Abstract
Rhesus monkeys are naturally social animals, and behavioral management strategies have focused on promoting pair-housing in laboratory settings as an alternative to individual or group housing. In humans, co-sleeping can have a major impact on bed partners’ sleep, raising the possibility that pair-housing also may influence sleep parameters in monkeys. In the present study, we investigated if pair-housing would impact home-cage partner’s sleep in female rhesus monkeys, and if nighttime separation using socialization panels would alter this pattern. Sleep parameters of 10 experimentally naïve adult female rhesus monkeys (5 pairs) were evaluated for 7 consecutive days using actigraphy monitors attached to primate collars. Paired animals then were separated by socialization panels during the night, and sleep-associated measures were evaluated for 7 consecutive days. The data showed that sleep efficiency was significantly lower when monkeys were pair-housed as compared with when they were separated. On the nights when subjects were pair-housed, a positive correlation was detected for sleep measures (both sleep latency and efficiency) of both members of a pair (R2’s = 0.16–0.5), suggesting that pair-housing influences sleep quality. On nights when subjects were separated, no correlations were observed for sleep measures between members of the pairs (R2’s = 0.004–0.01), suggesting that when separated, the home-cage partner’s sleep no longer influenced the partner’s sleep. Our results indicate that pair-housing has a strong impact on the home-cage partner’s sleep, and that this pattern can be prevented by nighttime separation using socialization panels. Studies evaluating sleep in pair-housed monkeys should consider the effects that the partner’s sleep may have on the subject’s sleep. Sleep is a biologic phenomenon and experimental outcome that affects physical and behavioral health and altered sleep due to pair-housing may affect a range of research outcomes.
Abbreviations: NHP, nonhuman primate(s)
Introduction
According to the Animal Welfare Act,1 any institution that houses nonhuman primates (NHP) must develop a plan to provide for the animals’ psychologic wellbeing and to address their social needs. Rhesus monkeys (Macaca mulatta) live in social groups in nature, and social environments have been shown to significantly improve the welfare of laboratory NHP, both behaviorally and physiologically.19,27 For instance, single housing has been associated with physiologic changes such as immunosuppression23 and higher blood pressure.11 Therefore, behavioral management strategies have focused on promoting pair-housing as a more feasible alternative to group housing,3 and research facilities have received increased regulatory pressure to pair-house NHP in indoor colonies.18 In fact, according to the most recent survey, among all research facilities in the United States, the percentage of laboratory NHP being housed in pairs (23%) is now higher than that of single-housed animals (16%).4
While promoting social housing (that is, both pair- and group-housing) in laboratory animals has been proposed as a way to increase the external validity of primate biomedical research,18 many experimental variables can be influenced by social-housing. Sleep, for instance, is a frequently neglected contributor to physical health and wellbeing,23,28,30 and sleep can be influenced by sleeping arrangements. In humans, co-sleeping can have a major impact on bed partner’s sleep and perhaps on their health and wellbeing.15,18,33 Given that NHP tend to huddle together when sleeping in naturalistic environments, the human literature on co-sleeping raises the possibility that social housing also may influence sleep parameters in monkeys. Altered sleep due to pair-housing might affect research outcomes, especially for studies in which sleep is a primary outcome measure. It may also affect the animals in other ways and consequently influence the external validity of primate biomedical research.
Intermittent pair-housing has been proposed as an alternative to continuous pair-housing to accommodate with research and management needs.20 Intermittent pair-housing involves temporary daily or weekly separations that last several hours.3,9 One group has shown that overnight separations may influence cortisol levels in pair-housed female monkeys.20 Because high cortisol levels are associated with poor sleep in humans,31 a reasonable assumption is that pair-housing and/or intermittent separation might also influence sleep in NHP.
The current study investigated whether pair-housing influences the partner’s sleep in pair-housed female rhesus monkeys, and whether nighttime separation alters this pattern. Sleep-like parameters were measured using actigraphy during 7 d while animals were pair-housed, and during 7 d of nighttime separation using socialization panels that allowed for visual and tactile contact between the subjects throughout the evening/night.
Materials and Methods
Subjects.
Ten adult female rhesus monkeys (Macaca mulatta), weighing between 6- 10 kg across the course of the study, served as subjects. Six of the subjects (3 pairs) were housed at the University of Mississippi Medical Center (UMMC), and 4 (2 pairs) were housed at the Yerkes National Primate Research Center, Emory University. Both institutions are AAALAC-accredited. All 10 subjects were experimentally naïve at the time of the experiments. UMMC monkeys were pair-housed originally by the behavioral management team from the Harlow Center for Biologic Psychology, University of Wisconsin-Madison, before being moved to UMMC; whereas Yerkes monkeys were pair-housed by the Yerkes behavioral management team. All monkeys had been pair-housed for at least 6 mo before the beginning of these studies. Subjects had visual, auditory, and olfactory contact with other monkeys in the room throughout the study, as well as access to chew toys. Subjects were maintained on a 12h light/12h dark cycle (lights on at 600h), at a temperature of 21 ± 2°C, with water available ad libitum and monkey diet available once/day, supplemented by fresh fruit and forage (seeds and dry fruit), and were weighed monthly during physical examinations. Animals were fitted with primate collars (Primate Products, FL) prior to the start of the studies.
