Abstract
Metabolic caging is an important tool for quantitative urine and feces collection in rodents, although significant limitations and problems accompany its use. Despite strong opinions among investigators regarding the effects of metabolic caging on energy and fluid homeostasis, careful quantitative analysis of the impact of this caging type—particularly when used for mice—is lacking. The current study assessed the effects of metabolic caging, with or without modifications such as plastic platform inserts, on ingestive behaviors, energy expenditure, accuracy of urine and fecal collection, and ambulatory activities in male C57BL/6J mice. Housing mice in metabolic cages, regardless of platform inclusion, increased energy expenditure without modifying food intake, presumably due to the inability of mice to perform normal thermoregulatory behaviors (burrowing and huddling). Surprisingly, mice in metabolic cages actively avoided platforms, and the inclusion of platforms modified the behavior of the mice and had position-dependent effects that reduced the accuracy of urine collection. Moving mice from cohousing to individual housing in home cages also increased ingestive behaviors and energy expenditure. We conclude that single housing of male C57BL/6J mice increases energy expenditure, that this increase is potentiated in metabolic caging conditions, and that platforms in metabolic cages alter mouse behavior and urine collection. Additional future work is needed to determine the potential benefits of using higher ambient temperature for studies of mice in metabolic caging and whether the above effects occur in females and other strains of mice and other rodent species.
Abbreviations: HDPE, high-density polyethylene
Introduction
Metabolic caging allows researchers to quantitate and collect urine and feces and is widely used across disciplines. Traditional metabolic cages house subjects on a metal grid raised above a funnel apparatus that allows separate collection of urine and feces. The use of this type of caging is generally considered to have some qualitative effects on cardiometabolic endpoints. For example, larger rodents (that is large rats, guinea pigs) housed long-term on wire flooring are at risk of developing ulcerative pododermatitis.2,11,13,15 The risk or actual development of lesions inherently limits the amount of time such animals can spend in metabolic cages, such that use of metabolic cages may be restricted to short-term studies. In addition, the quantitative effects of metabolic caging on fluid and energy homeostasis remain largely undefined, especially in mice.
Previous studies have supported the idea of promoting animal welfare through the addition of solid flooring, or “platforms,” to wire-bottom caging systems. For example, some data suggest that rats prefer solid flooring, especially when resting.9 Another study found that lesions, pain, or distress can result from prolonged housing of rats on wire-bottom cages such as used in metabolic caging.15 However, the quantitative impacts of inserting a solid platform inside a metabolic cage on energy and fluid balance physiology and other related behaviors are not known. The relative increase in the use of mice for metabolic and cardiovascular research over the previous several decades, together with the observation that mice do not appear to adapt to novel housing for 3 to 4 d after placement,18 support the need to quantitatively assess the effects of housing in metabolic cages on fluid and energy balance in mice.
We hypothesized that cage type, number of mice present, and the presence and position of a solid platform within metabolic cages would impact energy and fluid balance physiology and related behaviors in these mice. Our data support the general concept that metabolic caging creates a thermal challenge to mice, and thereby increases energy expenditure. The data also show unexpected modulatory effects of platform position on various endpoints, as the mice exhibited an apparent aversion to resting on the platforms. Furthermore, moving mice from cohousing to individual housing in home cages also affected fluid and energy balance in ways that were not paralleled by mice housed in metabolic cages. Collectively, these studies quantify the effects of metabolic caging on cardiometabolic physiology in male C57BL/6J mice and illustrate unintended negative consequences of an otherwise logical intervention to improve this caging approach.
Materials and Methods
Animals.
