Abstract
The pharmacokinetics, safety, and tolerability of oral moxifloxacin, a new 8-methoxy quinolone, were assessed in a randomized, double-blind, placebo-controlled study in which healthy male and female volunteers received either 400 mg of moxifloxacin once daily (n = 10) or a placebo once daily (n = 5) for 10 days. Plasma moxifloxacin concentrations on days 1 and 10 were measured by high-performance liquid chromatography and fluorometric detection. Standard pharmacokinetic parameters were estimated by noncompartmental methods. Natural logarithmic estimates for each pharmacokinetic variable of each subject were analyzed by a two-way analysis of variance. Hematology, blood chemistry, vital signs, and adverse events were monitored, and electrocardiograms (ECG) were performed. Plasma moxifloxacin concentrations of predicted therapeutic relevance were achieved in this study. For day 1, the mean maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve from 0 to 24 h (AUC0–24) were 3.4 mg/liter and 30.2 mg · h/liter, respectively. Corresponding means on day 10 were 4.5 mg/liter and 48 mg · h/liter, respectively. On day 10, the mean elimination half-life was approximately 12 h. Plasma moxifloxacin concentrations exceeded the MIC for Streptococcus pneumoniae throughout the 24-h dosing period. The day 1 and day 10 mean AUC/MIC ratios were 121 and 192, respectively, and the mean Cmax/MIC ratios were 13 and 18, respectively. Moxifloxacin was well tolerated; no clinically relevant changes in the standard laboratory tests, vital signs, or ECG were observed. Pharmacokinetic parameters demonstrated linearity, and estimates of pharmacokinetic/pharmacodynamic ratios (AUC/MIC and Cmax/MIC) indicate that the regimen of 400-mg once daily should be effective for treating a variety of infections. Moxifloxacin was found to be safe and well tolerated in healthy volunteers when it was given as a single daily 400-mg dose for 10 days.
Moxifloxacin (Bay 12-8039) is a new, enantiomerically pure 1-cyclopropyl-7-(2,8-diazabicyclo[4.3.0]nonane)-6-fluoro-8- methoxy-1,4-dihydro-4-oxo-3-quinoline carboxylic acid hydrochloride which has potent activity against an extensive spectrum of bacteria. Excellent in vitro activities against gram-positive cocci (including penicillin-resistant Streptococcus pneumoniae strains), beta-lactamase-negative and -positive Haemophilus influenzae, Moraxella catarrhalis, nonpseudomonal gram-negative bacilli, and anaerobic and atypical organisms (such as Legionella, Mycoplasma, and Chlamydia species) were observed (2, 5, 8–10, 17). In addition, moxifloxacin is bactericidal against respiratory tract pathogens by time-kill methodology (1). Accordingly, moxifloxacin would be potentially useful in the treatment of respiratory tract and other infections.
The pharmacokinetics and safety of moxifloxacin following single doses ranging from 50 to 800 mg and in multiple-dose trials with a dosage of up to 600 mg once daily for 10 days have been studied. Prior to this study, the pharmacokinetics and safety of the drug at a dosage of 400 mg once daily for 5 days had been studied (7, 13). Following the regimen of 400 mg once daily for healthy male volunteers, the mean steady state of the maximum concentration of drug in serum (Cmax) and the area under the concentration-time curve (AUC) on day 5 were 3.2 mg/liter and 34 mg · h/liter, respectively. Twenty-four hours following the fifth dose, the mean trough concentration in serum was 0.5 mg/liter, which is above the MIC for the most commonly isolated respiratory tract and skin pathogens. Moxifloxacin’s steady-state elimination half-life is approximately 12 h (7). The pharmacokinetic values from the single- and multiple-dose studies were linear for all dosing regimens investigated (7, 13). Overall, these early phase I studies also found moxifloxacin to have a good safety profile, with no clinically relevant changes in vital signs, standard hematology and chemistry tests, and electrocardiogram (ECG) and electroencephalogram evaluations.
Here, we investigated the pharmacokinetics, safety, and tolerability of 400 mg of moxifloxacin given once daily for a prolonged duration (10 days) in healthy male and female volunteers. A second goal was to predict the effectiveness of moxifloxacin with pharmacokinetic/pharmacodynamic ratios for potential efficacy (i.e., AUC/MIC and Cmax/MIC).