For the 6 subjects at UMMC, all animal use procedures were approved by the UMMC Animal Care and Use Committee. For the 4 subjects at Yerkes, all animal use procedures were approved by the Yerkes Animal Care and Use Committee. All protocols and animal care and handling followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Research Council, 2011).
Experimental procedures.
Actigraphy-based sleep parameters.
Actiwatch sensors (Mini Mitter, Bend, OR) were used to assess activity, as previously described.5,6 The Actiwatch consists of an omni-directional sensor that is sensitive to motion (recorded as activity counts) and has been previously shown to be a reliable, non-invasive method for activity monitoring.27 The monitors were programmed to record the total piezoelectric voltage generated over the preceding 15 s (i.e. epoch length = 15 s). The devices record intensity, amount and duration of movement in all three planes by producing a voltage that is subsequently converted to an arbitrary count and data logged (for review see Mann et al. 2005). While no studies to date have validated the use of actigraphy to investigate sleep in NHP using EEG-based sleep assessments, actigraphy has been validated for sleep studies in humans,13 showing a high agreement with polysomnography-based sleep. Specifically, the Actiwatch device consisted of an omni-directional sensor sensitive to motion (recorded as activity counts). The monitors were programmed to record the total piezo-electric voltage generated over the preceding 60 s (that is, epoch length = 60 s).
The devices recorded the intensity, amount, and duration of movement in all 3 planes. Subjects were adapted to wearing the activity monitors attached to their collars for at least 1 wk prior to the experiments, and adaptation was determined to occur if subjects no longer manipulated the collars and/or actigraphy cases. Nighttime activity data were generated in terms of the following behavioral sleep indices: sleep efficiency (that is, the percentage of the dark phase during which the animal was inactive), and sleep latency (that is, the time between “lights off” time and the first bout of inactivity). All parameters were calculated using the Actiware Sleep 3.4 software program (Mini-Mitter, Bend, OR).
Experimental design.
Before the separation phase, Actiwatches were attached to both monkeys’ collars and baseline sleep behavior was measured for 1 wk while the subjects were pair-housed. Activity recording continued for the duration of the experiments. During the separation phase, monkeys were separated overnight from 1600h until 800h by placement of a socialization panel that physically separated the animals but allowed visual and tactile contact through a wire mesh (UMMC) or through large holes (Yerkes). The pairs were separated nightly for 7 d, and their sleep measures were evaluated during the separation nights.
Data analysis.
Sleep data from monkeys while pair-housed and separated were averaged across days of pair-housing and separation (Figure 1) and were analyzed using 2-way repeated measures (RM) ANOVA followed by Bonferroni’s t test with time (days 1 through 7) and housing (paired compared with separated) as factors. To evaluate the influence of one monkey’s sleep on her cage-mate’s sleep, we conducted a series of correlations between facilities and across paired compared with separated conditions, as well as collapsed across both facilities. Correlational analyses were conducted using Pearson’s Correlation. All graphical data presentations were created, and all statistical tests were performed, using Prism 8 (GraphPad Software). Significance was accepted at an α of P ≤ 0.05. Descriptive data are expressed as mean with SEM.
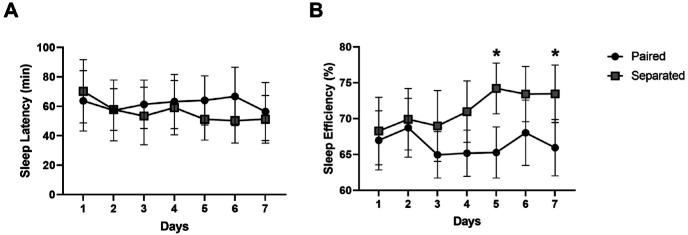
Figure 1.
Actigraphy-based (A) sleep latency and (B) sleep efficiency of 10 naïve adult female rhesus monkeys (5 pairs of monkeys) during nights when subjects remained paired and during nights when pairs were separated using socialization panels. Data are expressed as mean± SEM
*P < 0.05 compared with Paired, paired Student t test.
Results
Sleep parameters under paired and separated nighttime housing conditions.
Analysis of the average sleep data across the 7 days under paired and separated housing showed that nighttime separation with socialization panels significantly improved sleep measures. Separated monkeys showed a significantly lower sleep latency (sleep latency paired: 62 ± 17 min; sleep latency separated: 34 ± 9 min; paired t-test, [t(9) = 2.31, p < 0.05]) and significantly higher sleep efficiency (sleep efficiency paired: 66 ± 4%; sleep efficiency separated: 76 ± 4%) compared with the same monkeys when pair-housed paired t-test, [t(9) = 2.6, p < 0.05]. Sleep data also were analyzed across days under the 2 nighttime housing conditions using 2-way RM ANOVA (Figure 1). No significant differences were found for sleep latency across the 7 d of paired and separated housing (Interaction: [F(6,54) = 0.4016, P = 0.87], Figure 1A). Sleep efficiency showed a significant interaction between time and housing [F(6,54) = 2.814, P < 0.05], with a significantly higher sleep efficiency on Days 5 and 7 when subjects were separated as compared with when they were paired (Figure 1B).