All studies used male C57BL/6J mice (n = 51) purchased from the Jackson Laboratories at 5 to 6 wk of age (Stock Number 000664) and male mice harboring an intact but conditional allele for the endogenous melanocortin type 4 receptor (n = 20) (that is Mc4rFlox); these mice, originally obtained from Jackson Laboratories Mc4rtm2.2Lowl/J, Stock Number 023720, were obtained from an in-house breeding colony and maintained on the C57BL/6J background. No phenotypic differences were identified between C57BL/6J and Mc4rFlox and numbers of each strain were balanced across groups. The mouse disease surveillance program included a triannual PCR exhaust air dust panel (for cages maintained on ventilated racks) or serological testing (of soiled bedding sentinels from the colony cages on their respective racks) for detecting excluded pathogens and parasites. Excluded murine agents include Clostridium piliforme, Corynebacterium bovis, cilia-associated respiratory bacillus, ectromelia virus, Encephalitozoon caniculi, lymphocytic choriomeningitis virus, minute virus of mice, mouse adenovirus, mouse cytomegalovirus, mouse hepatitis virus, mouse parvovirus, mouse rotavirus, mouse thymic virus, Mycoplasma pulmonis, pneumonia virus of mice, polyoma virus, reovirus, Sendai virus, Theiler murine encephalomyelitis virus, and pinworms and fur mites. Murine norovirus and Helicobacter spp. are not specifically excluded from the area in which the subject mice were housed. All activities in these studies were approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee. The animal care and use program at the Medical College of Wisconsin is accredited by AAALAC International and conforms to the National Research Council’s Guide for the Care and Use of Laboratory Animals, 8th Edition.14
Before the study, mice were housed in ventilated microisolation caging on commercial racks (model no. MS75JU70MVPSHR-R, Allentown, Allentown, NJ) with between 2 to 5 mice per cage. Mice received ad libitum access to the natural ingredient diet Teklad 2920× – Irradiated soy protein-free extruded diet (Envigo, Indianapolis, IN) (3.13 kcal/g, purchased in both pelleted and powdered forms directly from the manufacturer to ensure consistent formulation) and reverse osmosis-filtered, chlorinated water via an automatic water system (Avidity Science, Waterford, WI). Animal rooms were maintained at 22 to 23°C (72 to 73°F) and on a 14:10h light:dark cycle. Studies to assess the effects of 5 different experimental housing conditions were carried out at 13.3 ± 0.2 wk of age (range 10.7 to 19.9 wk), with no age differences among the 5 treatment groups (one-way ANOVA, P = 0.7696).
Experimental housing conditions.
Mice were randomly assigned to 1 experimental housing condition for 4 consecutive overnight periods, as follows. Two groups were housed in freshly cleaned microisolation (“home”) cages (Figure 1A-C), either in pairs (that is, 2 mice per cage, with pairs having previously been cohoused in groups of 2 to 5 per cage) or singly (that is, 1 mouse per cage). Home cages (model JAG75, Allentown Caging, Allentown, NJ) contained hardwood chips (SaniChip, PJ Murphy Forest Products, Montville, NJ) and shredded paper as enrichment (Enviro-Dri, Shepard Specialty Papers, Watertown, TN), and were placed on Metro racks (InterMetro Industries Corporation, Wilkes-Barre, PA) with a water bottle to enable measurement of fluid intake. Mice remained in the same cage without bedding/cage change for 4 consecutive overnight periods to minimize cage-switch stresses. Daily cage servicing included weighing the food hopper and water bottle to determine intakes (and replenishing if needed), and sifting through bedding material to find any cached food pellets; food intake was determined daily based on the mass of food remaining in the food hopper and the bedding material. Food intake of pair-housed mice was determined by calculating total food consumed by the pair each day and dividing by 2; as a result, each pair of pair-housed mice only contributed n = 1 replicate to statistical comparisons.
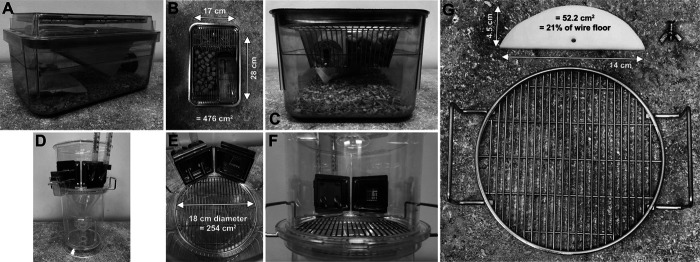
Figure 1.
Schematic of caging conditions. (A) Complete home cage. (B) Home cage viewed from above, illustrating total floor space of ≈476 cm2. (C) Water bottle and food presentation, from the perspective of the mouse. (D) Complete metabolic cage, custom-modified to allow instrumentation with 2 burettes. (E) Metabolic cage viewed from above, illustrating total floor space of ≈254 cm2. (F) Drink burettes and food hopper presentation, from the perspective of the mouse. (G) HDPE platform of 0.6 cm thickness, shaped as a circular segment to match the radius of the metabolic cage interior. As the platform covers ≈52.2 cm2, this represents ≈21% of the total floor space within the metabolic cage.
Three groups of mice were housed singly in metabolic cages (Nalgene type; Tecniplast, West Chester, PA, model number 3600M021; wire floor diameter 18 cm, yielding 254 cm2 total floor space). The mice had ad libitum access to a standard powdered-food hopper, and the cages had been custom-modified to provide two distinct drink choices (Figure 1D-F). One group was housed in metabolic cages fitted with a high-density polyethylene (HDPE) platform (52.2 cm2, equating to 21% of the wire floor area) covering the circular segment closest to the food hopper and water burettes (Figure 1G). The second group was housed in metabolic cages fitted with the HDPE platform covering the circular segment on the opposite side of the cage (away from the hopper and burettes). The third group was housed in metabolic cages with no platform installed. All mice were housed in the same room in order to avoid the presence of extraneous room-related stimuli other than the experimental cage type. Mice remained in the same metabolic cage for the 4 consecutive overnight periods under study. Daily cage servicing included weighing and replenishment of the powdered food hopper to determine intakes, replenishment of drinking water burettes, and cleaning of urine and feces collection tubes.