(This research was presented in part at the European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, May 1997.)
Fifteen healthy volunteers between the ages of 18 and 45 years were recruited by advertisement. Subjects qualified for the study if they had normal findings for a prestudy (up to 21 days prior to the administration of the study drug) medical history and a physical examination that included the following: assessment of vital signs; chest X ray (if not available within the prior year); ECG evaluation; urinalysis and urine drug screen; tests for serum human choriogonadotropin for female subjects, serum hepatitis B surface antigen, serum hepatitis C virus antibody, and serum human immunodeficiency virus; and standard hematology and chemistry tests. All subjects had to have a body weight within 15% of the upper or lower limit of the normal weight range (Metropolitan Life Insurance Company, New York, N.Y.). All subjects signed an informed consent form approved by the investigational review board of Innovex, Inc., Lenexa, Kans.
Fifteen subjects (12 Caucasian, 2 African-American, and 1 Asian), including 10 males and 5 females, were enrolled in the study. All 10 subjects who received moxifloxacin completed the study procedures, and their data were considered valid for pharmacokinetic and safety analysis. Five subjects received placebos. One of the placebo-treated subjects received only eight doses; his data were valid for the safety analysis. The other four placebo-treated subjects received 10 daily doses. Demographic and baseline features, by gender and treatment group, are shown in Table 1.
TABLE 1.
Demographics and baseline features of test subjects
| Gender | Treatment group | n | Mean (range)
|
||
|---|---|---|---|---|---|
| Age (yr) | Ht (cm) | Wt (kg) | |||
| Male | Placebo | 3 | 24.3 (19–30) | 177.0 (173–180) | 67.7 (62.5–73.6) |
| Moxifloxacin | 7 | 24.9 (19–31) | 179.4 (165–191) | 78.9 (64–100) | |
| Female | Placebo | 2 | 19 (19) | 167.5 (165–170) | 68.5 (61–76) |
| Moxifloxacin | 3 | 33.3 (25–41) | 165.7 (162–170) | 67.7 (55–86) | |
The trial was conducted with a single-center, randomized, double-blind, placebo-controlled design. Eligible subjects were confined to the clinic for a total of 12 consecutive days. All subjects were randomly assigned in a 2:1 ratio to receive either 400 mg of oral moxifloxacin (two 200-mg encapsulated tablets; Bayer Corporation, West Haven, Conn.) or two identical-looking placebo capsules. Each subject received 10 single daily doses of the study drug. All study medication was administered in the morning when the subjects were in a fasting state (from 10 p.m. the prior evening to 2 h postdose) and was swallowed, intact, with 180 ml of water.
In addition to the screening procedures performed prior to dosing, all subjects had a brief physical examination and their vital signs were measured just prior to receiving the first dose of assigned medication, and they had a complete physical examination, including laboratory tests and an ECG, at the end of the study. Concomitant medications, except for oral contraceptives and acetaminophen (maximum, 2,000 mg per day), were not permitted for the study’s duration. One subject took 250 mg of acetaminophen 3 days prior to the first dose of moxifloxacin. Therefore, it can be said that no one took acetaminophen during the moxifloxacin treatment. In addition, the allowed medications could not be taken in the 8-h period prior to the 2-h period after dosing on days 1 and 10.
Venous blood samples (5 ml) for the determination of plasma moxifloxacin concentrations were collected from an indwelling catheter or by venipuncture into heparinized tubes on days 1 and 10 immediately prior to dosing and then at the following times: 0.25, 0.75, 1.0, 1.5, 2.0, 2.5, 3.0, 4.0, 6.0, 8.0, 10.0, 12.0, 16.0, and 24.0 h. In addition, 36- and 48-h postdose samples were obtained from the subjects following the day 10 dose. Three venous blood samples (5 ml) were also collected into nonheparinized tubes on day 1 at 6, 8, and 10 h postdose for the determination of serum moxifloxacin concentrations. All blood samples were centrifuged within 1 h of collection; the plasma and serum were separated, transferred to polypropylene storage tubes, and stored upright at a temperature of −20°C or lower until assayed.
