Abstract
In vitro treatment with clarithromycin inhibited the expression of the matrix metalloproteinase-9, transforming growth factor β, and tumor necrosis factor alpha genes in 13762NF rat mammary adenocarcinoma cells. Transient enhancement, rather than inhibition, was observed for the interleukin-6 gene, and no significant change was observed for the tissue inhibitor of metalloproteinase-2 gene. Such an effect was not observed for cefotiam or gentamicin.
A variety of macrolide activities as biological response modifiers have already been reported on (1, 3, 6, 7, 9, 11, 14). We (13) also recently reported finding that in a rat tumor model, clarithromycin (CAM) has an activity which induces a beneficial therapeutic outcome, possibly by inhibiting the production of tumor-derived factors which induce cachexia (2, 5) or immunosuppression (15). In this study, we examined in more detail the effects of CAM on the production of cytokines by a tumor to define a characteristic property of CAM.
The tumor we used was the 13762NF (subclone MTLn3) mammary adenocarcinoma (12) originating from an F-344 rat, and it was maintained in vitro in RPMI 1640 medium containing 10% fetal calf serum (FCS). CAM (Abbott Co. Ltd.) was dissolved with 100% methanol (1 mg/ml) and then further diluted in RPMI culture medium to reach final concentrations. Gentamicin sulfate (GM) and cefotiam dehydrochloride (CTM) were also diluted in RPMI medium. Expression of genes was measured by the reverse transcription-PCR method as described previously (13). The primers used for the amplification of genes were as follows: matrix metalloproteinase-9 (MMP-9) gene, 5′-GGTCCCCCCACTGCTGGCCCTTCTACGGCC-3′ and 5′-GTCCTCAGGGCACTGGAGGATGTCATAGGT-3′; transforming growth factor β (TGF-β) gene, 5′-GCCCTGGACACCTATTGC-3′ and 5′-GCTGCACTTGCAGGAGCGCAC-3′; tumor necrosis factor alpha (TNF-α) gene, 5′-CAAGGAGGAGAAGTTCCCAA-3′ and 5′-CGGACTCCGTGATGTCTAAG-3′; interleukin-6 (IL-6) gene, 5′-ATGTAGCCGCCCCACACAGA-3′ and 5′-CATCCATCTTTTTCAGCCAT-3′; inhibitor of metalloproteinase (TIMP-2) gene, 5′-TGCAGCTGCTCCCCGGTGCAC-3′ and 5′-TTATGGGTCCTCGATGTCGAG-3′. As an internal control, a set of primers for the glyceraldehyde-3-phosphate dehydrogenase gene (5′-CAAAAGGGTCATCTCTG-3′ and 5′-CCTGCTTCACCACCTTCTTG-3′) was added to each sample. The degree of gene expression, determined with a densitometer, was expressed as a ratio to glyceraldehyde-3-phosphate dehydrogenase. Gelatinolytic activity in the culture medium was assayed by the method of Heussen and Dowdle (4), but in this assay, tumor cells were cultured in the presence of 2%, instead of 10%, FCS because significant gelatinolytic activity was found in the FCS. Data are shown as means ± standard errors, and statistical significance was evaluated by Student’s t test. A P value of less than 0.05 was judged to be significant.
