Immunomodulatory therapies are typically used to treat patients with moderate-to-severe atopic dermatitis (AD). Thus, it is critical to understand their effects on coronavirus disease 2019 (COVID-19) outcomes. We recently reported that patients with AD on dupilumab were more likely to be asymptomatic or have milder COVID-19 symptoms.1 However, the impact of dupilumab and systemic immunosuppressants on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 antibody levels in patients with AD remains unknown. We, thus, evaluated immunoglobulin (Ig)G antibody levels in unvaccinated patients with COVID-19 infection and after messenger RNA (mRNA) vaccination.
As part of a prospective registry related to COVID-19 in the Department of Dermatology at the Icahn School of Medicine at Mount Sinai, we collected serum samples from patients before vaccination and after mRNA vaccination between June 8, 2020 and October 14, 2021. Patients were enrolled under institutional review board–approved consent, and the study was conducted according to the Declaration of Helsinki. Inclusion criteria included being older than 12 years of age with a diagnosis of moderate-to-severe AD, defined as currently or previously on systemic therapy (including dupilumab, phototherapy, or oral immunomodulatory medications), or as candidates for systemic therapy. On the basis of reported COVID-19–related symptoms, each patient was given a COVID-19 symptom severity score from 0 to 2: 0 being “asymptomatic”; 1 being “mild disease” (no fever, no dyspnea, resolving in <7 days, resembling a common cold); and 2 being “moderate disease” (some fever, cough, or other lower respiratory symptoms, resolving at home in 7-14 days).
As we aimed to compare the effects of dupilumab treatment on antibody responses, we only included samples from patients on dupilumab for at least 2 months at the time of sample collection (to ensure enough time and treatment had passed to allow effects of dupilumab to manifest). Patients with positive IgG antibodies lacking vaccination were defined as COVID-19–infected. The SARS-CoV-2 IgG antibody levels were measured using the Mount Sinai Laboratory COVID-19 enzyme-linked immunosorbent assay IgG antibody test, which received emergency use authorization from the Food and Drug Administration (https://www.fda.gov/media/137029/download) but was used for research purposes in this study. Antibody levels were categorized into 4 groups on the basis of the Mount Sinai Laboratory predefined levels: negative (<5 arbitrary unit [AU]/mL), weak (5-15 AU/mL), moderate (16-39 AU/mL), and strong (≥40 AU/mL).
There were 3 treatment groups compared: (1) limited (topical therapy or no active treatment); (2) systemics (broad-acting treatments, namely: Janus kinase [JAK] inhibitors, prednisone, phototherapy); and (3) dupilumab. Antibody group proportions were compared using a 2-sided Fisher test, and log10 antibody level comparison was performed using multivariate linear regression models. Spearman correlations were used to determine whether the postvaccine antibody rate decreases over time.
A total of 54 serum samples (dupilumab, n = 23; systemics, n = 8; limited, n = 23) were collected from different patients before vaccination and 180 samples (dupilumab, n = 101; systemics, n = 15; limited, n = 64) were collected from 180 individuals at least 14 days after the second mRNA vaccine dose (either Pfizer or Moderna) and were included in the analysis. Systemics before vaccination included JAK inhibitors (n = 4), prednisone (n = 2), and phototherapy (n = 2) and after vaccination included JAK inhibitors (n = 9), prednisone (n = 1), and phototherapy (n = 5). No significant differences were observed in terms of age, sex, or race among the 3 treatment groups before vaccination (age: P = .07; sex: P = .10; race: P = .18) or after vaccination (age: P = .26; sex: P = .08; race: P = .45). Among the 54 COVID-19–positive samples prevaccination, decreased symptom severity was associated with lower IgG antibody levels across all treatments, consistent with studies associating more severe COVID-19 with greater SARS-CoV-2 IgG antibody levels.1, 2, 3, 4, 5 Asymptomatic patients and those with mild COVID-19 symptoms exhibited lower antibody titers than those with moderate symptoms (52.5 ± 20.3 vs 96.2 ± 36.1 [mean ± SE]; P = .03). Dupilumab-treated patients with AD had significantly lower antibody levels than those on systemics when comparing both the proportions of weak vs moderate/strong groups (8/23 for dupilumab vs 1/8 for systemics; P = .01) and age-adjusted quantitative antibody levels (29.0 ± 35.7 vs 170.5 ± 54.9 [mean ± SE]; P = .01). A trend toward lower levels was also observed in dupilumab-treated patients vs limited group (8/23 for dupilumab vs 4/23 for limited group for weak vs moderate/strong proportion comparison; P = .09).
