Abstract
The susceptibility of Neisseria gonorrhoeae to several medium-chain fatty acids and their 1-monoglycerides was tested at a short inactivation time of 1 min. The results indicate that monocaprin, a monoglyceride of capric acid (10 carbon atoms, no double bonds), causes the fastest and most effective killing of all strains of N. gonorrhoeae tested.
Although the incidence of gonorrhea has decreased in many countries in recent years (4, 6, 7), it is still a significant health care problem worldwide (1, 6) and is the second leading cause of pelvic inflammatory disease. A vaccine against Neisseria gonorrhoeae has not been developed, and other means of prevention, except for the use of condoms, are not available. In recent years there have been proposals to use microbicidal compounds against sexually transmitted diseases (STDs) (5), especially with regard to developing a product that can both prevent pregnancy and protect against infectious agents, including the human immunodeficiency virus type 1.
The microbicidal effects of a variety of lipids have been extensively studied in recent years. A number of free fatty acids and their 1-monoglycerides have a broad spectrum of microbicidal activity against enveloped viruses and various bacteria in vitro (8, 10, 13, 14, 16), including pathogens like group B streptococcus (3), herpes simplex virus (12, 14), and Chlamydia trachomatis (2). These lipids are commonly found in natural products, for example, in milk, and are therefore likely to be nontoxic to mucosas, at least at low concentrations. In nature, e.g., in milk and at mucosas, these compounds are considered to be potent inhibitory factors against infections by many human pathogens or parasites (8, 9). It has therefore been suggested that they might be useful as intravaginal microbicides for protection against STDs (9). In the present study, several fatty acids and their 1-monoglycerides were tested for their microbicidal activities against N. gonorrhoeae. This was done to define the range of active lipids against the bacterium. A short inactivation time of 1 min was selected as a criterion for a fast and effective killing of five strains of the bacterium.
Fatty acids and 1-monoglycerides (purest grade) were purchased from Sigma Chemical Co., St. Louis, Mo. Stock solutions were made in ethanol: 0.5 M for monomyristin and 1 M for all the other fatty acids and monoglycerides.
One N. gonorrhoeae strain (strain III) was obtained from the American Type Culture Collection (ATCC 49226). Four strains were clinical isolates. All were isolated from urethral swabs of male patients with gonorrhea; two clinical isolates were penicillin sensitive (strains I and IV) and two were penicillin resistant (strains II and V). Strains IV and V were recent isolates (1998) with a short passage history. The strains were identified by the oxidase test and Gram staining and were confirmed by standard sugar fermentation (11) and by staining with fluorescent monoclonal antibodies (Difco Laboratories, Detroit, Mich.). Cultures were prepared from frozen bacterial stock. For each experiment, the bacteria were streaked on a chocolate agar medium plate and were incubated at 37°C in an atmosphere of 5% CO2 for 24 h. The bacterial suspensions used in experiments were prepared by removing the colonies from the culture plate with a loop and suspending them in Trypticase soy broth by vortexing for a couple of minutes. If needed, broth was added to bring the suspension to a standard density of 108 to 109 CFU per ml.
Assay of antibacterial activity was performed by diluting stock solutions of fatty acids or monoglycerides in Trypticase soy broth to the desired concentration by vortexing at the highest speed at 37°C. The solutions showed a little turbidity which varied between lipids but was less for lipids with short or unsaturated fatty acid chains. The solutions were immediately tested against N. gonorrhoeae by thoroughly mixing 200 μl of a lipid solution and 200 μl of the N. gonorrhoeae inoculum adjusted to a density of 108 to 109 CFU per ml in a plastic tube. Bacteria mixed with broth alone and with 2% ethanol in broth were used as controls. The mixtures were incubated for 1 min at room temperature. Strain III was further tested after an incubation time of 10 min at 37°C. Samples were removed and diluted 10-fold in sterile physiological saline, and the number of viable bacteria was determined by streaking 10 μl of a 10−2 to 10−6 dilution and 100 μl of a 10−1 dilution on chocolate agar plates with a pipette tip. Each streaking was done in duplicate. Bacterial colonies were counted after incubation for 24 to 48 h at 37°C in a CO2 incubator. The titers (log10 CFU) of lipid-bacterium mixtures were subtracted from the titer of the control mixture, and the difference was used as a measure of the antibacterial activity of the lipids.