Plasma and serum moxifloxacin concentrations were determined by a validated high-performance liquid chromatography procedure which involved protein precipitation with acetonitrile and reversed-phase chromatographic separation of moxifloxacin from a structurally related quinolone internal standard (isopropyl analogue of ciprofloxacin) and plasma supernatant matrix, followed by fluorometric detection at 296-nm excitation and 504-nm emission wavelengths (12). The quantitation range of this procedure was 25 to 4,000 ng/ml. Samples having concentrations above 4,000 ng/ml were subsequently diluted with blank plasma and reassayed. The intraday accuracy ranged from 99.2 to 103.0%, and the intraday precision, reflected by the coefficient of variation (CV), ranged from 0.6 to 4.5%. The interday accuracy and precision were obtained with quality control samples, which were analyzed concurrently with each set of subject samples, and the observed CVs were 7.2% at 40 ng/ml, 2.5% at 750 ng/ml, and 2.4% at 3,000 ng/ml. The corresponding accuracies were 103% at 40 ng/ml, 97.8% at 750 ng/ml, and 97.2% at 3,000 ng/ml.
Each subject was closely monitored for any adverse events by using “wellness assessments,” vital sign assessments, and clinical laboratory tests. At days 7 and 30 following the last dose of the study drug, subjects were contacted by telephone for general safety follow-up. Each adverse event was categorized by the investigator in terms of its severity (mild, moderate, or severe) and its relationship to the study drug (probable, possible, remote, or none).
Plasma drug concentration data of the individual subjects were analyzed by standard noncompartmental methods. The individual Cmaxs, times to Cmax (Tmax), and minimum predose concentrations of drug in plasma were obtained by visual inspection of the plots of concentration in plasma versus time. In addition, the Cmax was normalized for dose and weight (Cmax,norm) and was calculated as follows: Cmax/(dose/weight). The primary pharmacokinetic parameters included the AUC from 0 h to infinity (AUC0–∞), the AUC0–∞ normalized for dose and weight (AUC0–∞,norm), the AUC from 0 to 24 h (AUC0–24), the AUC0–24 normalized for dose and weight (AUC0–24,norm), and elimination half-life. The AUC to the last measurable concentration was calculated with the log-linear trapezoidal rule, and AUC0–∞ was determined as follows: AUC0–tn + Ctn/ke, where AUC0–tn is the AUC to the last measurable concentration, Ctn is the last measurable concentration in plasma, and ke is the terminal elimination rate constant by linear regression of the terminal log-linear phase of the concentration in plasma versus time curve (6). The elimination half-life was calculated as follows: 0.693/ke. The accumulation ratio was estimated with the AUC0–24 of the last dose divided by the AUC0–24 of the first dose, and the linearity index was calculated with the AUC0–24 of the last dose divided by the AUC0–∞ of the first dose.
Summary statistics for the baseline values, demographics, vital signs, and ECGs, as well as the frequencies of adverse events, were obtained. For each pharmacokinetic variable, logarithmically (natural log) transformed estimates for each subject were analyzed by using a two-way analysis of variance with terms for day (1 versus 10) and subject. This analysis is equivalent to the paired t test, which can test whether the difference on the log scale between days 1 and 10 is significantly different from zero. This analysis can also be used to determine whether the ratio (day 10 to day 1) is different from one. Log-scale least-squares means and log-scale differences were exponentiated to obtain geometric least-squares mean and ratio estimates.
Pharmacokinetics.
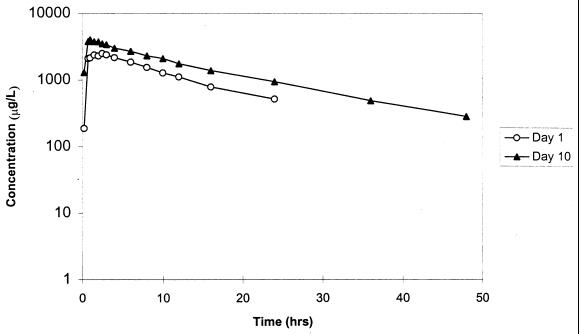
The time courses of the mean plasma moxifloxacin concentrations following single and multiple 400-mg daily doses are illustrated in Fig. 1. Model-independent pharmacokinetic parameters following day 1 and day 10 doses are summarized in Table 2. Because only three females received moxifloxacin, gender comparisons for moxifloxacin pharmacokinetics were not performed. Serum moxifloxacin values were not different from those of plasma. The geometric mean Cmaxs following a single dose and 10 once-daily doses were 3.36 and 4.52 mg/liter, respectively. The Tmaxs following the day 1 and day 10 doses were similar: 1.49 and 1.24 h, respectively. The similarities in trough concentrations (means ± standard deviations) immediately prior to the day 10 dose (0.95 ± 0.10 mg/liter) and 24 h following the dose (0.89 ± 0.12 mg/liter) indicate that the steady state had been achieved.
