AG), by Coroda Crop Care, The Netherlands, has been reported in previous studies, this emulsifier is expensive and time-consuming for preparation. Moreover, the solubility problems of active ingredients in the AG formulation and side effects on the host crop have been observed. Alternately, another formulation “Emulsifiable Concentrate (EC)” was developed by the UPL, India. The present study is carried out to test and evaluate the efficacy of this newly developed EC formulation of two very simple and efficient SL analogs under laboratory, greenhouse, and field conditions. We also compared the old AG formulations of both SL analogs on the suicidal germination activity with this new formulation. Besides, this is the first SL analogs formulation as suicidal agents, synthesized on a large-scale for real field application. This EC formulation is user-friendly, handy, water-soluble, highly compatible with SL analogs, and stable at room temperature (>2 years). To this end, our results will lead to developing a package of suicidal technology to combat Striga in Africa.
2. Results
2.1. Striga Seed Germination in Response to EC and AG Formulations of SL Analogs under Lab Conditions
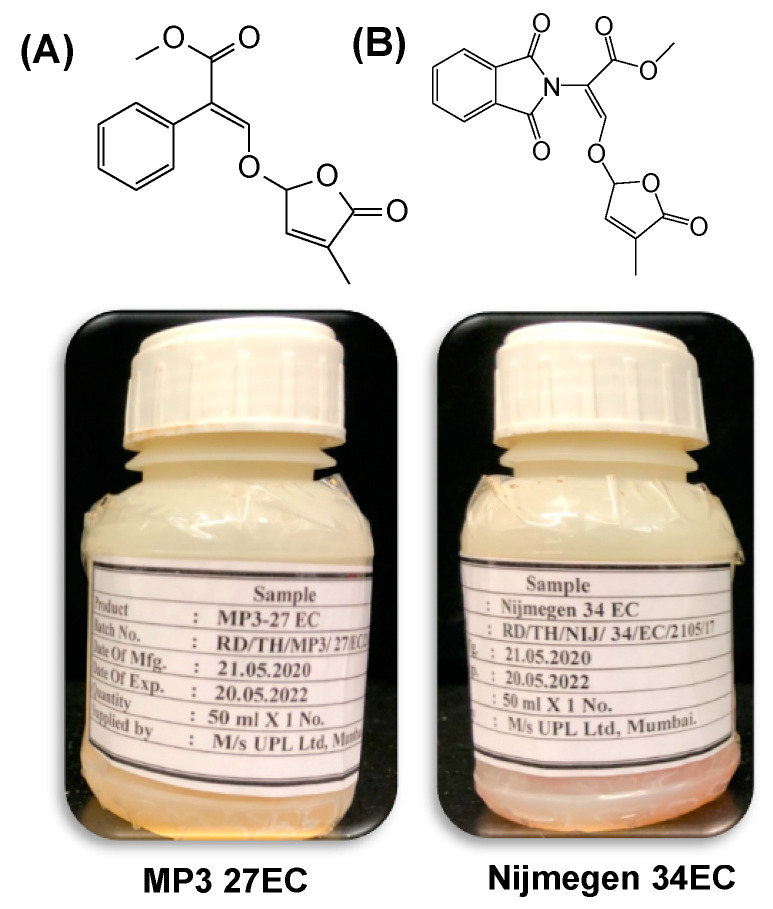
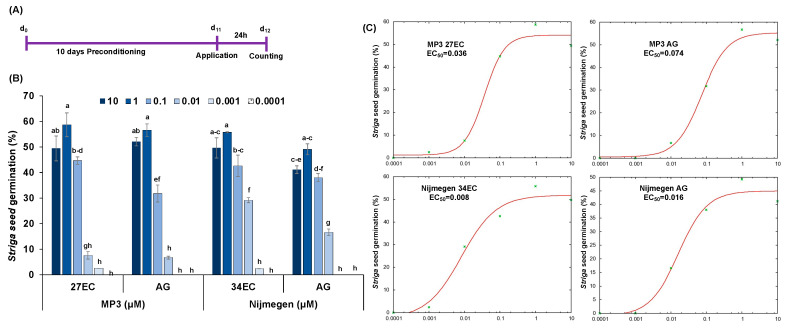
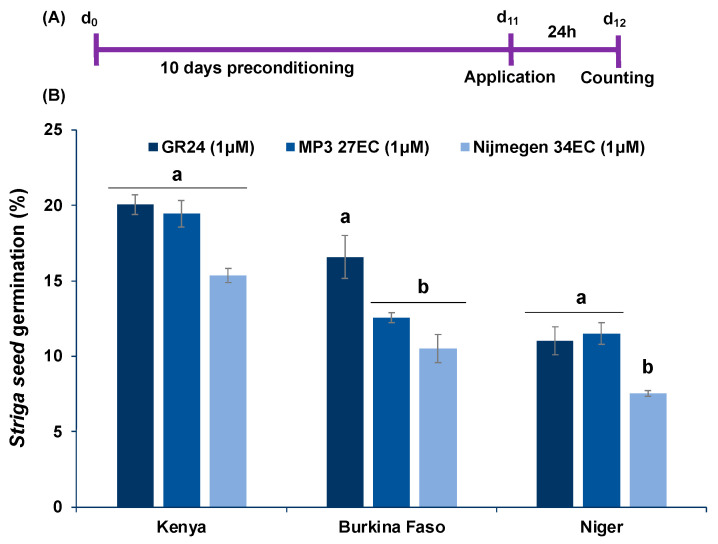
Structure of SL analogs and packing of Emulsifiable Concentrate (EC) formulation of MP3 27EC and Nijmegen 34EC are shown in Figure 1. The scheme of the experiment conducted for Striga seed germination bioassays is depicted in Figure 2A. The EC formulation of SL analogs MP3 27EC and Nijmegen 34EC induced about 56–59% Striga seed germination at a concentration of 1.0 µM, which was about 3–13% higher than AG formulation (49–57%) (Figure 2B). We also compared the activity of the two EC formulated analogs MP3 27EC and Nijmegen 34EC and observed both analogs at 1, 0.1, and 0.01 µM had similar activity, while Nijmegen 34EC at lower 0.001 µM showed a better activity and exhibited the lower EC50 value (0.008 vs. 0.036 µM) (Figure 2C). Importantly, EC formulation effectively reduced the value of EC50 in comparison to AG formulation (Figure 2C). Moreover, both EC formulated SL analogs induced seed germination of various Striga batches collected from Kenya, Niger, and Burkina Faso. Seed collected from Kenya showed about 9–15% Striga seed germination, followed by Burkina Faso batch with 11–13%, and Niger batch with 8–11% germination (Figure 3). The Striga seeds collected from Sudan appeared very active, showing maximum germination (~60%) as compared to seed populations collected from Burkina Faso and Niger (8–11%). However, these outcomes revealed that EC formulation of both SL analogs are still able to induce germination of various ecotypes of Striga seeds throughout African countries, depending upon seed viability and dormancy.