Mouse assessments.
Body composition (that is, fat compared with fat-free mass) was assessed on day 0 and on day 4 of the experiment using time-domain nuclear magnetic resonance (NMR; model LF110, Bruker, Billerica, MA), as previously described.5 Briefly, unanesthetized mice were placed in a restraint tube that was inserted into the analyzer for approximately 2 min. The mouse was then returned to its home cage.
The osmolality of urine collected in the metabolic cages was measured using freezing-point depression osmometry (OsmoPro; Advanced Instruments, Norwood, MA) after 1:10 dilution of urine with deionized (18.2 million ohm-cm) water.
To determine potential effects of platform positioning in metabolic cages on gross locomotor activity of mice, video recordings were made of the mice during the final 4 h of the light phase (that is, 3 to 7 PM) on day 3, with no personnel present in the room. A GoPro HERO7 Black camera (San Mateo, CA) was suspended above the cages. Recordings were subsequently analyzed using AnyMaze software (Stoelting, Wood Dale, IL).
Statistical analyses.
Throughout, independent t tests, 2-way repeated measures ANOVA, or 1-way ANOVA followed by the Fisher LSD procedure (to minimize type II hypothesis testing errors) were used to evaluate the effects of [experimental cage condition], [experimental day], and [cage condition] x [experimental day] interactions on endpoints described in the results section and figure legends. Prism 9.1.2 was used for all analyses (GraphPad Software, LLC, San Diego, CA). Two-tailed testing was used throughout, and P < 0.05 was considered statistically significant. All summary data are presented as mean± SE.
Results
Body mass in metabolic cages.
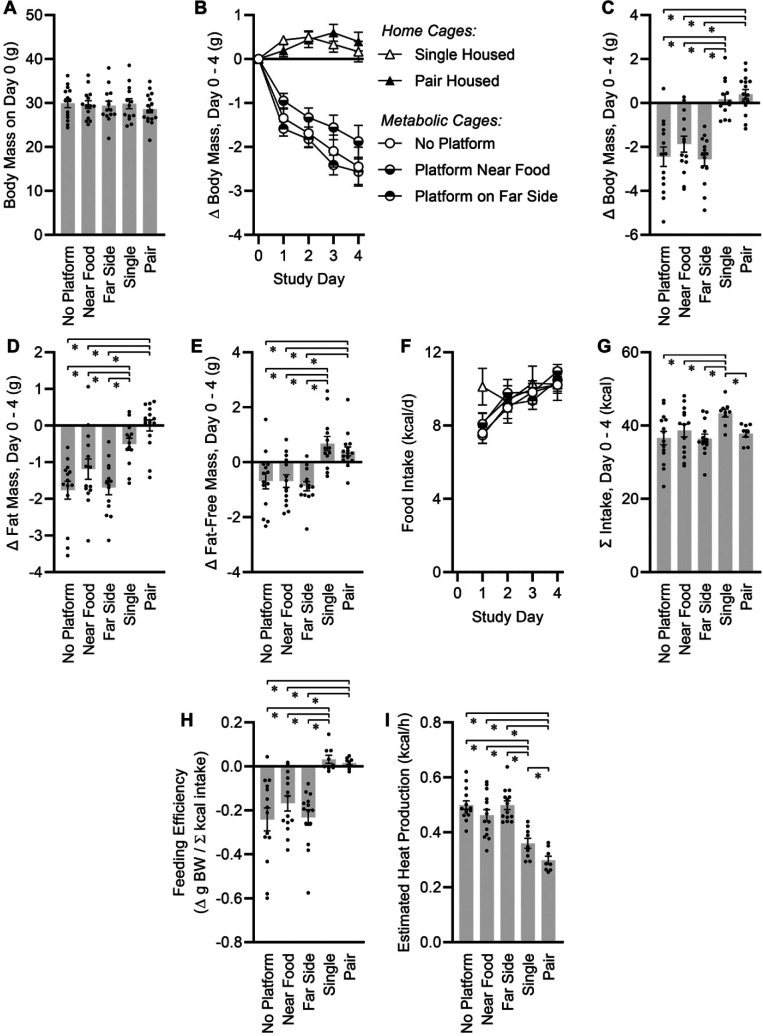
Random assignment of mice (n = 71) to the 5 experimental caging conditions resulted in similar average body masses and variance in body masses (29.5 ± 0.4 g, P = 0.8702) (Figure 2A), and similar body composition (25.1 ± 0.4% fat, P = 0.6456) across all groups. For 4 consecutive overnight periods, mice housed in metabolic cages showed significant reductions in body mass as compared with mice housed either singly or in pairs in standard home cages (Figures 2B-C). The platforms had no significant effect on the pattern of the weight loss. Weight loss was due to similar patterns of fat mass (Figure 2D) and fat-free mass loss in the 3 metabolic cage groups (Figure 2E).