FIG. 1.
Semilogarithmic plot of mean plasma moxifloxacin concentration versus time profiles following single (day 1) and multiple (day 10) once-daily 400-mg oral doses administered to 10 healthy volunteers.
TABLE 2.
Pharmacokinetic parameters of oral moxifloxacin (Bay 12-8039) obtained with a dosage of 400 mg once daily for 10 daysa
| Day | Cmax (mg/liter) | Cmax,norm [kg · h/liter] | Tmax (h) | AUC0–∞ (kg · h/liter) | AUC0–∞,norm [mg · h/liter] | AUC0–24 (kg · h/liter) | AUC0–24,norm [mg · h/liter] | t1/2 (h)b |
|---|---|---|---|---|---|---|---|---|
| 1 | 3.36 (21.5) | 0.63 (28.4) | 1.49 (62.2) | 36.68 (13.2) | 6.81 (12.4) | 30.24 (14.2) | 5.66 (10.5) | 9.30 (12.1) |
| 10 | 4.52 (12.2) | 0.85 (18.8) | 1.24 (48.0) | 47.97 (5.8) | 8.98 (16.9) | 11.95 (10.8) |
Values are geometric means, with percent CVs in parentheses.
t1/2, half-life.
The geometric mean AUC0–24s on days 1 and 10 and the mean AUC0–∞ on day 1 are given in Table 2. The mean accumulation ratio (AUC0–24 on day 10/AUC0–24 on day 1) was 1.59 (CV, 13.1%). The mean linearity index was 1.3 (CV, 12.8%). The mean elimination half-life was approximately 12 h under steady-state conditions. Overall, with the exception of the Tmaxs, there was little intersubject variation for most of the pharmacokinetic variables, as indicated by CVs that were less than 29% (Table 2).
Safety and tolerance.
Ten subjects received moxifloxacin, and all 15 subjects were included in the safety analysis. Overall, a total of 35 treatment-emergent adverse events were reported for 12 (80%) subjects, including 8 subjects who received moxifloxacin (Table 3). Most of these adverse events were mild (32 of 35 [91%]) and self-limiting and resolved spontaneously without intervention. No subject was discontinued from the study prematurely due to an adverse event, although one placebo-treated subject left the study for personal reasons. No clinically significant treatment-emergent elevations occurred in liver function tests or in other parameters assessed by laboratory studies, physical examination, vital signs or ECGs.
TABLE 3.
Subjects with treatment-emergent adverse events
| Body system and adverse event | No. of subjects in treatment group with adverse event
|
|
|---|---|---|
| Moxifloxacin, 400 mg (n = 10) | Placebo (n = 5) | |
| Any adverse event | 8 | 4 |
| Whole body (total) | 3 | 2 |
| Abdominal pain | 1 | 1 |
| Arm pain | 1 | 0 |
| Headache | 2 | 1 |
| Digestive system (total) | 6 | 2 |
| Diarrhea | 3 | 2 |
| Dry mouth | 2 | 0 |
| Dyspepsia | 1 | 0 |
| Flatulence | 1 | 0 |
| Nausea | 3 | 1 |
| Tooth pain | 1 | 0 |
| Nervous system, dizziness | 1 | 1 |
| Respiratory tract, rhinitis | 3 | 0 |
| Skin and appendages, acne | 1 | 0 |
Discussion.
