Abstract
A common consequence of acute ischemic stroke (AIS), stroke-associated pneumonia (SAP), might result in a poor prognosis after stroke. Based on the critical position of inflammation in SAP, this study aimed to explore the correlation between platelet-to-lymphocyte ratio (PLR) and the occurrence of SAP. We included 295 patients with acute ischemic stroke, 40 with SAP, and 255 without SAP. The area under the receiver operating characteristic curve was used to determine the diagnostic value of SAP risk factors using binary logistic regression analysis. The comparison between the two groups showed that age, the baseline National Institutes of Health Stroke Scale (NIHSS) score, and the proportion of dysphagia, atrial fibrillation, and total anterior circulation infarct were higher, and the proportion of lacunar circulation infarct was lower in the SAP group (P < 0.001). In terms of laboratory data, the SAP group had considerably greater neutrophil counts and PLR, while the non-SAP group (P < 0.001) had significantly lower lymphocyte counts and triglycerides. Binary logistic regression analysis revealed that older age (aOR = 1.062, 95% CI: 1.023–1.102, P = 0.002), atrial fibrillation (aOR = 3.585, 95% CI: 1.605–8.007, P = 0.019), and PLR (aOR = 1.003, 95% CI: 1.001–1.006, P = 0.020) were independent risk factors associated with SAP after adjusting for potential confounders. The sensitivity and specificity of PLR with a cutoff value of 152.22 (AUC: 0.663, 95% CI: 0.606–0.717, P = 0.0006) were 57.5% and 70.6%, respectively. This study showed that high PLR is an associated factor for SAP in AIS patients. Increased systemic inflammation is linked to SAP in ischemic stroke. Inflammatory biomarkers that are easily accessible may aid in the diagnosis of high-risk SAP patients.
1. Introduction
Stroke is a common and leading cause of death and disability worldwide, imposing a heavy burden on society and the economy [1, 2]. Stroke-associated pneumonia (SAP) often occurs after a stroke; approximately 7%–38% [3–8] of stroke patients are diagnosed with SAP during hospitalization. Once a patient develops SAP, it leads to a poor prognosis and a prolonged hospital duration [9–12].Early diagnosis and preventive treatment are necessary for patients at high risk of SAP. Therefore, an effective biomarker is needed for risk assessment and early detection of SAP. Neurogenic immunosuppression is one of the critical mechanisms of increased susceptibility to postinfection [13, 14].
Platelet-to-lymphocyte ratio (PLR) is a novel and inexpensive biomarker that reflects systemic inflammation response [15, 16], which is affected by changes in lymphocytes and platelets after acute stroke. PLR is a sensitive marker to predict the occurrence and prognosis of cardiovascular events [16, 17], cerebrovascular diseases [18], poststroke depression [19],and pneumonia [20]. However, the association of PLR with SAP is uncertain. In light of this, we investigated the relationship of PLR with SAP in AIS patients.
In the proposed investigation, we discovered that high PLR is linked to SAP in AIS patients. PLR could be a useful tool for identifying high-risk SAP patients. It is more beneficial to improve results if SAP is detected early and well prevented. PLR was found to be considerably greater in the SAP group than in the non-SAP group in this study.
In the following section, we discuss the section-wise study. Section 2 discusses the materials and methods and also discusses the study subjects, clinical variables collection, laboratory parameters and clinical features, statistical analysis, and results. Section 3 defines the conclusion of the study. Finally, the research work is concluded in Section 4.
2. Materials and Methods
The overall quality of any research dissemination output is evaluated in this section and it reveals to readers what techniques, methodologies, designs, and treatments are used in the research, allowing us to repeat the results.
2.1. Study Subjects
Acute ischemic stroke patients in the Department of Neurology, Beijing Shijitan Hospital, were included in this study between March 1, 2018, and July 31, 2019. The study protocol was approved by the Scientific Research Ethics Committee of Beijing Shijitan Hospital Affiliated with Capital Medical University.
The inclusion criteria for enrolled patients are as follows:
Hospitalization within 72 h after the onset of symptoms
Patients diagnosed as new acute ischemic stroke by imaging examination (cranial magnetic resonance imaging or computed tomography) at the time of admission
Modified Rankin scale (mRS) score<2 before admission
Patients who were not discharged within 3 days before hospitalization
The exclusion criteria are as follows:
The acute infections (vacuities, urinary, and infection of the upper or lower respiratory tract) within 2 weeks before the onset of the stroke
Medical history of cancer, hematologic disease, severe liver and kidney dysfunction, and autoimmune disease
2.2. Clinical Variables Collection
Demographic data obtained at admission include age, sex, and the length of hospitalization. Premorbid history relevant risk factors contain current smoking, coronary heart disease, hypertension, diabetes, previous stroke, and atrial fibrillation.
