Abstract
Purpose:
To explore the efficacy and toxicity of stereotactic body radiation therapy (SBRT) in high-risk prostate cancer (HRPCa) in a consortium of 7 institutional phase 2 trials and prospective registries.
Methods and Materials:
Individual patient data were pooled for 344 patients with a minimum follow-up of 24 months. Biochemical recurrence-free survival (BCRFS) and distant metastasis-free survival (DMFS) were estimated using a Kaplan-Meier framework. Fine and Gray competing risk and Cox proportional hazards regression models were developed to assess the association between time to BCR and time to distant metastasis and prespecified variables of interest. Logistic regression models were developed to evaluate associations between acute and late grade ≥2 genitourinary and gastrointestinal and the following a priorie-specified variables: age, dose per fraction, ADT use, and nodal radiation therapy.
Results:
Median follow-up was 49.5 months. Seventy-two percent of patients received ADT, with a median duration of 9 months, and 19% received elective nodal radiation therapy. Estimated 4-year BCRFS and DMFS rates were 81.7% (95% CI, 77.2%−86.5%) and 89.1% (95% CI, 85.3%−93.1%). The crude incidences of late grade ≥3 genitourinary and gastrointestinal toxicity were 2.3% and 0.9%.
Conclusions:
These data support a favorable toxicity and efficacy profile for SBRT for HRPCa. Further prospective studies are needed to evaluate the optimal dose and target volume in the context of SBRT for HRPCa. 2021 Elsevier Inc. All rights reserved.
Introduction
Stereotactic body radiation therapy (SBRT) is a form of ultrahypofractionated radiation therapy in which advanced treatment delivery techniques are used to deliver high doses of radiation over the course of 5 or fewer treatments. The 2020 National Comprehensive Cancer Network (NCCN) guidelines suggest that SBRT can be considered for patients with high-risk prostate cancer (HRPCa) provided they have social or medical hardships that preclude longer courses of radiation.1 The 2020 European Association of Urology guidelines are less supportive of this and note that the major evidence to support ultrahypofractionation for HRPCa comes from a subset of 126 patients enrolled on the randomized HYPO-RT-PC trial.2,3 These patients did not receive concurrent androgen deprivation therapy (ADT), which is now considered a standard of care for patients with HRPCa receiving definitive radiation therapy, and the authors conclude that their general conclusions of oncologic equivalency may not be applicable for patients with HRPCa. Other published prospective data supporting SBRT for HRPCa are limited to medium-term results from 2 small phase 2 trials and a small prospective database with short-term data.4–6
Methods and Materials
To evaluate efficacy and toxicity outcomes among men receiving SBRT for HRPCa in a larger cohort, we established a consortium and obtained patient-level data from 7 institutions with phase 2 studies and prospective databases. The site-specific distribution of patients and their treatment characteristics are shown in Table 1. Each institutional review board approved contribution of its data to the coordinating data center (University of California, Los Angeles). Analyses were limited to patients with ≥24 months of follow-up. Biochemical recurrence (BCR) was defined as a PSA increase >2 ng/mL higher than the lowest value after SBRT, per the Phoenix definition.7 Gastrointestinal (GI) and genitourinary (GU) toxicity were scored per the Common Terminology Criteria for Adverse Events (CTCAE) version 3.0 or version 4.0. Kaplan-Meier methods were used to obtain 4-year survival estimates of BCR-free survival (BCRFS) and distant metastasis-free survival (DMFS) with time to event measured from the final day of SBRT. Univariate and multivariable Fine and Gray competing risk and Cox proportional hazards regression models were developed to assess the association between time to BCR and time to distant metastasis. Multivariable models were adjusted for dose per fraction (categorical, with 8 Gy as the reference dose), age at treatment, clinical T stage (T3–4 vs T1–2), ln (initial prostate-specific antigen), and Gleason grade group (1–3 vs 4–5). Due to the nonuniform use of ADT and nodal radiation therapy and the consideration that important other variables that might confound potential associations, such as socioeconomic status, were not available, these variables were not included in the multivariable analyses. Multivariable logistic regression models were developed to evaluate associations between acute and late grade ≥2 GU and GI and the following a priori specified variables: age at treatment, dose per fraction (categorical, with 8 Gy per fraction as the reference dose), ADT use, and nodal radiation therapy. In this case, ADT use and nodal radiation therapy were included in the model because the impact of selection biases related to their use and the absence of information about important confounding variables was thought to be less important in investigating relationships with toxicity versus measures of efficacy. Due to the low event rate, Firth’s penalized likelihood method was used to estimate the relevant odds ratios (ORs) and hazard ratios (HRs). Cumulative incidence curves were developed using Allen estimator, and Gray’s test was used to compare the equality of cumulative incidence functions across strata.8 Analyses were completed using SAS (9.4 SAS Institute Inc, Cary, NC) and R, version 3.3.2. All P values were from 2-tailed tests, and results were deemed statistically significant at P < .05.
