Abstract
The contribution of the dosage of target enzyme P-450 14α-demethylase (14αDM) to fluconazole resistance in both Candida albicans and Saccharomyces cerevisiae remains unclear. Here, we show that overexpression of Saccharomyces P-450 14αDM in S. cerevisiae, under the control of the regulatable promoter GAL1, results in azole resistance.
Fluconazole, a useful azole antifungal, selectively inhibits the cytochrome P-450-dependent C-14 lanosterol α-demethylase or CYP51A1, encoded by the ERG11 gene (13) in Candida albicans. Lanosterol 14α-demethylase (14αDM) is a key enzyme in ergosterol biosynthesis in both C. albicans and Saccharomyces cerevisiae, a genetically tractable fungus closely related to C. albicans (3, 10, 13).
Three mechanisms of fluconazole resistance that often operate simultaneously in C. albicans have been described: reduced accumulation of fluconazole, a defect in Δ5,6 desaturation, and target-site (CYP51A1) alterations (13). The last mechanism can be the result of either point mutations of ERG11 (13) followed by loss of demethylation activity or high levels of CYP51A1 caused by the overexpression of ERG11 (13). The role of point mutations of ERG11 in fluconazole-resistant isolates of C. albicans has been documented (7, 11). However, the contribution of overexpression of ERG11 to azole resistance has been less clear (13). In the few fluconazole-resistant isolates of C. albicans where ERG11 is overexpressed that have been studied, the relatively low level of overexpression of ERG11, the concomitant presence of point mutations in ERG11, and the frequent overexpression of efflux pumps such as CDR or MDR1 complicate the matter (9, 12, 13).
Even though the importance of point mutations in the S. cerevisiae gene ERG11 which encodes for the P-450 14αDM or Erg11p (10) that results in the loss of 14αDM activity has been shown, the contribution of overexpression of P-450 14αDM in Saccharomyces is less clear (4). Heterologous overexpression and complementation with the closely related (5) C. albicans CYP51A1 in azole-sensitive Saccharomyces strains resulted in low and variable levels of overexpression; the effect of overexpression on azole resistance as measured by the MICs in liquid medium has been small (4, 5). As such, the effect of the level of P-450 14αDM on azole resistance in both C. albicans and S. cerevisiae remains unclear.
In the present study, we show that overexpression of Erg11p under the control of the GAL1 promoter results in azole resistance. The regulation of the genes required to metabolize galactose in S. cerevisiae has been extensively studied (2). If cells are growing on glucose, the expression of these genes is repressed (2). The regulation of GAL1 expression by carbon sources and the high expression of GAL1 in galactose from low levels in glucose makes GAL1 an effective regulatable promoter (2).
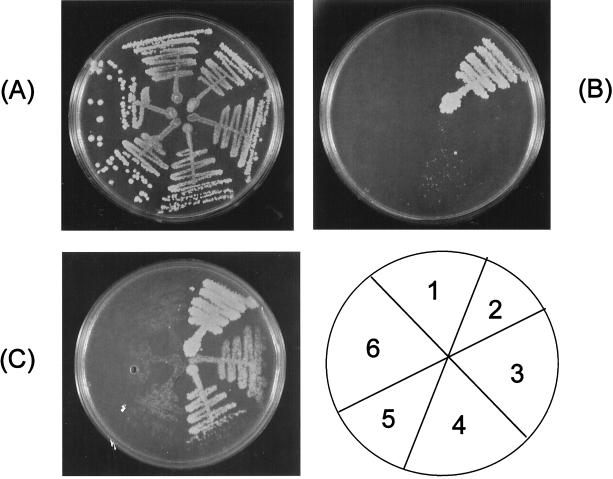
We transformed the Saccharomyces wild-type strain 10560-14C (MATa ura3-52 leu2::hisG his3::hisG) (Fink Laboratory, Whitehead Institute for Biomedical Research, Cambridge, Mass.) with a Saccharomyces URA3-based cDNA library (Fink Laboratory) under the control of the GAL1 promoter (cloned to centromeric plasmid PRS 316 [2, 8]) and selected the Ura+ transformants in synthetic complete medium lacking uracil (SC-uracil)-glucose plates. We used standard methods to prepare the yeast growth medium and to manipulate yeast (2). We then replica plated to SC-uracil–galactose plates, and we incubated these plates for 24 h at 30°C in order to allow the plasmid-dependent expression of cDNA in galactose medium. We then replica plated to SC-uracil–galactose–fluconazole (128 μg/ml) plates and looked for fluconazole-resistant colonies after 48 h of incubation. (Previous pilot experiments determined that the 10560-14C strain transformed by the URA3 centromeric plasmid PRS 316 fails to grow in SC-uracil–galactose–fluconazole (32 μg/ml) medium. We then retested the purified candidates by streaking them on SC-uracil–glucose–fluconazole (128 μg/ml) plates and on SC-uracil–galactose–fluconazole (128 μg/ml) plates. The true positive candidates were fluconazole sensitive and fluconazole resistant, respectively. We then cloned and sequenced one of the cDNA clones with the primer GAL1 (5′ TGGATAACCACTTTAACT 3′; position 690 to 707) that, when overexpressed, results in resistance to fluconazole. We found that the insert contained the ERG11 sequence. We then subcloned the insert into pBluescript SK(−) (Stratagene, La Jolla, Calif.) (1). The clone contained the full-length ERG11 cDNA and had no point mutations. This ERG11 cDNA was then retransformed to the wild-type S. cerevisiae 10560-14C. The transformants were, as was the initial 10560-14C–GAL1 cDNA clone, resistant to fluconazole in SC-uracil–galactose medium but not in SC-uracil–glucose medium. The following haploid strains (numbered 1 to 6) are shown in Fig. 1: 10560-14C transformed by PRS 316, a fluconazole-sensitive control (strain 1), 10560-14C–ERG11 cDNA, two independent colonies (strains 3 and 4), and 10560-14C transformed by two additional random GAL1 cDNAs that did not confer resistance to fluconazole, both controls (strains 5 and 6). PDR1-100, a fluconazole-resistant mutant transformed with the plasmid PRS 316, was used as the fluconazole-resistant control (strain 2). This mutant has a point mutation in the regulatory gene PDR1, and it overexpresses Pdr5p (6). PDR5 encodes for an ATP-binding cassette transporter whose overexpression is well known to be involved in azole resistance (3, 4). As shown in Fig. 1B and C, the overexpression of ERG11 cDNA in galactose results in fluconazole resistance. The GAL1 ERG11 cDNA also exhibited slight growth in SC-uracil–glucose–fluconazole (128 μg/ml) (Fig. 1B). The microcolonies seen predominantly in strain 4 but also in strain 3 (both ERG11 cDNA strains) could represent point mutations that result in ERG11 overexpression in a GAL1-independent fraction. Since this growth was not seen in the controls, this phenomenon could alternatively imply a partial degree of expression of GAL1 ERG11 cDNAs in glucose. The shadows seen in strains 1, 5, and 6 (fluconazole-sensitive controls) in the SC-uracil–galactose–fluconazole (128 μg/ml) plate (Fig. 1C) are a reflection of the density of the replica plating and do not constitute real growth. All growth was aerobic at 30°C for 2 days.