The moxifloxacin pharmacokinetic findings here are similar to previously reported results for young healthy male volunteers (7, 13). The AUC0–∞ and AUC0–∞,norm on day 1 in this study were comparable to day 1 values in a previous trial of 400 mg given once daily to healthy young male volunteers, 30.1 mg · h/liter and 5.5 kg · h/liter, respectively (7). A single-dose study conducted with young healthy males also produced a similar AUC0–∞ and AUC0–∞,norm (26.8 mg · h/liter and 5.3 kg · h/liter, respectively) (13). Similarly, the Cmax and Cmax,norm on day 1 in the present study were comparable with those of two earlier studies, although the values from our study were slightly higher than those in the German trials (7, 13). As expected, the Cmaxs and AUCs in this study were approximately double those reported for previously conducted studies with 200-mg single doses and quadruple those of studies with 100-mg single doses (7, 13). Similarly, these values were approximately half those obtained with an 800-mg dose. These results indicate that moxifloxacin exhibits linear pharmacokinetics over a 100- to 800-mg dose range. An important finding was that the mean trough concentration of moxifloxacin (approximately 0.5 mg/liter) following a single 400-mg dose (i.e., 24 h postdose) in this study, as well as in others, was above the MIC required for the most commonly encountered gram-positive and gram-negative pathogens. At the steady state, trough concentrations were approximately 0.9 mg/liter. The steady-state pharmacokinetic parameters of moxifloxacin in our 10-day study were somewhat higher than the corresponding pharmacokinetic values in the 5-day study conducted in Germany (7). The AUC0–24 and AUC0–24,norm in this study were different from the corresponding values, 33.9 mg · h/liter and 6.0 kg · h/liter, in the German study (7). These differences may be artifactual (i.e., small numbers of subjects enrolled in both trials) or may be based, in part, on different subject populations, since we included young females, as well as young males, and non-Caucasians, unlike the German study, which was limited to young Caucasian males. Previous studies of moxifloxacin indicated that body weight is a significant determinant of pharmacokinetic values (15). However, normalized pharmacokinetic values were also higher in the U.S. population. Accumulation ratios should theoretically approximate 1.3 for a drug with a terminal elimination half-life of 12 h that is given once daily (6). The accumulation ratio for the 5-day German study was 1.1 (7), while it was 1.59 in this study. Accordingly, the difference in these results is most likely explained by random variation associated with small sample sizes.
Because moxifloxacin will be used to treat respiratory tract infections, an important target organism is S. pneumoniae. The moxifloxacin MIC at which 90% of S. pneumoniae organisms are inhibited in vitro has been reported as 0.12 to 0.25 mg/liter, according to different studies (2, 5). Here, we found that plasma moxifloxacin concentrations exceeded the predicted MIC for S. pneumoniae for the entire 24-h dosing interval of the treatment course. Calculations of pharmacokinetic/pharmacodynamic ratio revealed that day 1 and day 10 mean AUC/MIC ratios for S. pneumoniae were 121 and 192, respectively, and mean Cmax/MIC ratios were 13 and 18, respectively. While not incontrovertibly applicable to infections caused by gram-positive organisms, similar values have been associated with clinical cure in both a mouse model and a clinical survey (4, 16).
Moxifloxacin was found to be safe and well tolerated in our 10 healthy volunteers. Importantly, this study includes the first information on safety for females that were administered multiple doses. While there were only three females who received moxifloxacin, their response in terms of safety was not obviously different from that of the male subjects. However, with the small number of subjects, limited conclusions can be made about gender effects in this study. There were only three adverse events reported as moderate in severity, and none were graded as severe. Two of these events were headache (one on day 1 and the other on day 8), and both resolved spontaneously without medication after a few hours. A third subject complained of abdominal pain on day 7, which lasted about 8 h and also resolved spontaneously without medication. An evaluation of adverse events in moxifloxacin phase I studies revealed that headache was not observed any more frequently in subjects taking moxifloxacin than in subjects taking a placebo (n = 130) (14). In the phase I database, the only symptom that appeared to be more frequent in subjects taking moxifloxacin was nausea of mild severity (7% with moxifloxacin versus 1% with the placebo). In our small study, three episodes of nausea graded as mild in severity were reported by moxifloxacin recipients, compared to one similar episode occurring in a placebo-treated subject. In this study, there were no obvious differences in the frequencies of adverse events between subjects taking moxifloxacin and those taking the placebo. Because of previous concerns with other fluoroquinolones (3), it is reassuring that no clinically relevant changes in laboratory parameters were noted, including glucose or hematologic parameters.
Overall, this study demonstrated that moxifloxacin was safe and well tolerated when administered to 10 healthy young males and females as a single daily 400-mg oral dose for a total of 10 days. Plasma moxifloxacin levels were consistent with those in previously conducted investigations and consistent with dose-proportional pharmacokinetics (11).
