Abstract
Background
CheckMate 9LA, a phase 3, randomized, open-label study in first-line advanced non-small cell lung cancer (NSCLC), showed significantly improved overall survival (OS) with nivolumab plus ipilimumab combined with 2 cycles of chemotherapy versus chemotherapy alone (4 cycles). We present results for the Asian subpopulation enrolled in Japan and China.
Methods
Patients aged ≥ 18 years with treatment-naive, histologically confirmed stage IV or recurrent NSCLC, Eastern Cooperative Oncology Group performance status 0–1 and no sensitizing EGFR/ALK mutations were randomized 1:1 to nivolumab [360 mg every 3 weeks (Q3W)] plus ipilimumab (1 mg/kg Q6W) combined with chemotherapy (Q3W for 2 cycles), or chemotherapy alone (Q3W for 4 cycles). Primary endpoint was OS; secondary endpoints included progression-free survival (PFS) and objective response rate (ORR).
Results
Twenty-eight patients received nivolumab plus ipilimumab combined with chemotherapy and 30 received chemotherapy. At a minimum follow-up of 12.7 months, median OS was not reached with nivolumab plus ipilimumab combined with chemotherapy versus 13.3 months with chemotherapy [hazard ratio (HR) 0.33; 95% confidence interval (CI) 0.14–0.80]. Median PFS was 8.4 versus 5.4 months (HR 0.47; 95% CI 0.24–0.92) and ORR was 57% versus 23%, respectively. Grade 3–4 treatment-related adverse events were observed in 57% versus 60% of patients, respectively.
Conclusion
Consistent with results in the all randomized population, nivolumab plus ipilimumab combined with chemotherapy improved efficacy in the Asian subpopulation versus chemotherapy alone and had a manageable safety profile, supporting its use as first-line treatment for advanced NSCLC in Asian patients.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10147-022-02120-0.
Keywords: Immunotherapy, Non-small cell lung cancer, Asia, Japan, Nivolumab, Ipilimumab
Introduction
Clinical trials of immunotherapy regimens have demonstrated improvements in clinical outcomes over traditional chemotherapy for patients with advanced non-small cell lung cancer (NSCLC) [1–5]. Immunotherapy treatments are now approved as monotherapy or in combination with other immunotherapy agents or chemotherapy for the first-line treatment of advanced NSCLC in many countries [6–8]. The immunotherapy agents nivolumab and ipilimumab have distinct but complementary mechanisms of action: nivolumab, a fully human immunoglobulin G4 programmed death (PD)-1 immune checkpoint inhibitor antibody, restores anti-tumor T-cell function [9–11] and ipilimumab, a fully human immunoglobulin G1 cytotoxic T-lymphocyte antigen-4 immune checkpoint inhibitor antibody, induces T-cell proliferation and de novo anti-tumor T-cell responses, including an increase in memory T cells [12–14].
First-line treatment with nivolumab plus ipilimumab provides durable long-term survival benefit for patients with advanced NSCLC, regardless of tumor PD-L1 expression and histology, as observed in CheckMate 227 [1, 15]. Addition of a limited course (2 cycles) of platinum-doublet chemotherapy to nivolumab plus ipilimumab was hypothesized to provide rapid initial disease control while potentially building on the durable benefits of nivolumab plus ipilimumab. CheckMate 9LA (NCT03215706), a phase 3, randomized, open-label study in first-line advanced NSCLC, showed significantly improved overall survival (OS), progression-free survival (PFS), and objective response rate (ORR) with nivolumab plus ipilimumab combined with 2 cycles of chemotherapy versus 4 cycles of chemotherapy alone, with a manageable safety profile [16, 17]. These data led to the approval of nivolumab plus ipilimumab combined with chemotherapy (2 cycles) in many countries, including the United States, the European Union, and several Asian countries such as Japan, South Korea, Taiwan, and Singapore, as first-line treatment for patients with metastatic or recurrent NSCLC, with no EGFR or ALK genomic tumor aberrations [7, 8, 18–22].
