Abstract
Using checkerboard and time-kill assays, we evaluated the in vitro activity of quinupristin-dalfopristin (RP 59500) alone and in combination with five other antimicrobial agents against 12 clinical strains of vancomycin-resistant Enterococcus faecium (VREF). In time-kill studies, six VREF strains exhibited synergism with the combination of quinupristin-dalfopristin and doxycycline and three exhibited synergism with quinupristin-dalfopristin plus ampicillin-sulbactam. Combinations of quinupristin-dalfopristin with these and other agents warrant further clinical evaluation for the treatment of serious VREF infections.
Enterococci are recognized to be important nosocomial pathogens, capable of causing serious and potentially life-threatening infections. Bacteremic enterococcal infections, such as endocarditis, are typically treated with a combination of antimicrobial agents in order to achieve bactericidal activity. The past decade has seen the emergence of enterococci increasingly resistant to ampicillin, high-level aminoglycosides, and glycopeptides (8). Such resistance has occurred most frequently in Enterococcus faecium and is typically associated with resistance to nearly all currently available antibiotics (7). Vancomycin-resistant E. faecium (VREF) has now become endemic in many hospitals. Treatment of serious infections caused by these organisms is problematic, and no single agent is reliably bactericidal against VREF. Consequently, newer agents or combinations of drugs with enhanced activities against vancomycin-resistant enterococci are needed.
Quinupristin-dalfopristin (RP 59500) is a semisynthetic injectable streptogramin compound which has been reported to possess good in vitro activity against gram-positive organisms, including VREF, although the drug has minimal activity against Enterococcus faecalis (2). Composed of two pristinamycin derivatives, quinupristin-dalfopristin inhibits protein synthesis by irreversibly binding to the 50S ribosomal subunit. It is the formation of this stable complex which may account for its often bactericidal and prolonged postantibiotic effects against a wide variety of gram-positive bacteria. However, quinupristin-dalfopristin has often been found to be bacteriostatic against enterococci (4), and bactericidal activity may require the use of this drug in combination with other antimicrobial agents. Therefore, the objective of this study was to assess the in vitro activities of quinupristin-dalfopristin alone and in combination with other antimicrobials against clinical strains of VREF by checkerboard and time-kill methodologies.
Twelve epidemiologically unrelated clinical isolates of VREF (four blood culture isolates, seven rectal swab isolates, and one urinary isolate) were used in this study. Bacterial identification to the species level was confirmed by standard laboratory methods. The isolates were characterized by pulsed-field gel electrophoresis (13) to confirm that they were genetically unrelated. The glycopeptide resistance genotypes (vanA, vanB, and vanC) were determined by a multiplex PCR assay (5, 16).
Checkerboard susceptibility testing was done by a previously described broth microdilution method (6) which also allowed for the concurrent determination of the MIC of each antimicrobial agent tested. Testing was performed with cation-adjusted Mueller-Hinton broth (CAMHB; Difco Laboratories, Detroit, Mich.), with a final inoculum concentration of approximately 5 × 105 CFU/ml. The broths were incubated in ambient air at 35°C for 24 h in accordance with the guidelines of the National Committee for Clinical Laboratory Standards (15). The antimicrobial agents (drug concentrations in parentheses) assayed included vancomycin (0.25 to 32.0 μg/ml), ampicillin-sulbactam (0.25 to 128.0 μg/ml), doxycycline (0.125 to 16.0 μg/ml), chloramphenicol (0.25 to 32.0 μg/ml), and sparfloxacin (0.06 to 8.0 μg/ml), each of which were used in combination with quinupristin-dalfopristin (0.06 to 8.0 μg/ml). Control strains for checkerboard susceptibility testing included Staphylococcus aureus ATCC 29213, E. faecalis ATCC 29212, Escherichia coli ATCC 35218, and Pseudomonas aeruginosa ATCC 27853. The fractional inhibitory concentration index (ΣFIC) was calculated for each drug combination. A ΣFIC of ≤0.5 was interpreted as indicating the presence of synergy; a ΣFIC of >0.5 but ≤1 was interpreted as indicating additivity; a ΣFIC of >1 but ≤4 was interpreted as indicating indifference; and a ΣFIC of >4 was interpreted as indicating the presence of antagonism (6, 12).