Table 1.
Individual prospective study characteristics
| Seminal | Intrafraction | Original | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Institution | No. of | Dose × | vesicle | Margins† | motion | Image | toxicity | |||
| NCT/IRB | or trial | patients | fraction | coverage* | Prescription | monitoring | guidance | Fractionation | scoring | |
|
| ||||||||||
| IRB#16-001851 | University of California, Los Angeles | 71 | 8 Gy × 5 | Proximal 1 cm | 5 mm/3 mm posterior | 100% of Rx to cover 95% of PTV Max: 105% | Yes | Cone beam CT before treatment; planar imaging during treatment, fiducials in place | Every other day | CTCAE v4.0 |
| IRB#12–1175 | Georgetown University | 104 | 7–7.5 Gy × 5 | Proximal 1 cm | 5 mm/3 mm posterior | 100% of Rx to cover 95% of PTV Max: 120%-128% | Yes | CyberKnife fiducial-based tracking | Every other day | CTCAE v4.0 |
| IRB#16-001851 | Genesis | 16 | 8 Gy × 5 | Proximal 1 cm | 5 mm/4 mm anterior and posterior | 100% of Rx to cover 95% of PTV Max: 200% | Yes | CyberKnife fiducial-based tracking | Daily | CTCAE v3.0 |
| Institutional registry | Katz | 45 | 7–7.25 Gy × 5 | Proximal 1 cm | 5 mm/3 mm posterior Max: 117%-121% | 100% of Rx to cover 95% of PTV | Yes | CyberKnife fiducial-based tracking | Daily | RTOG |
| Project ID 900550 | Tata Memorial Hospital | 28 | 7–7.5 Gy × 5 | Proximal 2.4 cm | 5 mm/3 mm posterior Max 107% | 95% of Rx to cover 98% of PTV | No | Cone beam CT before treatment, no fiducials | Every other day | RTOG |
| NCT01505075 ‡ | Sunnybrook pHART 8 | 29 | 8 Gy × 5 | Proximal 1 cm | 5 mm Max: 107% | 100% of Rx to cover 99% of CTV; 95% of Rx to cover 99% of PTV | No | Cone beam CT before treatment. fiducials in place | Weekly | CTCAE v3.0 |
| NCT01953055 ‡ | Sunnybrook SATURN | 30 | 8 Gy × 5 | Proximal 1 cm | 3 mm Max: 107% | 100% of Rx to cover 99% of CTV; 83% of Rx to cover 99% of PTV | No | Cone beam CT before treatment, fiducials in place | Weekly | CTCAE v3.0 |
| NCT01664130 | Cleveland Clinic Foundation | 21 | 7.25 Gy × 5 | Proximal 2 cm | 3 mm/0 mm posteriorly Max: unlimited | 100% of Rx to cover 95% of PTV | Yes | Triggered imaging every 30° with a 2-mm threshold, fiducials in place | Every other day | CTCAE v3.0 |
Abbreviations: CTCAE = common terminology criteria for adverse events; CT = computed tomography; CTV = clinical treatment volume; NCT/ IRB = national clinical trial/institutional review board; PTV = planning treatment volume; Rx = prescription.
Full seminal vesicle coverage was pursued if cT3b disease.
No patients had rectal spacers used.
No magnetic resonance imaging fusion used to guide contour delineation.