Differences in the treatment outcomes and safety profiles of various therapies have been observed between Asian and non-Asian patients with NSCLC [23–25]. It is therefore important to evaluate the clinical activities of therapies in Asian populations to optimize treatment strategies. Here, we report efficacy and safety data for the Asian subpopulation in CheckMate 9LA.
Patients and methods
Patients and treatment
The study design for CheckMate 9LA (NCT03215706) has been described previously [16]. Briefly, eligible patients were aged ≥ 18 years with treatment-naive, histologically confirmed stage IV or recurrent NSCLC, an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1, and no sensitizing EGFR or ALK mutations. Patients in this subanalysis were enrolled at 16 centers (14 centers in Japan and 2 in China).
Patients were randomly assigned (1:1) by an interactive web response system via permuted blocks (block size of 4) to either: nivolumab [360 mg intravenously every 3 weeks (Q3W)] plus ipilimumab (1 mg/kg intravenously Q6W) combined with histology-based, platinum-doublet chemotherapy (intravenously Q3W for 2 cycles), or histology-based chemotherapy alone (Q3W for 4 cycles). Randomization was stratified by tumor histology (squamous versus nonsquamous), sex, and tumor programmed death ligand-1 (PD-L1) expression (< 1% versus ≥ 1%). In either arm, patients with nonsquamous disease received pemetrexed plus cisplatin or pemetrexed plus carboplatin, and those with squamous disease received paclitaxel plus carboplatin. Patients with nonsquamous histology in the chemotherapy arm could receive optional pemetrexed maintenance until disease progression or unacceptable toxicity. For patients in the nivolumab plus ipilimumab combined with chemotherapy arm, immunotherapy treatment continued until disease progression, unacceptable toxicity, or completion per protocol (2 years).
Endpoints and assessments
The primary endpoint of CheckMate 9LA was OS in all randomly assigned patients. Secondary endpoints included PFS by blinded independent central review (BICR) and ORR by BICR. Safety was analyzed in all treated patients, and adverse events (AEs) were graded per the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.0). Treatment-related adverse events (TRAEs) and treatment-related select AEs, defined as AEs with potential immunologic cause, were collected between first dose and 30 days after last dose of study drug. Tumor PD-L1 expression was determined by the PD-L1 IHC 28-8 pharmDx assay (Agilent Technologies, Inc., Santa Clara, CA, USA). All endpoints described above were assessed for the Asian subpopulation in this analysis.
Statistical analyses
OS, PFS, and duration of response (DOR) were assessed by Kaplan–Meier method. Hazard ratios (HRs) for OS and PFS were calculated using a Cox proportional hazards model with treatment group as a single covariate. All subgroup analyses were descriptive; no formal statistical testing was done for the Asian subpopulation as these were post hoc analyses.
Results
Patients
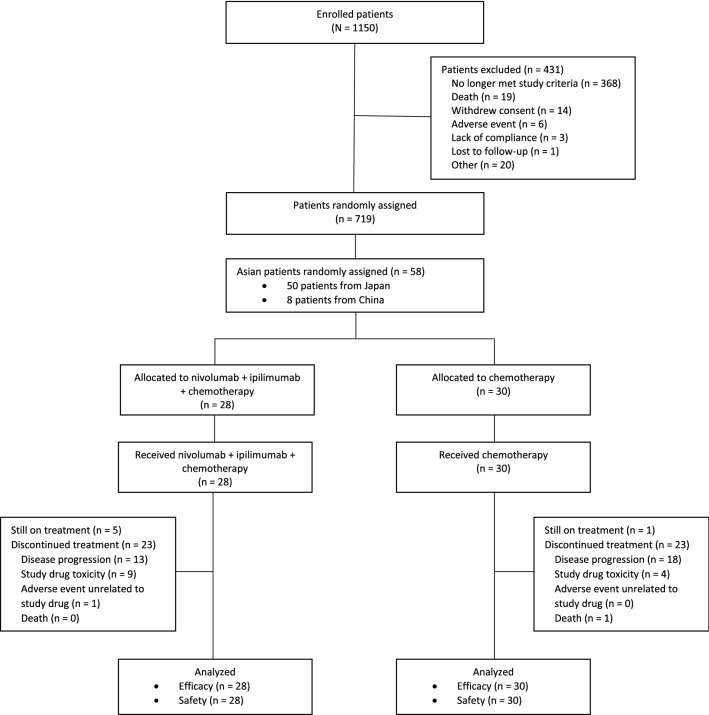
In the all randomized study population of CheckMate 9LA, 361 patients were randomized to nivolumab plus ipilimumab combined with chemotherapy and 358 were randomized to chemotherapy. In the Asian subpopulation, 28 patients (22 patients from Japan and 6 from China) were randomized to nivolumab plus ipilimumab combined with chemotherapy and 30 patients (28 patients from Japan and 2 from China) to chemotherapy (Fig. 1). This analysis was based on a database lock of March 9, 2020, with minimum follow-up of 12.7 months for OS and 12.2 months for all other