Abstract
Background
The most recent vancomycin monitoring guideline recommends targeting a value for area under the curve (AUC) of 400 to 600 mg*h/L, with an assumed minimum inhibitory concentration (MIC) of 1 mg/L. Few studies have investigated the effect of this method on vancomycin dosing regimens, relative to a target trough concentration of 15 to 20 mg/L.
Objective
To compare vancomycin dosing regimens generated with the 2 monitoring methods.
Methods
This retrospective chart review included hospitalized patients who received vancomycin between May 2019 and April 2020. The dosing regimens were compared, with the paired Student t test, in terms of unit dose, daily dose, and dosing interval. Variables of interest were collected from electronic medical charts. A pharmacy resident used first-order pharmacokinetic equations to determine dosing regimens based on AUC monitoring. Local pharmacists retrospectively determined dosing regimens for trough-based monitoring.
Results
Of 100 courses of treatment initially identified, 66 were included in the analysis. The unit dose was similar with the 2 methods (1086 mg with AUC-based monitoring versus 1100 mg with trough-based monitoring; p = 0.62). AUC monitoring was associated with a 12.8% lower daily dose (2294 mg versus 2630 mg; p < 0.001) and a 13.5% longer dosing interval (13.24 h versus 11.67 h; p < 0.001) relative to trough-based monitoring. AUC monitoring also generated a lower extrapolated trough concentration (12.90 mg/L versus 16.22 mg/L; p < 0.001).
Conclusions
A target trough concentration of 15 to 20 mg/L was confirmed as being unnecessarily high. AUC monitoring could allow a reduction in daily vancomycin dose and an extension of the dosing interval relative to trough-based monitoring.
Keywords: area under the curve, drug regimen, pharmacokinetics, pharmacodynamic, therapeutic drug monitoring, vancomycin
Mots-clés: surface sous la courbe, schéma thérapeutique, pharmacocinétique, pharmacodynamique, suivi thérapeutique médicamenteux, vancomycine
RÉSUMÉ
Contexte
La plus récente directive en matière de surveillance de la vancomycine recommande de cibler une valeur de surface sous la courbe (en anglais, AUC) de 400 à 600 mg*h/L, avec une concentration minimale inhibitrice (CMI) supposée de 1 mg/L. Peu d’études ont étudié l’effet de cette méthode sur les schémas posologiques de la vancomycine, par rapport à une concentration minimale cible de 15 à 20 mg/L.
Objectif
Comparer les schémas posologiques de la vancomycine générés avec les 2 méthodes de surveillance.
Méthodes
Cette revue rétrospective des dossiers comprenait des patients hospitalisés ayant reçu de la vancomycine entre mai 2019 et avril 2020. Un test de Student pour données appariées a été réalisé afin de comparer les schémas posologiques sur le plan de la dose unitaire, de la dose quotidienne et de l’intervalle de dosage. Les variables d’intérêt ont été recueillies à partir de dossiers médicaux électroniques. Un résident en pharmacie a utilisé des équations pharmacocinétiques de premier ordre pour déterminer les schémas posologiques en fonction de la surveillance de l’AUC. Les pharmaciens locaux ont déterminé rétrospectivement les schémas posologiques pour la surveillance basée sur la concentration résiduelle.
Résultats
Sur 100 cours de traitement initialement identifiés, 66 ont été inclus dans l’analyse. La dose unitaire était similaire avec les 2 méthodes (1086 mg avec surveillance basée sur l’AUC contre 1100 mg avec surveillance basée sur la concentration résiduelle; p = 0,62). La surveillance de l’AUC était associée à une dose quotidienne inférieure de 12,8 % (2294 mg contre 2630 mg; p < 0,001) et à un intervalle de dosage plus long de 13,5 % (13,24 h contre 11,67 h; p < 0,001) par rapport à la surveillance basée sur la concentration résiduelle. La surveillance de l’AUC a également généré une concentration minimale extrapolée plus faible (12,90 mg/L contre 16,22 mg/L; p < 0,001).
Conclusions
Une concentration résiduelle cible de 15 à 20 mg/L a été confirmée comme étant inutilement élevée. La surveillance de l’AUC pourrait permettre une réduction de la dose quotidienne de vancomycine et un allongement de l’intervalle de dosage par rapport à la surveillance basée sur la concentration résiduelle.