Figure 1.
Structure of selected SL analogs and packaging of Emulsifiable Concentrate (EC) formulation of strigolactone analogs, developed by UPL, India (A) MP3 27EC and (B) Nijmegen 34EC, prepared by UPL, India.
Figure 2.
Comparison of EC formulated SL analogs MP3 27EC and Nijmegen 34EC with AG formulated SL analogs for Striga seed germination. (A) The scheme of the experiment conducted for Striga seed germination bioassays. (B) Effect of various concentrations of both formulations (AG and EC) of the two SL analogs on Striga seed germination. The Striga seeds collected from a sorghum infested field during 2020 in Sudan were treated with the two formulations of SL analogs. (C) EC50 of Striga seed germination in response to the various concentrations of the two SL analogs in different formulations. Data are means ± SE (n = 3), treatments with various letters differ significantly according to one-way analysis of variance (ANOVA) and Tukey’s post hoc test (p < 0.05).
Figure 3.
Effect of EC formulated SL analogs (MP3 27EC and Nijmegen 34EC) on Striga seed germination collected from a maize field in Kenya, pearl millet fields in Burkina Faso, and Niger. (A) Scheme of the experiment conducted for Striga seed germination bioassays. (B) Striga seed germination of various ecotype in response to MP3 27EC and Nijmegen 34EC. Data are means ± SE (n = 7). For each SL analog, treatments with various letters differ significantly according to one-way analysis of variance (ANOVA) and Tukey’s post hoc test (p < 0.05).
2.2. Stability of EC and AG Formulations of Strigolactone Analogs
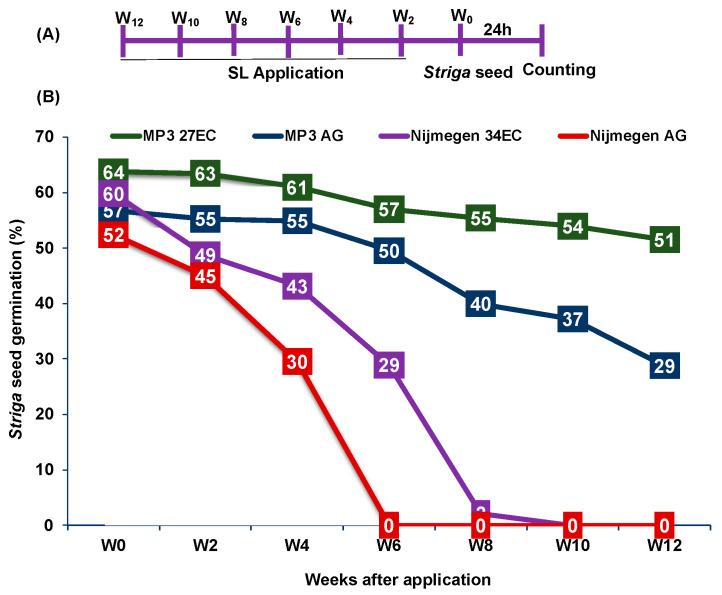
Fresh preparations of 1.0 µM SL analogs MP3 and Nijmegen with both formulations demonstrated about 52–64% Striga germination. EC formulation exhibited 60–64% germination when compared to AG formulation 52–57% (Figure 4). MP3 in EC formulation remained active even at 12 weeks after application, showing up to 51% Striga seed germination; while the AG formulation of MP3 only had a 29% activity on Striga germination on week 12. Surprisingly, Nijmegen in AG formulation completely lost its activity in week 6 (~30% Striga germination on Week 4); whereas EC formulation of Nijmegen was much more stable and remained active up to week 6 with ~29% Striga germination (Figure 4).
Figure 4.
Stability of EC and AG formulation of the two strigolactone analogs. (A) Scheme of the experiment conducted biweekly to test the stability of SL analogs. (B) Striga seeds germination in response to two formulations of MP3 and Nijmegen at two-week intervals. Each SL analog (10 mL) was applied in a Petri plate on 5 filter papers and incubated at 30 °C for two-week intervals up to 12 weeks. The Striga seeds collected from a sorghum field in Sudan were evaluated for germination in each Petri plate. Data are means ± SE (n = 5).
2.3. Effect of Various Formulations of Strigolactone Analogs on Striga Emergence in Pots under Greenhouse Conditions
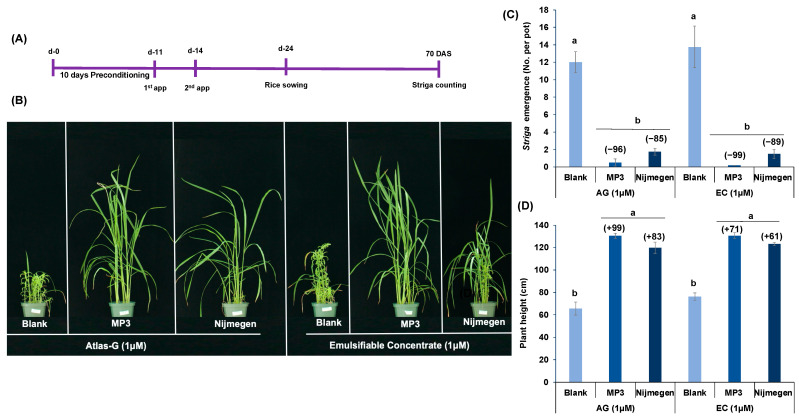
Both formulations of the two SL analogs (MP3 and Nijmegen) were further evaluated by applying at 1.0 µM concentration to Striga infested pot under greenhouse conditions (Figure 5A,B). Intriguingly, EC formulation of both analogs showed around 89–99% decrease of Striga emergence, which was about 3–5% higher than the reduction detected with the AG formulation (85–96%) (Figure 5C). However, no clear difference was obtained between the two formulated Nijmegen groups. Among the two EC formulated analogs, MP3 27EC had a larger decline in Striga emergence (99%) than Nijmegen 34EC (89%). In addition, a higher reduction in Striga emergence led to a better growth of the host plant, indicated by an increase of 61–99% in plant height of the host crop (Figure 5D).
Figure 5.
Effect of various formulations of strigolactone analogs on Striga emergence in pots under greenhouse conditions. (A) Scheme of the experiment conducted for Striga emergence in pots under greenhouse conditions. (B) View of the Striga infected pots. Each pot was filled with the soil infested with Striga seeds, collected from a sorghum field in Sudan. Both formulations of the two SL analogs (at 1.0 µM) were applied for two times in Striga infested pots. Striga emergence was counted at 70 days after sowing of rice. (C) Values of each bar showed average emergence of Striga per plot. (D) Average plant height of rice host plant measured at 70 DAS. Data are means ± SE (n = 4). For each SL analog, treatments with various letters differ significantly (p < 0.05). Values in parenthesis are showing the percentage increase (+) or decrease (−) over blank treatment.