endpoints. Baseline characteristics in Asian patients were generally balanced between treatment groups (Table 1), although the number of patients was small in some subgroups. Median age was 68 years in the nivolumab plus ipilimumab combined with chemotherapy group, and 66 years in the chemotherapy group. Most patients in both the nivolumab plus ipilimumab combined with chemotherapy and chemotherapy treatment arms were current/former smokers (93% and 87%, respectively) and had nonsquamous histology (61% and 70%, respectively). All patients in the Asian subpopulation had quantifiable tumor PD-L1 expression; most had tumor PD-L1 expression ≥ 1% (57% in each arm).
Fig. 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram of patient disposition in the Asian subpopulation of CheckMate 9LA
Table 1.
Baseline demographic and clinical characteristics of the Asian subpopulation in CheckMate 9LA
| NIVO + IPI + chemoa (n = 28) | Chemob (n = 30) | |
|---|---|---|
| Age, median (range), years | 68 (46–77) | 66 (31–77) |
| < 65 years, n (%) | 10 (36) | 11 (37) |
| ≥ 65 to < 75 years, n (%) | 16 (57) | 16 (53) |
| ≥ 75 years, n (%) | 2 (7) | 3 (10) |
| Female, n (%) | 3 (11) | 6 (20) |
| Region, n (%) | ||
| Japan | 22 (79) | 28 (93) |
| China | 6 (21) | 2 (7) |
| Disease stage, n (%) | ||
| Stage IV | 24 (86) | 27 (90) |
| Recurrent | 4 (14) | 3 (10) |
| ECOG performance status, n (%) | ||
| 0 | 10 (36) | 12 (40) |
| 1 | 18 (64) | 18 (60) |
| Smoking status, n (%) | ||
| Never smoker | 2 (7) | 4 (13) |
| Current/former smoker | 26 (93) | 26 (87) |
| Histology, n (%) | ||
| Squamous | 11 (39) | 9 (30) |
| Nonsquamous | 17 (61) | 21 (70) |
| Bone metastasis, n (%) | 9 (32) | 10 (33) |
| CNS metastasis, n (%) | 6 (21) | 5 (17) |
| Liver metastasis, n (%) | 2 (7) | 5 (17) |
| Tumor PD-L1 expression,c n (%) | ||
| < 1% | 12 (43) | 13 (43) |
| ≥ 1% | 16 (57) | 17 (57) |
| 1–49% | 9 (32) | 9 (30) |
| ≥ 50% | 7 (25) | 8 (27) |
Chemo chemotherapy, CNS central nervous system, ECOG Eastern Cooperative Oncology Group, IPI ipilimumab, n number of patients, NIVO nivolumab, PD-L1 programmed death ligand-1
aNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
bChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology)
cQuantifiable for 100% of Asian patients
Treatment exposure and subsequent therapy
At the database lock, 18% of Asian patients were still receiving nivolumab plus ipilimumab combined with chemotherapy; 3% of patients were still receiving chemotherapy (Table 2). The most common reasons for treatment discontinuation (in ≥ 10% of patients) were disease progression (46% of patients in the nivolumab plus ipilimumab combined with chemotherapy arm and 60% of patients in the chemotherapy arm) and study drug toxicity (32% and 13%, respectively). In the nivolumab plus ipilimumab combined with chemotherapy arm, median duration of therapy was 4.2 months and 93% of patients completed 2 cycles of chemotherapy. In the chemotherapy arm, median duration of therapy was 2.1 months; 63% of patients received 4 or more cycles of treatment; 57% of patients with nonsquamous histology received pemetrexed maintenance. In patients with a PFS event (progression or death or censored for initiation of subsequent systemic therapy), subsequent systemic therapy was received by 77% of patients in the nivolumab plus ipilimumab combined with chemotherapy arm and 79% of patients in the chemotherapy arm; subsequent immunotherapy was received by 9% and 69% of patients, respectively; and subsequent chemotherapy was received by 73% and 38% of patients, respectively (Table 3). Subsequent therapies received among all patients are reported in Table 3.