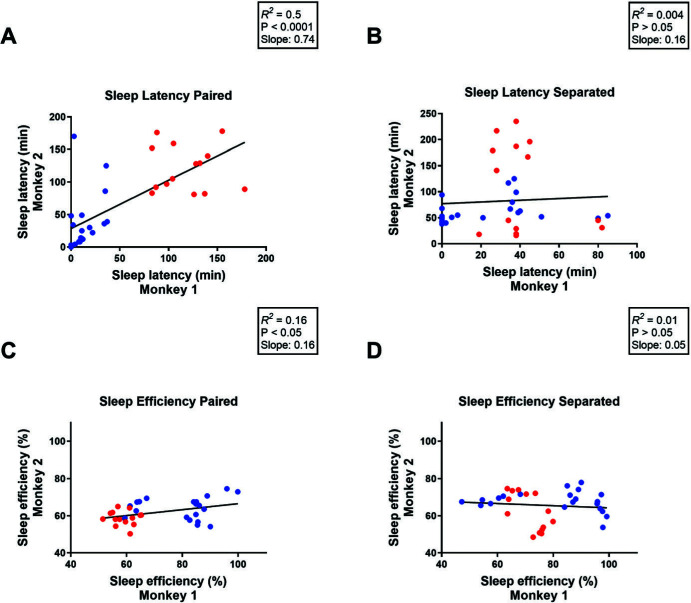
These studies were conducted in two different research centers (University of Mississippi Medical Center and Yerkes National Primate Research Center). Correlational analysis of the combined data showed a positive correlation between sleep measures of a monkey and its home-cage partner on the nights when subjects were pair-housed (sleep latency: [R2 = 0.5, P < 0.0001], slope = 0.74, Figure 2A; sleep efficiency: [R2 = 0.16, P < 0.05], slope = 0.16, Figure 2C and Table 2). The correlation for sleep efficiency was much weaker than that for sleep latency, although both were statistically significant. However, a significant correlation was not detected during separation nights for either sleep latency (R2 = 0.004, P > 0.05; slope = 0.16; Figure 2B) or sleep efficiency (R2 = 0.01, P > 0.05; slope = 0.05; Figure 2D).
Figure 2.
Correlational analysis for (A, B) sleep latency or (C, D) sleep efficiency of one monkey and its home-cage partner during nights when subjects remained (A, C) paired or during nights when subjects were (B, D) separated using socialization panels. Pearson Correlation. Blue dots represent monkeys housed at the University of Mississippi Medical Center; red dots represent monkeys housed at the Yerkes National Primate Research Center.
Table 2.
Correlations between actigraphy-based sleep measures for a monkey and cage mate while paired and following nighttime separation per facility
| Facility | Sleep Measure | Housing Condition | R2 | Slope | P value |
|---|---|---|---|---|---|
| UMMC | Sleep Latency | Paired | 0.15 | 1.02 | 0.07 |
| Separated | 0.03 | 0.18 | 0.40 | ||
| Sleep Efficiency | Paired | 0.32 | 0.26 | <0.01* | |
| Separated | 0.03 | -0.06 | 0.40 | ||
| Yerkes | Sleep Latency | Paired | 0.65 | 0.92 | <0.001* |
| Separated | 0.06 | -1.24 | 0.36 | ||
| Sleep Efficiency | Paired | 0.30 | 0.50 | <0.05* | |
| Separated | 0.42 | -1.11 | <0.05* |
Significant correlation (Pearson’s Correlation).
We also analyzed data separately for each facility. For monkeys from Yerkes, analysis of the averaged sleep data across the 7 days under paired and individual housing conditions showed that nighttime separation with socialization panels significantly improved sleep measures. At Yerkes, separated monkeys, as compared with pair-housing of the same monkeys, showed a significantly lower sleep latency (paired: 119 ± 3 min; separated: 51 ± 14 min; paired t-test, [t(3) = 5.58, P < 0.05]) and significantly higher sleep efficiency (paired: 59 ± 1%; separated: 68 ± 4%; paired t-test, [t(3) = 3.41, P < 0.05]). For monkeys from UMMC sample, nighttime separation improved sleep efficiency (paired: 71 ± 5%; separated: 80 ± 5%; paired t-test, [t(5) = 2.24, P < 0.05]) but not sleep latency (paired: 24 ± 11 min; separated: 23 ± 11 min; paired t-test, [t(5) = 0.25, P > 0.05]). Correlational analysis conducted to compare the two facilities found that even with the low sample sizes, relatively high correlations that were statistically significant or close to significance were detected when animals were paired, but not when they were separated (except for one significant negative correlation) (see Table 2 for descriptive data).
Individual subject data.
