Abstract
The spectrum of pulmonary parenchymal and vascular pathologies related to the COVID-19 have emerged. There is evidence of a specific susceptibility related to thrombotic microangiopathy in situ and a complex immune-inflammatory cascade, especially in the pulmonary vascular bed. The potential to lead to transient or self-correcting sequelae of pulmonary vascular injury will only become apparent with longer-term follow-up. In this review, we aimed to present the findings in a group of patients with severe pneumonia due to covid-19 complicated by acute pe documented by chest angiography, who during a follow-up of more than 3 months with oral anticoagulant met clinical, hemodynamic, and imaging criteria of chronic thromboembolic pulmonary hypertension. We present a brief review of the epidemiology, pathophysiology, clinical findings, comorbidities, treatment, and imaging findings of chronic thromboembolic pulmonary hypertension as a sequel of severe post-covid-19 pneumonia; and compared and discussed these findings with similar reports from the medical literature.
Introduction
As the initial wave of the COVID-19 pandemic has subsided, a spectrum of pulmonary parenchymal, and vascular pathologies related to the acute form of the disease have emerged. The combination of viral pneumonia and acute respiratory distress syndrome that characterizes the severe manifestations of acute COVID-19 disease is considered a possible precursor to pulmonary fibrosis.1 Another potential pulmonary morbidity is the tendency to thromboembolic phenomena, both in the systemic and pulmonary circulation.2 Apart from the risk of venous thromboembolism in hospitalized patients with acute illnesses, there is evidence of a specific susceptibility to COVID-19, related to thrombotic microangiopathy in situ and a complex immune-inflammatory cascade, especially in the pulmonary vascular bed.3 , 4 Many patients with proven COVID-19 thromboembolism have been patient reports in intensive care units, but pulmonary thromboembolism is now being reported in less critical patients in hospital and outpatient settings. Whether such pulmonary vascular injury is transient and self-correcting, or has the potential to lead to sequelae, will only become apparent with longer-term follow-up. Within the limitations of an insufficient knowledge base on the recovery of COVID-19, algorithms have been proposed for follow-up that integrate the performance of studies to identify possible vascular sequelae, and pulmonary interstitial disease.5
In this review, we aimed to present the findings in a group of patients with severe pneumonia due to COVID-19 complicated by acute pulmonary embolism (PE) documented by chest angiography, who during a follow-up of more than 3 months with oral anticoagulant met clinical, hemodynamic, and imaging criteria of chronic thromboembolic pulmonary hypertension (CTEPH). We compared and discussed these findings with similar reports in the medical literature.
Epidemiology
Following initial reports from China in December 2019 of atypical pneumonia caused by a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and confirmation of person-to-person transmission, coronavirus disease 2019 (COVID-19) rapidly progressed to a global pandemic.6 , 7 Several coronaviruses are endemic in the population: these viruses generally cause a mild respiratory tract infection in the winter months, although the disease can be severe in neonates, the elderly, and people with predisposing conditions. The highly pathogenic severe acute respiratory syndrome coronaviruses (SARS-CoV) and the Middle East respiratory syndrome coronavirus (MERS-CoV) caused outbreaks in humans, with a case fatality rate of 9% and 40%, respectively.8 However, these last 2 viruses are not easily transmitted between humans, and therefore containment of the outbreaks was relatively simple. In contrast, SARS-CoV-2 is intermediate in virulence between endemic coronaviruses and highly pathogenic viruses, but its high human-to-human transmissibility has led to the current human pandemic.
Pathophysiology
The development of diffuse interstitial lung disease has been reported in post-COVID-19 patients. An expected complication secondary to interstitial lung disease (ILD) post-COVID-19 is the development of pulmonary hypertension (PH), which has been reported in different publications.9 CTEPH is a rare but significant complication of acute PE. There is a strong and documented causal relationship between COVID-19 and venous thromboembolism. CTEPH results from obstruction of the pulmonary artery bed by organized thrombus after acute or recurrent pulmonary embolism (PE). Its pathogenesis includes small vessel vasculopathy. The resulting increase in pulmonary pressures can lead to right ventricular dysfunction and death.