INTRODUCTION
Vancomycin is an antibiotic widely used for severe gram-positive infections, especially in the treatment of infections caused by methicillin-resistant Staphylococcus aureus (MRSA), which can lead to more than 20% mortality.1 Therapeutic drug monitoring is essential for patients receiving vancomycin therapy to ensure that serum concentrations are sufficient to treat the infection.2 However, high serum trough concentrations of vancomycin are associated with an increased risk of acute kidney injury (AKI).2,3
In 2020, the US consensus guideline for therapeutic monitoring of vancomycin was revised on the basis of the best current evidence.4 According to the guideline, therapeutic drug monitoring of vancomycin should be based on the ratio of area under the curve over 24 hours to minimal inhibitory concentration (AUC24/MIC).4 An AUC24/MIC ratio of 400 to 600 (assuming vancomycin broth microdilution MIC of 1 mg/L) should be advocated to achieve efficacy and patient safety for serious MRSA infections.4
Previously, the 2009 version of the guideline advised targeting a serum trough concentration of 15 to 20 mg/L for severe infections as a surrogate marker for AUC24/MIC ratio above 400.5 This method was easy to use and required only 1 blood sample. However, it was later shown that the trough concentration does not adequately predict AUC in at least 25% of cases, leading to an overestimation of the required dose and thereby increasing patients’ exposure to vancomycin.6,7 Several studies also showed an increased risk of AKI with this approach.8,9 Some patients may not fully recover from vancomycin nephrotoxicity, and even patients with mild AKI have significantly increased morbidity, length of stay, and health care costs.10
Trough-based monitoring is still widely used by physicians and pharmacists in health care centres in Canada and the United States. A national survey of vancomycin monitoring was conducted in the United States in 2019, with responses from 78 representatives of hospital pharmacy departments.11 The study showed that 77% of medical centres were still using trough-based monitoring and that the main barrier to the implementation of AUC monitoring was a lack of knowledge. Other barriers identified were a lack of time, the impression that the AUC method has not proven superior, and other logistical reasons, such as frequent errors in the timing of blood samples.11
Until recently, our Canadian centre was still determining vancomycin dosing regimens according to target trough concentration, with pharmacists monitoring serum trough concentrations of vancomycin for all patients treated with this antibiotic. A transition to monitoring based on AUC24/MIC would be a significant change of practice and represented a great challenge.
Although software using Bayesian methods can estimate AUC from a single serum concentration, acquisition of such a software program is not currently being considered at our centre. Therefore, first-order pharmacokinetic equations are required to estimate the AUC. This approach relies on the determination of 2 serum concentrations from samples obtained at or near steady-state, which increases the need for nursing time and laboratory resources.12
Despite growing data on the subject, few studies have looked at the differences between dosing regimens generated with these 2 monitoring methods. Some data on cumulative exposure are available, but not information on the dosing intervals used.13 We performed a local study to determine the impact of implementing routine AUC24/MIC monitoring on dosing regimen adjustments made by pharmacists.
The primary objective of this study was to compare vancomycin dosing regimens generated with trough-based monitoring and AUC24/MIC monitoring. The dosing regimens generated were compared in terms of unit dose, total daily dose, dosing interval, extrapolated trough concentration, and extrapolated AUC. The secondary objectives were to describe the relationship between the trough concentration and the AUC and to describe the prevalence of AKI associated with vancomycin therapy under current practice at our centre.
METHODS
This retrospective chart review was conducted at a 250-bed teaching hospital in Canada, which serves as the regional referral centre for patients with peripheral vascular disease. The study included hospitalized patients who received IV vancomycin between May 2019 and April 2020 and was approved by the hospital’s research ethics board. Courses of therapy were systematically selected after application of specific inclusion and exclusion criteria, described below. To improve the sample size, a given patient was eligible for selection if they received multiple courses of vancomycin treatment (e.g., during different hospital stays).
Courses of therapy were eligible for inclusion if the patients were 18 years of age or older and had been treated with vancomycin for a suspected or confirmed pathogen that required this antibiotic. In addition, to allow performance of AUC calculations, eligibility required at least 2 measured values for serum vancomycin concentration: a postdistributional peak concentration (0.5–3 hours) and a trough concentration at the end of the dosing interval (0–0.5 hours). Samples for determination of serum concentration had to have been drawn near steady state, as defined by a peak obtained after the third (or a later) vancomycin dose. Patients with a pre- existing need for renal replacement therapy,14 those receiving vancomycin by a route other than IV, and those with central nervous system infection were excluded.12,15 Bone cement loaded with vancomycin, which may be applied locally during surgery, also affects serum concentration of the drug; patients treated with this cement were also excluded.16
All data were collected by a single reviewer, a pharmacy resident (A.D.S.), using a standardized data collection tool. In May 2020, demographic data, weight, indication for antibiotic therapy, total duration of treatment, medication administration record, and laboratory values were collected from the patients’ electronic medical charts. The indication for vancomycin therapy was obtained from the pharmacist’s note or the final discharge summary (if not clearly mentioned in the note). The dates and times of administration of vancomycin, the infusion rate, and the dosing regimens were obtained from the medication administration record. Other potentially nephrotoxic medications were also identified. The following drugs were considered nephrotoxic: aminoglycosides, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, amphotericin B, IV contrast dyes, furosemide, nonsteroidal anti-inflammatory drugs, piperacillin–tazobactam, and vasopressors.3
For determination of AUC, first-order pharmacokinetic equations developed by Pai and others were used, which allow AUC to be reliably estimated from 2 blood samples17,18 (detailed equations are available in Supplement 1, available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/208). A local spreadsheet (Office 365 Excel, Microsoft Corporation) was used to generate the dosing regimens from 2 blood samples. The target AUC24/MIC was 400 to 600.4 For this study, the MIC was presumed to be 1 mg/L, rather than being measured, because vancomycin resistance is rare at our centre (less than 1% for the period 2016–2019). This assumption also corresponds to international MRSA surveillance data and current guideline recommendations for monitoring vancomycin.4,19,20
For trough-based monitoring, a panel of 3 pharmacists, including the pharmacy resident (A.D.S.), determined the dosing regimens for purposes of the study. Trough values, current dosing regimen, age, and estimated glomerular filtration rate, as collected from the medical charts, were provided to the pharmacists, with peak values blinded. The pharmacists were instructed to adjust the dose of vancomycin on the basis of their clinical experience, assuming that the sample for determination of trough concentration was drawn at steady state and the target trough concentration was 15 to 20 mg/L. The pharmacists independently determined each patient’s adjusted dosing regimen. Any disagreements were resolved by discussion and consensus. No additional information was given to or considered by the panel. This approach closely approximates the method currently used for dosage adjustments in our centre.