Results
Overall, 344 patients were included in this analysis, with a median follow-up of 49.5 months (interquartile range, 35.8–61.9 months) (Table 2). A total of 248 patients (72%) received ADT, with a median duration of 9 months (inter-quartile range, 9–18 months). Estimated 4-year BCRFS and DMFS rates were 81.7% (95% CI, 77.2%−86.5%) and 89.1% (95% CI, 85.3%−93.1%), respectively. Overall, 59 patients (17%) experienced a BCR and 26 patients (8%) experienced a distant metastasis (DM). On multivariable competing risk analyses, 7 Gy versus 8 Gy per fraction was significantly associated with increased risk of BCR (sub-distribution hazard ratio [sHR] 2.15; 95% CI, 1.07–4.32; P = .03), as was ln-iPSA (sHR 1.42; 95% CI, 1.06–1.9; P =.02) (Table 3). No statistically significant predictors of time to DM were identified (Table 3). Cause-specific models had similar results for BCR and DM; additionally, 1-year increase in age at treatment was (HR 1.04; 95% CI, 1–1.07; P = .035) (Tables E1 and E2).
Table 2.
Clinical, demographic, and treatment characteristics
| Parameter | Distribution |
|---|---|
|
| |
| Age (median, IQR), y | 72.3 (67–78.5) |
| Initial prostate-specific antigen | |
| Median, IQR | 11 (7–21.3) |
| Mean (SD) | 18.8 (25.9) |
| <10 | 146 (42%) |
| 10–20 | 94 (27%) |
| >20 | 103 (30%) |
| T stage T1 |
151 (45%) |
| T2 | 144 (43%) |
| T3a | 25 (7%) |
| T3b | 15 (4%) |
| T4 | 3 (1%) |
| Gleason grade group | |
| 1 | 25 (7%) |
| 2 | 43 (12%) |
| 3 | 38 (11%) |
| 4 | 156 (45%) |
| 5 | 82 (24%) |
| Androgen deprivation therapy | |
| Use | 248 (72%) |
| Duration (median, IQR) | 9 (9–18) |
| Nodal radiation therapy | 66 (19%) |
| Dose per fraction 7 |
67 (19%) |
| 7.5 | 124 (36%) |
| 8 | 153 (44%) |
| Acute GU grade ≥ 2 | |
| Yes | 44 (18%) |
| No | 196 (82%) |
| Acute GI grade ≥ 2 | |
| Yes | 12 (5%) |
| No | 228 (95%) |
| Late GU grade ≥ 2 | |
| Yes | 64 (19%) |
| No | 279 (81%) |
| Late GI grade ≥ 2 | |
| Yes | 32 (9%) |
| No | 311 (91%) |
Abbreviations: GI = gastrointestinal; GU = genitourinary; IQR = interquartile range.
Table 3.
Competing risk regression analysis for predictors of biochemical recurrence and distant metastasis
| Variable | sHR (95% CI) | P value |
|---|---|---|
|
| ||
| Biochemical recurrence | ||
| Age at treatment (1-y increase) | 1.04 (1–1.08) | .067 |
| Natural log iPSA | 1.42 (1.06–1.9) | .021 |
| Gleason grade group 4–5 vs 1–3 | 1.06 (0.57–1.97) | .845 |
| T3/4 (yes vs no) | 0.5 (0.15–1.62) | .245 |
| Dose/fraction (ref = 8 Gy) 7 vs 8 Gy |
2.15 (1.07–4.32) | .033 |
| 7.25 vs 8 Gy | 1.29 (0.64–2.6) | .473 |
| Distant metastasis | ||
| Age at treatment | 1.02 (0.97–1.08) | .344 |
| Natural log iPSA | 1.2 (0.79–1.84) | .39 |
| Gleason grade group 4–5 vs 1–3 | 2.31 (0.81–6.59) | .118 |
| T3/4 no vs yes | 1.97 (0.6–6.4) | .262 |
| Dose/fraction (ref = 8 Gy) | ||
| 7 vs 8 Gy | 1.37 (0.46–4.06) | .566 |
| 7.25 vs 8 Gy | 0.72 (0.27–1.97) | .526 |
Abbreviations: CI = confidence interval; HR = hazard ratio; iPSA = initial prostate-specific antigen.