All vascular wall components from the lumen to the perivascular regions may be affected and contribute to the general pathobiology of COVID-19.10 The lumen may contain fresh microthrombi and chronic thrombotic lesions with acute or chronic inflammatory cells and fibroblasts, showing varying degrees of organization. However, the frequency of microthrombi in COVID-19 patients is surprisingly varied in different case series. Reports in the literature highlight both a high incidence of macrovascular injury (eg, acute PE) and a microvascular injury in COVID-19. In a study by Klok et al.,2 thrombotic complications in 184 intensive care unit (ICU) patients with COVID-19 was 31%. Anecdotal reports from large critical care units in the UK suggest potentially higher numbers. Autopsy findings of the first 12 consecutive deaths from COVID-19 in a single German academic medical center revealed deep vein thrombosis in 58% in whom no venous thromboembolism was suspected, with PE being a direct cause of death in 4 patients; however, COVID-19 has also been associated with microvascular coagulopathy, with findings similar to those seen in disseminated intravascular coagulopathy.11 , 12
Clinical Findings
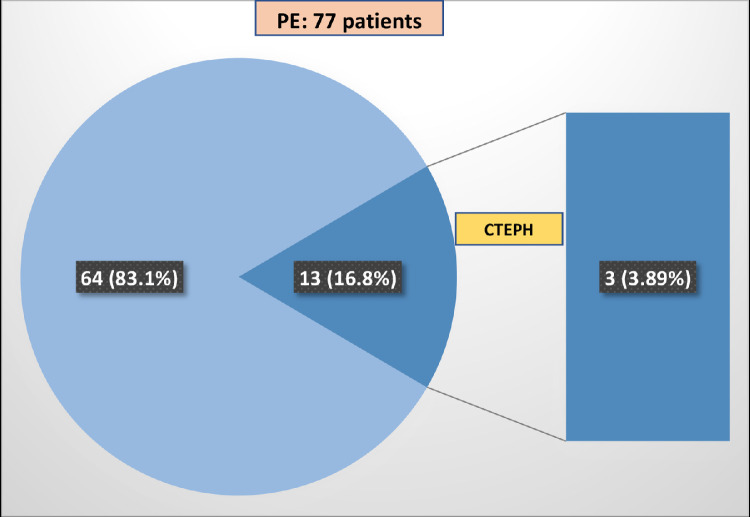
During a period of 11 months (August 2020-July 2021), 77 new cases of PE were documented by CT angiography; in 13 (16.8%), PE was established in the context of severe pneumonia due to COVID-19 with Positive RT-PCR during their hospitalization (Fig 1 ).
FIG 1.
The proportion of patients with PE that developed CTEPH in the context of severe pneumonia due to COVID-19.
According to the pulmonary hypertension guidelines, CTEPH is a type of precapillary PH according to the hemodynamic definition. The working group of the 6th World Symposium on PH recently proposed a new definition of precapillary PH that includes PAPm >20 mm Hg, pulmonary artery wedge pressure <15 mm Hg, and pulmonary vascular resistance (PVR) >3 Wood units (WU).13
All 13 patients were receiving prophylactic anticoagulation at the time of acute PE. Three patients (23.07%) met the criteria for CTEPH, given that they received oral anticoagulation an average of 5 months before performing right heart catheterization (RHC), Table 1 . All patients had dyspnea on exertion, and the perfusion lung scan reported multiple segmental and lobar defects compatible with CTEPH. It should be noted that our 3 patients were free of chronic respiratory manifestations before COVID-19.
TABLE 1.
Clinical features of 3 patients who met the criteria for CTEPH after severe pneumonia due to COVID-19
| Variables | Case 1 | Case 2 | Case 3 |
|---|---|---|---|
| Age / Sex | 45 y / Male | 45 y/Female | 74 y/Female |
| Comorbidities | SAH | MD/SAH | Hypothyroidism/SAH |
| Risk stratification | High medium EP | High-risk EP | High medium EP |
| Treatment of the acute event | LMWH | HBPM + ST (100%) Mechanical thrombo-fragmentation | LMWH |
| Treatment | Rivaroxaban | Rivaroxaban | Rivaroxaban |
| COVID-19-PCR time | Four mo | Six mo | Five mo |
| Actual state | Alive | Alive | Alive |
Comorbidities
Several clinical characteristics and comorbidities are associated with a poor prognosis and an increased risk of mortality from COVID-19. These include pre-existing health problems such as hypertension, diabetes, cardiovascular disease, obesity, cancer, chronic kidney, liver, lung diseases, advanced age, male sex, smoking, and race.14 It is currently unclear if these risk factors are also predictors of longer-term COVID-19 outcomes. The comorbidity reported in the 3 patients was subarachnoid hemorrhage (SAH).