2.3. Laboratory Parameters and Clinical Features
Within 24 h after admission, peripheral venous blood was collected from the participants and used for immediate biomarker detection. Laboratory data consisted of neutrophils, lymphocytes, platelets, total cholesterol (TC), triglycerides (TG), and low-density lipoprotein cholesterol (LDL-C). The PLR (defined as platelet/lymphocyte) was calculated. The clinical features of patients included dysphagia, the Oxfordshire Community Stroke Project (OCSP), and baseline National Institutes of Health Stroke Scale (NIHSS) score on admission.
According to clinical and laboratory examinations and chest X-ray or CT scan results retrospectively gathered from medical records, SAP was defined as a lower respiratory tract infection within the first 7 days of hospitalization after a stroke [21, 22].
2.4. Statistical Analysis
Normally distributed continuous variables were expressed as the mean ± standard deviation, while nonnormally distributed variables were expressed as the median (interquartile range). The Mann–Whitney U test was used to compare the clinical characteristics of continuous variables between groups. The chi-square test was used for categorical variables. Variables with P < 0.05 from the results of the univariate analyses were considered confounders in the multivariable logistic regression analysis. The association between different PLR and SAP risks was estimated using univariate analysis and binary logistic regression analysis. All patients were classified into two groups according to their median PLR (126.67), and the potential confounders of PLR were identified. We adjusted all potential confounding factors (parameters with P < 0.05 in univariate analysis). The results are presented in the form of an adjusted odds ratio (aOR) and 95% confidence interval (CI).
We conducted the receiver operative curve (ROC) and the area under the curve (AUC) to measure the specificity and sensitivity of PLR and obtained the optimal cutoff value of PLR. Results were considered significant at P < 0.05. The analysis of ROC was performed with MedCalc 15.6 (MedCalc Software, Belgium). Other statistical analyses were carried out using SPSS v23 (SPSS Inc., Chicago, IL).
3. Results
A total of 295 consecutive AIS patients were retrospectively included from March 1, 2018, and July 31, 2019, according to the inclusion and exclusion criteria, 40 (13.6%) of whom were diagnosed with SAP in the final analysis (Figure 1). The average age of patients was 69.5 ± 12.0 years, and 196 (66.4%) were male. The median length of hospitalization was 14.0 (11.0, 17.0) days. The comparison of demographic, clinical, and laboratory characteristics is given in Table 1. There were significant differences in age (P < 0.001) and baseline NIHSS score (P < 0.001) between the SAP group and the non-SAP group. The presence of dysphagia (P < 0.001), atrial fibrillation (P < 0.001), total anterior circulation infarction (TACI) (P < 0.001), and lacunar circulation infarction (LACI) (P < 0.001) were significantly different between the two groups. Neutrophil counts and PLR were significantly increased (P ≤ 0.001; Figure 2), whereas lymphocyte counts and TG were significantly decreased in the SAP group (P < 0.001). The number of platelets in the patients with SAP was decreased, although not statistically significant.
Figure 1.
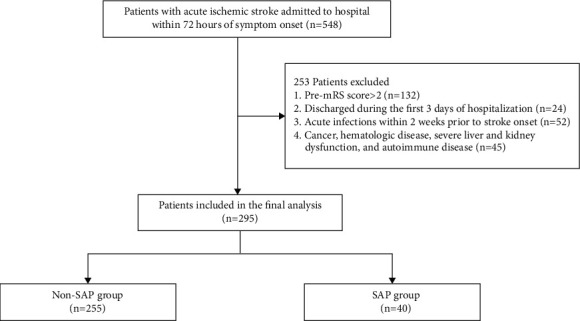
Flow diagram of the patient selection process.
Table 1.