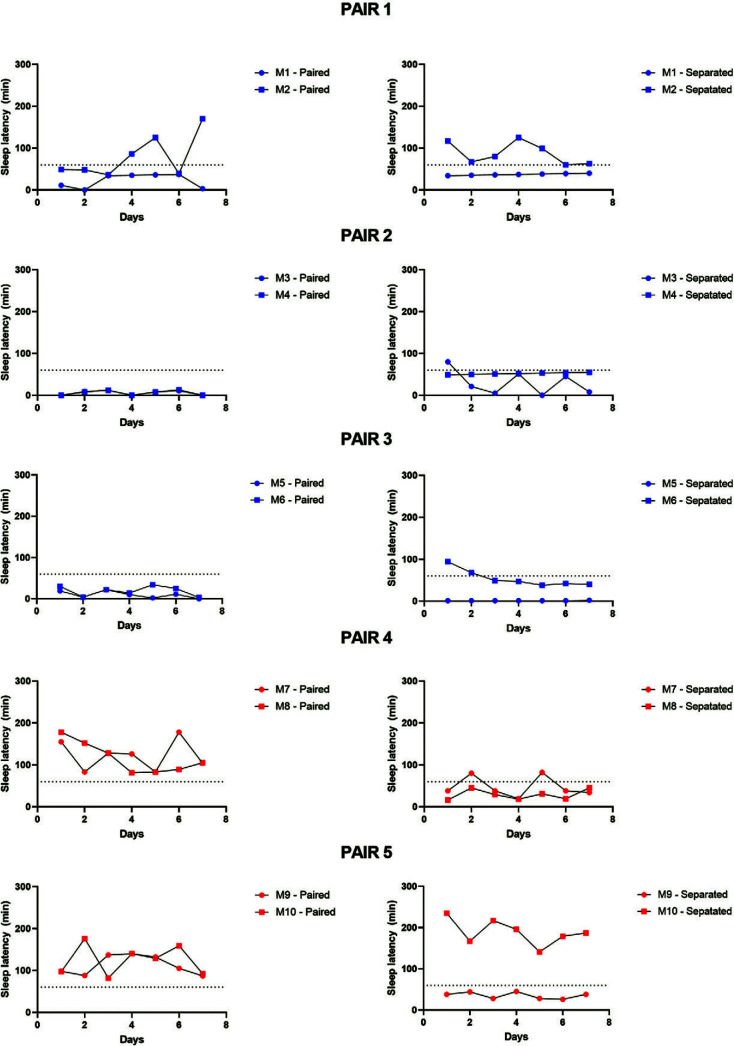
Individual subject data across experimental days are shown in Figure 3 (sleep latency) and Figure 4 (sleep efficiency). For most pairs, an obvious difference in their sleep parameters is seen after subjects are separated during the nighttime. We have previously grouped monkeys at our UMMC colony as “normal sleepers” or “short sleepers” based on individual monkeys’ sleep phenotypes.7 Based on this classification, “short sleepers” are monkeys in which sleep latency is longer and sleep efficiency values are less than typical values compared with the larger pool of available monkeys in our colony.7 Using this approach, our “short-duration sleep” cohort differs significantly in their sleep parameters compared with our larger colony. Adult female monkeys presenting the short sleep phenotype consistently show shorter sleep duration (sleep efficiency ≤ 70%) and longer time to fall asleep (sleep latency ≥ 60 min) compared with adult female normal sleepers. Therefore, in the present study, we also compared individual normal and short sleepers based on their sleep phenotypes when monkeys were separated.
Figure 3.
Individual subject sleep latency data for the 5 pairs of female rhesus monkeys across the 7 d of sleep recording under paired (left panels) and nighttime separation (right panels) conditions. Dotted lines (60min) represent the sleep latency cut off for short compared with normal sleep (≥60min = short sleeper). Blue dots represent monkeys housed at the University of Mississippi Medical Center; red dots represent monkeys housed at the Yerkes National Primate Research Center.
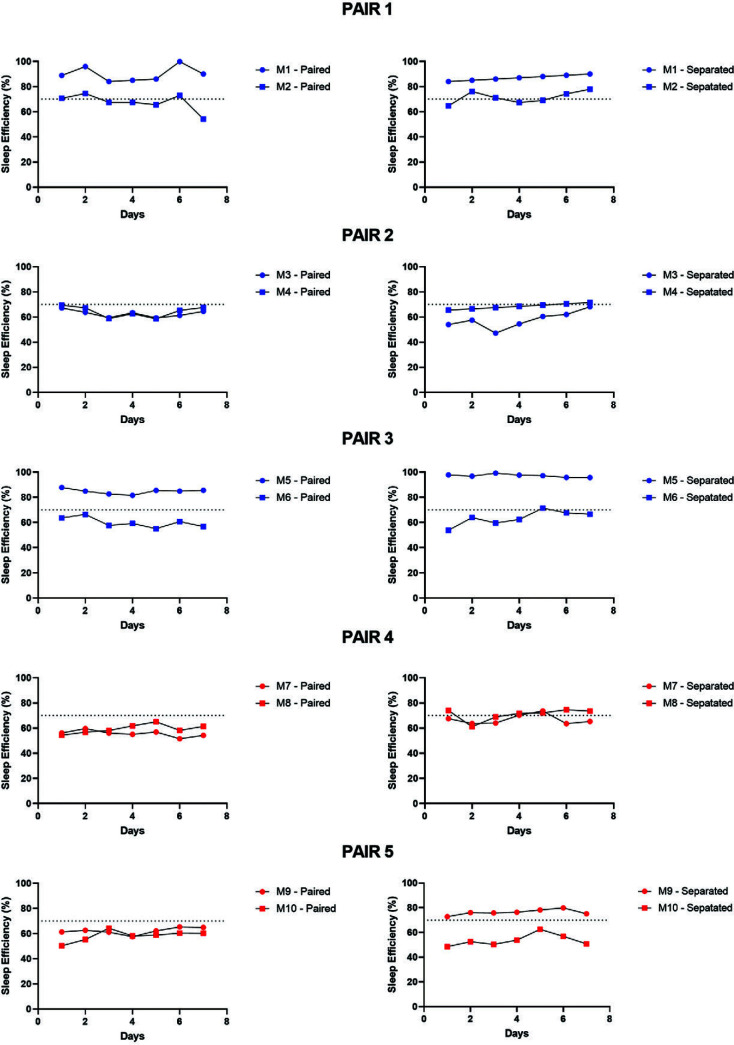
Figure 4.