2.4. Effect of Various Formulations of Strigolactone Analogs on Striga Infection under Mini-Field Conditions
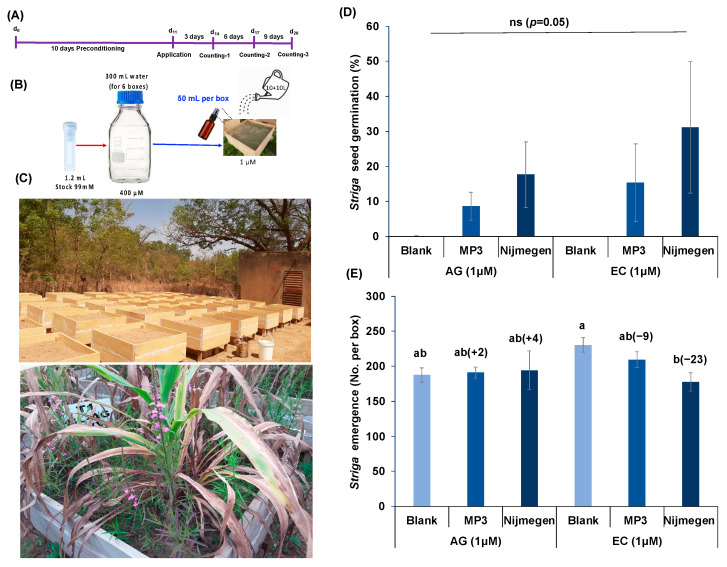
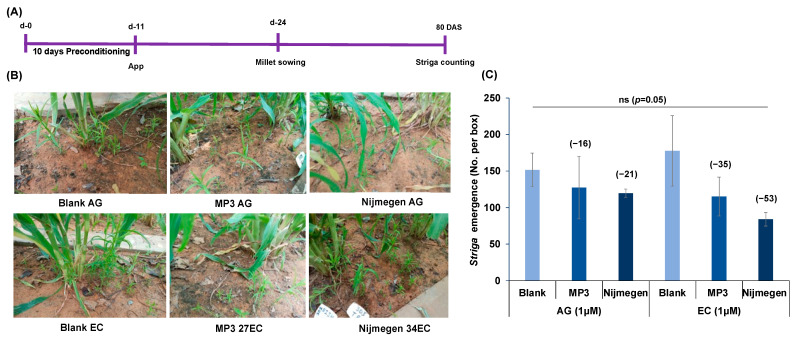
To fulfill the practical purpose of developing EC formulation for alleviating Striga, the efficacy of the two SL analogs MP3 27EC and Nijmegen 34EC was tested under mini-field conditions (Figure 6A–C) in INERA, Burkina Faso and ICRISAT, Niger. At INERA, although a huge variation among replications resulted in a non-significant impact on Striga germination (Figure 6D), apparently, we observed more Striga germination upon the application of MP3 27EC and Nijmegen 34EC (at 1.0 µM) compared to AG formulation. EC formulation of both SLs showed about 15–31% Striga germination and the Nijmegen 34EC appeared more active than MP3 27EC (Figure 6D). In addition to Striga germination, EC formulation of both SL analogs showed 9–23% reduction in Striga emergence, whereas we did not observe any reduction in Striga emergence after AG formulation treatment (Figure 6E). Similarly, we noticed a huge variation among replicated mini-boxes from ICRISAT, Niger, making the results non-significant. However, we still observed 35–53% reduction in Striga emergence by EC formulation of MP3 27EC and Nijmegen 34EC which was better than the AG formulation (16–21%). Additionally, Nijmegen 34EC showed better activity than MP3 27EC (Figure 7).
Figure 6.
Effect of various formulations of SL analogs on Striga infection under mini-field conditions at Burkina Faso. (A) Scheme of the experiment conducted for Striga germination and emergence. (B) Experimental protocol adopted to conduct mini-field trials. Each box was filled with the soil, infested with Striga seeds collected from a pearl millet field in Burkina Faso. (C) View of the miniboxes and Striga infestation. (D) Average number of Striga seed germination in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 µM) were applied in Striga infested mini-boxes. Striga germination was counted at 6 and 9 days after application. (E) Average number of Striga emergence in response to formulated MP3 and Nijmegen application. Striga emergence was counted after 80 days of pearl millet planting. Values of each bar showed average of Striga germination/emergence per minibox. Data are means ± SE (n = 6). For each SL analog, treatments with various letters differ significantly (p < 0.05). Values in parenthesis are showing the percentage increase (+) or decrease (−) over blank treatment. ns: non-significant.
Figure 7.
Effect of various formulations of SL analogs on Striga emergence under mini-field conditions at ICRISAT, Niger. (A) Scheme of the experiment conducted for Striga germination and emergence. (B) View of miniboxes and Striga infestation. Each box was filled with the soil, infested with Striga seeds collected from a pearl millet field in Niger. (C) Average number of Striga emergence in response to formulated MP3 and Nijmegen application. Striga emergence was counted after 80 days of pearl millet planting. Values of each bar showed average of Striga emergence per minibox. Data are means ± SE (n = 3). Values in parenthesis are showing the percentage increase (+) or decrease (−) over blank treatment. ns: non-significant.