Comparison of the characteristics of AIS patients according to the onset of SAP.
| Characteristics | Total (N = 295) | Non-SAP (N = 255) | SAP (N = 40) | P value |
|---|---|---|---|---|
| Age, years, mean ± SD | 69.5 ± 12.0 | 68.1 ± 11.7 | 77.5 ± 10.6 | <0.001∗ |
| Male, n (%) | 196 (66.4) | 171 (67.1) | 25 (62.5) | 0.570 |
| Length of hospitalization, days, median (IQR) | 14.0 (11.0, 17.0) | 14.0 (11.0, 16.0) | 14.5 (9.0, 33.0) | 0.241 |
| Baseline NIHSS, median (IQR) | 3 (1, 7) | 3 (1, 5) | 14 (7, 18) | <0.001∗ |
| Dysphagia, n (%) | 49 (16.6) | 15 (5.8) | 34 (85.0) | <0.001∗ |
| Current smoking, n (%) | 96 (32.5) | 85 (33.3) | 11 (27.5) | 0.464 |
| Coronary artery disease, n (%) | 88 (29.8) | 72 (28.2) | 16 (40.0) | 0.131 |
| Hypertension, n (%) | 229 (77.6) | 196 (76.9) | 33 (82.5) | 0.426 |
| Diabetes, n (%) | 106 (35.9) | 96 (37.6) | 10 (25.0) | 0.121 |
| Previous stroke, n (%) | 81 (27.5) | 66 (25.9) | 15 (37.5) | 0.126 |
| Atrial fibrillation, n (%) | 46 (15.6) | 29 (11.4) | 17 (42.5) | <0.001∗ |
| OCSP | ||||
| TACI, n (%) | 15 (5.1) | 5 (2.0) | 10 (25.0) | <0.001∗ |
| PACI, n (%) | 90 (30.5) | 78 (30.6) | 12 (30.0) | 0.940 |
| POCI, n (%) | 39 (13.2) | 30 (11.8) | 9 (22.5) | 0.062 |
| LACI, n (%) | 151 (51.2) | 142 (55.7) | 9 (22.5) | <0.001∗ |
| TC, mmol/L, median (IQR) | 4.2 (3.5, 5.0) | 4.3 (3.6, 5.0) | 4.0 (3.1, 4.9) | 0.086 |
| TG, mmol/L, median (IQR) | 1.2 (0.9, 1.7) | 1.3 (1.0, 1.8) | 0.9 (0.7, 1.3) | <0.001∗ |
| LDL-C, mmol/L, median (IQR) | 2.4 (1.8, 3.0) | 2.4 (1.8, 3.0) | 2.2 (1.8, 2.8) | 0.306 |
| Neutrophils, ×109/L, median (IQR) | 4.3 (3.3, 6.0) | 4.0 (3.1, 5.3) | 7.3 (6.0, 9.6) | <0.001∗ |
| Lymphocytes, ×109/L, median (IQR) | 1.6 (1.3, 2.1) | 1.8 (1.4, 2.1) | 1.1 (0.9, 1.6) | <0.001∗ |
| PLT, ×109/L, median (IQR) | 216.0 (179.0, 253.0) | 216.0 (180.0, 253.0) | 210.5 (152.3, 247.8) | 0.137 |
| PLR, median (IQR) | 126.7 (99.6, 175.3) | 123.2 (98.0, 163.8) | 161.2 (114.1, 209.2) | 0.001∗ |
NIHSS, National Institute of Health Stroke Scale; OCSP, Oxfordshire Community Stroke Project; TACI, total anterior circulation infarct; PACI, partial anterior circulation infarct; POCI, posterior circulation infarct; LACI, lacunar circulation infarct; TC, total cholesterol; TG, triglycerides; LDL-C, low-density lipoprotein cholesterol; PLT, platelet; PLR, platelet-to-lymphocyte ratio; SD, standard deviation; IQR, interquartile range; SAP, stroke-associated pneumonia. ∗P values less than 0.05 were considered statistically significant.
Figure 2.
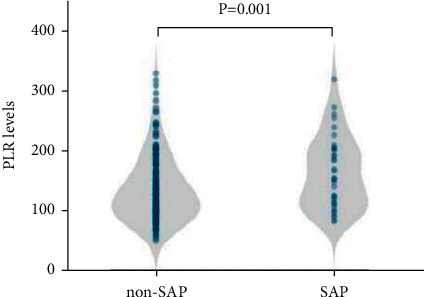
PLR levels and SAP. Violin plot of PLR distribution in patients with SAP and without SAP.
The demographic and clinical characteristics (parameters with P < 0.05 in univariate analysis) in the high and low PLR groups are given in Table 2. We found that patients with the high PLR had high baseline NIHSS scores (P = 0.047) and low TG (P = 0.004). The prevalence of dysphagia (P = 0.020) was significantly higher in the high PLR group than in the low PLR group.