Individual subject sleep efficiency data for the 5 pairs of female rhesus monkeys across the 7 d of sleep recording under paired (left panels) and nighttime separation (right panels) conditions. Dotted lines (70%) represent the sleep efficiency cut off for short compared with normal sleepers (≤70% = short sleeper). Blue dots represent monkeys housed at the University of Mississippi Medical Center; red dots represent monkeys housed at the Yerkes National Primate Research Center.
In a sample of 10 female monkeys, we classified 6 as normal sleepers (M1, M4, M5, M7, M8, M9) and 4 as short sleepers (M2, M3, M6, M10) based on the average 7-d sleep data under separated conditions. This distribution is consistent with that of sleep phenotypes across our colony, with approximately 40% of females showing a short sleep phenotype. Therefore, most of our pairs (M1-M2, M3-M4, M5-M6, M9-M10) include both normal and short sleepers, with only one pair (M7-M8) being comprised of 2 normal sleepers. Based on these classifications, we also can determine whether the subjects’ sleep parameters improved (decreased sleep latency, increased sleep efficiency), worsened (increased sleep latency, decreased sleep efficiency) or unchanged with nighttime separation. Table 1 shows the summary of the individual subject sleep phenotype and sleep changes after separation. In general, normal sleepers showed either no change or an improvement in at least one sleep parameter after separation. Only one subject, a short sleeper, showed longer sleep latency and lower sleep efficiency after separation. Two of the 4 short sleepers showed no change after separation. The 4th short sleeper showed with worsened (longer) sleep latency and improved (longer) sleep efficiency after separation.
Table 1.
Individual subject sleep phenotype and sleep change observed after separation.
| Subject | Sleep Phenotype | Sleep change after separation |
|---|---|---|
| M1 | Normal | No change |
| M2 | Short | No change |
| M3 | Short | Worsened |
| M4 | Normal | Latency: worsened; Efficiency: improved |
| M5 | Normal | Improved |
| M6 | Short | Latency: worsened; Efficiency: improved |
| M7 | Normal | Improved |
| M8 | Normal | Improved |
| M9 | Normal | Improved |
| M10 | Short | No change |
Normal: normal sleep phenotype; short: short sleep phenotype (sleep efficiency ≤ 70%; sleep latency ≥ 60min). Improved: decreased sleep latency, increased sleep efficiency. Worsened: increased sleep latency, decreased sleep efficiency.
Discussion
The promotion of social housing in laboratory animals has been proposed to increase the wellbeing of laboratory NHP and, consequently, the external validity of primate biomedical research. However, many experimental variables can be influenced by changes in the social environment. The frequency of changes in social housing that laboratory rhesus monkeys experience is an established risk factor for developing abnormal behaviors.17 Moreover, changes in the social environment can alter hormonal and immune responses.19 Therefore, scientists, veterinarians, and colony managers should understand the full impact of social housing and housing changes on research outcomes and animal wellbeing. Sleep is a biologic phenomenon and an experimental outcome that affects physical and behavioral health and can itself be affected by the social environment. Therefore, sleep changes during pair-housing may affect a range of research outcomes.
Because sleep induces decreased awareness and vigilance,12 it can be viewed as an attachment behavior that is optimized under conditions of physical and emotional safety and security.32 Therefore, one might expect that pair-housing would improve sleep in NHP. However, the present study shows that sleep efficiency was significantly worse when monkeys were pair-housed as compared with when subjects were separated at night with socialization panels. Our findings show that sleep can be negatively influenced by co-sleeping, either as a primary effect across several days or, in the case of sleep efficiency, as a more gradual change. The effects observed in the present study may be related to the indoor living situation with artificial light cycles. Further studies of sleep in group- or pair-housed NHP living in outdoor colonies are warranted to understand the effects of the social context of sleep under conditions that more closely mimic what NHP experience in naturalistic environments.