Kaplan-Meier curves of BCRFS and DMFS stratified by ADT use are shown in Figure 1. BCRFS was significantly greater among patients receiving ADT (P = .009 by log-rank), but DMFS was not significantly different (P value.097 by log-rank). Similar curves stratified by nodal RT and iPSA are shown in Figures E1 and E2, respectively. Cumulative incidences of BCR and DM, stratified by ADT use, are shown in Figure E3. The cumulative incidence of BCR was significantly lower among patients receiving ADT (P = .017 by Gray’s test), and the cumulative incidence of DM was no different (P = .36 by Gray’s test). Meaningful analysis of ADTduration was precluded by the low event rate within any given ADT duration (none vs ≤9 vs 9–18 vs >18 months) as well as selection biases inherent to the duration of ADT provided, given the heterogeneity in practice patterns.
Fig. 1.
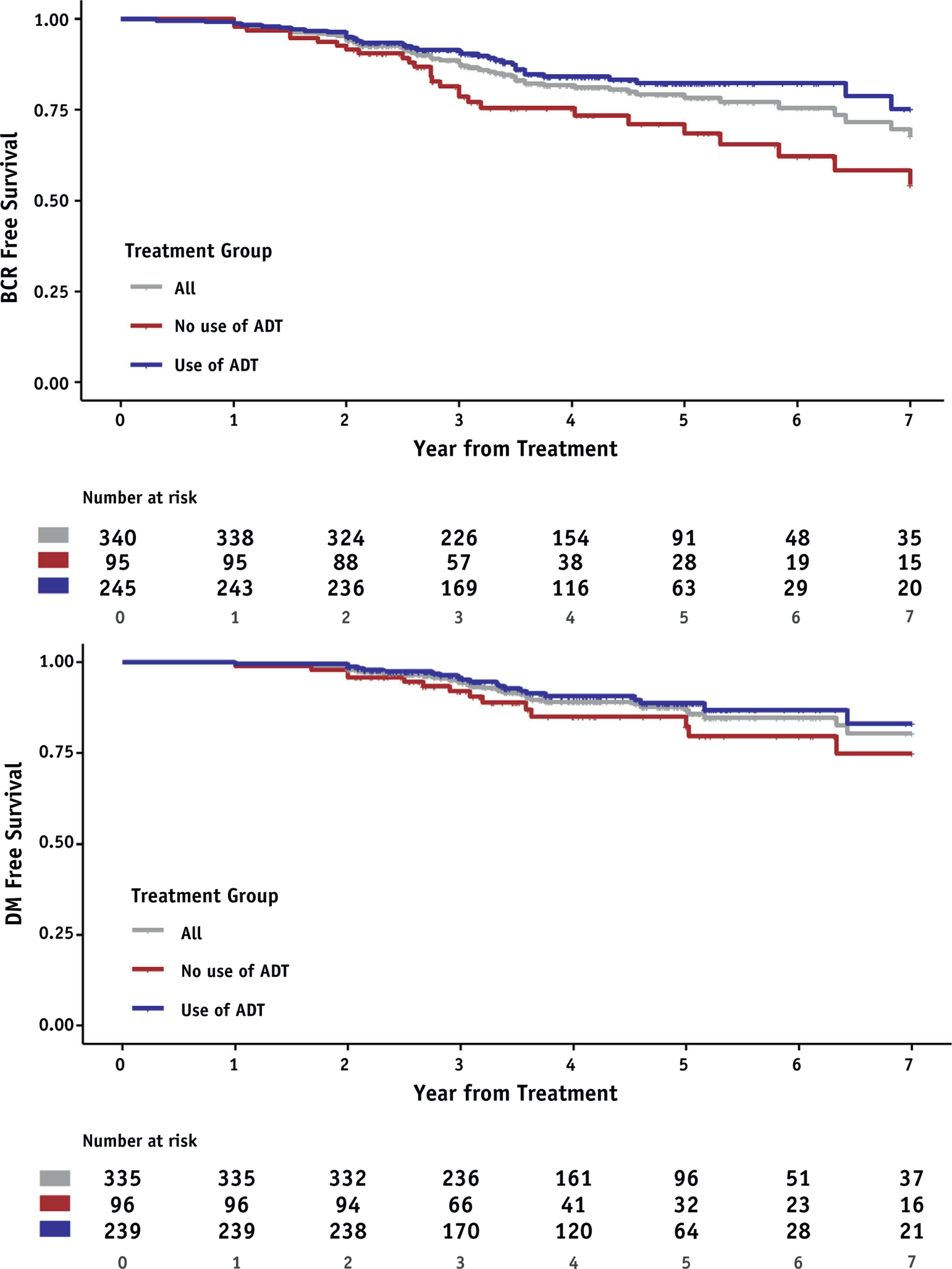
Biochemical recurrence-free survival and distant metastasis-free survival among patients receiving stereotactic body radiation therapy (SBRT) with or without androgen deprivation therapy (ADT). Abbreviations: BCR = biochemical recurrence; DM = distant metastasis.