Time-kill studies were performed with selected antimicrobial combinations based on the checkerboard susceptibility test results and in accordance with National Committee for Clinical Laboratory Standards guidelines (14). Overnight cultures were inoculated onto CAMHB and incubated for 3 h at 35°C to achieve logarithmic growth phase. The culture was adjusted to a 0.5 McFarland standard and was further diluted 10-fold and used to inoculate tubes containing either a single antimicrobial agent or the antibiotic in combination with quinupristin-dalfopristin in CAMHB. The antimicrobial agents were tested at subinhibitory concentrations which were no greater than one-half the MICs of quinupristin-dalfopristin, doxycycline, and ampicillin-sulbactam. Vancomycin was tested at a concentration of 20 μg/ml, a concentration that is readily achievable in serum when standard doses of the drug are administered. All cultures contained an initial inoculum concentration of approximately 5 × 105 CFU/ml. Tubes were incubated in ambient air without shaking at 35°C and assayed at 0, 4, 8, and 24 h. At each time point, aliquots were obtained and serially diluted in 0.85% saline and plated onto 5% Columbia blood agar plates (PML Microbiologicals, Mississauga, Ontario, Canada). Colony counts were determined following 48 h of incubation in ambient air at 35°C, and results were plotted on semilogarithmic paper as a function of time. By this method, colony counts as low as 1.6 log10 CFU/ml could be detected. A subset of time-kill studies were done in duplicate in order to ascertain the reproducibility of the results. Synergy was defined as a ≥2-log10-unit reduction in colony count in comparison with the colony count for the most active single antimicrobial after 24 h of incubation.
The MICs of the antimicrobial agents tested and the glycopeptide resistance genotypes as determined by multiplex PCR are summarized in Table 1. Eight of the isolates possessed a vanA genotype; for these isolates vancomycin MICs were >32 μg/ml. The other four isolates possessed a vanB genotype; for these isolates vancomycin MICs ranged from 8 to >32 μg/ml. The quinupristin-dalfopristin MICs for the 12 isolates ranged from 0.25 to 4.0 μg/ml.
TABLE 1.
Genotypic characterization and microbroth dilution MICs for vancomycin-resistant E. faecium study isolates
| Strain | Genotype | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| Vancomycin | Quinupristin-dalfopristin | Ampicillin-sulbactam | Doxycycline | Chloramphenicol | Sparfloxacin | ||
| 1 | vanA | >32 | 0.5 | 64 | 8.0 | 8.0 | >8.0 |
| 2 | vanA | >32 | 1.0 | 128 | ≤0.125 | 16 | >8.0 |
| 3 | vanA | >32 | 0.5 | 128 | 8.0 | 8.0 | >8.0 |
| 4 | vanA | >32 | 0.5 | 16 | 8.0 | 8.0 | 0.5 |
| 5 | vanB | 16 | 1.0 | 16 | 4.0 | 8.0 | 0.5 |
| 6 | vanB | 8.0 | 0.5 | 32 | ≤0.125 | 8.0 | >8.0 |
| 7 | vanA | >32 | 0.5 | 64 | 8.0 | 8.0 | >8.0 |
| 8 | vanA | >32 | 0.25 | 64 | 8.0 | 8.0 | >8.0 |
| 9 | vanA | >32 | 1.0 | 16 | ≤0.125 | 8.0 | >8.0 |
| 10 | vanA | >32 | 4.0 | 4.0 | >16 | 8.0 | 1.0 |
| 11 | vanB | >32 | 0.25 | 64 | 8.0 | 8.0 | >8.0 |
| 12 | vanB | 8.0 | 0.5 | 64 | ≤0.125 | 8.0 | >8.0 |
In checkerboard titration studies, all 12 strains of VREF demonstrated synergism when quinupristin-dalfopristin was combined with ampicillin-sulbactam at concentrations achievable in serum (Table 2). Synergism was also seen with one VREF strain when quinupristin-dalfopristin was combined with doxycycline at achievable concentrations. Antagonism was observed with nine isolates when they were tested with quinupristin-dalfopristin in combination with sparfloxacin. All other antimicrobial combinations resulted in either additive or indifferent effects.