The present study showed that on the nights when female rhesus monkeys were pair-housed, a positive correlation was observed between sleep measures of a monkey relative to its home-cage partner, further suggesting that pair-housing influences sleep quality. These data are in agreement with human studies showing that co-sleeping can have a major impact on bed partner’s sleep.32 For instance, obstructive sleep apnea has been referred to as a “disease of listeners”2 because, in addition to affecting the patient’s sleep quality, the associated snoring and increased arousals often adversely affect the bed partner’s sleep.10,24,34 Although this has been known for decades, only recently has sleep research shifted toward studying the social context of sleep, as opposed to focusing on sleep primarily as an individual behavior. By showing for the first time that co-sleeping also has a clear impact on the sleep of pair-housed laboratory female rhesus monkeys, our study adds to this literature by demonstrating the effect in a NHP species and emphasizes the importance of considering sleep as a biologic and experimental variable that may be influenced by social-housing.
Our findings have important implications for strategies to increase wellbeing and design research studies using pair-housed rhesus monkeys. Sleep is a frequently neglected contributor to physical health and wellbeing25,28,30 and, in humans, co-sleeping can negatively affect bed partner’s health and wellbeing.15,18,33 Rodent studies also have shown that housing conditions can influence sleep parameters in mice,17 which prompted researchers to discuss best animal care practices related to experiments on sleep-wake cycles in rodents.9 However, the literature on the influence of NHP housing on sleep is scarce. Therefore, the fact that pair-housing can negatively impact sleep in female rhesus monkeys indicates that this is an aspect of pair-housing that may negatively impact wellbeing and physical health. Given the evidence indicating that social housing promotes wellbeing in laboratory NHP,19 the benefits of pair-housing on wellbeing may surpass the negative effects on sleep, although little evidence in the literature addresses this fundamental aspect of NHP husbandry. In fact, many of the physiologic changes reported after changes in social and housing arrangements in laboratory NHP18 may be at least partially associated with changes in sleep patterns (for example, hypothalamic-pituitary-adrenal (HPA) axis-related changes).26
Our findings also have important implications for research that uses macaques. The most relevant situation is research studying sleep as a primary outcome measure, particularly if the research is conducted in pair-housed animals in which co-sleeping will most certainly affect sleep outcomes. Sleep quality can also affect many other physiologic processes in NHP, ranging from hormonal responses16,22,29 to cognition.14 Although no studies are available specifically in NHP, rodent and human studies show that sleep impairment can negatively influence inflammatory21 and immunologic processes.8 Given the extensive use of rhesus monkeys in immunologic research such as vaccine development, more information on how sleep affects immune function in this species is warranted.
On the nights during which pairs were separated, not only did subjects have improved sleep efficiency and latency, but these sleep measures were not correlated between the pairs. These data suggest that when separated, the home-cage partner’s sleep no longer influenced the other monkeys’ sleep, even though the pair could still have visual and tactile contact through the socialization panel. Intermittent pair-housing, including daily or weekly separations of pair-housed monkeys for several hours,3,9 has been proposed as an alternative to continuous pair-housing to comply with research and management needs.20 Here we show that nighttime intermittent pair-housing can disrupt the pattern of sleep interdependency observed in pair-housed female rhesus monkeys and may be a better alternative to continuous pair-housing for promoting healthy sleep patterns.
Although grouped data show improved sleep efficiency after separation, not all subjects showed this effect after separation as compared with when they were paired (see Table 1). While normal sleepers tend to show improvement in sleep quality upon separation, the only subject (M3) that showed overall worsened sleep quality after separation was classified as a short sleeper. In addition, the only pair in which both subjects showed improvements for both sleep measures after separation was also the only pair in which both subjects were normal sleepers. These data suggest that changes in sleep quality due to pair-housing may depend on the subject’s baseline sleep quality and the sleep quality of its cage mate. This observation emphasizes the importance of future studies investigating how sleep phenotypes influence pair-housing-induced changes in sleep quality. Finally, our data also indicate that sleep phenotypes should be considered as potentially important factors when selecting subjects for pair-housing.
In summary, our results show that pair-housing has a significant impact on the home-cage partner’s sleep in female rhesus monkeys, and that this pattern can be disrupted by nighttime separation with socialization panels. Altered sleep due to pair-housing may affect a range of research outcomes, but particularly studies in which sleep is the primary measure of interest. The finding that pair-housing can negatively impact sleep indicates that this frequently neglected aspect of pair-housing may also negatively impact wellbeing and physical health. Our studies included subjects from 2 different institutions with similar housing conditions (including identical light/dark cycles and no access to windows in the colony room). This indicates that the findings may generalize across institutions and suggests that the housing arrangement may influence the results. However, the present study had a small sample size, and we were unable to control for facility in our statistical analysis. Finally, an important limitation is the use of female rhesus monkeys; results may differ in male monkeys. Future multicenter studies with larger sample sizes are needed to explore sex differences in the influence of pair-housing on sleep, environmental influences on those effects, and how physiologic responses induced by changes in social environment affect sleep-wake patterns.