The dosing regimens generated with both monitoring methods were then entered in a spreadsheet containing pharmacokinetic equations to determine the extrapolated trough concentration and extrapolated AUC based on the patient’s medication half-life and volume of distribution previously calculated from the 2 blood samples. Given the retrospective design of this study, the patients did not actually receive the dosing regimens generated by either monitoring method.
For determination of AKI prevalence at our centre, we defined a nephrotoxic event as an increase in serum creatinine of 44 μmol/L or greater than 50% relative to pretreatment values for more than 2 consecutive days during vancomycin treatment.5 Trough-based monitoring was routinely used at our centre during the study period.
Where appropriate, descriptive statistics, such as means with standard deviations and medians, were calculated for quantitative variables of the dosing regimens and to describe the study population. Mean unit doses, daily doses, weight-based daily doses, and dosing intervals were compared with the paired Student t test to meet the primary objective, namely the comparison of dosing regimens determined with the 2 monitoring methods. The extrapolated trough and AUC values obtained for the new dosing regimens determined with the 2 monitoring methods were also compared with the paired Student t test. A scatter plot of these 2 markers was prepared to describe the relationship between trough concentration and AUC. For calculating the prevalence of nephrotoxicity, each patient was included only once, regardless of the number of courses of vancomycin treatment. All statistical tests were 2-sided, and p values less than 0.05 were considered statistically significant. Statistical analyses were performed with SPSS software (version 25.0; IBM Corporation).
RESULTS
One hundred courses of vancomycin therapy administered during the study period (May 2019 to April 2020) were screened. Of these, 34 courses were excluded, mainly because they did not reach a steady state before blood sampling (n = 16) or because the sample for peak concentration was drawn at the wrong time, mostly during the infusion period (n = 13). Other courses of therapy were excluded because the charts were incomplete (n = 4 with no data for time of blood sampling) or because vancomycin cement was used during surgery (n = 1). After these exclusions, a total of 66 courses of treatment, received by 51 patients, met the inclusion criteria. Patients’ ages ranged from 26 to 84 years. Baseline demographic and clinical characteristics of the study population are shown in Table 1.
TABLE 1.
Demographic and Clinical Characteristicsa
| Variable | No. (%) of Treatment Coursesb (n = 66) |
|---|---|
| Age (years) (mean ± SD) | 64.9 ± 11.9 |
|
| |
| Sex, male | 43 (65) |
|
| |
| Weight (kg) (mean ± SD) | 76.56 ± 16.31 |
|
| |
| BMI (mean ± SD)c | 26.85 ± 5.31 |
|
| |
| Baseline eGFR (mL/min/1.73 m2) | |
| < 30 | 0 (0) |
| 30–59 | 5 (8) |
| 60–89 | 20 (30) |
| ≥ 90 | 41 (62) |
|
| |
| Length of hospital stay (days) (median and range) | 12 (2–90) |
|
| |
| Total length of treatment (days) (median and range) | 6 (1–38) |
|
| |
| ICU admission | 34 (52) |
|
| |
| Septic shock | 7 (11) |
|
| |
| Infection-related death | 3 (5) |
|
| |
| Bacteria | |
| Known MRSA carrier | 2 (3) |
| Proven MRSA infection | 7 (11) |
| Staphylococcus epidermidis | 14 (21) |
| Enterococcus | 10 (15) |
| Other | 15 (23) |
| Culture unavailable | 20 (30) |
|
| |
| Indication for vancomycin | |
| Bacteremia | 5 (8) |
| Abdominal infection | 5 (8) |
| Skin and soft tissue infection | 7 (11) |
| Pneumonia | 4 (6) |
| Bone and joint infection | 8 (12) |
| Urinary tract infection | 2 (3) |
| Endocarditis | 8 (12) |
| Endovascular prosthesis | 10 (15) |
| Postoperative wound | 6 (9) |
| Prosthetic joint | 4 (6) |
| Unknown | 7 (11) |
BMI = body mass index, eGFR = estimated glomerular filtration rate, ICU = intensive care unit, MRSA = methicillin-resistant Staphylococcus aureus, SD = standard deviation.