2.5. Effect of EC and AG Formulation of Strigolactone Analogs on Striga Infection under African Field Conditions
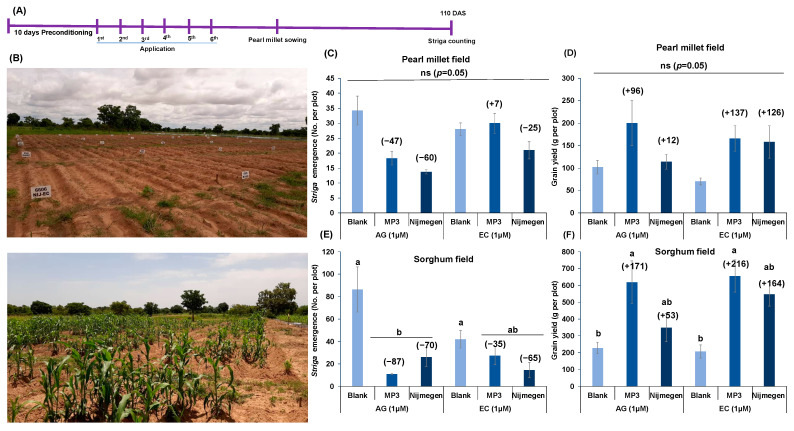
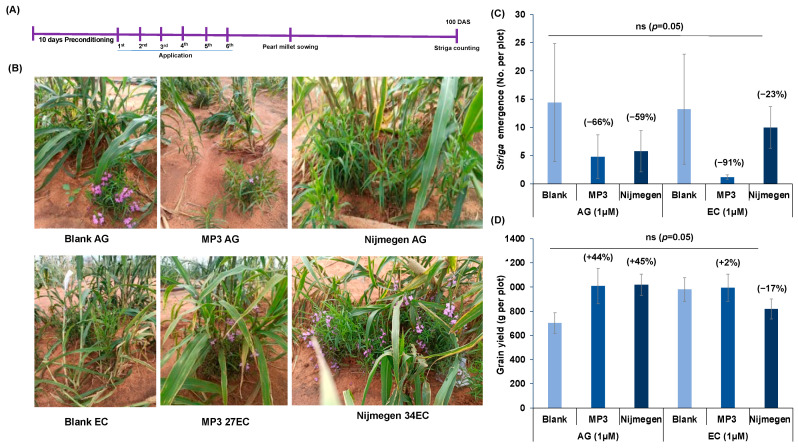
Finally, we investigated the efficacy of MP3 27EC and Nijmegen 34EC under naturally infested farmer-field conditions in INERA, Burkina Faso (Figure 8). In the pearl millet field, we observed 47–60% reduction in Striga emergence upon AG formulation of the two SL analogs application compared to blank treatment (Figure 8C). The EC formulation of Nijmegen 34EC showed about 25% reduction in Striga emergence in comparison to blank. Surprisingly, EC formulation of both SL analogs had a positive impact on pearl millet grain yield (126–137%). Moreover, MP3 in EC formulation showed a significant increase in grains per panicle over blank treatment (Supplementary Figure S1). In the sorghum farmer field, we observed 70–87% reduction in Striga emergence by SL analogs in AG formulation while EC formulation of MP3 and Nijmegen showed 35–65% reduction in Striga emergence (Figure 8E). This reduction in Striga emergence revealed a close association with Striga biomass, collected at final harvest from sorghum field (Supplementary Figure S2). We also observed 56–76% reduction in Striga biomass over blank treatment in AG formulated SL analogs while the reduction in EC formulated plots was 62–68% over blank treatment (Supplementary Figure S2). EC formulation treated plots of both SL analogs showed an increase in sorghum grain yield (164–216%) (Figure 8F). AG formulated MP3 showed considerable increase in yield components of sorghum compared to blank treatment (Supplementary Figure S3). Likewise, in ICRISAT, Niger, we observed a 91% reduction in Striga emergence by EC formulated MP3 27EC which was 27% higher than AG formulation of MP3 (66%) (Figure 9). Although MP3 27EC showed a better activity than Nijmegen 34EC, we observed a huge variation in Striga infestation among the plots of each treatment.
Figure 8.
Effect of various formulations of SL analogs on Striga emergence and grain yield under naturally infested farmers field conditions at INERA, Burkina Faso. (A) Scheme of the experiment conducted for Striga emergence under farmers field conditions during 2020. (B) View of the Striga infested farmers field at Fada N’gourma, Burkina Faso. (C) Average number of Striga emergence in the pearl millet field in response to formulated MP3 and Nijmegen application. (D) Average grain yield per plot from the pearl millet field in response to formulated MP3 and Nijmegen application. (E) Average number of Striga emergence in the sorghum field in response to formulated MP3 and Nijmegen application. (F) Average grain yield per plot from the sorghum field in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 µM) were applied in the Striga infested pearl millet and sorghum farmer fields. Striga emergence was counted after 110 days of pearl millet and sorghum planting. Values of each bar showed average of Striga emergence per plot. Data are means ± SE (n = 4). For each SL analog, treatments with various letters differ significantly (p < 0.05). Values in parenthesis are showing the percentage increase (+) or decrease (−) over blank treatment. ns: non-significant.
Figure 9.
Effect of EC and AG formulations of the two SL analogs (MP3 and Nijmegen) on Striga emergence under artificially infested field conditions at ICRISAT, Niger. (A) Scheme of the experiment conducted for Striga emergence under field conditions. (B) View of the Striga infested farms under various treatments during 2020. (C) Average number of Striga emergence in the pearl millet field in response to formulated MP3 and Nijmegen application. (D) Average grain yield per plot from the pearl millet field in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 µM) were applied in the Striga infested pearl millet field. Striga emergence was counted after 103 days of pearl millet planting. Values of each bar showed average of Striga emergence per plot. Data are means ± SE (n = 5). Values in parenthesis are showing the percentage increase (+) or decrease (−) over blank treatment. ns: non-significant.
3. Discussion
Striga, an obligate parasitic plant, attaches to the root system of most cereal crops in Africa while its subterranean nature of damage has made its control very difficult [5,35]. Developing suitable control strategies to minimize these underground losses has been proposed and advocated during the past few decades [28,36]. However, the underground damage of the host crop can be decreased only by reducing the seed bank density of Striga in the infested soil [37]. The “Suicidal Germination” technology has been suggested and tested in some field studies but with a lot of obstacles and limited success [38,39]. We have been working for the last five years to overcome the challenges of suicidal technology. We found two potent SL analogs MP3 and Nijmegen that can be applied into the field as suicidal agents [8,9]. In addition to the simple and efficient germination stimulants, the selection of suitable formulation is critical to facilitate large-scale field application [23]. A good formulation of SL analogs not only enhances the efficacy but also increases the stability and shelf-life after application into the field [22,40]. Atlas G-1086, a polyoxyethylene sorbitol hexaoleate mixed in cyclohexanone, has been used to formulate SL analogs in some past studies [23,25,26]. Previously, this emulsifier had been used to formulate Nijmegen-1 to apply in tobacco fields parasitized by Orobanche ramosa [22,41]. Moreover, some other potent SL analogs were also formulated with AG and used in Striga infested pearl millet and sorghum farmers field in Burkina Faso, showing a promising impact on reduction in Striga emergence [23,25]. However, there were few drawbacks of using AG to formulate SL analogs. We have been working closely with our partner, UPL, India, on developing suitable, handy, effective, and easily accessible formulated SL analogs that confer not only stability but also the ability to deplete easily of the seed bank in the infested soil. For this purpose, a new formulation “Emulsifiable Concentrate (EC)” of MP3 and Nijmegen (Figure 1) has been prepared by UPL, India.