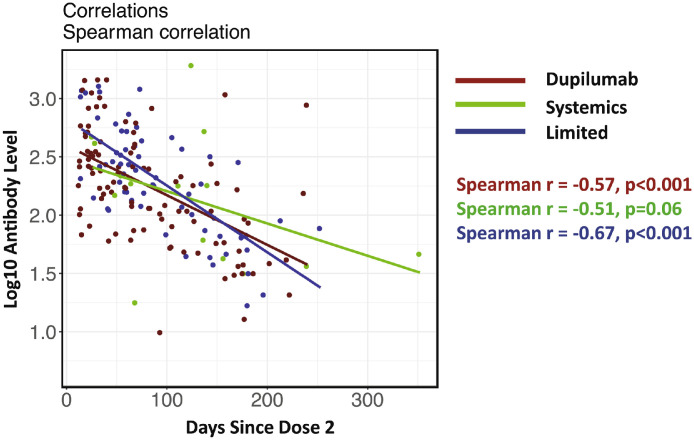
To assess if lower antibody levels were caused by treatment-based modulation of antibody production or from differential responses to the SARS-CoV-2 virus itself, we then assessed antibody levels after mRNA vaccination. Overall, we found similar rate decreases in antibody levels over time among treatment groups (Fig 1 ). Correspondingly, no differences were observed among groups regarding antibody level groups (weak/moderate/strong). Furthermore, using a linear regression model adjusted for age and time after vaccination, we detected no significant differences in antibody concentrations among any treatment groups (P > .18).
Figure 1.
Spearman correlation between log10 antibody levels and days since the second vaccine dose in each treatment group.
Overall, this study found that patients had significantly lower antibody levels after COVID-19 infection when treated with dupilumab vs systemic therapies (P = .01), and lower levels (approaching significance, P = .09) compared with patients receiving limited/no therapy, paralleling our previous finding that dupilumab-treated patients were more likely to have milder symptoms COVID-19 symptoms compared with patients on broad-acting treatments and also those receiving limited/no treatment.1 However, there were no differences in antibody levels among treatment groups after mRNA vaccination. This suggests that dupilumab does not impair antibody responses, but rather reduces COVID-19 symptom severity and downstream IgG levels. The limitations of this study include unknown COVID-19 infection dates (often because of lack of symptoms and, therefore, testing), the smaller number of systemic patients that tested positive to COVID-19 matching our practice prescribing tendencies, and the lack of a control group without AD. Further studies to characterize T cell components of COVID-19 immune responses with different immunomodulatory treatments are needed. Taken together with our previous publication reporting ameliorated COVID-19 symptoms with dupilumab treatment in AD, these results provide reassurance that specific TH2-targeting in patients with AD does not affect antibody levels after mRNA vaccination and supports continuing dupilumab treatment during the COVID-19 pandemic irrespective of vaccination status.
Footnotes
Disclosures: Dr Guttman-Yassky is an employee of Mount Sinai and has received research funds (grants paid to the institution) from Abbvie, Amgen, AnaptysBio, AstraZeneca, Boehringer-Ingelheim, Cara Therapeutics, Innovaderm, Janssen, KAO, Kyowa Kirin, Leo Pharma, Pfizer, Regeneron Pharmaceuticals, Inc, and UCB; and is a consultant for Abbvie, Almirall, Amgen, Arena, Asana Biosciences, AstraZeneca, Boehringer-Ingelheim, Bristol-Meyers Squibb, Cara Therapeutics, Connect Pharma, Eli Lilly, EMD Serono, Evidera, Galderma, Ichnos Sciences, Incyte, Janssen Biotech, Kyowa Kirin, Leo Pharma, Pandion Therapeutics, Pfizer, Ribon, RAPT Therapeutics, Regeneron Pharmaceuticals, Inc, Sanofi, SATO Pharmaceutical, Siolta Therapeutics, Target PharmaSolutions, UCB, and Ventyx Biosciences. Dr Pavel is an employee of the University of Mississippi and has a research contract with Mount Sinai. The remaining authors have no conflicts of interest to report.
Funding: This work was supported by the Department of Dermatology at the Icahn School of Medicine at Mount Sinai and a grant from Regeneron and Sanofi. Patients were recruited from within the Department of Dermatology at the Icahn School of Medicine. All funding sources reviewed and accepted the study design and the manuscript, with minimal input from Regeneron and Sanofi. Research reported in this publication was also supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number U01AI152036. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ungar B, Glickman JW, Golant AK, Dubin C, Marushchak O, Gontzes A, et al. COVID-19 symptoms are attenuated in moderate-to-severe atopic dermatitis patients treated with Dupilumab. J Allergy Clin Immunol Pract. 2022;10(1):134–142. doi: 10.1016/j.jaip.2021.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widjaja G, Turki Jalil A, Sulaiman Rahman H, Abdelbasset WK, Bokov DO, Suksatan W, et al. Humoral immune mechanisms involved in protective and pathological immunity during COVID-19. Hum Immunol. 2021;82(10):733–745. doi: 10.1016/j.humimm.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo L, Wang Y, Kang L, Hu Y, Wang L, Zhong J, et al. Cross-reactive antibody against human coronavirus OC43 spike protein correlates with disease severity in COVID-19 patients: a retrospective study. Emerg Microbes Infect. 2021;10(1):664–676. doi: 10.1080/22221751.2021.1905488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu L, To KK, Chan KH, Wong YC, Zhou R, Kwan KY, et al. High neutralizing antibody titer in intensive care unit patients with COVID-19. Emerg Microbes Infect. 2020;9(1):1664–1670. doi: 10.1080/22221751.2020.1791738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, et al. Antibody responses to SARS-CoV-2 in patients with novel coronavirus Disease 2019. Clin Infect Dis. 2020;71(16):2027–2034. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]