The data represent 51 individual patients, with some patients contributing data for more than 1 course of treatment.
Except where indicated otherwise.
n = 64 (data missing for 2 courses of therapy).
Patients received 3 to 12 doses of vancomycin before sampling for the peak value used for this study (third dose for 38 courses of therapy, fourth dose for 17 courses of therapy, and fifth or subsequent dose for 11 courses of therapy). The mean daily vancomycin dose was 29.94 mg/kg at that time. The mean elimination half-life in the study population was 9.64 hours, and the mean volume of distribution was 0.74 L/kg. Detailed characteristics can be found in Supplement 2 (available at: https://www.cjhp-online.ca/index.php/cjhp/issue/view/208).
For the primary outcome, vancomycin dosing regimens generated with the 2 monitoring methods are shown in Table 2. A significantly lower daily dose was obtained with AUC monitoring than with trough-based monitoring, but the mean unit doses were similar with the 2 methods.
TABLE 2.
Vancomycin Dosing Regimens with Therapeutic Drug Monitoring Based on Trough Concentration and AUC (n = 66 Treatment Courses)a
| Variable | TDM Method; Mean ± SD | Mean Difference (95% CI) | Relative Difference (%) | p Value | |
|---|---|---|---|---|---|
| Trough | AUC | ||||
| Unit dose (mg) | 1100 ± 191 | 1086 ± 197 | −14 (−70 to −42) | −1.27 | 0.62 |
| Daily dose (mg) | 2630 ± 907 | 2294 ± 901 | −336 (−460 to −212) | −12.76 | < 0.001 |
| Daily dose, weight-based (mg/kg)b | 35.05 ± 12.34 | 30.24 ± 10.60 | −4.81 (6.48 to −3.15) | −13.72 | < 0.001 |
| Interval (h) | 11.67 ± 6.28 | 13.24 ± 6.76 | +1.58 (−0.77 to 2.38) | +13.45 | <0.001 |
| Extrapolated trough (mg/L) | 16.22 ± 3.28 | 12.90 ± 2.49 | −3.32 (−4.25 to −2.38) | −20.47 | <0.001 |
| Extrapolated AUC (mg*h/L) | 594.77 ± 104.36 | 509.48 ± 58.64 | −85.29 (−111.61 to −58.98) | −14.34 | <0.001 |
AUC = area under the curve, CI = confidence interval, SD = standard deviation, TDM = therapeutic drug monitoring.
Comparison analyzed by paired t test.
Divided by total body weight in kilograms.
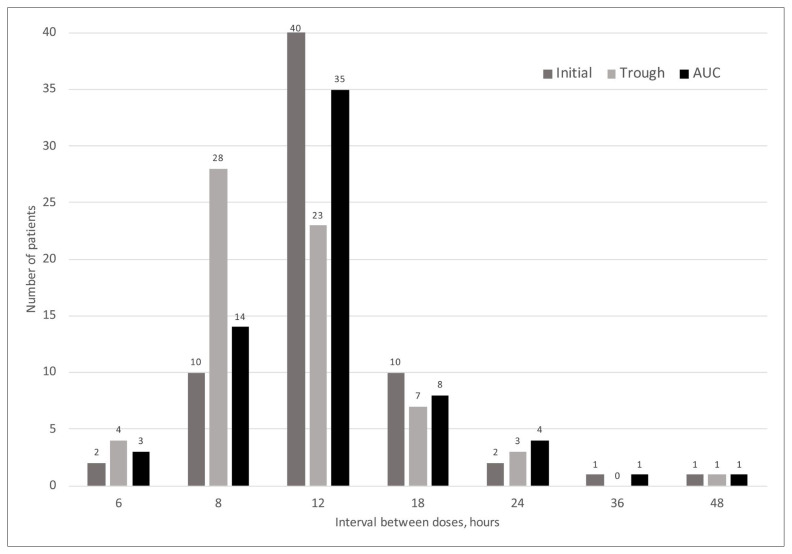
The dosing intervals were significantly longer with AUC monitoring than with trough-based monitoring. The distributions of dosing intervals with the 2 monitoring methods are shown in Figure 1.
FIGURE 1.
Vancomycin dosing interval with the initial dose and the 2 monitoring methods after dosing adjustment (n = 66 treatment courses). Trough = monitoring on the basis of trough concentration of medication, AUC = monitoring on the basis of area under the curve.
First-order equations were used to calculate the extrapolated trough and AUC values for each dosing regimen obtained with each monitoring method. The results are also shown in Table 2. The mean extrapolated trough concentration with trough-based monitoring was 16.22 mg/L, which correlated with pharmacists’ instructions to achieve a trough concentration between 15 and 20 mg/L.