Figure 2.
Energy balance effects of caging conditions. (A) Body mass at start of study. One-way ANOVA P = 0.8702. (B) Change in body mass from day 0. Two-way RM ANOVA caging P < 0.0001, day P < 0.0001, cage x day P < 0.0001. (C) Total change in body mass, day 0 to day 4. One-way ANOVA P < 0.0001. (D) Total change in fat mass, day 0 to day 4. One-way ANOVA P < 0.0001. (E) Total change in fat-free mass, day 0 to day 4. One-way ANOVA P < 0.0001. (F) Food intake per day. Two-way RM ANOVA caging P = 0.5671, day P < 0.0001, cage x day P = 0.3062. (G) Cumulative food intake, day 0 to day 4. One-way ANOVA P = 0.0337. (H) Feeding efficiency, day 0 to day 4. One-way ANOVA P < 0.0001. (I)Total heat production rate, estimated by integrated energy flux and body composition changes across the experimental period. One-way ANOVA P < 0.0001. For all panels, no platform n = 14, near food n = 14, far side n = 14, single n = 13, paired n = 16. Summary data presented as mean± SE; *P < 0.05 by Fisher LSD.
Feeding behavior, caging type, and single compared with pair-housing.
Food intake was measured daily in all mice (Figure 2F). Mice housed singly in standard cages showed a significantly greater cumulative food intake over the 4-d experiment, driven primarily by a greater increase in the first 24-h period as compared with the other 4 groups (Figure 2G). Food intake per mouse was similar in all 3 metabolic cage groups and in mice housed in pairs despite different patterns of body mass loss among these groups. The absence of urine or feces in the food hoppers was confirmed visually for all mice housed in metabolic caging.
Feeding efficiency and energy expenditure.
To estimate energy expenditure, feeding efficiency (weight gain per calorie consumed, and thereby an inverse metric of energy expenditure) was calculated. Whereas no difference in feeding efficiencies was observed between single- and pair-housed mice in home cages, mice in metabolic cages showed significant reductions in feeding efficiency consistent with increased energy expenditure (Figure 2H). This effect was not modified by the inclusion of a platform.
In addition, total metabolic rate of the mice was estimated from body composition changes and caloric intake across the experimental period, as we demonstrated previously.17 Estimating the caloric value of fat mass at 9 kcal/g and fat-free mass at 4 kcal/g and NMR-based measures of fat and fat-free mass changes over the experimental period, we can approximate the calories used for growth (or liberated by weight loss). In our previous bomb calorimetry studies analyzing the digestive efficiency of adult mice fed Teklad 2920× diet, we estimated that approximately 80% of calories ingested were absorbed by the mice.5 Multiplying total caloric intake by 80% and subtracting calories used for growth, we can estimate calories expended over the 96-h experimental period. Such calculations yield total 24 h energy expenditure estimates for the 5 groups (Figure 2I). Mice housed in metabolic cages exhibited similarly increased expenditure, with or without platforms present, relative to mice housed in home cages. Further, mice housed singly in home cages exhibited increased energy expenditure compared to mice housed in pairs.
Fluid intake.