Treatment
We have learned a great deal about the broad spectrum of illnesses due to COVID-19 in the past 18 months, ranging from asymptomatic infection to severe pneumonia, respiratory failure, and death.15 There is growing concern about whether COVID-19 survivors will have long-term lung sequelae, including fibrotic interstitial lung disease, and the presence of pulmonary hypertension.
Using the usual stratification guidelines, 2 patients correspond to high intermediate risk, and one to high risk. Two patients received low-molecular-weight heparin (LMWH) at a 1.5 mg/kg (enoxaparin). The third patient initially received systemic thrombolysis with 100 mg of alteplase; however, he was hypotensive, with amine requirements and significant thrombotic load. He underwent mechanical fragmentation thrombolysis and catheter-guided thrombolysis with an additional injection dose of 10 mg of alteplase. The RHC findings in each patient meet precapillary PH criteria (Figs. 2 , 3 , and 4 and Table 2 ). All patients are still alive.
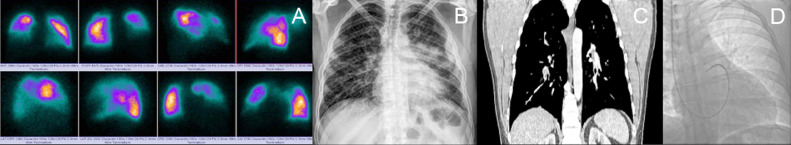
FIG 2.
(A) Perfusion lung scan q shows multiple bilateral segmental and right lobar defects. (B) chest radiograph with predominantly left bilateral opacities. (C) Coronal CT angiography with filling defects in the left lobar and right segmental artery. (D) flotation catheter in pulmonary artery with the hemodynamic report of case 1 in Table 2.
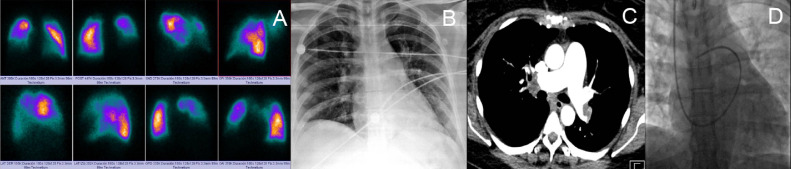
FIG 3.
(A) perfusion lung scan q shows multiple segmental defects. (B) chest radiograph with left opacity. (C) chest angiotomography axial section with intraluminal defect from the main right pulmonary artery extends to the inferior lobar and middle lobar arteries. Left pulmonary artery, with intraluminal defect at the level of the bifurcation toward the upper, and lower lobar with involvement of the segmental ones. (D) flotation catheter in the pulmonary artery and the hemodynamic report of case 2 in Table 2.
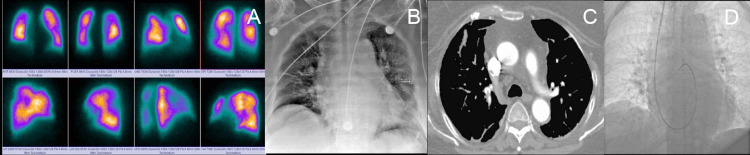
FIG 4.
(A) perfusion lung scan q shows multiple bilateral segmental defects. (B) chest radiograph with cardiomegaly and predominantly left bilateral infiltrate. (C) axial CT angiography with filling defects in the right basal segmental artery. (D) flotation catheter in the pulmonary artery, and the hemodynamic report of case 3 in Table 2.
TABLE 2.