Acute grade ≥2 GU and GI toxicity were seen in 18% and 5% of patients, respectively; no acute grade ≥3 GU or GI toxicities were seen. Results of multivariable logistic regression models for acute grade ≥2 GU or GI toxicities are shown in Table E3. A dose per fraction of 7 Gy versus 8 Gy and ADT use were associated with lower and higher odds of acute grade ≥2 GU toxicity, respectively (OR 0.09 [95% CI, 0.02–0.48], P = .005 for dose per fraction 7 Gy vs 8 Gy and 4.1 [95% CI 1.3–13.4], P = .02 for ADT use). No significant predictors of acute GI toxicity were identified. Cumulative incidence curves late grade ≥2 GU and GI toxicity are shown in Figure 2. The 4-year cumulative incidence estimates for late grade ≥2 GU and GI toxicity were 17.6% (95% CI, 13.6%−21.9%) and 6.4% (95% CI, 3.7%−10.1%), respectively. The crude incidence of late grade 3 GU toxicity was 2.3% (median time to onset 21 months), and the crude incidence for late grade 3 GI toxicity was 0.9% (median time to onset 22 months).
Fig. 2.
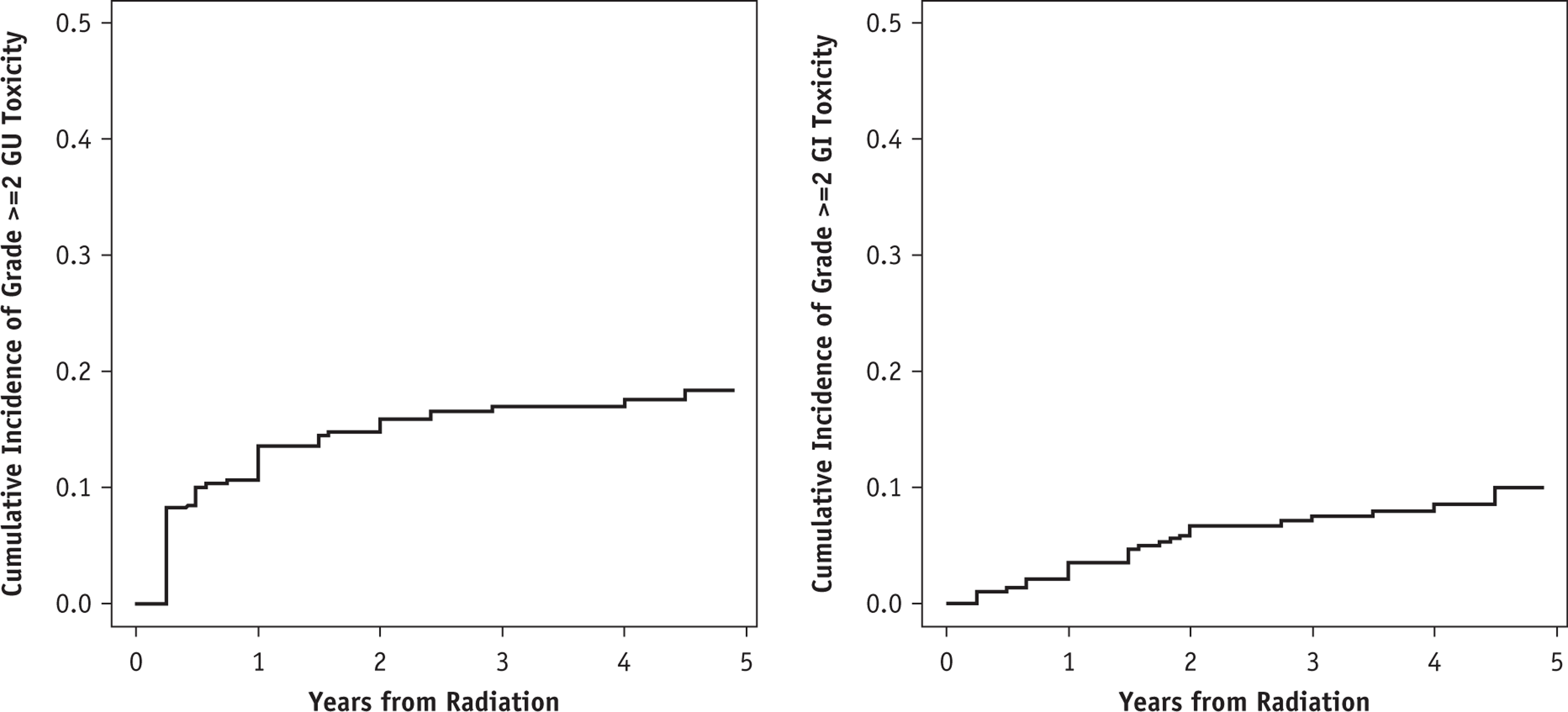
Cumulative incidence of late grade ≥ 2 genitourinary (GU) toxicity (left) and late grade ≥ 2 gastrointestinal (GI) toxicity (right) in patients receiving stereotactic body radiation therapy (SBRT).