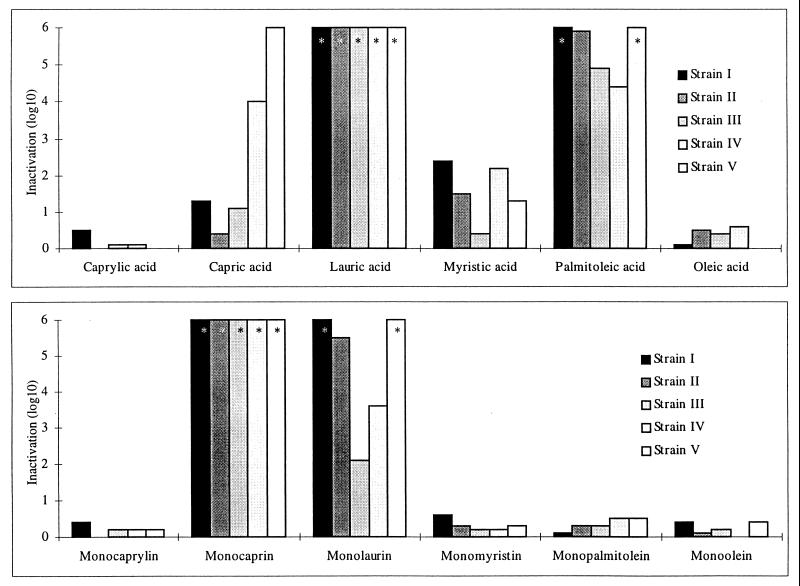
A comparison of the bactericidal activities of six fatty acids is presented in Fig. 1. The bars represent the reduction in infectivity titers (log10 CFU) of the five strains of N. gonorrhoeae after incubation with 2.5 mM (361 to 706 μg/ml) fatty acid for 1 min at room temperature. Caprylic acid (eight carbon atoms, no double bonds [8:0]) and oleic acid (18:1) caused only minor inactivations of the bacteria with reductions in titer ranging from zero to 0.5 log10 CFU. Myristic acid (14:0) and capric acid (10:0) showed higher activities, with reductions in infectivity titer ranging from 0.4 to 6.0 log10 CFU, varying between the strains. In contrast, lauric acid (12:0) reduced the titer by greater than 6.0 log10 CFU, and the unsaturated palmitoleic acid (16:1) reduced the titer by about 6 log10 CFU for strains I, II, and V, and 4.9 and 4.4 log10 CFU for strains III and IV, respectively. After incubation for 10 min (only done for strain III), the activities of capric acid (5.7 log10 CFU), myristic acid (≥6.6 log10 CFU), and oleic acid (4.0 log10 CFU), increased. Caprylic acid continued to have no effect after 10 min of incubation.
FIG. 1.
Inactivation of N. gonorrhoeae by incubation with 2.5 mM solutions of fatty acids and monoglycerides for 1 min at room temperature. The bars represent reduction of CFU. In the bars, the asterisks indicate that the reduction of titer was equal to or greater than 6.0 log10 CFU. Caprylic acid and monocaprylin (8:0) (number of carbon atoms/number of double bonds), capric acid and monocaprin (10:0), lauric acid and monolaurin (12:0), myristic acid and monomyristin (14:0), palmitoleic acid and monopalmitolein (16:1), and oleic acid and monoolein (18:1) are shown.
To further compare the activities of lauric acid and palmitoleic acid, these fatty acids were tested at lower concentrations for an incubation time of 1 min. Table 1 shows that lauric acid lost almost all of its activity when diluted to a concentration of 0.31 mM but still showed a significant activity against strains I, II, and IV at a 0.63 mM concentration and against all the strains at a concentration of 1.25 mM. The activity of palmitoleic acid showed a different pattern than lauric acid. At higher concentrations (2.5 and 1.25 mM), lauric acid was more active, while palmitoleic acid was more active at the lower concentration of 0.31 mM. This difference between lauric acid and palmitoleic acid became more pronounced after 10 min of incubation with strain III. Palmitoleic acid caused reductions in titer of greater than 6 log10 CFU at concentrations of 0.31 mM and higher, and even at 0.16 mM the infectivity titer was reduced by 4.7 log10 CFU. In contrast, after 10 min, lauric acid caused only a 4.6 log10 CFU reduction in titer at 0.63 mM and showed no activity at lower concentrations.
TABLE 1.