RHC findings in 3 patients who met the criteria for precapillary PH after severe pneumonia due to COVID-19
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| HR | 73 | 69 | 75 |
| RA mm Hg | 1 | 2 | 3 |
| RVs/d mm Hg | 84/5 | 52/2 | 54/1 |
| APs/d/m mm Hg | 54/18/42 | 53/23/29 | 54/18/35 |
| CI l/min/m2 | 3 | 3 | 3.7 |
| PCWP mm Hg | 4 | 10 | 13 |
| PVR mm Hg | 6.6 | 3.7 | 3.3 |
| PvO2 mm Hg | 39 | 40 | 39 |
| SvO2 mm Hg | 74% | 65% | 62% |
| PaO2 mm Hg | 69 | 75 | 64 |
| SaO2 mm Hg | 93% | 94% | 89% |
Imaging Findings
A main pulmonary artery (PA) diameter greater than 30-31 mm should raise the suspicion of PH; however, in the context of established pulmonary fibrosis, a ratio between the pulmonary artery, and adjacent ascending aorta >1.1 may be a more reliable predictor.16 It has also been shown that a greater pulmonary artery to bronchus ratio in at least 3 vessels has a high specificity for PH diagnosis.17 A ratio between the right ventricle and the left ventricle (RV: LV) >1.0 has been shown consistently as a helpful risk stratification biomarker and predictor of poor outcome in acute PE, CTEPH, and interstitial lung disease; however, the R: LV relationship is substantially underreported, as highlighted in the 2019 National Confidential Outcome and Death Investigation.18 , 19 Together with reflux of contrast medium in the hepatic veins, these findings can be present in all forms of PH, regardless of etiology. In CTED and CTEPH, the pulmonary arteries should be checked for vascular networks, and occlusions. DECT can also show multiple peripheral perfusion defects. The lung parenchyma can often show mosaic attenuation with geographic areas of low attenuation associated with peripheral scarring, the residue of previous infarction. With CT and scintigraphy, subtle pulmonary vascular abnormalities, particularly those seen in the more distal chronic thromboembolic disease, can be challenging to detect. The study of suspected PH, ventilation, and perfusion (VQ) should be considered a screening tool image.
In addition to changes in the lung parenchyma, such as diffuse alveolar damage with hyaline membranes and acute pneumonia, significant vascular changes have been observed in patients with COVID-19. Vascular changes affect the entire pulmonary vascular tree, from large-caliber vessels to capillaries.20 In patients with severe COVID-19, enlargement of the large vessels proximal to lung opacities was seen on pulmonary angiograms during the acute phase of the disease. In a UK study that also used CT pulmonary angiography, 21 of 33 COVID-19 patients and at least 2 evaluable lobes with no evidence of acute pulmonary emboli had dilated peripheral vessels, with 100% of patients showing defects of the perfusion on dual-energy CT, suggesting microvascular dysfunction.
Discussion
Every physician following post-COVID-19 patients must keep the possibility of PH in mind. CTEPH is particularly underdiagnosed; in 2015, it was estimated that, on average, only 16% of patients in the United States, Europe, and Japan who had CTEPH were diagnosed.21
Our findings in this study represent the first series of patients described in the literature that reflects the local incidence of CTEPH in a series of patients with acute PE after COVID-19 pneumonia and who, during follow-up, meet the criteria to consider CTEPH. The problems will continue because we do not know if the current standards for acute PE that evolve to CTEPH should be used. We also do not know if the benefits of pulmonary balloon angioplasty will be superior to pulmonary endarterectomy due to the segmental or subsegmental location described in PD associated with COVID-19. Finally, what is the role of specific drug treatment in this group of patients? They will be candidates for guanylate cyclase stimulators, benefiting from phosphodiesterase 5 inhibitors or endothelin antagonists. What anticoagulant will be the ideal? Now our 3 patients are receiving phosphodiesterase 5 inhibitors and anticoagulation permanently. Finally, we will have to wait for new similar works where the true incidence of HPTEC can be known. Of the total number of patients with acute PE in 11 months (77 Patients), Three (3.8%) met the CTEPH criteria, which is like the original series reported by Pengo.22
Therefore, it seems reasonable to suggest that, although patients may develop acute PE secondary to severe illness and hospitalization, the pronounced inflammatory response seen in a cohort of COVID-19 patients results in a marked prothrombotic tendency. There is not enough literature on the use of dual-energy CT (DECT) in COVID-19 to know whether this technique provides an additional assessment of microvascular disease in the form of “perfusion abnormalities” on iodine maps; however, Lang et al.23 highlighted the presence of perfusion defects (in the absence of visible PE) in DECT in 12 patients.