Table 2.
Comparison of the characteristics between subgroups based on the median PLR.
| Characteristics | Low PLR | High PLR | P value |
|---|---|---|---|
| N = 147 (PLR<126.67) | N = 148 (PLR≥126.67) | ||
| Age, years, mean ± SD | 68.8 ± 12.2 | 70.0 ± 11.7 | 0.346 |
| Baseline NIHSS, median (IQR) | 3 (1, 5) | 4 (2, 8) | 0.047∗ |
| Dysphagia | 17 (11.6) | 32 (21.6) | 0.020∗ |
| Atrial fibrillation, n (%) | 17 (11.6) | 29 (19.6) | 0.057 |
| TG, mmol/L, median (IQR) | 1.2 (1.0, 1.8) | 1.1 (0.8, 1.6) | 0.004∗ |
NIHSS, National Institute of Health Stroke Scale; TG, triglycerides; PLR, platelet-to-lymphocyte ratio; SD, standard deviation; IQR, interquartile range. ∗P values less than 0.05 were considered statistically significant.
In the univariate analysis, the variables with P < 0.05 were used as covariates, and SAP was used as a dependent variable. As given in Table 3, after adjusting for the confounders of PLR, multivariate logistic regression analysis showed that PLR (aOR = 1.003, 95% CI: 1.001–1.006, P = 0.020), age (aOR = 1.062, 95% CI: 1.023–1.102, P = 0.002), and atrial fibrillation (aOR = 3.585, 95% CI: 1.605–8.007, P = 0.019) remained associated with SAP. In the ROC analysis, the best cutoff level of PLR was 152.22 (P = 0.0006), with 0.575 sensitivity and 0.706 specificity. The area under the ROC curve was 0.663 (95% CI: 0.606–0.717) (Figure 3).
Table 3.
Multivariate logistic regression analysis of the associations between PLR and SAP.
| Risk factors | B | SE | Wald | P value | aOR | aOR (95% CI) |
|---|---|---|---|---|---|---|
| Age | 0.060 | 0.019 | 9.819 | 0.002∗ | 1.062 | 1.023–1.102 |
| Atrial fibrillation | 1.277 | 0.410 | 9.699 | 0.019∗ | 3.585 | 1.605–8.007 |
| PLR | 0.003 | 0.002 | 5.393 | 0.020∗ | 1.003 | 1.001–1.006 |
PLR, platelet-to-lymphocyte ratio; SAP, stroke-associated pneumonia; SE, standard error; aOR, adjusted odds ratio. ∗P values less than 0.05 were considered statistically significant.
Figure 3.
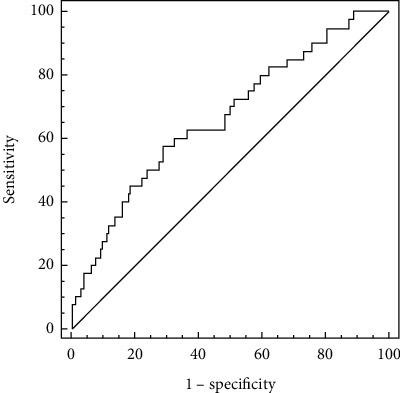
The receiver operating characteristic (ROC) curve analysis of platelet-to-lymphocyte ratio for predicting SAP.
4. Discussion
Based on increasing evidence, researchers reported the predictive effectiveness of PLR in the prognosis of artery diseases [16–18] and inflammation diseases. With reference to the studies by Turkmen et al. and Uslu et al. [23, 24] in the present study, we found that high PLR is associated with SAP in AIS patients. The results of multivariate analysis showed that PLR, a marker of systemic inflammation, is an independent risk factor for SAP, which further confirmed high PLR may be used as an effective indicator of inflammatory diseases.
Platelets have the function of regulating inflammation and immune responses, which play an important role in the formation of thrombosis in the arterial circulation. When AIS occurs, abnormal platelets are overactivated and accumulated [25], which may lead to thrombosis and vascular blockage and then vascular events [26]. Changes in circulating platelet counts are uncertain in diverse diseases. Platelets can increase in many inflammations with an acute phase. In the case of sepsis, the platelet count will increase due to the accelerated expression of platelets after the increase in their breakdown [27]. However, we found the platelet counts were decreased in AIS patients with SAP. There could be three reasons for this observation. The first reason is the particularities of AIS patients. When arterial platelet-fibrin thrombi are formed in AIS patients, a reduction of the circulation platelet counts occurs due to their increased consumption in the infarction area [28]. The second reason is that platelet count in AIS patients may correlate with disease severity. Ming Yang et al. showed that AIS patients in the lowest platelet count quintile had a higher risk of poor functional outcome [29]. In this study, SAP patients were severe, with high admission NIHSS scores, which lead to the reduction of platelet count. The third reason may be the effect of antithrombotic therapy of acute AIS on platelets. IVT using rt-PA influences peripheral blood platelet counts and lymphocyte concentrations, resulting in enhancing infection risk [30].