In this report, we investigated the newly developed EC formulation of MP3 and Nijmegen with in vitro lab bioassays studies and the results indicated that MP3 and Nijmegen would be the potent SL analogs on seed germination (Figure 2). Both analogs with EC formulation showed better induction of Striga germination and EC50 over AG formulation. The induction of germination of various batches of Striga seeds by EC formulation of both SL analogs suggested that this formulation can be equally effective against all ecotypes of Striga in various parts of Africa (Figure 3). However, efficacy of SL analogs for both formulations varied for different Striga seed populations, with highest germination (60%) in Sudan batch and lowest germination (11%) in Niger batch. This variation among various populations might be attributed to seed viability, dormancy, as well as response to germination stimulants. The bioactivity of EC formulated MP3 for a longer period of time also indicates its potential as a good suicidal agent for field application in African soil. The EC formulated SL analogs possess longer shelf life and stability that is the desired characteristics for real field applications (Figure 4). In our previous studies, we observed several adverse effects of AG formulation on plant development and growth, which can be overcome by the EC formulation. Moreover, it is hard to dissolve SL analogs in AG formulation as compared to EC formulation. We also validated our lab outcome in a pot study under greenhouse conditions. Both SL analogs with EC and AG formulation have shown 85–99% decrease in Striga emergence (Figure 5).
However, we failed to get a significant impact on the reduction of Striga emergence under mini-field conditions both at INERA, Burkina Faso and ICRISAT, Niger (Figure 6). In fact, we had only one application in the mini-field, which might not have been enough to successfully reach to the Striga seeds to induce germination. In addition, a high variation among mini-fields might be due to several reasons, including high Striga density in the infested soils, low efficacy of applied treatments, soil type, leakage of water along the side of mini-fields, insufficient gap between application time, and host planting. It is recommended to enhance the number of applications in the mini box (>1) and to give enough time for suicidal death after Striga germination. Indeed, when we increased the number to six applications under naturally infested farmer fields in Burkina Faso, we observed very encouraging results, particularly in the sorghum field (Figure 8). Surprisingly, AG formulated SL analogs showed 70–87% reduction in Striga emergence in sorghum field and 47–60% in pearl millet field while EC formulated SL analogs did not reveal any significant impact on Striga emergence. A possible reason behind this low activity of EC formulated SL analogs is that the host crop was planted just after one week of the last application. Since EC formulated SL analogs are more stable so they may remain active in the soil for a longer period of time, which might continuously induce Striga germination that is easily attached to the host root. It is recommended that the host crop should not be grown just after the last application of EC-formulated SL analogs. We should consider enough time (3–4 months) or the whole rainy season for maximum induction of suicidal germination of Striga seeds. In spite of this, reduction in Striga infection led to better yield of both sorghum and pearl millet crops (Figure 8). In ICRISAT, Niger although it is an artificial infested field, we observed a huge variation among replicated plots (Figure 9). The emergence of Striga is limited such that was is hard to make conclusions from these findings. Low viability of Striga seeds, seed dormancy, cross-contamination among the plots, insect attack on the host, and poor growth of host plant can be possible reasons. We will repeat these field trials and expand our work in Kenya and Sudan to validate the findings, which will also expand our technology tackles different Striga ecotypes among Africa.
In summary, our results clearly demonstrate the effectiveness of the newly developed EC formulation of two potent and simple synthetic SL analogs as suicidal agents for practical field application. The new EC formulation of the two SL analogs appeared to be very bioactive, showing significant Striga reduction of 89–99% in rice under greenhouse conditions and 35–65% reduction in sorghum under field conditions in Burkina Faso. The reduction in Striga infection also led to increase rice plant height (61–71%) and pearl millet grain yield (>200%) as compared to blank treatment. The new EC formulation of the two SL analogs appeared to be very bioactive in terms of Striga reduction and host yield increase. In addition, the other advantages of EC formulation are simplicity, large-scale easy synthesis, stability, friendly use, easy packing/transportation and storage at normal room temperature. The development of proposed EC formulated simple SL analogs is the first step towards the large-scale synthesis of suicidal agents for field application and we believe this product will bring a breakthrough in suicidal technology to combat Striga in Africa.
4. Materials and Methods
4.1. Plant Materials and Chemicals
The SLs analogs MP3 and Nijmegen were synthesized and provided by Prof. Binne Zawanenburg, Radboud University, The Netherlands. Atlas G-1086 (provided by Croda Crop Care, Gouda, The Netherlands) was mixed with cyclo-hexanone (1:4) to make conventional formulation of SL analogs, used previously in the field. The new formulation “Emulsifiable Concentrate” (EC) of the two SL analogs (MP3, Nijmegen, The Netherlands) were prepared by UPL, India and Safety Data Sheet (SDS) has been attached (Supplementary Files S1 and S2). MP3 27EC means that 27 mg MP3 has been dissolved in 1.0 mL EC solvent (98.4 mM) while Nijmegen 34EC means that 34 mg Nijmegen has been added in 1.0 mL EC solvent (99 mM). Striga hermonthica seeds were collected from a sorghum (Sorghum bicolor) field during 2020 in Sudan (provided by Prof. A. G. Babiker), a maize (Zea mays) field during 2018 in Kenya (provided by Prof. Steven Runo, Kenyata University, Nairobi, Kenya), a pearl millet (Pennisetum glaucum) field during 2020 in Burkina Faso (provided by Dr. Djibril Yonli, INERA) and a pearl millet field during 2019 in Niger (provided by Dr. Mohammed Riyazaddin, ICRISAT, Niamey, Niger). Seeds of rice IAC-165 were obtained from Africa Rice, Tanzania (Courtesy of Dr. Jonne Rodenburg).
4.2. Striga Seed Germination Bioassays
First of all, the two formulations of both SL analogs were tested under lab conditions. Striga germination bioassays was conducted by following the procedure as described before [42]. The Striga seeds (collected from a sorghum, maize, and pearl millet infested field in Sudan, Kenya, Burkina Faso, and Niger, respectively) were first pre-conditioned before treating with SL analogs. For this purpose, the seeds were surface sterilized with 50% commercial bleach for 6–7 min and washed subsequently six times with MiliQ water in a laminar flow cabinet. The surface sterilized seeds (~50–100) were spread uniformly on a glass fiber filter paper disc (9 mm). Then 3 mL of sterilized MiliQ water was added in a plastic Petri plate containing one sterilized Whatman filter paper and 12 discs with Striga seeds. The Petri plates were sealed with parafilm, wrapped in aluminum foil, and incubated at 30 °C for 10 days. On the 11th day, the discs were dried in a laminar flow cabinet and the SL analogs (50 μL) were applied on each disc with various concentrations ranging from 10−5 M to 10−11 M. After application, the Striga seeds were induced to germinate in the dark for 24 h at 30 °C. The discs were scanned under a binocular microscope and germinated, and non-germinated seeds were counted by SeedQuant [43] and germination rate (in %) was calculated.