FIG. 1.
GAL1 ERG11 cDNA confers a galactose-dependent resistance to fluconazole. The growth responses of the GAL1 ERG11 cDNA clone in the following media are shown: SC-uracil–glucose (A), SC-uracil–glucose–fluconazole (128 μg/ml) (B), and SC-uracil–galactose–fluconazole (128 μg/ml) (C). The schematic drawing of a plate (lower right) indicates the location of the plated strains (1 to 6). The strains are described in the text.
This work supports the concept that overexpression of Erg11p may result in azole resistance. This regulated system of Erg11p overexpression (galactose = on; glucose = off) may provide an additional tool for the dissection of the interrelated mechanisms of azole resistance in Saccharomyces. The implications of this study for overexpression of the Candida Erg11p in C. albicans need to be addressed with future work.
Acknowledgments
This work was supported by the Cancer Center (Core) Grant (CA16672) from The University of Texas M. D. Anderson Cancer Center to D.P.K. and by NIH1 RO1 GM 57427 to K.H. We thank NIH-CHRC 5P30 HD 27823 for sequence analysis.
We thank Denise Barrientos for excellent secretarial support.
REFERENCES
- 1.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: Greene Publishing Associates/Wiley Interscience; 1998. [Google Scholar]
- 2.Guthrie C, Fink G R, editors. Guide to yeast genetics and molecular biology. New York, N.Y: Academic Press; 1991. [Google Scholar]
- 3.Joseph-Horne T, Hollomon D W. Molecular mechanisms of azole resistance in fungi. FEMS Microbiol Lett. 1997;149:141–149. doi: 10.1111/j.1574-6968.1997.tb10321.x. [DOI] [PubMed] [Google Scholar]
- 4.Kelly S L, Arnoldi A, Kelly D E. Molecular genetic analysis of azole antifungal mode of action. Biochem Soc Trans. 1993;21:1034–1038. doi: 10.1042/bst0211034. [DOI] [PubMed] [Google Scholar]
- 5.Kirsh D R, Lai M H, O’Sullivan J. Isolation of the gene for cytochrome P450 L1A1 (lanosterol 14α-demethylase) from Candida albicans. Gene. 1988;68:229–237. doi: 10.1016/0378-1119(88)90025-x. [DOI] [PubMed] [Google Scholar]
- 6.Kontoyiannis, D. P. Pdr5p could modulate sterol homeostasis in Saccharomyces cerevisiae. Submitted for publication.
- 7.Lamb D C, Kelly D E, Schunk W-H, Shyadehi A Z, Akhtar M, Lowe D J, Baldwin B C, Kelly S L. The mutation T315A in Candida albicans sterol 14α-demethylase causes reduced enzyme activity and fluconazole resistance through reduced affinity. J Biol Chem. 1997;272:5682–5688. doi: 10.1074/jbc.272.9.5682. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Krizek J, Bretscher A. Construction of a GAL1-regulated yeast cDNA expression library and its application to the identification of genes whose overexpression causes lethality in yeast. Genetics. 1992;132:665–673. doi: 10.1093/genetics/132.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez-Ribot J L, McAtee R K, Lee L N, Kirkpatrick W R, White T C, Sanglard D, Patterson T F. Distinct patterns of gene expression associated with development of fluconazole resistance in serial Candida albicans isolates from human immunodeficiency virus-infected patients with oropharyngeal candidiasis. Antimicrob Agents Chemother. 1998;42:2932–2937. doi: 10.1128/aac.42.11.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paltauf F, Kohlwein S D, Henry S A. Regulation and compartmentalization of lipid synthesis in yeast. In: Jones E W, Pringle J R, Broach J R, editors. The molecular and cellular biology of the yeast Saccharomyces. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 415–500. [Google Scholar]
- 11.Sanglard D, Ischer F, Koymans L, Bille J. Amino acid substitutions in the cytochrome P-450 lanosterol 14α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob Agents Chemother. 1998;42:241–253. doi: 10.1128/aac.42.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.White T C. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1997;41:1482–1487. doi: 10.1128/aac.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.White T C, Marr K A, Bowden R A. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev. 1998;11:382–402. doi: 10.1128/cmr.11.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]