Results of multivariable logistic regression models for late grade ≥2 GU or GI toxicities are shown in Table 4. Dose per fraction of 7 Gy and 7.25 Gy vs 8 Gy and ADT use were associated with lower and higher odds of late recurrence; DM = distant metastasis. grade ≥2 GU toxicity, respectively (OR 0.18 [95% CI, 0.06–0.54], P = .002 for 7 Gy vs 8 Gy, 0.25 [95% CI, 0.11–0.56], P = .001 for 7.25 Gy vs 8 Gy, and 4.34 [95% CI 1.68–11.2], P = .002 for ADT use) (Table 4). The same variables were also associated with lower odds of late grade ≥ 2 GI toxicity (OR 0.1 [95% CI, 0.02–0.54], P = .008 and 0.2 [95% CI, 0.07–0.57], P = .002 for dose per fraction 7 Gy and 7.25 Gy vs 8 Gy and OR 0.11 [95% CI 0.02–0.58], P = .009 for ADT use) (Table 4).
Table 4.
Multivariable logistic regression for late grade ≥ 2 toxicity
| Variable | OR (95% CI) | P value |
|---|---|---|
|
| ||
| Genitourinary toxicity | ||
| Age at treatment (1-y increase) | 1.02 (0.98–1.06) | .343 |
| Dose/fraction (ref = 8 Gy) | ||
| 7 vs 8 Gy | 0.18 (0.06–0.54) | .002 |
| 7.25 vs 8 Gy | 0.25 (0.11–0.56) | .001 |
| ADT use (yes vs no) | 4.34 (1.68–11.2) | .002 |
| Nodal radiation therapy (yes vs no) | 1.53 (0.75–3.13) | .243 |
| Gastrointestinal toxicity | ||
| Age at treatment (1-y increase) | 0.98 (0.93–1.03) | .475 |
| Dose/fraction (ref = 8 Gy) | ||
| 7 vs 8 Gy | 0.1 (0.02–0.54) | .008 |
| 7.25 vs 8 Gy | 0.2 (0.07–0.57) | .002 |
| ADT use (yes vs no) | 0.11 (0.02–0.58) | .009 |
| Nodal radiation therapy (yes vs no) | 0.66 (0.28–1.59) | .358 |
Abbreviations: ADT = androgen deprivation therapy; CI = confidence interval; OR = odds ratio.