REFERENCES
- 1.Federici J A, Herrington J A, Painter B G, Remy J M, Thurberg B E. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. The bactericidal activity of Bay 12-8039 against respiratory tract pathogens, abstr. F13; p. 102. [Google Scholar]
- 2.Felmingham D, Robbins M J, Leakey A, Salman H, Dencer C, Clark S, Ridgway G L, Grüneberg R N. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. In vitro activity of BAY12-8039 against bacterial respiratory tract pathogens, mycoplasmas and obligate anaerobic bacteria, abstr. F8; p. 101. [Google Scholar]
- 3.Finch R G. The withdrawal of temafloxacin. Drug Saf. 1993;8:9–11. doi: 10.2165/00002018-199308010-00002. [DOI] [PubMed] [Google Scholar]
- 4.Forrest A, Nix D E, Ballow C H, Goss T F, Birmingham M C, Schentag J J. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother. 1993;37:1073–1081. doi: 10.1128/aac.37.5.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Georgopoulous A, Buxbaum A, Graninger W. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. Activity of BAY 12-8039 against 1154 clinical isolates of Streptococcus pneumoniae, abstr. F5; p. 100. [Google Scholar]
- 6.Gibaldi M, Perrier D. Pharmacokinetics. 2nd ed. New York, N.Y: Marcel Dekker, Inc.; 1982. [Google Scholar]
- 7.Kubitza D, Stass H H, Wingender W, Kuhlmann J. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. BAY 12-8039 (I), a new 8-methoxy-quinolone: safety (S), tolerability (T) and steady state pharmacokinetics (PK) in healthy male volunteers, abstr. F25; p. 104. [Google Scholar]
- 8.Renaudin H, Bebear C M, Boudjadja A, Bebear C. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. In vitro activity of Bay 12-8039, a new fluoroquinolone, against mycoplasmas, abstr. F9; p. 101. [Google Scholar]
- 9.Roblin P M, Hammerschlag M R. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. Activity of a new quinolone, Bay 12-8039 against Chlamydia pneumoniae in vitro, abstr. F11; p. 101. [Google Scholar]
- 10.Ruckdeschel G, Lob S. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. In vitro activity of a new 8-methoxyquinolone, BAY 12-8039, against Legionella spp. in comparison to ciprofloxacin (CIP), erythromycin (ERY) and rifampicin (RIF), abstr. F7; p. 101. [Google Scholar]
- 11.Stass H, Dalhoff A, Kubitza D, Schühly U. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob Agents Chemother. 1998;42:2060–2065. doi: 10.1128/aac.42.8.2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stass H, Dalhoff A. Determination of BAY 12-8039, a new 8-methoxyquinolone, in human body fluids by high-performance liquid chromatography with fluorescence detection using on-column focusing. J Chromatogr B. 1997;702:163–174. doi: 10.1016/s0378-4347(97)00371-x. [DOI] [PubMed] [Google Scholar]
- 13.Stass H H, Dalhoff A, Kubitza D, Ahr G. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. BAY 12-8039, a new 8-methoxy-quinolone: first pharmacokinetic (PK) results in healthy male volunteers, abstr. F24; p. 104. [Google Scholar]
- 14.Sullivan J T, Kubitza D, Schubly U, Wingender W. Proceedings of the 20th International Congress of Chemotherapy. 1997. Safety of the new quinolone BAY 12-8039 in 130 healthy volunteers, abstr. 3355. [Google Scholar]
- 15.Sullivan J T, Lettieri J, Hogan C H, Heller A H. Age and gender effect on pharmacokinetics (PK) of BAY 12-8039. Clin Pharmacol Ther. 1997;61:148. . (Abstract.) [Google Scholar]
- 16.Sullivan M C, Cooper B W, Nightingale C H, Quintiliani R, Lawlor M T. Evaluation of the efficacy of ciprofloxacin against Streptococcus pneumoniae by using a mouse protection model. Antimicrob Agents Chemother. 1993;37:234–239. doi: 10.1128/aac.37.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise R, Andrews J M, Brenwald N. Abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C.: American Society for Microbiology; 1996. In vitro activity of a new fluoroquinolone Bay 12-8039 in comparison with other fluoroquinolones and β-lactams against recent isolates and Chlamydia, abstr. F10; p. 101. [Google Scholar]