Inactivation of N. gonorrhoeae by lipids after 1 min of incubation at room temperature
| Fatty acid | Straina | Reduction of CFU (log10) at concentrations (mM)b:
|
||||
|---|---|---|---|---|---|---|
| 2.50 | 1.25 | 0.63 | 0.31 | 0.16 | ||
| Lauric acid (12:0)c | I | ≥6.6d | ≥6.6 | 3.1 | 0.4 | NDe |
| II | ≥6.0 | ≥6.0 | 3.9 | 0.2 | ND | |
| III | ≥7.0 | 5.1 | 0.4 | 0.1 | ND | |
| IV | 6.3 | 5.1 | 2.8 | 0.6 | ND | |
| V | 6.7 | 6.7 | 0.3 | 0.0 | ND | |
| Palmitoleic acid (16:1) | I | ≥6.4 | 5.1 | 3.2 | 1.5 | 0.1 |
| II | 5.9 | 6.1 | 3.7 | 1.8 | 1.0 | |
| III | 4.9 | 4.5 | 1.8 | 0.8 | 0.4 | |
| IV | 4.4 | 4.0 | 2.8 | 1.7 | 0.5 | |
| V | ≥6.4 | 6.0 | 2.6 | 0.7 | 0.1 | |
| Monocaprin (10:0) | I | ≥6.6 | ≥6.6 | ≥6.6 | 0.3 | ND |
| II | ≥6.0 | ≥6.0 | 6.0 | 1.2 | ND | |
| III | ≥6.6 | ≥6.6 | 6.4 | 0.7 | 0.1 | |
| IV | 6.0 | 5.0 | 4.4 | 0.6 | 0.2 | |
| V | ≥6.7 | ≥6.7 | ≥6.7 | 1.3 | 0.2 | |
| Monolaurin (12:0) | I | 6.6 | ≥6.6 | 6.0 | 3.1 | 0.0 |
| II | 5.4 | 6.0 | 4.9 | 3.2 | 1.4 | |
| III | 2.1 | 1.3 | 0.4 | 0.3 | 0.1 | |
| IV | 3.6 | 2.8 | 1.7 | 0.5 | 0.2 | |
| V | ≥6.7 | 6.7 | 5.9 | 4.3 | 0.8 | |
Strains are defined in the text.
Final concentration of lipids.
Number of carbon atoms/number of double bonds.
≥ Indicates that no colonies were detectable in 100 μl of the 10−1 dilution, which was the lowest dilution tested.
ND, not done.
The antibacterial activities of six 1-monoglycerides are presented in Fig. 1. They were tested in the same way as the corresponding fatty acids, at concentrations of 2.5 mM (546 to 891 μg/ml). Monocaprylin (8:0), monomyristin (14:0), monopalmitolein (16:1), and monoolein (18:1) had only small effects, causing reductions in titer of up to 0.6 log10 CFU after 1 min of incubation. Monocaprin (10:0), on the other hand, caused reductions in titer of greater than 6.0 log10 CFU in all strains, whereas monolaurin (12:0) had high activities against strains I, II, and V but much less (2.1 and 3.6 log10 CFU) against strain III.
As was done for the fatty acids showing the highest activities, the two most active 1-monoglycerides were further analyzed. Table 1 shows that monocaprin was still very active at 0.63 mM, causing reductions in titer of 6.0 log10 CFU or greater in all strains but strain IV (4.4 log10 CFU). However, at 0.31 mM, the activity was almost lost. Of all the lipids tested, monolaurin showed the highest activity at the concentration of 0.31 mM, namely 3.1, 3.2, and 4.3 log10 CFU for strains I, II, and V, respectively. In contrast, monolaurin had very little activity against strains III and IV, with reductions of only 2.1 and 3.6 log10 CFU at 2.5 mM and 1.3 and 2.8 log10 CFU at 1.25 mM, respectively. This demonstrates a variability between the different strains of N. gonorrhoea, with the ATCC strain (strain III) being the least susceptible. After 10 min of incubation of strain III with the various lipids (data not shown), monomyristin and monopalmitolein became slightly more effective, whereas no increase in efficacy was seen for monocaprin. With monolaurin, the activity of concentrations of 0.625 mM and higher was greatly increased (4.9 log10 CFU).
In this study, we have shown that N. gonorrhoeae, a gram-negative coccus and a STD pathogen, is effectively killed by exposure for 1 min to 2.5 mM lauric acid (12:0), palmitoleic acid (16:1), or monocaprin (10:0), and is therefore exceptionally susceptible to these lipids when compared to other microbes. Furthermore, when the activity profile of N. gonorrhoeae is compared to these microbes (2, 3), only two of the lipids tested, namely monocaprin and lauric acid, are highly effective against all of the bacteria. Only two of the lipids, i.e., capric acid and monolaurin, showed significant variability in activity against all five different strains of N. gonorrhoeae.
Our hypothesis is that the lipids kill the bacteria by disruption of their cell membrane(s). This has been shown for other organisms, both viral (14) and bacterial (2), by electron microscopy. Because of the lipid action on biological membranes, we deem the emergence of resistant strains very unlikely. A similar disruption of cell membranes could be seen when cell cultures were exposed to high concentrations of lipids and analyzed by electron microscopy (14). Although toxic in cell cultures, monocaprin at a concentration of 5 mg per ml (20 mM) has been shown to not cause irritation of the vaginal mucosa of mice and rabbits (15).