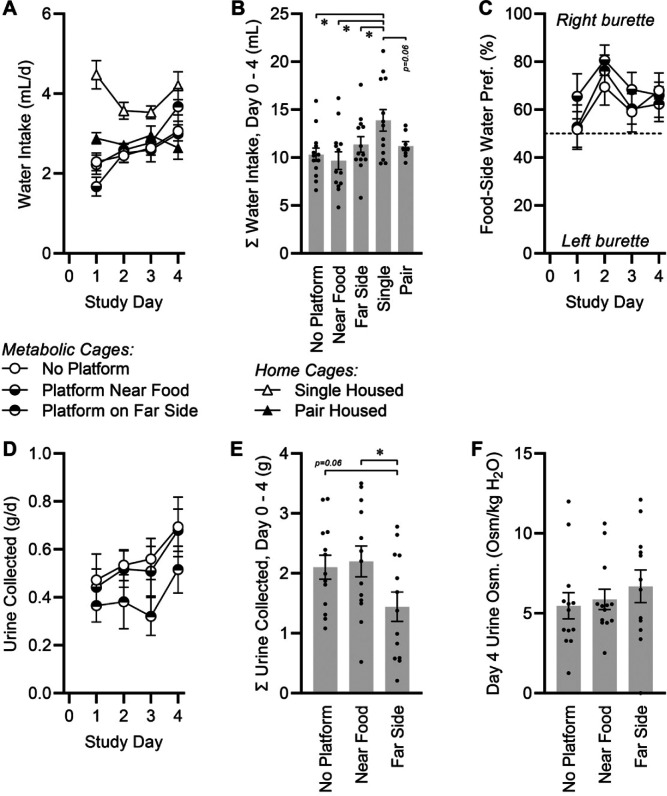
Water intake was measured daily in all mice (Figure 3A). Water intake per mouse was not different among metabolic cage groups and pair-housed mice, but singly-housed mice in standard cages had higher fluid intake (Figure 3B). As metabolic cages were instrumented with 2 identical graduated burettes (Figure 1F), a side preference could be calculated between the burette located closer to the food hopper (right burette, from the animal’s perspective) and the more distally located burette (left burette, from animal’s perspective). A consistent and statistically significant preference was detected for the food-adjacent burette (65 ± 3%, P < 0.05 as compared with 50%) (Figure 3C). However, no differences were observed between mice living in metabolic cages with or without platforms.
Figure 3.
Fluid balance effects of caging conditions. (A) Water intake per day. Two-way RM ANOVA caging P < 0.0001, day P = 0.0004, cage x day P < 0.0001. (B) Cumulative water intake, day 0 to day 4. One-way ANOVA P = 0.0115. (C) Fraction of water consumed from bottle located closer to food source. Two-way RM ANOVA caging P = 0.3774, day P = 0.0192, cage x day P = 0.9381. All groups P < 0.05 compared with 50% by one-sample t test. (D) Urine collected per day. Two-way RM ANOVA caging P = 0.1630, day P = 0.0182, cage x day P = 0.9517. (E) Cumulative urine collected, day 0 to day 4. One-way ANOVA P = 0.0605. (F) Osmolality of urine collected on day 4. One-way ANOVA P = 0.5843. For all panels, no platform n = 14, near food n = 14, far side n = 14, single n = 13, paired n = 16. Summary data presented as mean± SE; *P < 0.05 by Fisher LSD.
Urine and feces collection in metabolic cages.
We assessed the impact of including platforms on the accuracy of collecting both urine and feces. Daily urine collected per mouse increased over time (Figure 3D), in association with progressive increases in water intake (Figure 3A). The efficacy of urine collection in the metabolic cages was significantly affected by the presence of a platform especially if the platform was located on the opposite side of the cage, away from the food and water (Figure 3E). In contrast to urine collection kinetics, total feces collected did not differ across groups in metabolic cages (n = 42, 0.74 ± 0.22 g/d, P = 0.4068). Finally, the quality of collected urine was assessed by determining the osmolality of urine that was captured. No significant difference in urine osmolality was detected among the groups (Figure 3F).
Platform placement and behavior.
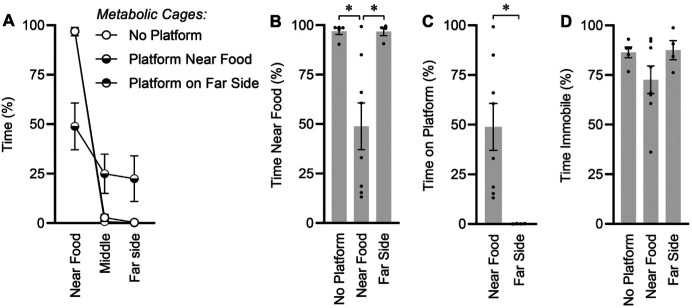
Locomotor activity was assessed by a video camera in a subset of mice during the last 4 h of the light phase. This timeframe was selected so that no personnel were present in the testing room during this period, the mice were in freshly serviced cages (that is, no urine/feces had yet accumulated on platforms, which could have secondary effects such as inducing avoidance behavior), and quality video capture was possible without the use of infrared or similar technologies. The distribution of time spent in the 21% of the cage near the food and water sources, the 58% of the cage in the center, or the 21% of the cage on the far side of the cage during this 4-h period was quantified by automated video tracking (Figure 4A). Mice housed in metabolic cages with no platform spent roughly 97% of their time huddled near the food hopper, and only 3% of time in the remaining 79% of the cage area (Figure 4B). In addition, the placement of a platform closest to the food area of the cage induced a redistribution of time spent in various regions of the cage because mice did not rest on the platform. Furthermore, placing a platform on the opposite side of the cage did not draw the mice toward this region of the cage (Figure 4C). Placement of the platform near the food and water sources did not significantly alter total immobile time (P= 0.0942), and (Figure 4D), and no difference in total distance traveled during this 4-h period was detected among groups (n = 17, 22.1 ± 3.8 m/4 h, P = 0.7149).