The lymphocytes belong to the adaptive immune cells, which influence the regulation of the inflammatory response. Lymphocytes were suggested to be a pivotal subtype that determined the severity of neuroinflammation in acute brain injuries. Urra et al. [31] suggested that lymphopenia in the early stages of stroke is a sign of persistent brain damage, stress response, and a greater possibility of infection. In addition, it has been reported that lymphopenia after reperfusion in vascular events can effectively predict the existence of microvascular occlusion in the early stage [32]. Konstantin et al. [33] reported apoptosis of lymphocytes after stroke, and catecholamine-mediated lymphocyte defect played an important role in stroke-associated infection. Recent studies in rodent models have shown that T cells accumulate early in the damaged area within the first 24 hours after the onset of a stroke, which causes lymphopenia in peripheral blood [34]. The stroke-induced lymphocyte apoptosis results in immune system suppression and inflammation [35, 36], shifting from a proinflammatory Th1 type response to an anti-inflammatory Th2 type response. Therefore, stroke causes local inflammation and leads to stroke-induced immunosuppressive syndrome (SIDS) [14]. SIDS then leads to the systemic inflammatory response through endocrine pathways involving the hypothalamic-pituitary-adrenal axis or activation of the sympathetic nervous system [33, 37, 38]. Meanwhile, SIDS results in decreased peripheral blood lymphocyte counts and functional T cell inactivation [39]. The results of this study further supported that lymphopenia caused by immunosuppression after stroke has a potential effect on SAP susceptibility.
PLR is a systemic inflammation index, which is calculated by dividing the platelet count by lymphocyte count. PLR represents the burden of systemic inflammation and combines platelet counts and lymphocytes counts to predict the prognosis of cardiovascular diseases [40] and ischemic stroke [41]. Altintas et al. found that high PLR values could reflect the infarcted size and poor recanalization rate in patients with thrombectomy therapy, supporting the view that PLR represents a prothrombotic inflammatory state [40]. In addition, PLR may provide important information for pulmonary diseases. Kumar et al. [42] showed that PLR could predict the 90-day mortality in patients with acute exacerbations of COPD. The PLR value can guide in diagnosing hypersensitivity pneumonia [43]. Kartal et al. showed that utilization of PLR may differentiate patients with community-acquired pneumonia [44]. Moreover, Jong-Han Lee et al. detected PLR might have diagnostic utilities such as CRP in the evaluation of the severity of pneumonia patients [45]. CRP has been reported to have a significant prognostic value and a useful adjunctive test as well as being a marker of treatment response in inflammation disease. The ability of PLR to predict infectious diseases is further proved by this indirect evidence. PLR was found to be considerably greater in the SAP group than in the non-SAP group in this study. However, Ahmet Adiguzel et al. found no correlation between the PLR level and SAP [46]. The possible reason for the different results is the difference in the severity of the stroke patients included. They included stroke patients with NIHSS >10 who were sicker than the patients in our study. Based on the discussion above, we speculated that there may be a correlation between high PLR and the occurrence of SAP, helping clinicians assess high-risk SAP in clinical works. The followings could be viewed as potential limitations to the study. First, selection bias is a concern because we undertook a single-center retrospective study. This study would have been more convincing if our sample size was enlarged. Second, as a retrospective study, some acute inflammation biomarkers were not incorporated for analysis. This could have a certain impact on the result. We will conduct prospective studies in the future to further prove this conclusion. Third, PLR was only measured at admission. Given the limitations related to the retrospective design, no data evaluating the dynamic change of PLR were available. In future studies, the hematologic markers during hospitalization should be dynamically measured to evaluate the predictive value of PLR for SAP. Fourth, we cannot rule out residual confounding or the effect of unmeasured confounders. Further studies need to record more potential risk factors for SAP.