4.3. Stability Analysis
The stability of both the formulations of two selected SL analogs was observed in an in vitro bioassay. Five filter papers were added in plastic Petri plates (9 cm). Then, 10 mL of MP3 or Nijmegen with EC- and AG formulations at 1.0 μM concentration were added in each Petri plate and sealed with parafilm to incubate in the dark at 30 °C for two-week intervals for up to 12 weeks. On the final week, five glass fiber filter paper discs, containing 50–100 pre-conditioned Striga seeds were added in each plate and incubated at 30 °C for 24 h. The discs were scanned under a binocular microscope and germinated and non-germinated seeds were counted by SeedQuant [43] and germination rate (in %) was calculated.
4.4. Striga Emergence in Pots under Greenhouse Conditions
The biological activity of the two formulations of the SL analogs was further tested in pots under greenhouse conditions as described [25,32,44]. A sand and soil (Stender, Basissubstrat) mixture (1:3 ratio) was prepared. About 0.5 L of this mixture without Striga seeds was added in the bottom of a 3 L perforated plastic pot. Then about 20 mg (Approximately 8000) Striga seeds (collected from a sorghum field during 2020 in Sudan) were thoroughly mixed in 1.5 L soil mixture and added on the top of clean soil in the same pot. The pots were given light irrigation under greenhouse conditions at 30 °C for pre-conditioning of Striga seeds for 10 days. Then each pot was treated with 500 mL (1.0 μM) of various treatments to allow Striga seeds to germinate without host for another 10 days. Then one week old three rice seedlings (IAC-165) were planted in the middle of each pot. The rice plants were allowed to grow under normal growth condition (30 °C, 65% RH). After 70 days of sowing, Striga emergence was observed in each pot and compared with mock treatment.
4.5. Striga Emergence under Mini-Box Conditions
The selected two SL analogs with both formulations were also evaluated in mini-boxes at INERA, Burkina Faso and at ICRISAT, Niger. At INERA, a 1 m × 1 m and 40 cm deep wooden box was used while in ICRISAT, Niger a mini-field, made of cemented bricks measuring 1 m × 2 m was used to assess Striga germination and emergence in response to SL treatments. The top 10 cm of soil in the box was infested with 50 mg of Striga seeds. Moreover, nine eplee bags containing surface sterilized Striga seeds (30–50 on average) were buried at a 10-cm depth in the box in an equidistant position and allowed them to pre-condition under hot and humid conditions for 10 days. At the end of the pre-conditioning of Striga seeds, both formulations of the SL analogs were applied (at 1.0 µM) and blank treatments (AG-1086 and EC) were included for comparison. Three eplee bags were taken out at 3, 6, and 9 days after SL application to count germinated and non-germinated seeds. After two weeks of application, pearl millet was sown in each box and emerged Striga plants were counted at 80 days after sowing (DAS).
4.6. Striga Emergence under Field Conditions
The two formulations of the candidate SL analogs were further assessed under naturally infested farmers field conditions in Eastern Burkina Faso and artificially infested field in ICRISAT, Niger. In Burkina Faso, two highly Striga-infested fields located near Kouaré research station (11°58′49″ N, 0°18′30″ E) of INERA were selected. In each field trial, the plots (4 × 4 m2) with various treatments were laid out by following randomized complete block design (RCBD) with six replications. Each plot comprised of five ridges spaced 80-cm apart. All of the plots were spaced with four (4) blank ridges to avoid any contamination of the treatments. The two formulations (EC vs. AG) of MP3 and Nijmegen were applied (25 mL/m2 at 400 µM) for six times, after onset of rainfall (≥10 mm) to make the final concentration of 1.0 µM. We included blank treatment as a control to compare the treatment effects. In the blank, we added the same amount of EC or AG emulsifier without SL analogs (active ingredients). Pearl millet (local cultivar Idipiéni) and sorghum (local cultivar Itchoari) crops were sown at 2 weeks after the last application. Striga emergence was counted at 110 days after crop planting. In ICRISAT, Niger the field was prepared with ploughing and planking and ridges with 80-cm spaces were made. Each plot consisted of 4 ridges (4 × 4 m2) and each ridge was infested with ~1.0 g Striga seeds. The trial was laid out by following RCBD with six replications. The field was artificially irrigated for pre-conditioning of Striga seeds for 10 days. All of the plots were treated with various treatments for six times and pearl millet (SOSTA-C88-P10) was sown at two weeks after the last application. Striga emergence was counted at 103 days after host planting.
4.7. Statistical Analysis
All of the data were collected by following standard procedure and collected data were analyzed statistically using statistical software package R (version 3.2.2). One-way analysis of variance (ANOVA) with Least Significant Difference (LSD) multiple range test and unpaired t-test were used for analyzing the effect of two formulations of the SL analogs on Striga infestation.
Acknowledgments
We are grateful to Abdel Gabar Babiker, The National Research Center, Sudan; Steven Runo, Kenyatta University, Kenya for Striga seeds; We are thankful to Jonne Rodenburg, Africa Rice, Tanzania for providing seeds of rice IAC-165.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants11060808/s1, Supplementary Figure S1: Effect of various formulations of SL analogs on number of grains per panicle, panicle dry biomass, total stalk yield and harvest index in Pearl millet field at INERA, Burkina Faso. (A) Average grains per panicle (B) Average weight of dry panicle (C) Average stalk yield and (D) Average of harvest index from Striga infested Pearl millet field in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 μM) were applied in Striga infested Pearl millet. Yield and yield component were measured after 110 days of pearl millet planting. Data are means ± SE (n = 5). For each SL analog, treatments with various letters differ significantly (p < 0.05).Values in parenthesis are showing the percentage increase (+) or decrease (-) over blank treatment. ns: non-significant. Figure S2:Effect of various formulations of SL analogs on Striga dry biomass at Burkina Faso. Average Striga dry biomass emerged in Sorghum field in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 μM) were applied in Striga infested Sorghum fields. Striga seedlings were collected from Sorghum infested field and Striga dry biomass was measured at final harvest. Values of each bar showed average of Striga dry biomass emerged per plot. Data are means ± SE (n=5). For each SL analog, treatments with various letters differ significantly (p < 0.05). Values in parenthesis are showing the percentage increase (+) or decrease (-) over blank treatment. ns: non-significant. Figure S3: Effect of various formulations of SL analogs on number of grains per panicle, panicle dry biomass, total stalk yield and harvest index from Striga infested Sorghum field at INERA, Burkina Faso. (A) Average grains per panicle, (B) Average weight of panicle, (C) Average stalk yield and (D) Average of harvest index from Striga infested Sorghum field in response to formulated MP3 and Nijmegen application. Both SL analogs of EC and AG formulations (at 1.0 μM) were applied in Striga infested Sorghum field. Yield and yield component were measured after 110 days of Sorghum planting. Values of each bar showed average of Striga emergence per plot. Data are means ± SE (n = 5). For each SL analog, treatments with various letters differ significantly (p < 0.05). Values in parenthesis are showing the percentage increase (+) or decrease (-) over blank treatment. ns: non-significant.