TABLE 2.
Checkerboard and time-kill assay results for the seven vancomycin-resistant E. faecium strains exhibiting synergism in time-kill experiments
| Strain | Lowest FIC index in checkerboard assays in which quinupristin-dalfopristin was combined with:
|
Drugs and drug concns resulting in synergism in time-kill experimentsa | ||
|---|---|---|---|---|
| Ampicillin-sulbactam | Vancomycin | Doxycycline | ||
| 1 | 0.25 | 1.0 | 0.06 | Q/d (0.5 × MIC) + A/s (0.25 × MIC) |
| Q/d (0.5 × MIC) + Dox (0.25 × MIC) | ||||
| 2 | 0.19 | 1.0 | 1.06 | Q/d (0.25 × MIC) + Dox (0.5 × MIC) |
| 3 | 0.19 | 1.25 | 0.75 | Q/d (0.5 × MIC) + A/s (0.25 × MIC) |
| Q/d (0.5 × MIC) + Dox (0.25 × MIC) | ||||
| 4 | 0.19 | 1.0 | 0.75 | Q/d (0.5 × MIC) + Dox (0.25 × MIC) |
| Q/d (0.5 × MIC) + Van (0.3 × MIC) | ||||
| 5 | 0.16 | 1.5 | 0.5 | Q/d (0.25 × MIC) + Dox (0.5 × MIC) |
| 6 | 0.19 | 1.125 | 1.125 | Q/d (0.5 × MIC) + A/s (0.25 × MIC) |
| Q/d (0.5 × MIC) + Dox (0.5 × MIC) | ||||
| 12 | 0.25 | 1.0 | 1.125 | Q/d (0.25 × MIC) + Van (2.0 × MIC) |
Q/d, quinupristin-dalfopristin; A/s, ampicillin-sulbactam; Dox, doxycycline; Van, vancomycin.
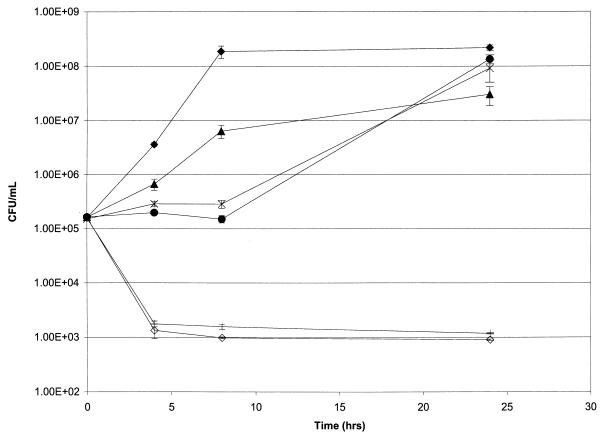
Time-kill experiments were done with quinupristin-dalfopristin in combination with either doxycycline, vancomycin, or ampicillin-sulbactam. Quinupristin-dalfopristin alone was not bactericidal against any of the VREF strains. A synergistic and bactericidal effect was found with at least one of the drug combinations in 7 of the 12 strains evaluated (Table 2). Six strains exhibited synergism with the combination of quinupristin-dalfopristin and doxycycline, three strains exhibited synergism when ampicillin-sulbactam was used, and two strains exhibited synergism with vancomycin. All other strains and drug combinations resulted in either an additive or an indifferent effect. Figure 1 illustrates time-kill study results, with one strain (strain 3) demonstrating synergism with quinupristin-dalfopristin plus doxycycline and with quinupristin-dalfopristin combined with ampicillin-sulbactam.
FIG. 1.
Time-kill curves for vancomycin-resistant E. faecium (strain 3) without a drug (⧫) or with ampicillin-sulbactam (32 μg/ml) (▴), doxycycline (2 μg/ml) (●), quinupristin-dalfopristin (0.25 μg/ml) (✠), ampicillin-sulbactam (32 μg/ml) plus quinupristin-dalfopristin (0.25 μg/ml) (|), and doxycycline (2.0 μg/ml) plus quinupristin-dalfopristin (0.25 μg/ml) (◊).