Table 2.
Treatment exposure in the Asian subpopulation of CheckMate 9LA
| NIVO + IPI + chemoa (n = 28) | Chemob (n = 30) | |
|---|---|---|
| Duration of therapy, median (range), months | 4.2 (0.0–17.6) | 2.1 (0.0–15.9) |
| Number of doses received, median (range) | ||
| Nivolumab | 7 (1–25) | NA |
| Ipilimumab | 4 (1–13) | |
| Cycles of platinum-based chemotherapy received, n (%) | ||
| 1 | 2 (7) | 1 (3) |
| 2 | 26 (93) | 5 (17) |
| 3 | 0 | 5 (17) |
| ≥ 4c | 0 | 19 (63) |
| Patients receiving pemetrexed maintenance therapy,d n (%) | NA | 12e (40) |
| Patients still on treatment, n (%) | 5 (18) | 1 (3) |
aNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
bChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology)
cIncludes patients who completed 4 cycles of platinum-doublet chemotherapy and patients with nonsquamous histology who received pemetrexed maintenance
dPemetrexed maintenance therapy was optional for patients with nonsquamous histology
e57% of patients with nonsquamous histology
Chemo chemotherapy, IPI ipilimumab, n number of patients, NA not available, NIVO nivolumab
Table 3.
Subsequent therapya received in the Asian subpopulation of CheckMate 9LA
| Subsequent therapy, n (%) | Asian patients | Asian patients with a PFS event per BICRd | ||
|---|---|---|---|---|
| NIVO + IPI + chemob (n = 28) | Chemoc (n = 30) | NIVO + IPI + chemob (n = 22) | Chemoc (n = 29) | |
| Any subsequent therapy | 18 (64) | 25 (83) | 18 (82) | 25 (86) |
| Subsequent radiotherapy | 6 (21) | 11 (37) | 6 (27) | 11 (38) |
| Subsequent systemic therapy | 17 (61) | 23 (77) | 17 (77) | 23 (79) |
| Immunotherapy | 2 (7) | 20 (67) | 2 (9) | 20 (69) |
| Anti–PD-1 | 0 | 15 (50) | 0 | 15 (52) |
| Anti–PD-L1 | 1 (4) | 4 (13) | 1 (4) | 4 (14) |
| Other immunotherapy | 1 (4) | 2 (7) | 1 (4) | 2 (7) |