Figure 4.
Behavioral analysis during last 4 h of light phase. (A) Distribution of time spent in fractions of cage near the food source, in the middle of the cage, or on the far side of the cage. Note that the “Platform on far side” group almost completely overlaps and is therefore visually obscured by the “No platform” group. (B) Time spent near food source. One-way ANOVA P = 0.0037. (C) Time spent on platform. Independent t test P = 0.0174. (D) Total immobile time. One-way ANOVA P = 0.1770. For all panels, no platform n = 5, near food n = 8, far side n = 4. Summary data presented as mean± SE; *P < 0.05 by Fisher LSD.
Discussion
Data presented here indicate that short-term housing in metabolic caging caused increased energy expenditure of the mice, which was unaffected by the presence or absence of solid platform inserts. Furthermore, food intake of mice singly housed in metabolic cages appeared similar to that of mice housed in pairs in standard cages, whereas mice singly housed in standard cages consumed more than the other groups. Fluid intake was similarly modified by single housing in home cages, but not metabolic cages; the inclusion of platforms in metabolic cages also had no modulatory effect on fluid intake. However, including a platform in metabolic cages did have place-dependent modulatory effects on the quantity (and thus, accuracy and precision) of the collected urine. Including a platform also had major modulatory effects on the distribution of time spent in various portions of a metabolic cage, as mice appeared to actively avoid the platforms regardless of its location in the cage.
Placing individual mice in metabolic cages without enrichment could negatively affect normal habituative behaviors. A previous study found that BALB/c mice housed in metabolic cages for a 3-wk period exhibited progressively increasing creatinine excretion and increased markers of oxidative stress, along with differences in core body temperature.6 In typical group-housing conditions, mice reduce heat loss and optimize thermogenesis by huddling together, thereby reducing the area of exposed body surface.1 This behavioral regulation is impossible for mice placed alone in metabolic caging. Metabolic cages also deprive the mice of the opportunity to exhibit burrowing and nesting behaviors. Mice with bedding and nesting material that support these behaviors have higher core body temperatures, further supporting the concept that housing in metabolic cages at typical room temperature (22 to 23°C, which is below the thermoneutral zone for mice4) is a physiologically significant thermal challenge for mice.8
As expected, energy balance physiology and behaviors were affected by housing conditions, although the effect of housing on energy balance was not simple or unidimensional. Mice housed singly in standard cages had greater food intake than did all other groups. However, mice housed in metabolic caging and mice housed in pairs in standard cages showed no differences in food intake. These findings highlight complex interactive consequences of cage type (standard cage compared with metabolic) and cohousing compared with single housing on food intake.
In addition to an influence of caging type on food intake, our findings related to feeding efficiency and the estimation of total energy expenditure based on body composition changes also support differences in energy expenditure. Our estimates of heat production in the standard-cage groups are similar to those that we previously reported for adult male mice of this body mass range, using Promethion (Sable Systems International) and OxyMax (Columbus Instruments International) multiplexed phenotyping systems,17 increasing confidence in these estimates. Thus, these data support the conclusions that energy expenditure is higher in mice housed individually in metabolic cages, that inclusion of a platform, at least if not used, has no effect on this increased expenditure, and that individual housing of mice in standard cages increases energy expenditure as compared with that of mice housed in pairs. Whether these complex effects on feeding and energy expenditure could be alleviated by performing metabolic cage studies in a room or environmental chamber that maintained the ambient temperature within the thermoneutral zone for mice requires future study.
The observation that food intakes by mice housed in metabolic caging covered the same range of intakes as for mice in standard cages supports the conclusion that ineffective or incomplete access to food in metabolic caging was not a confounding issue. In addition, the formulation of the diet (that is, powdered or pelleted) did not appear to have a major effect on intake. For example, food intake was similar between mice fed powdered diet while housed in metabolic cages, compared with mice fed pelleted chow while housed in standard cages. Further, food intake differed between mice fed pelleted food while housed singly or in pairs in standard cages. Thus, we conclude that food intake was most likely influenced by caging type more than by the formulation of the food (powdered compared with pelleted). Future studies including the use of powdered food in standard cages, and pelleted food in metabolic cages, could be performed to further dissect these relationships.
Fluid balance and intake were also impacted by housing conditions. As for food intake patterns, mice individually housed in standard cages differed from mice housed in pairs in standard cages or mice in any of the metabolic cage groups. These results are consistent with previous observations that food and fluid ingestion in rodents are typically positively correlated;3,16 however, the cause of increased ingestion of food and water by singly housed mice in standard cages remains unclear. Within metabolic cage groups, inclusion of a platform had no effect on fluid intake behaviors, regardless of position.