It has long been recognized that the treatment of serious bacteremic enterococcal infections, such as endocarditis, typically requires synergistic combination drug therapy in order to obtain bactericidal activity. With the emergence of glycopeptide and high-level aminoglycoside resistance in enterococci, this has been difficult to achieve. Several drugs and drug combinations, including newer investigational agents, have been evaluated in vitro and in animal models (1, 3, 9, 11, 12). However, these studies have generally used a relatively small number of VREF strains, and the results of these evaluations have been varied. In a checkerboard assay, the combination of quinupristin-dalfopristin and chloramphenicol was found to have an additive effect against 20 strains of VREF, but synergism was not demonstrated (12). The combination of ampicillin plus vancomycin was not found to be synergistic against 11 strains of VREF by a time-kill test method (3). Ampicillin plus ciprofloxacin exhibited in vitro activity against two VREF strains in time-kill studies, but this combination resulted in only modest efficacy when it was used in a rabbit model of experimental endocarditis (9). Combinations of quinupristin-dalfopristin with either LY333328 (a new glycopeptide) or CL331,002 (a glycylcycline) resulted in a synergistic effect with two of four VREF strains in a time-kill study (11).
In the present study, quinupristin-dalfopristin combined with ampicillin-sulbactam was found to have synergistic activity in the checkerboard analysis. However, these results should be interpreted with caution as they were confirmed with only 3 of the 12 strains by the time-kill test method. Moreover, checkerboard testing uses inhibition of bacterial growth as an endpoint, whereas time-kill studies have a bactericidal endpoint. The time-kill studies revealed that synergism resulting in bactericidal activity occurred most often (in 6 of 12 strains) when quinupristin-dalfopristin was combined with doxycycline. These results are similar to those reported by Aeschlimann et al. (1), who found that in a simulated endocardial-vegetation model, there was more rapid killing of VREF with quinupristin-dalfopristin plus doxycycline than with either compound used alone. In addition, use of the combination of drugs appeared to prevent the emergence of resistance to quinupristin-dalfopristin.
Treatment of endocarditis, and possibly of other bacteremic infections due to enterococci, requires the use of antimicrobial agents with bactericidal activities against the organism. Although in vitro checkerboard and time-kill studies may indicate drug combinations with synergistic and bactericidal activities against VREF, clinical correlation is essential. Combined therapy with quinupristin-dalfopristin, doxycycline, and rifampin was found to be synergistic in vitro and was effective in the treatment of a case of VREF endocarditis (10). The results of this study also suggest that the combination of quinupristin-dalfopristin with doxycycline, and to a lesser extent with ampicillin-sulbactam, may possess bactericidal activity against strains of VREF. Further evaluations of these and other drug combinations involving quinupristin-dalfopristin are warranted.
Acknowledgments
This study was supported, in part, by a grant provided by Rhône-Poulenc Rorer Inc.
We thank D. E. Low for providing clinical isolates of vancomycin-resistant enterococci and L. Cook for excellent secretarial services.