| VEGFR inhibitors | 6 (21) | 6 (20) | 6 (27) | 6 (21) |
| Other systemic therapy—experimental | 1 (4) | 1 (3) | 1 (4) | 1 (3) |
| Other systemic therapy—chemotherapy | 16 (57) | 11 (37) | 16 (73) | 11 (38) |
BICR blinded independent central review, Chemo chemotherapy, IPI ipilimumab, n number of patients, NIVO nivolumab, PD-1 programmed death-1, PD-L1 programmed death ligand-1, PFS progression-free survival, VEGFR vascular endothelial growth factor receptor
aDefined as therapy initiated on or after first dosing date (or randomization date if the patient was never treated); patients may have received more than 1 type of subsequent therapy
bNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
cChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology)
dIncludes patients that had an event of progression or death as well as being censored for subsequent systemic therapy
Efficacy outcomes
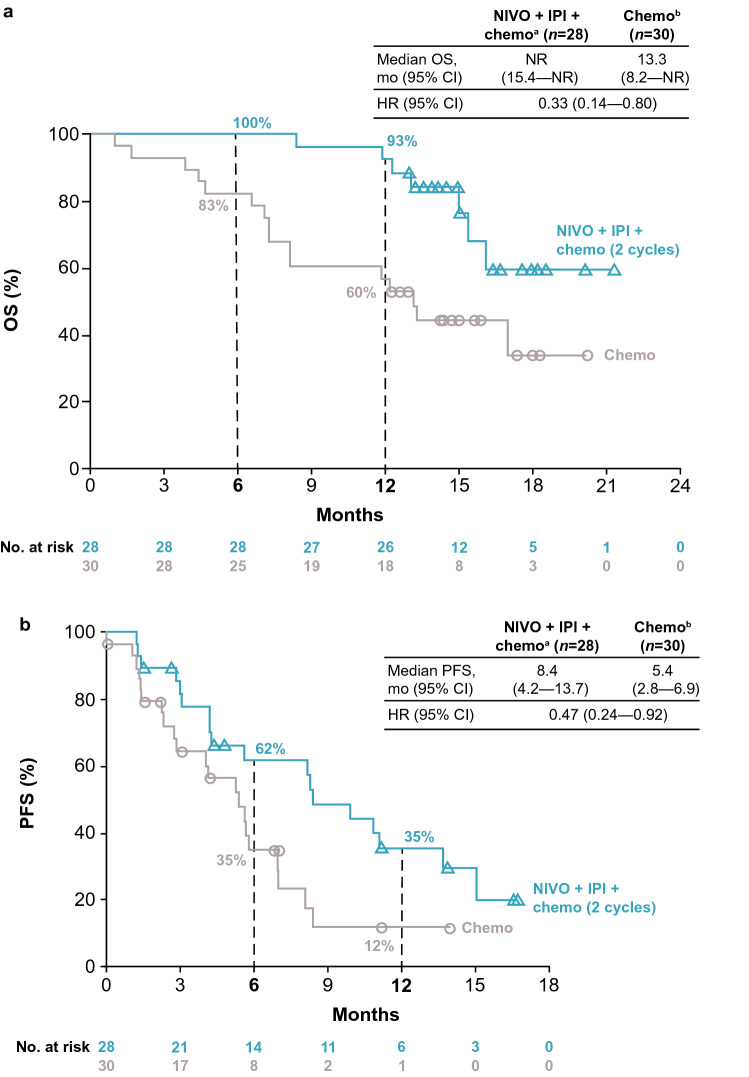
OS was improved with nivolumab plus ipilimumab combined with chemotherapy versus chemotherapy alone among Asian patients (Fig. 2a). Median OS was not reached [95% confidence interval (CI) 15.4–not reached] for patients in the nivolumab plus ipilimumab combined with chemotherapy arm versus 13.3 months (8.2–not reached) for patients in the chemotherapy arm; HR 0.33 (95% CI 0.14–0.80); 6-month and 1-year OS rates were 100% (95% CI 100.0–100.0) and 93% (95% CI 74.3–98.2) with nivolumab plus ipilimumab combined with chemotherapy versus 83% (95% CI 64.5–92.7) and 60% (95% CI 40.5–75.0) with chemotherapy, respectively. OS by histology (squamous and nonsquamous) and tumor PD-L1 expression (< 1% and ≥ 1%) also favored nivolumab plus ipilimumab combined with chemotherapy compared with chemotherapy (Online Resource 1).
Fig. 2.