Inclusion of platforms in metabolic cages had significant, position-dependent effects on the quantity and accuracy of urine collection but not on urine quality. The collected urine had similar osmolality regardless of the presence or position of platforms, but the total volume collected was greatly reduced when the platform was located on the side of the cage opposite the food and water sources. These findings are consistent with the conclusion that the mice primarily discharge urine in the region of the cage away from food and water sources, regardless of the presence or absence of a platform in this position. Subjectively, we noted that relatively large amounts of urine were routinely observed on platforms in cages when the platform was located on the side of the cage opposite the food and water sources, whereas only negligible amounts of urine were infrequently observed on platforms located near the food and water sources. Thus, these quantitative and subjective findings are consistent with previous findings that laboratory mice segregate cage space between clean and dirty areas.7 Because quantitative urine collection is typically the primary motivation for researchers to use metabolic caging, placing a platform on the opposite side of the cage from the food and water appears antithetical to the use of such caging. Future studies are needed to investigate possible effects of platform inclusion and placement on urine collection efficiency in other metabolic cage designs (such as those with smaller floor and funnel areas intended to reduce evaporation).
Preliminary analysis of locomotor activity demonstrated that platform location relative to food source influenced the positional preferences of mice. Specifically, video analyses of mice in the cage during the final few hours of the light phase found that mice housed in metabolic cages without platforms spent the majority of their time sitting in the region of the cage closest to the food and water sources. Placing a platform on the opposite side of the cage did not change this behavior, but placement of a platform closest to the food hopper and water burettes had unexpected and major modulatory effects on mouse behavior. In brief, placement of a platform near the food caused reduced time spent in this strongly preferred portion of the cage (from 97% to 49%). Thus, we conclude that the mice actively avoided resting on the platform and instead rested on the wire grid. This discovery indicates that although rats may prefer platforms during rest,9,10 mice do not. Furthermore, inclusion of a platform may robustly change mouse behavior.
The design of the current study had several limitations. First, only male C57BL/6J mice (or male mice on a C57BL/6J background) were used for the study, and interactive effects of cage type, sex, and strain may exist. Our previous work with mice housed in multiplexed phenotyping systems from different manufacturers, for example, found interactions among cage design, diet, and sex on metabolic physiology.17 Future studies are needed to document the interactive effects of such variables in metabolism cages. Second, we used platforms created from opaque white HDPE plastic sheeting. The use of a different color platform may alter the behavior of the mice, either enhancing or minimizing the observed effects. Third, the use of a different type of material, such as metal, may also alter mouse responses to the platform. Fourth, ventilated home cages are not designed to enable studies of food intake, and food spillage and caching of partially chewed food pellets are often observed.12 Although we purposefully sifted through bedding materials daily to attempt to control for this problem, we cannot rule out the potential (likely, minor) overestimation of food intake in the single- and pair-housed mice in standard cages in the current study. Fifth, we studied only 2 platform locations in the cage, close to the food and water sources and on the opposite side of the cage. Differently shaped or sized platforms or placement in a different location in the cage could alter mouse behaviors in unique ways. Finally, the current study purposefully focused on the effects of various housing designs over a 4-d testing period, as this testing period is not unusual in the literature,5 and major effects of housing were apparent even over this relatively short testing period. We and others have previously demonstrated that longer testing periods in metabolic caging result in animals reaching a steady-state for many variables;5 the effects of different housing types may similarly be sensitive to test duration. By extension, the current study supports the investigation and establishment of standard test durations and the use of acclimation periods when changing animal housing types.
In summary, these studies highlight robust effects of housing mice in different cage conditions, ranging from single housing in standard cages through metabolic cages that contain platforms in different parts of the cage, on food and water intake and locomotor activity, all of which may have implications for animal wellbeing and study outcomes. The data prompt greater consideration of cage design as a physiologically significant experimental variable that can affect endpoints of interest. Caging design can synergistically interact with other variables under study to modify quantitative and qualitative outcomes. For example, placing HDPE platforms in metabolic caging did not improve metabolic endpoints, negatively influenced urine collection depending on platform position in the cage, and were actively avoided by the mice. More effort is needed to continue to improve experimental caging to simultaneously better mimic typical features of natural habitats while enabling the quantitative collection of biologic specimens including urine and feces. Finally, these studies illustrate that empirical, quantitative validations must be performed in a species- and strain-specific manner before well-intentioned and otherwise logical interventions are adopted in animal research.