Author Contributions
S.A.-B. and M.J. conceived and designed the experiments. M.J., J.Y.W., L.B. and G.-T.E.C. performed lab and greenhouse studies. D.Y., O.M., H.T., M.R. and P.G. conducted mini-box and field experiments. R.H.P., S.E.B. and B.Z. helped in synthesis of SL analogs. J.Y.W., M.J. and S.A.-B. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Bill & Melinda Gates Foundation (grant number OPP1136424 to S.A.), and King Abdullah University of Science and Technology (KAUST), Saudi Arabia.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
We are providing financial support to UPL company from our project funded by Bill & Melinda Gates Foundation. There is not any conflict of interest with UPL company.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ejeta G. Breeding for Striga Resistance in Sorghum: Exploitation of an Intricate Host–Parasite Biology. Crop Sci. 2007;47:216–229. doi: 10.2135/cropsci2007.04.0011IPBS. [DOI] [Google Scholar]
- 2.Parker C. Observations on the Current Status of Orobanche and Striga Problems Worldwide. Pest Manag. Sci. 2009;65:453–459. doi: 10.1002/ps.1713. [DOI] [PubMed] [Google Scholar]
- 3.Spallek T., Mutuku M., Shirasu K. The Genus Striga: A Witch Profile. Mol. Plant Path. 2013;14:861–869. doi: 10.1111/mpp.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamil M., Kountche B.A., Al-Babili S. Current Progress in Striga Management. Plant Physiol. 2021;185:1339–1352. doi: 10.1093/plphys/kiab040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scholes J.D., Press M.C. Striga Infestation of Cereal Crops–an Unsolved Problem in Resource Limited Agriculture. Curr. Opin. Plant Biol. 2008;11:180–186. doi: 10.1016/j.pbi.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Rodenburg J., Riches C.R., Kayeke J.M. Addressing Current and Future Problems of Parasitic Weeds in Rice. Crop Prot. 2010;29:210–221. doi: 10.1016/j.cropro.2009.10.015. [DOI] [Google Scholar]
- 7.Ejeta G., Gressel J. Integrating New Technologies for Striga Control: Towards Ending the Witch-Hunt. World Scientific; Singapore: 2007. pp. 3–6. [Google Scholar]
- 8.Atera E.A., Itoh K., Azuma T., Ishii T. Farmers’ Perspectives on the Biotic Constraint of Striga hermonthica and its Control in Western Kenya. Weed Biol. Manag. 2012;12:53–62. doi: 10.1111/j.1445-6664.2012.00435.x. [DOI] [Google Scholar]
- 9.Pennisi E. Armed and Dangerous. Science. 2010;327:804–805. doi: 10.1126/science.327.5967.804. [DOI] [PubMed] [Google Scholar]
- 10.Pennisi E. How Crop-Killing Witchweed Senses its Victims. Science. 2015;350:146–147. doi: 10.1126/science.350.6257.146. [DOI] [PubMed] [Google Scholar]
- 11.Rubiales D., Verkleij J., Vurro M., Murdoch A.J., Joel D. Parasitic Plant Management in Sustainable Agriculture. Weed Res. 2009;49:1–5. doi: 10.1111/j.1365-3180.2009.00741.x. [DOI] [Google Scholar]
- 12.Berner D., Kling J., Singh B. Striga Research and Control. A Perspective from Africa. Plant Dis. 1995;79:652–660. doi: 10.1094/PD-79-0652. [DOI] [Google Scholar]
- 13.Hearne S.J. Control—the Striga Conundrum. Pest Manag. Sci. 2009;65:603–614. doi: 10.1002/ps.1735. [DOI] [PubMed] [Google Scholar]
- 14.Bebawi F.F., Eplee R.E., Harris C.E., Norris R.S. Longevity of Witchweed (Striga asiatica) Seed. Weed Sci. 1984;32:494–497. doi: 10.1017/S0043174500059403. [DOI] [Google Scholar]
- 15.Abayo G.O., English T., Eplee R.E., Kanampiu F.K., Ransom J.K., Gressel J. Control of Parasitic Witchweeds (Striga spp.) on Corn (Zea mays) Resistant to Acetolactate Synthase Inhibitors. Weed Sci. 1998;46:459–466. doi: 10.1017/S0043174500090901. [DOI] [Google Scholar]
- 16.Matusova R., Rani K., Verstappen F.W., Franssen M.C., Beale M.H., Bouwmeester H.J. The Strigolactone Germination Stimulants of the Plant-Parasitic Striga and Orobanche spp. are Derived from the Carotenoid Pathway. Plant Physiol. 2005;139:920–934. doi: 10.1104/pp.105.061382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matusova R., van Mourik T., Bouwmeester H.J. Changes in the Sensitivity of Parasitic Weed Seeds to Germination Stimulants. Seed Sci. Res. 2004;14:335–344. doi: 10.1079/SSR2004187. [DOI] [Google Scholar]
- 18.Al-Babili S., Bouwmeester H.J. Strigolactones, a Novel Carotenoid-Derived Plant Hormone. Ann. Rev. Plant Biol. 2015;66:161–186. doi: 10.1146/annurev-arplant-043014-114759. [DOI] [PubMed] [Google Scholar]
- 19.Xie X.N., Yoneyama K., Yoneyama K. The Strigolactone Story. Ann. Rev. Phytopath. 2010;48:93–117. doi: 10.1146/annurev-phyto-073009-114453. [DOI] [PubMed] [Google Scholar]
- 20.Fiorilli V., Wang J.Y., Bonfante P., Lanfranco L., Al-Babili S. Apocarotenoids: Old and New Mediators of the Arbuscular Mycorrhizal Symbiosis. Front. Plant Sci. 2019;10:1186–1195. doi: 10.3389/fpls.2019.01186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sibhatu B. Review on Striga Weed Management. Int. J. Life Sci. Sci. Res. 2016;2:110–120. [Google Scholar]
- 22.Zwanenburg B., Mwakaboko A.S., Kannan C. Suicidal Germination for Parasitic Weed Control. Pest Manag. Sci. 2016;72:2016–2025. doi: 10.1002/ps.4222. [DOI] [PubMed] [Google Scholar]
- 23.Kountche B.A., Jamil M., Yonli D., Nikiema M.P., Blanco-Ania D., Asami T., Zwanenburg B., Al-Babili S. Suicidal Germination as a Control Strategy for Striga hermonthica (Benth.) in Smallholder Farms of Sub-Saharan Africa. Plants People Planet. 2019;1:107–118. doi: 10.1002/ppp3.32. [DOI] [Google Scholar]