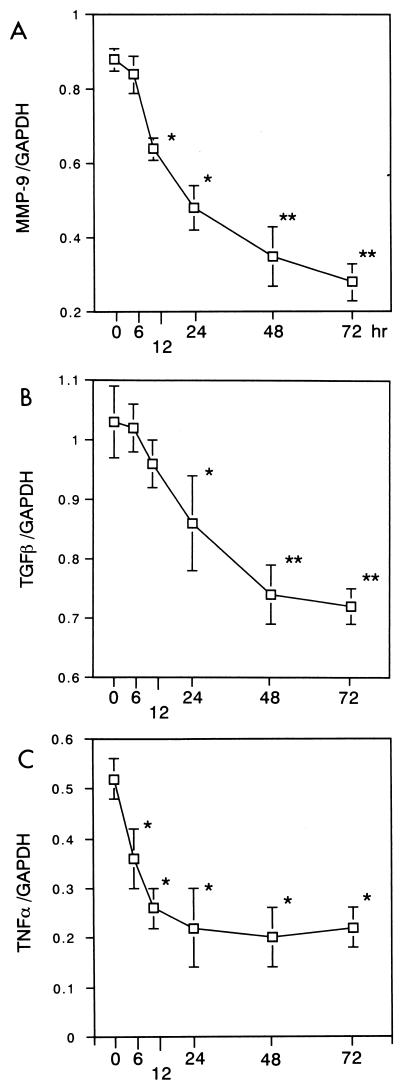
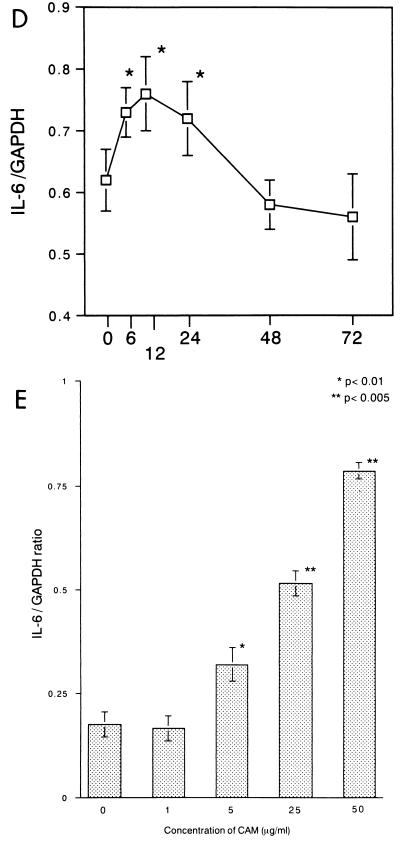
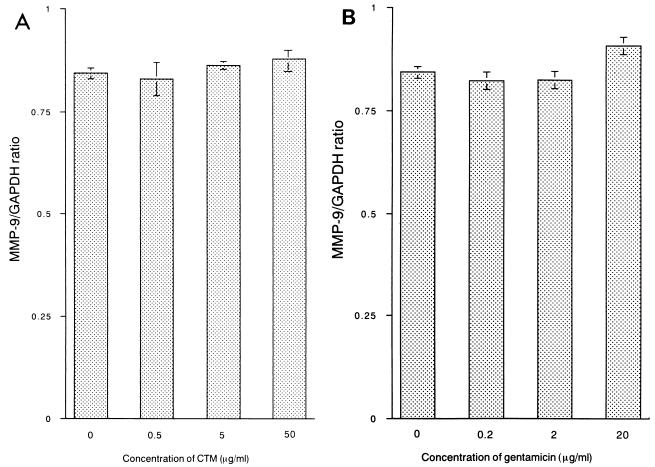
13762NF tumor cells were treated with CAM at 5 μg/ml, and total RNAs were extracted from the tumor cells at 6, 12, 24, 48, and 72 h. Expression of the MMP-9, TGF-β, and TNF-α genes was shown to be inhibited significantly by treatment with CAM (Fig. 1A to C). Conversely, transient enhancement was observed for the IL-6 gene (Fig. 1D). Next, tumor cells were treated for 24 h with different concentrations of CAM (1 to 50 μg/ml). Significant decreases in expression were observed for the MMP-9, TGF-β, and TNF-α genes (data not shown), and an increase was observed for the IL-6 gene (Fig. 1E). The gelatinolytic activity in the culture medium was shown to be inhibited by treatment of tumor cells with CAM (data not shown). We also examined the effects of two other antimicrobial agents, CTM and GM, on the expression of the MMP-9 gene (24 h), and no significant effect was observed for these two agents (Fig. 2). We further examined the effect of CAM on the expression of the TIMP-1 or TIMP-2 gene (8) in 13762NF tumor cells. In the tumor cells, the TIMP-2 gene was found to be expressed highly but the TIMP-1 gene was not (data not shown). As shown in Fig. 3, no significant change due to CAM (5 μg/ml) treatment was observed.
FIG. 1.
Effects of CAM (5 μg/ml) treatment time on expression of the MMP-9 (A), TGF-β (B), TNF-α (C), and IL-6 (D) genes. 13762NF tumor cells were treated with CAM at 5 μg/ml for different lengths of time, and then total RNAs were extracted for analysis. (E) Effect of CAM concentration on expression of the IL-6 gene. 13762NF tumor cells were treated with different concentrations of CAM for 24 h, and then total RNAs were extracted for analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FIG. 2.
Effect of treatment with CTM (A) or GM (B) on expression of the MMP-9 gene in 13762NF tumor cells. 13762NF tumor cells were treated with CTM (A) or GM (B) for 24 h, and then total RNAs were extracted for analysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FIG. 3.