REFERENCES
- 1.Aeschlimann J R, Zervos M J, Rybak M J. Treatment of vancomycin-resistant Enterococcus faecium with RP 59500 (quinupristin-dalfopristin) administered by intermittent or continuous infusion, alone or in combination with doxycycline, in an in vitro pharmacodynamic infection model with simulated endocardial vegetations. Antimicrob Agents Chemother. 1998;42:2710–2727. doi: 10.1128/aac.42.10.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brumfitt W, Hamilton-Miller J M T, Shah S. In-vitro activity of RP 59500, a new semisynthetic streptogramin antibiotic, against Gram-positive bacteria. J Antimicrob Chemother. 1992;30:29–37. doi: 10.1093/jac/30.suppl_a.29. [DOI] [PubMed] [Google Scholar]
- 3.Cercenado E, Eliopoulos G M, Wennersten C B, Moellering R C., Jr Absence of synergistic activity between ampicillin and vancomycin against highly vancomycin-resistant enterococci. Antimicrob Agents Chemother. 1992;36:2201–2203. doi: 10.1128/aac.36.10.2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collins L A, Malanoski G J, Eliopoulos G M, Wennersten C B, Ferraro M J, Moellering R C., Jr In vitro activity of RP 59500, an injectable streptogramin antibiotic, against vancomycin-resistant gram-positive organisms. Antimicrob Agents Chemother. 1993;37:598–601. doi: 10.1128/aac.37.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutka-Malen S, Evers S, Courvalin P. Detection of glycopeptide resistance genotypes and identification to the species level of clinically relevant enterococci by PCR. J Clin Microbiol. 1995;33:24–27. doi: 10.1128/jcm.33.1.24-27.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eliopoulos G M, Moellering R C., Jr . Antimicrobial combinations. In: Lorian V, editor. Antibiotics in laboratory medicine. 4th ed. Baltimore, Md: Williams and Wilkins; 1996. pp. 330–396. [Google Scholar]
- 7.Eliopoulos G M, Wennersten C B, Gold H S, Schülin T, Souli M, Farris M G, Cerwinks S, Nadler H L, Dowzicky M, Talbot G H, Moellering R C., Jr Characterization of vancomycin-resistant Enterococcus faecium isolates from the United States and their susceptibility in vitro to dalfopristin-quinupristin. Antimicrob Agents Chemother. 1998;42:1088–1092. doi: 10.1128/aac.42.5.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones R N, Sader H S, Erwin M E, Anderson S C the Enterococcus Study Group. Emerging multiply resistant enterococci among clinical isolates. I. Prevalence data from 97 medical center surveillance study in the United States. Diagn Microbiol Infect Dis. 1995;21:85–93. doi: 10.1016/0732-8893(94)00147-o. [DOI] [PubMed] [Google Scholar]
- 9.Landman D, Quale J M, Mobarakai N, Zaman M M. Ampicillin plus ciprofloxacin therapy of experimental endocarditis caused by multidrug-resistant Enterococcus faecium. J Antimicrob Chemother. 1995;36:253–258. doi: 10.1093/jac/36.1.253. [DOI] [PubMed] [Google Scholar]
- 10.Matsumura S, Simor A E. Treatment of endocarditis due to vancomycin-resistant Enterococcus faecium with quinupristin/dalfopristin, doxycycline, and rifampin: a synergistic drug combination. Clin Infect Dis. 1998;27:1554–1556. doi: 10.1086/517755. [DOI] [PubMed] [Google Scholar]
- 11.Mercier R-C, Penzak S R, Rybak M J. In vitro activities of an investigational quinolone, glycylcycline, glycopeptide, streptogramin, and oxazolidinone tested alone and in combinations against vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother. 1997;41:2573–2575. doi: 10.1128/aac.41.11.2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Messick C R, Pendland S L. In vitro activity of chloramphenicol alone and in combination with vancomycin, ampicillin, or RP 59500 (quinupristin/dalfopristin) against vancomycin-resistant enterococci. Diagn Microbiol Infect Dis. 1997;29:203–205. doi: 10.1016/s0732-8893(97)81811-1. [DOI] [PubMed] [Google Scholar]
- 13.Miranda A G, Singh K V, Murray B E. DNA fingerprinting of Enterococcus faecium by pulsed-field gel electrophoresis may be a useful epidemiologic tool. J Clin Microbiol. 1991;29:2752–2757. doi: 10.1128/jcm.29.12.2752-2757.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents. Vol. 12 1992. , no. 19. Tentative guideline M26-T. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 15.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing; eighth informational supplement. Vol. 18 1998. , no. 1. Approved standard M100-S8. National Committee for Clinical Laboratory Standards, Wayne, Pa. [Google Scholar]
- 16.Tyrrell G J, Bethune R N, Willey B, Low D E. Species identification of enterococci via intergenic ribosomal PCR. J Clin Microbiol. 1997;35:1054–1060. doi: 10.1128/jcm.35.5.1054-1060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]