Acknowledgments
We thank the Veterinary and Behavioral Management staff from the UMMC Center for Comparative Research and the Yerkes National Primate Research Center for their constant care for our animals. This work was supported by the National Institutes of Health [DA010344, DA031246, ODP51OD11132, AA029023, DA011792, DA043204, DA046778 and DA049886] and Associação Fundo de Incentivo à Pesquisa.
References
- 1.Animal Welfare Act as Amended. 2008. 7 USC §2131–2156.
- 2.Ashtyani H, Hutter DA. 2003. Collateral damage: the effects of obstructive sleep apnea on bed partners. Chest 124:780–781. 10.1378/chest.124.3.780. [DOI] [PubMed] [Google Scholar]
- 3.Baker KC, Bloomsmith MA, Oettinger B, Neu K, Griffis C, Schoof V, Maloney M. 2012. Benefits of pair housing are consistent across a diverse population of rhesus macaques. Appl Anim Behav Sci 137:148–156. 10.1016/j.applanim.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bennett BT. 2016. Association of Primate Veterinarians 2014 nonhuman primate housing survey. J Am Assoc Lab Anim Sci 55:172–174. [PMC free article] [PubMed] [Google Scholar]
- 5.Berro LF, Andersen ML, Howell LL. 2017. Assessment of tolerance to the effects of methamphetamine on daytime and nighttime activity evaluated with actigraphy in rhesus monkeys. Psychopharmacology (Berl) 234:2277–2287. 10.1007/s00213-017-4654-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berro LF, Shields H, Odabas-Geldiay M, Rothbaum BO, Andersen ML, Howell LL. 2018. Acute effects of 3,4-methylenedioxymethamphetamine (MDMA) and R(-) MDMA on actigraphy-based daytime activity and sleep parameters in rhesus monkeys. Exp Clin Psychopharmacol 26:410–420. 10.1037/pha0000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berro LF, Moreira-Junior E, Rowlett JK. 2021. The dual orexin receptor antagonist almorexant blocks the sleep-disrupting and daytime stimulant effects of methamphetamine in rhesus monkeys. Drug Alcohol Depend 227:108930. 10.1016/j.drugalcdep.2021.108930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Besedovsky L, Lange T, Haack M. 2019. The sleep-immune crosstalk in health and disease. Physiol Rev 99:1325–1380. 10.1152/physrev.00010.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bittman EL, Kilduff TS, Kriegsfeld LJ, Szymusiak R, Toth LA, Turek FW. 2013. Animal care practices in experiments on biological rhythms and sleep: report of the Joint Task Force of the Society for Research on Biological Rhythms and the Sleep Research Society. J Am Assoc Lab Anim Sci. 52(4):437-443. [PMC free article] [PubMed] [Google Scholar]
- 10.Capitanio JP, Blozis SA, Snarr J, Steward A, McCowan BJ. 2015. Do “birds of a feather flock together” or do “opposites attract”? Behavioral responses and temperament predict success in pairings of rhesus monkeys in a laboratory setting. Am J Primatol 79:e22464. 10.1002/ajp.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cartwright RD, Knight S. 1987. Silent partners: the wives of sleep apneic patients. Sleep 10:244–248. 10.1093/sleep/10.3.244. [DOI] [PubMed] [Google Scholar]
- 12.Coelho AM, Jr, Carey KD, Shade RE. 1991. Assessing the effects of social environment on blood pressure and heart rates of baboons. Am J Primatol 23:257–267. 10.1002/ajp.1350230406. [DOI] [PubMed] [Google Scholar]
- 13.Dahl RE. 1996. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol 8:3–27. 10.1017/S0954579400006945. [DOI] [Google Scholar]
- 14.de Souza L, Benedito-Silva AA, Pires ML, Poyares D, Tufik S, Calil HM. 2003. Further validation of actigraphy for sleep studies. Sleep 26:81–85. 10.1093/sleep/26.1.81. [DOI] [PubMed] [Google Scholar]
- 15.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. 2007. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci 27:14239–14247. 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elsey T, Keller PS, El-Sheikh M. 2019. The role of couple sleep concordance in sleep quality: attachment as a moderator of associations. J Sleep Res 28:e12825. 10.1111/jsr.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Febinger HY, George A, Priestley J, Toth LA, Opp MR. 2014. Effects of housing condition and cage change on characteristics of sleep in mice. J Am Assoc Lab Anim Sci. 53(1):29-37. [PMC free article] [PubMed] [Google Scholar]
- 18.Giannella-Neto D, Quabbe HJ, Witt I. 1981. Pattern of thyrotropin and thyroxine plasma concentrations during the 24-hour sleep-wake cycle in the male rhesus monkey. Endocrinology 109:2144–2151. 10.1210/endo-109-6-2144. [DOI] [PubMed] [Google Scholar]
- 19.Gómez-González B, Domínguez-Salazar E, Hurtado-Alvarado G, Esqueda-Leon E, Santana-Miranda R, Rojas-Zamorano JA, Velázquez-Moctezuma J. 2021. Role of sleep in the regulation of the immune system and the pituitary hormones. Ann N Y Acad Sci. 1261:97-106. [DOI] [PubMed] [Google Scholar]