5. Conclusion
In conclusion, our study showed that high PLR is associated with SAP in AIS patients. PLR may be a promising indicator to identify high-risk SAP patients. High PLR was found to be a risk factor for SAP in AIS patients in this investigation. In ischemic stroke, increased systemic inflammation is connected to SAP. Easy-to-find inflammatory biomarkers could aid in the diagnosis of high-risk SAP patients. It is more beneficial to improve results if SAP is detected early and well prevented.
Data Availability
The datasets used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.GBD 2017 Disease and Injury Incidence and Prevalence. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32279-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Feigin V. L., Norrving B., Mensah G. A. Global burden of stroke. Circulation Research . 2017;120(3):439–448. doi: 10.1161/circresaha.116.308413. [DOI] [PubMed] [Google Scholar]
- 3.Finlayson O., Kapral M., Hall R., Asllani E., Selchen D., Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology . 2011;77(14):1338–1345. doi: 10.1212/wnl.0b013e31823152b1. [DOI] [PubMed] [Google Scholar]
- 4.Harms H., Grittner U., Dröge H., Meisel A. Predicting post-stroke pneumonia: the PANTHERIS score. Acta Neurologica Scandinavica . 2013;128(3):178–184. doi: 10.1111/ane.12095. [DOI] [PubMed] [Google Scholar]
- 5.Ji R., Shen H., Pan Y., et al. Novel risk score to predict pneumonia after acute ischemic stroke. Stroke . 2013;44(5):1303–1309. doi: 10.1161/strokeaha.111.000598. [DOI] [PubMed] [Google Scholar]
- 6.Meisel A., Smith C. J. Preventive antibiotics for stroke-associated pneumonia. Nature Reviews Neurology . 2015;11(12):672–673. doi: 10.1038/nrneurol.2015.220. [DOI] [PubMed] [Google Scholar]
- 7.Smith C. J., Bray B. D., Hoffman A., Meisel A., Heuschmann P. U., Wolfe C. D. Can a novel clinical risk score improve pneumonia prediction in acute stroke care? A UK multicenter cohort study. Journal of American Heart Association . 2015;4(1) doi: 10.1161/jaha.114.001307.e001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui R., Zhang L. Risk factors of stroke-associated pneumonia in Chinese patients. Neurological Research . 2011;33(5):508–513. doi: 10.1179/016164111x13007856084205. [DOI] [PubMed] [Google Scholar]
- 9.Kishore A. K., Vail A., Bray B. D., et al. Clinical risk scores for predicting stroke-associated pneumonia: a systematic review. European Stroke Journal . 2016;1(2):76–84. doi: 10.1177/2396987316651759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Y., Zhang Y., Ma L., Niu X., Chang J. Risk of stroke-associated pneumonia during hospitalization: predictive ability of combined A2DS2 score and hyperglycemia. BMC Neurology . 2019;19(1):p. 298. doi: 10.1186/s12883-019-1497-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teh W. H., Smith C. J., Barlas R. S., et al. Impact of stroke-associated pneumonia on mortality, length of hospitalization, and functional outcome. Acta Neurologica Scandinavica . 2018;138(4):293–300. doi: 10.1111/ane.12956. [DOI] [PubMed] [Google Scholar]
- 12.Yang J., Dai Y., Zhang Z., Chen Y. Value of combination of the A2DS2 score and IL-6 in predicting stroke-associated pneumonia. Neuropsychiatric Disease and Treatment . 2020;16:2353–2359. doi: 10.2147/ndt.s268878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chamorro A., Urra X., Planas A. M. Infection after acute ischemic stroke. Stroke . 2007;38(3):1097–1103. doi: 10.1161/01.str.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann S., Harms H., Ulm L., et al. Stroke-induced immunodepression and dysphagia independently predict stroke-associated pneumonia - the PREDICT study. Journal of Cerebral Blood Flow and Metabolism . 2017;37(12):3671–3682. doi: 10.1177/0271678x16671964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin B., Ma N., Tang Q., et al. Neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) were useful markers in assessment of inflammatory response and disease activity in SLE patients. Modern Rheumatology . 2016;26(3):372–376. doi: 10.3109/14397595.2015.1091136. [DOI] [PubMed] [Google Scholar]