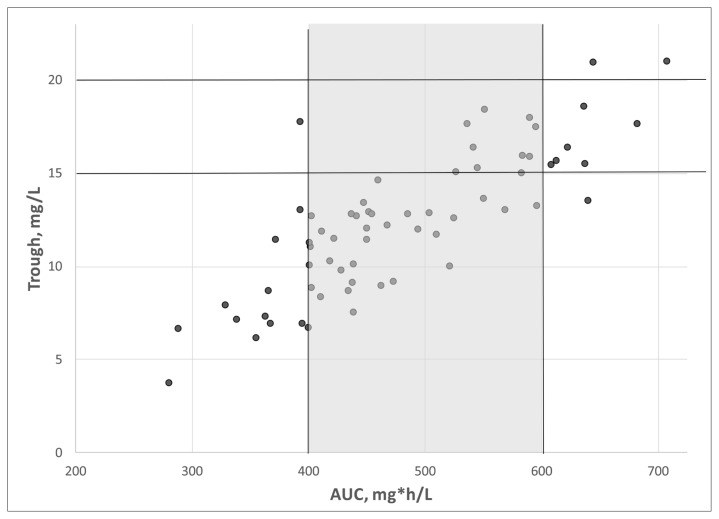
The relationship between the trough concentration of vancomycin and AUC is shown in Figure 2. Of the 17 patients with a trough concentration between 15 and 20 mg/L, 10 (59%) had an AUC in the therapeutic target of 400 to 600 mg*h/L, whereas 6 (35%) had an AUC of more than 600 mg*h/L. Conversely, of the 28 patients with a trough concentration between 10 and 14 mg/L, 25 (89%) had an AUC of 400 to 600 mg*h/L, and only 2 (7%) had an AUC below 400 mg*h/L.
FIGURE 2.
Relationship between trough concentration of medication and area under the curve (AUC) in patients receiving vancomycin (n = 66 treatment courses).
The prevalence of AKI during vancomycin treatment in the study population was another secondary outcome of this study, and results related to this outcome are displayed in Table 3. Most patients (n = 37/51, 73%) were receiving at least 1 concomitant nephrotoxic medication; in relation to the 66 courses of treatment, the most frequent nephrotoxic medications were contrast dye (n = 20/66, 30%) and furosemide (n = 16/66, 24%). At our centre, 6 of the 51 patients had AKI during their vancomycin treatment; as noted above, the monitoring method used at the time was mostly trough-based monitoring. Patients with AKI were treated with vancomycin for a median of 14 days (range 5–28 days). Subgroup analysis could not be performed because of the low number of events.
TABLE 3.
Acute Kidney Injury during Vancomycin Treatment
| Variable | No. (%) of Patientsa (n = 51) |
|---|---|
| Total length of treatment (days) (median and range) | 6.00 (1–38) |
|
| |
| Nephrotoxic event | 6 (12) |
|
| |
| Concomitant nephrotoxic drugs | n = 6 events |
| 0 or 1 | 0 (0) |
| 2 | 4 (67) |
| ≥ 3 | 2 (33) |
|
| |
| Concomitant drug therapy | n = 6 events |
| Furosemide | 4 (67) |
| ACE inhibitor or ARB | 3 (50) |
| Piperacillin–tazobactam | 2 (33) |
| Contrast dye | 2 (33) |
| Vasopressors | 2 (33) |
ACE = angiotensin-converting enzyme, ARB = angiotensin receptor blocker.
Except where indicated otherwise.
DISCUSSION
To our knowledge, this is the first study to compare vancomycin dosing regimens determined retrospectively by 2 different methods for the same patients, which ensured similarity of the comparator groups, with limited confounding. Our results show that use of AUC for therapeutic drug monitoring would allow a significant reduction in daily dose of vancomycin and a significant lengthening of the dosing interval, which would expose patients to a lower trough concentration and lower AUC.
We hope that our data, combined with those from other studies, will prove to physicians and pharmacists that the trough concentration is not a good surrogate marker for AUC and that its use may lead to overtreatment of patients. Indeed, in our study, more patients had the dosing regimen adjusted upward with trough-based monitoring (relative to AUC-based monitoring) to achieve the unnecessarily high target trough concentration of 15 to 20 mg/L, which is consistent with many observations reported in the literature.17,21 For example, in a Monte Carlo simulation of 5000 patients, Pai and others17 showed that 60% of patients could reach a therapeutic AUC (> 400 mg*h/L) with a trough concentration of less than 15 mg/L. In our study, the proportion was even higher than that, with 89% of patients who had a trough concentration between 10 and 14 mg/L having an AUC of 400 to 600 mg*h/L.