Discussion
The results of this consortium analysis highlight several important points. First, these prospective data underscore the efficacy of this approach. The estimated 4-year BCRFS rate of 81.7% for patients receiving SBRT in this consortium is similar to the 5-year BCRFS rates for HRPCa patients enrolled on ASCENDE-RT who received a brachytherapy boost (85.5%) or dose-escalated conventionally fractionated radiation therapy alone (83.6%) along with 12 months of ADT, despite the inclusion of patients in the present consortium who either received no ADT or received shorter durations of ADT.9
Second, overall toxicity rates were low and consistent with prior SBRT reports in low- and intermediate-risk disease.10 The estimated 4-year cumulative incidence of late grade ≥2 GU toxicity was 17.6% in this study, versus 5-year cumulative incidences of late grade ≥2 GU toxicity of 53.3% with a brachytherapy boost and 26.4% with dose-escalated conventionally fractionated radiation therapy alone in ASCENDE-RT (though that trial did not use intensity modulated radiation therapy). Similarly, the estimated 4-year cumulative incidence of late grade ≥2 GI was 6.4% in this study, versus 5-year cumulative incidences of late grade ≥2 GI toxicity of 40.4% with a brachytherapy boost and 23.4% with dose-escalated conventionally fractionated radiation therapy alone in ASCENDE-RT. These rates of late grade ≥2 GU and GI toxicity are also comparable to the 5-year cumulative incidences of toxicity seen in prospective randomized trials evaluating moderate hypofractionation, including CHHiP, which identified a 11.7% and 11.9% rate of late grade ≥2 GU and GI toxicity in the 60 Gy arm, respectively.11 Caution must be exercised when comparing these toxicity rates because ASCENDERT and CHHiP used single-protocol prospective data collection methods while our pooled cohort may underreport due to the disparate nature of data collection. Nevertheless, the low incidence of grade 3 GI and GU toxicity in this cohort remains encouraging. We did find that dose per fraction and ADT were associated with increased toxicity, consistent with prior studies.12,13 The etiology of the relationship between ADT use and toxicity is not clear, but increased frequencies of both late GI and GU toxicity have been reported in the setting of ADT, and the phase 3 NRG-GU003 trial investigating GI and GU outcomes in the setting of postprostatectomy radiation includes ADT as a prespecified stratification factor.14–17
Third, nodal radiation therapy was associated with neither improved outcomes nor increased toxicity. A significantly smaller analysis of 2 trials included in the present study did identify a difference in cumulative incidence of BCR favoring nodal radiation therapy, but this finding may have been biased by the small sample size.5
This study has several limitations. First, this is a consortium analysis of multiple single-arm phase 2 studies and prospective registries and therefore cannot provide level I evidence to support SBRT for HRPCa due to its nonrandomized nature. Second, questions regarding the association of ADT or nodal radiation therapy cannot be answered by the current multivariable analyses because, in addition to the selection biases associated with ADT use and duration (as well as nodal radiation therapy use), important variables, including details of socioeconomic status, gland size, and geographic considerations, were not available. These limitations also affect the multivariable analyses that were performed regarding factors associated with toxicity. Third, heterogeneity in contouring, planning, and treatment delivery introduce additional uncertainty when attempting to pool results from disparate studies and institutions. Fourth, additional patient- and treatment-specific covariates that may have affected toxicity, such as prostate size or rectal dose, were unavailable for analysis. Fifth, patient-reported quality of life indices were not available for analysis, and nor were doses received by normal tissues-both would help inform our understanding of toxicity. Finally, the median follow-up of 48 months must be taken in context of the long natural history of prostate cancer, and as such, these should be considered medium-term rather than long-term results.
Conclusions
SBRT has shown promising efficacy in patients with HRPCa in a multi-institutional, international setting. Further prospective studies are needed to verify these results and investigate the optimal dose and target volume in the context of SBRT. The ongoing randomized PACE-C trial is expected to provide additional level I evidence concerning the efficacy of SBRT versus conventional radiation therapy among patients with HRPCa.18
Supplementary Material
Acknowledgments
ASTRO-PCF Career Development Award. This research was supported by NIH National Center for Advancing Translational Science (NCATS) UCLA CTSI Grant Number UL1TR001881.
Footnotes
Disclosures: A.L. has advisory board membership with AbbVie, Sanofi, and TerSera. S.P.C. is a consultant for Accuray. M.L.S. is a consultant for ViewRay.
Data Availability: Research data are not available at this time. Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2021.01.016.
References
- 1.National Comprehensive Cancer Network. Prostate cancer (2.2020). Available at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed June 18, 2020.