OS (a) and PFS per BICR (b) in the Asian subpopulation of CheckMate 9LA. aNivolumab plus ipilimumab combined with chemotherapy (2 cycles); bChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology). BICR blinded independent central review, chemo chemotherapy, CI confidence interval, HR hazard ratio, IPI ipilimumab, mo months, n number of patients, NIVO nivolumab, NR not reached, OS overall survival, PFS progression-free survival
PFS per BICR in Asian patients was improved with nivolumab plus ipilimumab combined with chemotherapy versus chemotherapy alone. Median PFS was 8.4 months (95% CI 4.2–13.7) for patients in the nivolumab plus ipilimumab combined with chemotherapy arm versus 5.4 months (95% CI 2.8–6.9) for patients in the chemotherapy arm; HR 0.47; 95% CI 0.24–0.92 (Fig. 2b); 6-month and 1-year PFS rates were 62% (95% CI 40.2–77.3) and 35% (95% CI 17.1–53.9) with nivolumab plus ipilimumab combined with chemotherapy versus 35% (95% CI 17.0–53.0) and 12% (95% CI 2.1–29.9) with chemotherapy, respectively.
ORR and DOR per BICR are presented in Table 4. ORR was higher with nivolumab plus ipilimumab combined with chemotherapy (16 of 28 patients, 57%) versus chemotherapy alone (7 of 30 patients, 23%). Two patients (7%) achieved complete response with nivolumab plus ipilimumab combined with chemotherapy; no complete responses were reported in the chemotherapy arm. Median time to response for patients who received nivolumab plus ipilimumab combined with chemotherapy was 1.5 months versus 2.8 months for those who received chemotherapy. Median DOR was longer with nivolumab plus ipilimumab combined with chemotherapy [7.0 months (95% CI 2.8–11.0)] than with chemotherapy [4.4 months (95% CI 2.8–not reached)].
Table 4.
ORR and DOR per BICR in the Asian subpopulation of CheckMate 9LA
| NIVO + IPI + chemoa (n = 28) | Chemob (n = 30) | |
|---|---|---|
| ORR, n (%) | 16 (57) | 7 (23) |
| Best overall response, n (%) | ||
| Complete response | 2 (7) | 0 |
| Partial response | 14 (50) | 7 (23) |
| Stable disease | 9 (32) | 16 (53) |
| Progressive disease | 3 (11) | 5 (17) |
| Could not be determined | 0 | 2 (7) |
| Disease control rate, n (%) | 25 (89) | 23 (77) |
| Time to response, median (range), months | 1.5 (1.2–9.5) | 2.8 (1.2–5.4) |
| DOR, median (95% CI), months | 7.0 (2.8–11.0) | 4.4 (2.8–NR) |
BICR blinded independent central review, Chemo chemotherapy, CI confidence interval, DOR duration of response, IPI ipilimumab, n number of patients, NIVO nivolumab, NR not reached, ORR objective response rate
aNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
bChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology)
Safety
TRAEs of any grade among Asian patients were reported in 28 (100%) patients treated with nivolumab plus ipilimumab combined with chemotherapy and in 29 (97%) patients treated with chemotherapy (Table 5). The most common (in ≥ 40% of patients) any-grade TRAEs included decreased appetite (46%) and constipation (43%) in patients treated with nivolumab plus ipilimumab combined with chemotherapy; and nausea (60%), constipation (57%), anemia (50%), and decreased appetite (40%) in patients treated with chemotherapy. Grade 3–4 TRAEs were reported in 16 (57%) patients treated with nivolumab plus ipilimumab combined with chemotherapy and in 18 (60%) patients treated with chemotherapy. The most common (in ≥ 10% of patients) grade 3–4 TRAEs included decreased neutrophil count (18%), maculopapular rash (11%), and decreased white blood cell count (11%) in patients treated with nivolumab plus ipilimumab combined with chemotherapy; and anemia (23%), decreased neutrophil count (17%), and decreased appetite (10%) in patients treated with chemotherapy. TRAEs of any grade leading to discontinuation of any component of the treatment regimen were reported in 21% of patients treated with nivolumab plus ipilimumab combined with chemotherapy versus 17% of patients treated with chemotherapy. Any-grade and grade 3–4 serious TRAEs were reported in 36% and 21% of patients treated with nivolumab plus ipilimumab combined with chemotherapy, and in 30% and 20% of patients treated with chemotherapy, respectively. No treatment-related deaths occurred in the nivolumab plus ipilimumab combined with chemotherapy arm; 1 grade 5 AE was reported in the chemotherapy arm as potentially related to treatment, but cause of death was recorded as unknown.