Our results are also useful to help health care professionals better understand the difference between the 2 monitoring methods, through provision of specific clinical variables, such as the unit dose and the dosing interval. Few other studies have examined actual differences between dosing regimens. In a retrospective, quasi-experimental study of 1280 hospitalized patients, Finch and others13 compared cumulative doses of vancomycin determined with AUC- and trough-based monitoring. AUC-based monitoring allowed 4% to 7% lower cumulative vancomycin doses at 24, 48, and 72 hours than trough-based monitoring. This difference was statistically significant but appears small relative to our results, which would have allowed a significant reduction in vancomycin dose of about 13%. However, the previous authors did not directly compare the dosing regimens generated by the 2 methods. Also, the 2 cohorts in the study by Finch and others13 were not similar: the AUC-based monitoring group had a higher infection severity score and comorbidity index, which limited the interpretation of results for cumulative doses.
Of note, the pharmacists involved in determining dosing regimens with trough-based monitoring in our study mentioned that, in clinical practice, they would tend to tolerate trough concentrations slightly below 15 mg/L, as they would expect the accumulation of vancomycin and rising trough concentrations with monitoring early in therapy. During our study, pharmacists were instructed to design a dosing regimen that would necessarily generate a trough concentration between 15 and 20 mg/L without consideration of other variables; this might have increased the difference in daily doses determined by the 2 methods.
In our study, we found that the dosing regimens with AUC- and trough-based monitoring differed more in terms of the dosing intervals than in terms of the unit dose. Nix and others22 studied the impact of dosing interval in limiting vancomycin AUC with trough-based monitoring. Their data suggested that maintaining a longer dosing interval and escalating unit doses to achieve a target trough concentration results in excessive vancomycin exposure (as reflected in the AUC), with potentially high peak concentrations. They recommended shortening the dosing interval with the trough-based method to maintain a therapeutic AUC. This recommendation contrasts with our data: even if our pharmacists tended to shorten dosing intervals when the target trough concentration of 15 to 20 mg/L was not reached (rather than increasing the unit dose), the mean predicted AUC was still high, just below the nephrotoxicity threshold of 600 mg*h/L.
The prevalence of nephrotoxic events in this study was 12%, greater than other recent data showing that AKI occurs in about 5% of patients exposed to vancomycin.23,24 The prevalence of AKI observed in our study occurred in the context of trough-based monitoring, the method used at our centre at the time of data collection. This finding concurred with several recent studies showing that higher vancomycin exposure and trough-based monitoring are linked with nephrotoxicity.3,8,13,23 The risk of AKI is potentiated by the concomitant use of nephrotoxic drugs, which was high in our study, with 73% of patients taking at least 1 concomitant nephrotoxic drug. It would be relevant to compare the prevalence of nephrotoxicity at our centre before and after large-scale implementation of the AUC-based monitoring method.
This study had some limitations. It was a single-centre, retrospective study; however, there were very few missing data for the selected patients. Some blood sampling times may have been incorrect, because nurses in our centre often enter timing data into the electronic records before actually taking the sample. However, given that the same data were used to compare the 2 methods, we do not expect that differences in sampling time would lead to significant differences in our results. Patients with unstable renal function or presenting with AKI before or during treatment were not excluded from the study; this might have affected the data obtained, given that steady state is usually not achieved in these cases.25 Also, critical care patients frequently have unstable vancomycin clearance, which prevents achievement of a steady state.26 In 52% of the courses of therapy in our study, the patients were admitted to the intensive care unit during their vancomycin treatment, but few had septic shock, and creatinine levels were stable in most patients. In addition, the mean volume of distribution for vancomycin in the study population was 0.74 L/kg, which is similar to what has been described in the literature.27,28
Our institution-specific practice (with a high rate of endovascular infections) and the study’s exclusion criteria (with exclusion of patients undergoing renal replacement therapy, those with central nervous system infection, and pediatric patients) limit extrapolation of data to these populations. In addition, our study did not aim to compare Bayesian-determined dosing regimens.
Our study compared dosing regimens in terms of pharmacokinetic parameters; it was not designed to evaluate clinical efficacy. Only 11% of the treatment courses included in the analysis were for proven MRSA infection, which may bring the relevance of our results into question; according to the monitoring guideline, there is insufficient evidence to provide recommendations on vancomycin monitoring for patients with infections other than MRSA.4 However, our study data compared well with the most extensive prospective study of AUC-guided vancomycin dosing in adults, in which, similarly, only 10% of the study population had microbiologically proven MRSA infections.29 In that study, there was no difference in clinical efficacy between AUC- and trough-based monitoring.29 Further research is warranted in this area, as prospective data for vancomycin monitoring in infections other than MRSA are still rare.
CONCLUSION
AUC-based monitoring could allow a significant reduction in daily vancomycin doses and a significant lengthening of dosing intervals relative to trough-based therapeutic drug monitoring. Extrapolated trough concentrations and AUC values were also considerably lower with AUC-based monitoring, and we confirmed that a target trough concentration of 15 to 20 mg/L is unnecessarily high. These benefits have the potential to reduce nephrotoxicity at our centre, and AUC-guided dosing for vancomycin should therefore replace trough-based monitoring.