Effect of CAM on expression of the TIMP-2 gene in 13762NF tumor cells. 13762NF tumor cells were treated with CAM (5 μg/ml) for different lengths of time (given in hours on the x axis). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
As a consequence, three patterns were observed after CAM treatment: suppression (MMP-9, TGF-β, and TNF-α), transient enhancement (IL-6), and no change (TIMP-2). It is difficult to explain why these different patterns appeared following CAM treatment. Such a modulatory effect was not observed for CTM or GM. We suppose that the effect observed in this study is specific to the macrolides, probably specific to the 14-membered ring macrolides. For example, Yatsunami et al. (Kyushu University School of Medicine) showed, in the B16 tumor-C57BL6 mouse model, that CAM and roxithromycin (14-membered ring) could inhibit angiogenesis but josamycin (16-membered ring) and azithromycin (15-membered ring) could not (personal communication). We have no explanation for the difference in action among the 14-, 15-, and 16-membered ring macrolides.
It is interesting that macrolide antibiotics cause a beneficial therapeutic outcome in hosts bearing cancer (3, 10, 13). We hope that CAM may become a beneficial tool for the treatment of certain cancers.
REFERENCES
- 1.Bailly S, Pocidalo J J, Fay M, Pocidalo M A G. Differential modulation of cytokine production by macrolides: interleukin-6 production is increased by spiramycin and erythromycin. Antimicrob Agents Chemother. 1991;35:2016–2019. doi: 10.1128/aac.35.10.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Espat N J, Copeland E M, Moldawer L L. Tumor necrosis factor and cachexia: a current perspective. Surg Oncol. 1994;3:255–262. doi: 10.1016/0960-7404(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 3.Hamada K, Kita E, Sawaki M, Mikasa K, Narita N. Antitumor effect of erythromycin in mice. Chemotherapy. 1995;41:59–69. doi: 10.1159/000239325. [DOI] [PubMed] [Google Scholar]
- 4.Heussen C, Dowdle E B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980;102:196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- 5.Kishimoto T. The biology of interleukin 6. Blood. 1989;74:1–10. [PubMed] [Google Scholar]
- 6.Kita E, Sawaki M, Nishikawa F, Mikasa K, Yagyu Y, Takeuchi S, Yasui K, Narita N, Kashiba S. Enhanced interleukin production after long-term administration of erythromycin stearate. Pharmacology. 1990;41:177–183. doi: 10.1159/000138716. [DOI] [PubMed] [Google Scholar]
- 7.Kobayashi H. Airway biofilm disease: clinical manifestation and therapeutic possibilities using macrolides. J Infect Chemother. 1995;1:1–15. doi: 10.1016/s0002-9343(99)80282-4. [DOI] [PubMed] [Google Scholar]
- 8.Kugler A, Hemmerlein B, Thelen P, Kallerhoff M, Radzun H J, Ringert R H. Expression of metalloproteinase 2 and 9 and their inhibitors in renal cell carcinoma. J Urol. 1998;160:1914–1918. [PubMed] [Google Scholar]
- 9.Majima T, Mikasa K, Hamada K, Konishi M, Maeda K, Sakamoto M, Yoshimoto E, Murakawa K, Ueda K, Kita E, Narita N. Changes of cytokine mRNA in peripheral blood mononuclear cells from unresectable non-small cell lung cancer patients before and after clarithromycin therapy. Jpn J Chemother. 1999;47:345–348. . (In Japanese.) [Google Scholar]
- 10.Mikasa K, Sawaki M, Kita E, Hamada K, Teramoto S, Sakamoto M, Maeda K, Konishi M, Narita N. Significant survival benefit to patients with advanced non-small-cell lung cancer from treatment with clarithromycin. Chemotherapy. 1997;43:288–296. doi: 10.1159/000239580. [DOI] [PubMed] [Google Scholar]
- 11.Morikawa K, Oseko F, Morikawa S, Iwamoto K. Immunomodulatory effects of three macrolides, midecamycin acetate, josamycin, and clarithromycin, on human T-lymphocyte function in vitro. Antimicrob Agents Chemother. 1994;38:2643–2647. doi: 10.1128/aac.38.11.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakajima M, Welch D R, Wynn D M, Tsuruo T, Nicolson G L. Serum and plasma Mr 92,000 progelatinase levels correlate with spontaneous metastasis of rat 13762NF mammary adenocarcinoma. Cancer Res. 1993;53:5802–5807. [PubMed] [Google Scholar]
- 13.Sassa K, Mizushima Y, Fujishita T, Oosaki R, Kobayashi M. Therapeutic effect of clarithromycin on a transplanted tumor in rats. Antimicrob Agent Chemother. 1999;43:67–72. doi: 10.1128/aac.43.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawaki M, Kita E, Narita N. Clarithromycin as a potent anti-angiogenesis agent: possible application for antitumor agent. Can J Infect Dis. 1995;6(Suppl. c):213. [Google Scholar]
- 15.Sporn M B, Roberts A B, Wakefield L M, de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-β. J Cell Biol. 1986;105:1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]