In the experience of our group, most of our patients with COVID-19 and PD have often developed PE in segmental and subsegmental vessels rather than in main or lobar pulmonary arteries. It is possible (but still unknown) that people recovering from COVID-19 may develop the chronic thromboembolic disease (CTED), CTEPH, and pulmonary hypertension (PH) secondary to lung disease, for example, diffuse interstitial lung disease.
As can be seen, the emerging landscape of intense interaction between COVID-19 and chronic pulmonary hypertension globally is expected to have a significant effect on the future of both diseases.
Footnotes
Declaration of Competing Interest: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Spagnolo P, Balestro E, Aliberti S, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. 2020;8:750–752. doi: 10.1016/S2213-2600(20)30222-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klok FA, Kruip M, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lax SF, Skok K, Trauner M. Pulmonary arterial thrombosis as an important complication of COVID-19 pulmonary disease: letter to the editor. Virchows Arch. 2020;477:467–468. doi: 10.1007/s00428-020-02896-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teuwen LA, Geldhof V, Pasut A, Carmeliet P. Author Correction: COVID-19: the vasculature unleashed. Nat Rev Immunol. 2020;20:448. doi: 10.1038/s41577-020-0356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dhawan RT, Gopalan D, Howard L, et al. Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med. 2021;9:107–116. doi: 10.1016/S2213-2600(20)30407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Y, Grunewald M, Coronaviruses Perlman S. An updated overview of their replication and pathogenesis. Methods Mol Biol. 2020;2203:1–29. doi: 10.1007/978-1-0716-0900-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cueto-Robledo G, Porres-Aguilar M, Puebla-Aldama D, et al. Severe pulmonary hypertension: an important sequel after severe post-acute COVID-19 pneumonia. Curr Probl Cardiol. 2022;47 doi: 10.1016/j.cpcardiol.2021.101004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wichmann D. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. 2020;173:1030. doi: 10.7326/L20-1206. [DOI] [PubMed] [Google Scholar]
- 12.Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135:2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frost A, Badesch D, Gibbs JSR, Gopalan D, Khanna D, Manes A, et al. Diagnosis of pulmonary hypertension. Eur Respir J. 2019;53 doi: 10.1183/13993003.01904-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York city area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grosse A, Grosse C, Lang I. Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One. 2018;13 doi: 10.1371/journal.pone.0201468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Devaraj A, Wells AU, Meister MG, Corte TJ, Wort SJ, Hansell DM. Detection of pulmonary hypertension with multidetector CT and echocardiography alone and in combination. Radiology. 2010;254:609–616. doi: 10.1148/radiol.09090548. [DOI] [PubMed] [Google Scholar]
- 18.Ema R, Sugiura T, Kawata N, et al. The dilatation of main pulmonary artery and right ventricle observed by enhanced chest computed tomography predict poor outcome in inoperable chronic thromboembolic pulmonary hypertension. Eur J Radiol. 2017;94:70–77. doi: 10.1016/j.ejrad.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Srivastava V, McPherson SJ, Smith NCE, Koomson D, Mason M. National Confidential Enquiry into Patient Outcome and Death (NCEPOD); London, Uk: 2019. The National Confidential Enquiry into Patient Outcome and Death. Know the Score.https://www.ncepod.org.uk/about.html Accessed at: March 20, 2022. Accessed from: [Google Scholar]
- 20.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gall H, Hoeper MM, Richter MJ, Cacheris W, Hinzmann B, Mayer E. An epidemiological analysis of the burden of chronic thromboembolic pulmonary hypertension in the USA, Europe and Japan. Eur Respir Rev. 2017;26 doi: 10.1183/16000617.0121-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med. 2004;350:2257–2264. doi: 10.1056/NEJMoa032274. [DOI] [PubMed] [Google Scholar]
- 23.Lang M, Som A, Mendoza DP, et al. Hypoxaemia related to COVID-19: vascular and perfusion abnormalities on dual-energy CT. Lancet Infect Dis. 2020;20:1365–1366. doi: 10.1016/S1473-3099(20)30367-4. [DOI] [PMC free article] [PubMed] [Google Scholar]