Supplementary Information
Acknowledgements
The authors thank Guylaine Morneau (CHU de Québec-Université Laval) for comments on the manuscript in advance of submission, Mahukpe Narcisse Ulrich Singbo (CHU de Québec-Université Laval) for assistance with planning the statistical analysis, and Stephanie Viel (CHU de Québec-Université Laval) for presubmission language editing.
Footnotes
Competing interests: None declared.
Note: This article contains supplementary material (Supplements 1 and 2), available at https://www.cjhp-online.ca/index.php/cjhp/issue/view/208
Funding: None received.
References
- 1. Dantes R, Mu Y, Belflower R, Aragon D, Dumyati G, Harrison LH, et al. Emerging Infections Program–Active Bacterial Core Surveillance MRSA Surveillance Investigators. National burden of invasive methicillin-resistant Staphylococcus aureus infections, United States, 2011. JAMA Intern Med. 2013;173(21):1970–8. doi: 10.1001/jamainternmed.2013.10423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ye ZK, Tang HL, Zhai SD. Benefits of therapeutic drug monitoring of vancomycin: a systematic review and meta-analysis. PLoS One. 2013;8(109):e77169. doi: 10.1371/journal.pone.0077169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Filippone EJ, Kraft WK, Farber JL. The nephrotoxicity of vancomycin. Clin Pharmacol Ther. 2017;102(3):459–69. doi: 10.1002/cpt.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rybak MJ, Le J, Lodise TP, Levine DP, Bradley JS, Liu C, et al. Therapeutic monitoring of vancomycin for serious methicillin-resistant Staphylococcus aureus infections: a revised consensus guideline and review by the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the Society of Infectious Diseases Pharmacists. Am J Health Syst Pharm. 2020;77(11):835–64. doi: 10.1093/ajhp/zxaa036. [DOI] [PubMed] [Google Scholar]
- 5. Rybak MJ, Lomaestro BM, Rotschafer JC, Moellering RC, Jr, Craig WA, Billeter M, et al. Therapeutic monitoring of vancomycin in adults summary of consensus recommendations from the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Pharmacotherapy. 2009;29(11):1275–9. doi: 10.1592/phco.29.11.1275. [DOI] [PubMed] [Google Scholar]
- 6. Neely MN, Youn G, Jones B, Jelliffe RW, Drusano GL, Rodvold KA, et al. Are vancomycin trough concentrations adequate for optimal dosing? Antimicrob Agents Chemother. 2014;58(1):309–16. doi: 10.1128/AAC.01653-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Prybylski JP. Vancomycin trough concentration as a predictor of clinical outcomes in patients with Staphylococcus aureus bacteremia: a meta-analysis of observational studies. Pharmacotherapy. 2015;35(10):889–98. doi: 10.1002/phar.1638. [DOI] [PubMed] [Google Scholar]
- 8. Aljefri DM, Avedissian SN, Rhodes NJ, Postelnick MJ, Nguyen K, Scheetz MH. Vancomycin area under the curve and acute kidney injury: a meta-analysis. Clin Infect Dis. 2019;69(11):1881–7. doi: 10.1093/cid/ciz051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stoessel AM, Hale CM, Seabury RW, Miller CD, Steele JM. The impact of AUC-based monitoring on pharmacist-directed vancomycin dose adjustments in complicated methicillin-resistant Staphylococcus aureus infection. J Pharm Pract. 2019;32(4):442–6. doi: 10.1177/0897190018764564. [DOI] [PubMed] [Google Scholar]
- 10. Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol. 2005;16(11):3365–70. doi: 10.1681/ASN.2004090740. [DOI] [PubMed] [Google Scholar]
- 11. Kufel WD, Seabury RW, Mogle BT, Beccari MV, Probst LA, Steele JM. Readiness to implement vancomycin monitoring based on area under the concentration-time curve: a cross-sectional survey of a national health consortium. Am J Health Syst Pharm. 2019;76(12):889–94. doi: 10.1093/ajhp/zxz070. [DOI] [PubMed] [Google Scholar]
- 12. Heil EL, Claeys KC, Mynatt RP, Hopkins TL, Brade K, Watt I, et al. Making the change to area under the curve-based vancomycin dosing. Am J Health Syst Pharm. 2018;75(24):1986–95. doi: 10.2146/ajhp180034. [DOI] [PubMed] [Google Scholar]
- 13. Finch NA, Zasowski EJ, Murray KP, Mynatt RP, Zhao JJ, Yost R, et al. A quasi-experiment to study the impact of vancomycin area under the concentration-time curve-guided dosing on vancomycin-associated nephrotoxicity. Antimicrob Agents Chemother. 2017;61(12):e01293–17. doi: 10.1128/AAC.01293-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crew P, Heintz SJ, Heintz BH. Vancomycin dosing and monitoring for patients with end-stage renal disease receiving intermittent hemodialysis. Am J Health Syst Pharm. 2015;72(21):1856–64. doi: 10.2146/ajhp150051. [DOI] [PubMed] [Google Scholar]