The rapid in vitro killing of bacteria and viruses by lipids is an essential prerequisite for the possible use of lipids in the prevention of STDs. Also, due to the high in vitro efficacy of monocaprin against a number of different microbes, this lipid may be useful as a microbicidal agent against some human pathogens, both as a treatment and as a chemical barrier.
Acknowledgments
This work was supported by a grant from the Research Fund of the University of Iceland.
We thank Sigfús M. Karlsson and Erla Sigvaldadóttir for their help in this work.
REFERENCES
- 1.Australian Gonococcal Surveillance Programme. Annual report 1997. Commun Dis Intell. 1998;22:212–216. [PubMed] [Google Scholar]
- 2.Bergsson G, Arnfinnsson J, Karlsson S M, Steingrímsson Ó, Thormar H. In vitro inactivation of Chlamydia trachomatis by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1998;42:2290–2294. doi: 10.1128/aac.42.9.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergsson G, Steingrímsson Ó, Thormar H. Microbicidal effect of lipids against gram-positive and gram-negative cocci. Icelandic Med J. 1998;84(Suppl. 37):118. [Google Scholar]
- 4.Bjekic M, Vlajinac H, Sipetic S. Incidence of gonorrhea in Belgrade, 1988–1994. J Infect. 1998;37:44–47. doi: 10.1016/s0163-4453(98)90518-7. [DOI] [PubMed] [Google Scholar]
- 5.Elias C J, Heise L L. Challenges for the development of female-controlled vaginal microbicides. AIDS. 1994;8:1–9. doi: 10.1097/00002030-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Fox K K, Whittington W L, Levine W C, Moran J S, Zaidi A A, Nakashima A K. Gonorrhea in the United States, 1981–1996. Demographic and geographic trends. Sex Transm Dis. 1998;25:386–393. doi: 10.1097/00007435-199808000-00011. [DOI] [PubMed] [Google Scholar]
- 7.Hiltunen-Back E, Rostila T, Kautiainen H, Paavonen J, Reunala T. Rapid decrease of endemic gonorrhea in Finland. Sex Transm Dis. 1998;25:181–186. doi: 10.1097/00007435-199804000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Isaacs C E, Litov R E, Thormar H. Antimicrobial activity of lipids added to human milk, infant formula, and bovine milk. Nutr Biochem. 1995;6:362–366. doi: 10.1016/0955-2863(95)80003-u. [DOI] [PubMed] [Google Scholar]
- 9.Isaacs C E, Kim K S, Thormar H. Inactivation of enveloped viruses in human bodily fluids by purified lipids. Ann N Y Acad Sci. 1994;724:457–464. doi: 10.1111/j.1749-6632.1994.tb38947.x. [DOI] [PubMed] [Google Scholar]
- 10.Kabara J J. Fatty acids and derivatives as antimicrobial agents. In: Kabara J J, editor. The pharmacological effect of lipids. St. Louis, Mo: The American Oil Chemists Society; 1978. pp. 1–14. [Google Scholar]
- 11.Knapp J S, Rice R J. Neisseria and Branhamella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 324–340. [Google Scholar]
- 12.Kristmundsdóttir, T., S. Árnadóttir, G. Bergsson, and H. Thormar. Development and evaluation of microbicidal hydrogels containing monoglycerides as the active ingredient. J. Pharm. Sci., in press. [DOI] [PubMed]
- 13.Shibasaki I, Kato N. Combined effects on antibacterial activity of fatty acids and their esters against gram-negative bacteria. In: Kabara J J, editor. The pharmacological effects of lipids. St. Louis, Mo: The American Oil Chemists Society; 1978. pp. 15–24. [Google Scholar]
- 14.Thormar H, Isaacs C E, Brown H R, Barshatzky M R, Pessolano T. Inactivation of enveloped viruses and killing of cells by fatty acids and monoglycerides. Antimicrob Agents Chemother. 1987;31:27–31. doi: 10.1128/aac.31.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thormar H, Bergsson G, Gunnarsson E, Georgsson G, Witvrouw M, Steingrímsson Ó, De Clercq E, Kristmundsdóttir T. Hydrogels containing monocaprin have potent microbicidal activities against sexually transmitted viruses and bacteria in vitro. Sex Transm Infect. 1999;75:181–185. doi: 10.1136/sti.75.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Welsh J K, Arsenakis M, Coelen R J, May J T. Effect of antiviral lipids, heat, and freezing on the activity of viruses in human milk. J Infect Dis. 1979;140:322–328. doi: 10.1093/infdis/140.3.322. [DOI] [PubMed] [Google Scholar]