Acknowledgments
The authors gratefully acknowledge husbandry support by the staff of the Medical College of Wisconsin Biomedical Resource Center. This work was supported by grants from the NIH (HL134850, HL084207), the American Heart Association (18EIA33890055), the Medical College of Wisconsin Clinical & Translational Science Institute “Obesity” Ensemble (UL1TR001436), the Advancing a Healthier Wisconsin Endowment to Medical College of Wisconsin, and the Medical College of Wisconsin’s Departments of Pediatrics and Physiology.
References
- 1.Alberts JR. 1978. Huddling by rat pups: group behavioral mechanisms of temperature regulation and energy conservation. J Comp Physiol Psychol 92:231–245. 10.1037/h0077459. [DOI] [PubMed] [Google Scholar]
- 2.Fullerton PM, Gilliatt RW. 1967. Pressure neuropathy in the hind foot of the guinea–pig. J Neurol Neurosurg Psychiatry 30:18–25. 10.1136/jnnp.30.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gannon KS, Smith JC, Henderson R, Hendrick P. 1992. A system for studying the microstructure of ingestive behavior in mice. Physiol Behav 51:515–521. 10.1016/0031-9384(92)90173-Y. [DOI] [PubMed] [Google Scholar]
- 4.Gordon CJ. 1993. Temperature regulation in laboratory rodents. Cambridge (UK): Cambridge University Press. 10.1017/CBO9780511565595 [DOI] [Google Scholar]
- 5.Grobe JL. 2017. Comprehensive assessments of energy balance in mice. Methods Mol Biol 1614:123–146. 10.1007/978-1-4939-7030-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalliokoski O, Jacobsen KR, Darusman HS, Henriksen T, Weimann A, Poulsen HE, Hau J, Abelson KS. 2013. Mice do not habituate to metabolism cage housing––a three week study of male BALB/c mice. PLoS One 8:e58460. 10.1371/journal.pone.0058460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makowska IJ, Franks B, El-Hinn C, Jorgensen T, Weary DM. 2019. Standard laboratory housing for mice restricts their ability to segregate space into clean and dirty areas. Sci Rep 9: 6179. 10.1038/s41598-019-42512-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maloney SK, Fuller A, Mitchell D, Gordon C, Overton JM. 2014. Translating animal model research: does it matter that our rodents are cold? Physiology (Bethesda) 29:413–420. 10.1152/physiol.00029.2014. [DOI] [PubMed] [Google Scholar]
- 9.Manser CE, Elliot H, Morris TH, Broom DM. 1996. The use of a novel operant test to determine the strength of preference for flooring in laboratory rats. Lab Anim 30:1–6. 10.1258/002367796780744974. [DOI] [PubMed] [Google Scholar]
- 10.Manser CE, Morris TH, Broom DM. 1995. An investigation into the effects of solid or grid cage flooring on the welfare of laboratory rats. Lab Anim 29:353–363. 10.1258/002367795780740023. [DOI] [PubMed] [Google Scholar]
- 11.Mecklenburg L, Kusewitt D, Kolly C, Treumann S, Adams ET, Diegel K, Yamate J, Kaufmann W, Müller S, Danilenko D, Bradley A. 2013. Proliferative and non–proliferative lesions of the rat and mouse integument. J Toxicol Pathol 26 3_Suppl:27S–57S. 10.1293/tox.26.27S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mohamed AA, Kravitz AV. 2018. Challenges in quantifying food intake in rodents. Brain Res. 1693B: 188-191. 10.1016/j.brainres.2018.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow DT, Robinette LR, Saubert CW, Van Hoosier GL. 1977. Poditis in the rat as a complication of experiments in exercise physiology. Lab Anim Sci 27:679–681. [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 15.Peace TA, Singer AW, Niemuth NA, Shaw ME. 2001. Effects of caging type and animal source on the development of foot lesions in Sprague Dawley rats (Rattus norvegicus). Contemp Top Lab Anim Sci 40:17–21. [PubMed] [Google Scholar]
- 16.Smith JC. 2000. Microstructure of the rat’s intake of food, sucrose and saccharin in 24–hour tests. Neurosci Biobehav Rev 24:199–212. 10.1016/S0149-7634(99)00073-1. [DOI] [PubMed] [Google Scholar]
- 17.Soto JE, Burnett CML, Ten Eyck P, Abel ED, Grobe JL. 2019. Comparison of the effects of high–fat diet on energy flux in mice using two multiplexed metabolic phenotyping systems. Obesity (Silver Spring) 27:793–802. 10.1002/oby.22441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stechman MJ, Ahmad BN, Loh NY, Reed AA, Stewart M, Wells S, Hough T, Bentley L, Cox RD, Brown SD, Thakker RV. 2010. Establishing normal plasma and 24–hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab Anim 44:218–225. 10.1258/la.2010.009128. [DOI] [PubMed] [Google Scholar]