- 15. Gregory ER, Burgess DR, Cotner SE, VanHoose JD, Flannery AH, Gardner B, et al. Vancomycin area under the curve dosing and monitoring at an academic medical center: transition strategies and lessons learned. J Pharm Pract. 2020;33(6):774–8. doi: 10.1177/0897190019834369. [DOI] [PubMed] [Google Scholar]
- 16. Wahl P, Guidi M, Benninger E, Rönn K, Gautier E, Buclin T, et al. The levels of vancomycin in the blood and the wound after the local treatment of bone and soft-tissue infection with antibiotic-loaded calcium sulphate as carrier material. Bone Joint J. 2017;99-B(11):1537–44. doi: 10.1302/0301-620X.99B11.BJJ-2016-0298.R3. [DOI] [PubMed] [Google Scholar]
- 17. Pai MP, Neely M, Rodvold KA, Lodise TP. Innovative approaches to optimizing the delivery of vancomycin in individual patients. Adv Drug Deliv Rev. 2014;77:50–7. doi: 10.1016/j.addr.2014.05.016. [DOI] [PubMed] [Google Scholar]
- 18. Turner RB, Kojiro K, Shephard EA, Won R, Chang E, Chan D, et al. Review and validation of Bayesian dose-optimizing software and equations for calculation of the vancomycin area under the curve in critically ill patients. Pharmacotherapy. 2018;38(12):1174–83. doi: 10.1002/phar.2191. [DOI] [PubMed] [Google Scholar]
- 19.Sous-comité de surveillance de l’utilisation des antibiotique. Rapport: antibiogrammes cumulatifs. CHU de Québec-Université Laval. 2018. [cited 2020 Dec 10]. Available from: https://www.chudequebec.ca/chudequebec.ca/files/d8/d8428f8f-a8b9-48eb-89e3-e0c38336065a.pdf.
- 20. Diaz R, Afreixo V, Ramalheira E, Rodrigues C, Gago B. Evaluation of vancomycin MIC creep in methicillin-resistant Staphylococcus aureus infections—a systematic review and meta-analysis. Clin Microbiol Infect. 2018;24(2):97–104. doi: 10.1016/j.cmi.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 21. Bel Kamel A, Bourguignon L, Marcos M, Ducher M, Goutelle S. Is trough concentration of vancomycin predictive of the area under the curve? A clinical study in elderly patients. Ther Drug Monit. 2017;39(1):83–7. doi: 10.1097/FTD.0000000000000359. [DOI] [PubMed] [Google Scholar]
- 22. Nix DE, Villanueva JE, Matthias KR. The importance of dosing interval in limiting vancomycin AUC with trough monitoring. Am J Health Syst Pharm. 2020;77(6):487–92. doi: 10.1093/ajhp/zxz180. [DOI] [PubMed] [Google Scholar]
- 23. Chavada R, Ghosh N, Sandaradura I, Maley M, Van Hal SJ. Establishment of an AUC0-24 threshold for nephrotoxicity is a step towards individualized vancomycin dosing for methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2017;61(5):e02535–16. doi: 10.1128/AAC.02535-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Luque Y, Mesnard L. Néphrotoxicité de la vancomycine: fréquence et mécanismes. [Vancomycin nephrotoxicity: frequency and mechanistic aspects]. Nephrol Ther. 2018;14(Suppl 1):S133–8. doi: 10.1016/j.nephro.2018.02.009. [DOI] [PubMed] [Google Scholar]
- 25.MacDougall C. Protein synthesis inhibitors and miscellaneous antibacterial agents. In: Brunton LL, Hilal-Dandan R, Knollmann BC, editors. Goodman & Gilman’s: the pharmacological basis of therapeutics. 13th ed. McGraw-Hill Education; 2017. Electronic version accessed 2020 Dec 10 through an institutional subscription. [Google Scholar]
- 26. Matzke GR, Zhanel GG, Guay DR. Clinical pharmacokinetics of vancomycin. Clin Pharmacokinet. 1986;11(4):257–82. doi: 10.2165/00003088-198611040-00001. [DOI] [PubMed] [Google Scholar]
- 27. Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis. 2006;42(Suppl 1):S35–9. doi: 10.1086/491712. [DOI] [PubMed] [Google Scholar]
- 28. Marsot A, Boulamery A, Bruguerolle B, Simon N. Vancomycin: a review of population pharmacokinetic analyses. Clin Pharmacokinet. 2012;5:1–13. doi: 10.2165/11596390-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 29. Neely MN, Kato L, Youn G, Kraler L, Bayard D, van Guilder M, et al. Prospective trial on the use of trough concentration versus area under the curve to determine therapeutic vancomycin dosing. Antimicrob Agents Chemother. 2018;62(2):e02042–17. doi: 10.1128/AAC.02042-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.