- 24.Uraguchi D., Kuwata K., Hijikata Y., Yamaguchi R., Imaizumi H., Sathiyanarayanan A., Rakers C., Mori N., Akiyama K., Irle S. A Femtomolar-Range Suicide Germination Stimulant for the Parasitic Plant Striga hermonthica. Science. 2018;362:1301–1305. doi: 10.1126/science.aau5445. [DOI] [PubMed] [Google Scholar]
- 25.Jamil M., Kountche B.A., Wang J.Y., Haider I., Jia K.-P., Takahashi I., Ota T., Asami T., Al-Babili S. A New Series of Carlactonoic Acid Based Strigolactone Analogs for Fundamental and Applied Research. Front. Plant Sci. 2020;11:1–13. doi: 10.3389/fpls.2020.00434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Samejima H., Babiker A.G., Takikawa H., Sasaki M., Sugimoto Y. Practicality of the Suicidal Germination Approach for Controlling Striga hermonthica. Pest Manag. Sci. 2016;72:2035–2042. doi: 10.1002/ps.4215. [DOI] [PubMed] [Google Scholar]
- 27.Mwakha F.A., Budambula N.L., Neondo J.O., Gichimu B.M., Odari E.O., Kamau P.K., Odero C., Kibet W., Runo S. Witchweed’s Suicidal Germination: Can Slenderleaf Help? Agronomy. 2020;10:873. doi: 10.3390/agronomy10060873. [DOI] [Google Scholar]
- 28.Parker C., Riches C. Parasitic Weeds of the World: Biology and Control. CAB International; Wallingford, UK: 1993. pp. 1–74. [Google Scholar]
- 29.Fukui K., Ito S., Asami T. Selective Mimics of Strigolactone Actions and Their Potential Use for Controlling Damage Caused by Root Parasitic Weeds. Mol. Plant. 2013;6:88–99. doi: 10.1093/mp/sss138. [DOI] [PubMed] [Google Scholar]
- 30.Blanco-Ania D., Mateman J.J., Hylova A., Spichal L., Debie L.M., Zwanenburg B. Hybrid-type Strigolactone Analogues Derived from Auxins. Pest Manag. Sci. 2019;75:3113–3121. doi: 10.1002/ps.5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jamil M., Kountche B.A., Haider I., Wang J.Y., Aldossary F., Zarban R.A., Jia K.-P., Yonli D., Hameed U.F.S., Takahashi I., et al. Methylation at the C-3’ in D-Ring of Strigolactone Analogs Reduces Biological Activity in Root Parasitic Plants and Rice. Front. Plant Sci. 2019;10:1–14. doi: 10.3389/fpls.2019.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamil M., Kountche B.A., Haider I., Guo X.J., Ntui V.O., Jia K.-P., Ali S., Hameed U.F.S., Nakamura H., Lyu Y., et al. Methyl Phenlactonoates are Efficient Strigolactone Analogs with Simple Structure. J. Exp. Bot. 2018;69:2319–2331. doi: 10.1093/jxb/erx438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jia K.-P., Kountche B.A., Jamil M., Guo X., Ntui V.O., Rüfenacht A., Rochange S., Al-Babili S. Nitro-phenlactone, a Carlactone Analog with Pleiotropic Strigolactone Activities. Mol. Plant. 2016;9:1341–1344. doi: 10.1016/j.molp.2016.05.017. [DOI] [PubMed] [Google Scholar]
- 34.Zwanenburg B., Blanco-Ania D. Strigolactones: New Plant Hormones in the Spotlight. J. Exp. Bot. 2018;69:2205–2218. doi: 10.1093/jxb/erx487. [DOI] [PubMed] [Google Scholar]
- 35.Jamil M., Charnikhova T., Verstappen F., Bouwmeester H. Carotenoid Inhibitors Reduce Strigolactone Production and Striga hermonthica Infection in Rice. Arch. Biochem. Biophys. 2010;504:123–131. doi: 10.1016/j.abb.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Kim S., Adetimirin V., The C., Dossou R. Yield Losses in Maize Due to Striga hermonthica in West and Central Africa. Int. J. Pest Manag. 2002;48:211–217. doi: 10.1080/09670870110117408. [DOI] [Google Scholar]
- 37.Oswald A. Striga Control—Technologies and Their Dissemination. Crop Prot. 2005;24:333–342. doi: 10.1016/j.cropro.2004.09.003. [DOI] [Google Scholar]
- 38.Wigchert S., Kuiper E., Boelhouwer G., Nefkens G., Verkleij J., Zwanenburg B. Dose−Response of Seeds of the Parasitic Weeds Striga and Orobanche toward the Synthetic Germination Stimulants GR24 and Nijmegen 1. J. Agric. Food Chem. 1999;47:1705–1710. doi: 10.1021/jf981006z. [DOI] [PubMed] [Google Scholar]
- 39.Yoneyama K., Awad A.A., Xie X., Yoneyama K., Takeuchi Y. Strigolactones as Germination Stimulants for Root Parasitic Plants. Plant Cell Physiol. 2010;51:1095–1103. doi: 10.1093/pcp/pcq055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kgosi R.L., Zwanenburg B., Mwakaboko A.S., Murdoch A.J. Strigolactone Analogues Induce Suicidal Seed Germination of Striga spp. in Soil. Weed Res. 2012;52:197–203. doi: 10.1111/j.1365-3180.2012.00912.x. [DOI] [Google Scholar]
- 41.Zwanenburg B., Mwakaboko A.S., Reizelman A., Anilkumar G., Sethumadhavan D. Structure and Function of Natural and Synthetic Signalling Molecules in Parasitic Weed Germination. Pest Manag. Sci. 2009;65:478–491. doi: 10.1002/ps.1706. [DOI] [PubMed] [Google Scholar]
- 42.Jamil M., Kanampiu F.K., Karaya H., Charnikhova T., Bouwmeester H.J. Striga hermonthica Parasitism in Maize in Response to N and P Fertilisers. Field Crops Res. 2012;134:1–10. doi: 10.1016/j.fcr.2012.03.015. [DOI] [Google Scholar]
- 43.Braguy J., Ramazanova M., Giancola S., Jamil M., Kountche B.A., Zarban R.A.Y., Felemban A., Wang J.Y., Lin P.-Y., Haider I., et al. SeedQuant: A Deep Learning-Based Tool for Assessing Stimulant and Inhibitor Activity on Root Parasitic Seeds. Plant Physiol. 2021;186:1632–1644. doi: 10.1093/plphys/kiab173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J.Y., Jamil M., Lin P.-Y., Ota T., Fiorilli V., Novero M., Zarban R.A., Kountche B.A., Takahashi I., Martínez C. Efficient Mimics for Elucidating Zaxinone Biology and Promoting Agricultural Applications. Mol. Plant. 2020;13:1654–1661. doi: 10.1016/j.molp.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.