- 20.Gottlieb DH, Capitanio JP, McCowan B. 2013. Risk factors for stereotypic behavior and self-biting in rhesus macaques (Macaca mulatta): animal’s history, current environment, and personality. Am J Primatol 75:995–1008. 10.1002/ajp.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gunn HE, Buysse DJ, Hasler BP, Begley A, Troxel WM. 2015. Sleep concordance in couples is associated with relationship characteristics. Sleep 38:933–939. 10.5665/sleep.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hannibal DL, Bliss-Moreau E, Vandeleest J, McCowan B, Capitanio J. 2016. Laboratory rhesus macaque social housing and social changes: Implications for research. Am J Primatol 79:e22528. 10.1002/ajp.22528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hannibal DL, Cassidy LC, Vandeleest J, Semple S, Barnard A, Chun K, Winkler S, McCowan B. 2018. Intermittent pair-housing, pair relationship qualities, and HPA activity in adult female rhesus macaques. Am J Primatol 80:e22762. 10.1002/ajp.22762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irwin MR, Olmstead R, Carroll JE. 2016. Sleep disturbance, sleep duration, and inflammation: a systematic review and meta-analysis of cohort studies and experimental sleep deprivation. Biol Psychiatry 80:40–52. 10.1016/j.biopsych.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacoby JH, Smith E, Sassin JF, Greenstein M, Weitzman ED. 1975. Altered growth of hormone secretory pattern following prolonged sleep deprivation in the rhesus monkey. Neuroendocrinology 18:9–15. 10.1159/000122378. [DOI] [PubMed] [Google Scholar]
- 26.Lilly AA, Mehlman PT, Higley JD. 1999. Trait-like immunological and hematological measures in female rhesus across varied environmental conditions. Am J Primatol 48:197–223. . [DOI] [PubMed] [Google Scholar]
- 27.Mann TM, Williams KE, Pearce PC, Scott EA. 2005. A novel method for activity monitoring in small non-human primates. Lab Anim 39(2):169-177. [DOI] [PubMed] [Google Scholar]
- 28.McArdle N, Kingshott R, Engleman HM, Mackay TW, Douglas NJ. 2001. Partners of patients with sleep apnoea/hypopnoea syndrome: effect of CPAP treatment on sleep quality and quality of life. Thorax 56:513–518. 10.1136/thx.56.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moore PJ, Adler NE, Williams DR, Jackson JS. 2002. Socioeconomic status and health: the role of sleep. Psychosom Med 64:337–344. 10.1097/00006842-200203000-00018. [DOI] [PubMed] [Google Scholar]
- 30.Nicolaides NC, Vgontzas AN, Kritikou I, Chrousos G. 2000. HPA axis and sleep. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dungan K, Grossman A, Hershman JM, Hofland J, Kaltsas G, Koch C, Kopp P, Korbonits M, McLachlan R, Morley JE, New M, Purnell J, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. South Dartmouth (MA): MDText.com, Inc. [Google Scholar]
- 31.Olsson IAS, Westlund K. 2007. More than numbers matter: the effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Anim Behav Sci 103:229–254. 10.1016/j.applanim.2006.05.022. [DOI] [Google Scholar]
- 32.Pilcher JJ, Ott ES. 1998. The relationships between sleep and measures of health and well-being in college students: a repeated measures approach. Behav Med 23:170–178. 10.1080/08964289809596373. [DOI] [PubMed] [Google Scholar]
- 33.Quabbe HJ, Gregor M, Bumke-Vogt C, Eckhof A, Witt I. 1981. Twenty-four-hour pattern of growth hormone secretion in the rhesus monkey: studies including alterations of the sleep/wake and sleep stage cycles. Endocrinology 109:513–522. 10.1210/endo-109-2-513. [DOI] [PubMed] [Google Scholar]
- 34.Tavernier R, Willoughby T. 2014. A longitudinal examination of the bidirectional association between sleep problems and social ties at university: the mediating role of emotion regulation. J Youth Adolesc 44:317–330. 10.1007/s10964-014-0107-x. [DOI] [PubMed] [Google Scholar]
- 35.Terán-Pérez G, Arana-Lechuga Y, Esqueda-León E, Santana-Miranda R, Rojas-Zamorano J, Velázquez Moctezuma J. 2012. Steroid hormones and sleep regulation. Mini Rev Med Chem 12:1040–1048. 10.2174/138955712802762167. [DOI] [PubMed] [Google Scholar]
- 36.Troxel WM. 2010. It’s more than sex: exploring the dyadic nature of sleep and implications for health. Psychosom Med 72:578–586. 10.1097/PSY.0b013e3181de7ff8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Troxel WM, Braithwaite SR, Sandberg JG, Holt-Lunstad J. 2016. Does improving marital quality improve sleep? results from a marital therapy trial. Behav Sleep Med 15:330–343. 10.1080/15402002.2015.1133420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulfberg J, Carter N, Talbäck M, Edling C. 2000. Adverse health effects among women living with heavy snorers. Health Care Women Int 21:81–90. 10.1080/073993300245311. [DOI] [PubMed] [Google Scholar]