- 16.Temiz A., Gazi E., Güngör Ö, et al. Platelet/lymphocyte ratio and risk of in-hospital mortality in patients with ST-elevated myocardial infarction. Medical Science Monitor : International Medical Journal of Experimental and Clinical Research . 2014;20:660–665. doi: 10.12659/MSM.890152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozcan Cetin E. H., Cetin M. S., Aras D., et al. Platelet to lymphocyte ratio as a prognostic marker of in-hospital and long-term major adverse cardiovascular events in ST-segment elevation myocardial infarction. Angiology . 2016;67(4):336–345. doi: 10.1177/0003319715591751. [DOI] [PubMed] [Google Scholar]
- 18.Altintas O., Tasal A., Niftaliyev E., Kucukdagli O. T., Asil T. Association of platelet-to-lymphocyte ratio with silent brain infarcts in patients with paroxysmal atrial fibrillation. Neurological Research . 2016;38(9):753–758. doi: 10.1080/01616412.2016.1210357. [DOI] [PubMed] [Google Scholar]
- 19.Hu J., Zhou W., Zhou Z., Han J., Dong W. Elevated neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios predict post-stroke depression with acute ischemic stroke. Experimental and Therapeutic Medicine . 2020;19(4):2497–2504. doi: 10.3892/etm.2020.8514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akilli N. B., Yortanlı M., Mutlu H., et al. Prognostic importance of neutrophil-lymphocyte ratio in critically ill patients: short- and long-term outcomes. The American Journal of Emergency Medicine . 2014;32(12):1476–1480. doi: 10.1016/j.ajem.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Smith C. J., Kishore A. K., Vail A., et al. Diagnosis of stroke-associated pneumonia. Stroke . 2015;46(8):2335–2340. doi: 10.1161/strokeaha.115.009617. [DOI] [PubMed] [Google Scholar]
- 22.Cheng H.-R., Song J.-Y., Zhang Y.-N., et al. High monocyte-to-lymphocyte ratio is associated with stroke-associated pneumonia. Frontiers in Neurology . 2020;11 doi: 10.3389/fneur.2020.575809.575809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Turkmen K., Erdur F. M., Ozcicek F., et al. -----stage renal disease patients. Hemodialysis International . 2013;17(3):391–396. doi: 10.1111/hdi.12040. [DOI] [PubMed] [Google Scholar]
- 24.Uslu A. U., Küçük A., Şahin A., et al. Two new inflammatory markers associated with Disease Activity Score-28 in patients with rheumatoid arthritis: neutrophil-lymphocyte ratio and platelet-lymphocyte ratio. International Journal of Rheumatic Diseases . 2015;18(7):731–735. doi: 10.1111/1756-185x.12582. [DOI] [PubMed] [Google Scholar]
- 25.Wang X., Yue T.-L., Young P. R., Barone F. C., Feuerstein G. Z. Expression of interleukin-6, c-fos, and zif268 mRNAs in rat ischemic cortex. Journal of Cerebral Blood Flow and Metabolism . 1995;15(1):166–171. doi: 10.1038/jcbfm.1995.18. [DOI] [PubMed] [Google Scholar]
- 26.Franks Z. G., Campbell R. A., Weyrich A. S., Rondina M. T. Platelet-leukocyte interactions link inflammatory and thromboembolic events in ischemic stroke. Annals of the New York Academy of Sciences . 2010;1207(1):11–17. doi: 10.1111/j.1749-6632.2010.05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.von Hundelshausen P., Weber C. Platelets as immune cells. Circulation Research . 2007;100(1):27–40. doi: 10.1161/01.res.0000252802.25497.b7. [DOI] [PubMed] [Google Scholar]
- 28.D’Erasmo E., Aliberti G., Celi F. S., Romagnoli E., Vecci E., Mazzuoli G. F. Platelet count, mean platelet volume and their relation to prognosis in cerebral infarction. Journal of Internal Medicine . 1990;227(1):11–14. doi: 10.1111/j.1365-2796.1990.tb00111.x. [DOI] [PubMed] [Google Scholar]
- 29.Yang M., Pan Y., Li Z., et al. Platelet count predicts adverse clinical outcomes after ischemic stroke or TIA: subgroup Analysis of CNSR II. Frontiers in Neurology . 2019;10:p. 370. doi: 10.3389/fneur.2019.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Draxler D. F., Lee F., Ho H., Keragala C. B., Medcalf R. L., Niego B. e. t-PA suppresses the immune response and aggravates neurological deficit in a murine model of ischemic stroke. Frontiers in Immunology . 2019;10:p. 591. doi: 10.3389/fimmu.2019.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urra X., Cervera Á., Villamor N., Planas A. M., Chamorro Á. Harms and benefits of lymphocyte subpopulations in patients with acute stroke. Neuroscience . 2009;158(3):1174–1183. doi: 10.1016/j.neuroscience.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 32.Bodí V., Sanchis J., Núñez J., et al. Post-reperfusion lymphopenia and microvascular obstruction in ST-segment elevation acute myocardial infarction. Revista Española de Cardiología . 2009;62(10):1109–1117. doi: 10.1016/s1885-5857(09)73325-0. [DOI] [PubMed] [Google Scholar]