- 2.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet 2019;394:385–395. [DOI] [PubMed] [Google Scholar]
- 3.Mottet N, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancerd–2020 update. Part 1: screening, diagnosis, and local teatment with curative intent. Eur Urol. 2021;79:243–262. [DOI] [PubMed] [Google Scholar]
- 4.Murthy V, Gupta M, Mulye G, et al. Early results of extreme hypo-fractionation using stereotactic body radiation therapy for high-risk, very high-risk and node-positive prostate cancer. Clin Oncol (R Coll Radiol) 2018;30:442–447. [DOI] [PubMed] [Google Scholar]
- 5.Alayed Y, Cheung P, Vesprini D, et al. SABR in high-risk prostate cancer: Outcomes from 2 prospective clinical trials with and without elective nodal irradiation. Int J Radiat Oncol Biol Phys 2019;104:36–41. [DOI] [PubMed] [Google Scholar]
- 6.Loblaw A Stereotactic ablative body radiotherapy for intermediate- or high-risk prostate cancer. Cancer J 2020;26:38–42. [DOI] [PubMed] [Google Scholar]
- 7.Roach M, Hanks G, Thames H, et al. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int J Radiat Oncol Biol Phys 2006;65:965–974. [DOI] [PubMed] [Google Scholar]
- 8.Aalen O Nonparametric estimation of partial transition probabilities in multiple decrement models. Ann Stat 1978;6:534–545. [Google Scholar]
- 9.Morris WJ, Tyldesley S, Rodda S, et al. Androgen suppression combined with elective nodal and dose escalated radiation therapy (the ASCENDE-RT trial): An analysis of survival endpoints for a randomized trial comparing a low-dose-rate brachytherapy boost to a dose-escalated external beam boost for high- and intermediate-risk prostate cancer. Int J Radiat Oncol Biol Phys 2017;98:275–285. [DOI] [PubMed] [Google Scholar]
- 10.Kishan AU, Dang A, Katz AJ, et al. Long-term outcomes of stereotactic body radiotherapy for low-risk and intermediate-risk prostate cancer. JAMA Netw Open 2019;2:e188006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dearnaley D, Syndikus I, Mossop H, et al. Conventional versus hypofractionated high-dose intensity-modulated radiotherapy for prostate cancer: 5-year outcomes of the randomised, non-inferiority, phase 3 CHHiP trial. Lancet Oncol 2016;17:1047–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musunuru HB, Quon H, Davidson M, et al. Dose-escalation of five-fraction SABR in prostate cancer: Toxicity comparison of two prospective trials. Radiother Oncol 2016;118:112–117. [DOI] [PubMed] [Google Scholar]
- 13.Roach M, Moughan J, Lawton CAF, et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): Long-term results of a randomised, phase 3 trial. Lancet Oncol 2018;19:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feigenberg SJ, Hanlon AL, Horwitz EM, et al. Long-term androgen deprivation increases grade 2 and higher late morbidity in prostate cancer patients treated with three-dimensional conformal radiation therapy. Int J Radiat Oncol Biol Phys 2005;62:397–405. [DOI] [PubMed] [Google Scholar]
- 15.Roach M, DeSilvio M, Lawton C, et al. Phase III trial comparing whole-pelvic versus prostate-only radiotherapy and neoadjuvant versus adjuvant combined androgen suppression: Radiation Therapy Oncology Group 9413. J Clin Oncol 2003;21:1904–1911. [DOI] [PubMed] [Google Scholar]
- 16.Lawton CA, Bae K, Pilepich M, et al. Long-term treatment sequelae after external beam irradiation with or without hormonal manipulation for adenocarcinoma of the prostate: Analysis of Radiation Therapy Oncology Group studies 85–31, 86–10, and 92–02. Int J Radiat Oncol Biol Phys 2008;70:437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Library of Medicine. Hypofractionated radiation therapyor conventional radiation therapy after surgery in treating patients with prostate cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT03274687. Accessed February 1, 2021.
- 18.US National Library of Medicine, International randomised study of prostatectomy vs stereotactic body radiotherapy (SBRT) and conventional radiotherapy vs SBRT for organ-confined prostate cancer. Available at: https://clinicaltrials.gov/ct2/show/NCT01584258. Accessed February 13, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