- 33.Prass K., Meisel C., Höflich C, et al. Stroke-induced immunodeficiency promotes spontaneous bacterial infections and is mediated by sympathetic activation reversal by poststroke T helper cell type 1-like immunostimulation. Journal of Experimental Medicine . 2003;198(5):725–736. doi: 10.1084/jem.20021098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin R., Yang G., Li G. Inflammatory mechanisms in ischemic stroke: role of inflammatory cells. Journal of Leukocyte Biology . 2010;87(5):779–789. doi: 10.1189/jlb.1109766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heffernan D. S., Monaghan S. F., Thakkar R. K., Machan J. T., Cioffi W. G., Ayala A. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Critical Care . 2012;16(1):p. R12. doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menges T., Engel J., Welters I., et al. Changes in blood lymphocyte populations after multiple trauma. Critical Care Medicine . 1999;27(4):733–740. doi: 10.1097/00003246-199904000-00026. [DOI] [PubMed] [Google Scholar]
- 37.Hannawi Y., Hannawi B., Rao C. P. V., Suarez J. I., Bershad E. M. Stroke-associated pneumonia: major advances and obstacles. Cerebrovascular Diseases . 2013;35(5):430–443. doi: 10.1159/000350199. [DOI] [PubMed] [Google Scholar]
- 38.Li L., Zhang L.-h., Xu W.-p., Hu J.-m. Risk assessment of ischemic stroke associated pneumonia. World Journal of Emergency Medicine . 2014;5(3):209–213. doi: 10.5847/wjem.j.issn.1920-8642.2014.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klehmet J., Harms H., Richter M., et al. Stroke-induced immunodepression and post-stroke infections: lessons from the preventive antibacterial therapy in stroke trial. Neuroscience . 2009;158(3):1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 40.Altintas O., Altintas M. O., Tasal A., Kucukdagli O. T., Asil T. The relationship of platelet-to-lymphocyte ratio with clinical outcome and final infarct core in acute ischemic stroke patients who have undergone endovascular therapy. Neurological Research . 2016;38(9):759–765. doi: 10.1080/01616412.2016.1215030. [DOI] [PubMed] [Google Scholar]
- 41.Chen C., Gu L., Chen L., et al. Neutrophil-to-Lymphocyte ratio and platelet-to-lymphocyte ratio as potential predictors of prognosis in acute ischemic stroke. Frontiers in Neurology . 2020;11 doi: 10.3389/fneur.2020.525621.525621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kumar P., Law S., Sriram K. B. Evaluation of platelet lymphocyte ratio and 90-day mortality in patients with acute exacerbation of chronic obstructive pulmonary disease. Journal of Thoracic Disease . 2017;9(6):1509–1516. doi: 10.21037/jtd.2017.05.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Üçsular F., Polat G., Karadeniz G., et al. Predictive value of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with hypersensitivity pneumonia. Sarcoidosis Vasculitis and Diffuse Lung Diseases : Official Journal of WASOG . 2020;37(4) doi: 10.36141/svdld.v37i4.9966.e2020012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kartal O., Kartal A. T. Value of neutrophil to lymphocyte and platelet to lymphocyte ratios in pneumonia. Bratislavske Lekarske Listy . 2017;118(9):513–516. doi: 10.4149/BLL_2017_099. [DOI] [PubMed] [Google Scholar]
- 45.Lee J.-H., Song S., Yoon S.-Y., Lim C. S., Song J.-W., Kim H.-S. Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic markers for pneumonia severity. British Journal of Biomedical Science . 2016;73(3):140–142. doi: 10.1080/09674845.2016.1209898. [DOI] [PubMed] [Google Scholar]
- 46.Adiguzel A., Arsava E. M., Topcuoglu M. A. Temporal course of peripheral inflammation markers and indexes following acute ischemic stroke: prediction of mortality, functional outcome, and stroke-associated pneumonia. Neurological Research . 2022;44(3):224–231. doi: 10.1080/01616412.2021.1975222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used to support the findings of this study are available from the corresponding author upon request.