Table 5.
Treatment-related adverse events in the Asian subpopulation of CheckMate 9LA
| NIVO + IPI + chemoa (n = 28) | Chemob (n = 30) | |||
|---|---|---|---|---|
| Any grade | Grade 3–4 | Any grade | Grade 3–4 | |
| Total patients with an event,c n (%) | 28 (100) | 16 (57) | 29 (97) | 18 (60) |
| TRAEs occurring in ≥ 15% of patients in either treatment arm, n (%) | ||||
| Decreased appetite | 13 (46) | 2 (7) | 12 (40) | 3 (10) |
| Constipation | 12 (43) | 0 | 17 (57) | 0 |
| Nausea | 11 (39) | 0 | 18 (60) | 0 |
| Neutrophil count decreased | 10 (36) | 5 (18) | 8 (27) | 5 (17) |
| Fatigue | 8 (29) | 1 (4) | 7 (23) | 0 |
| Malaise | 8 (29) | 0 | 8 (27) | 0 |
| Maculopapular rash | 8 (29) | 3 (11) | 2 (7) | 0 |
| Anemia | 8 (29) | 1 (4) | 15 (50) | 7 (23) |
| Rash | 7 (25) | 2 (7) | 0 | 0 |
| Alopecia | 6 (21) | 0 | 8 (27) | 0 |
| White blood cell count decreased | 6 (21) | 3 (11) | 6 (20) | 2 (7) |
| Diarrhea | 5 (18) | 0 | 3 (10) | 1 (3) |
| Pyrexia | 5 (18) | 0 | 2 (7) | 0 |
| Platelet count decreased | 4 (14) | 1 (4) | 7 (23) | 1 (3) |
| Peripheral sensory neuropathy | 3 (11) | 0 | 9 (30) | 0 |
| Hiccups | 3 (11) | 0 | 8 (27) | 0 |
| TRAEs leading to treatment discontinuationd, n (%) | 6 (21) | 3 (11) | 5 (17) | 2 (7) |
| Serious TRAEse, n (%) | 10 (36) | 6 (21) | 9 (30) | 6 (20) |
| Treatment-related deathsf, g, n | 0 | 0 | ||
Chemo chemotherapy, IPI ipilimumab, n number of patients, NIVO nivolumab, TRAE treatment-related adverse event
aNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
bChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology)
cIncludes events reported between first dose and 30 days after last dose of study drug
dIncludes discontinuation of any component of the regimen
eSerious adverse events are defined as any untoward medical occurrence that, at any dose, result in the following: death or risk of death at the time of the event; inpatient hospitalization or prolongation of existing hospitalization, with the following exceptions: a visit to the emergency room or other hospital departments < 24 h, elective surgery (planned prior to signing consent), admissions as per protocol for a planned medical/surgical procedure, routine health assessment requiring admission for baseline/trending of health status, medical/surgical admission other than to remedy ill health and planned prior to entry into the study, admission for another life circumstance that is unrelated to health status and requires no medical/surgical intervention, and admission for administration of anticancer therapy in the absence of any other serious adverse event; persistent or significant disability/incapacity; and congenital abnormalities/birth defects. Important medical events (defined as events that may not be immediately life threatening or result in death or hospitalization but, based upon appropriate medical and scientific judgement, may jeopardize the participant or require intervention to prevent other serious outcomes listed above) are also classified as serious adverse events
fWithin 100 days of last dose
gOne grade 5 TRAE was reported in the chemotherapy arm, but cause of death was recorded as unknown
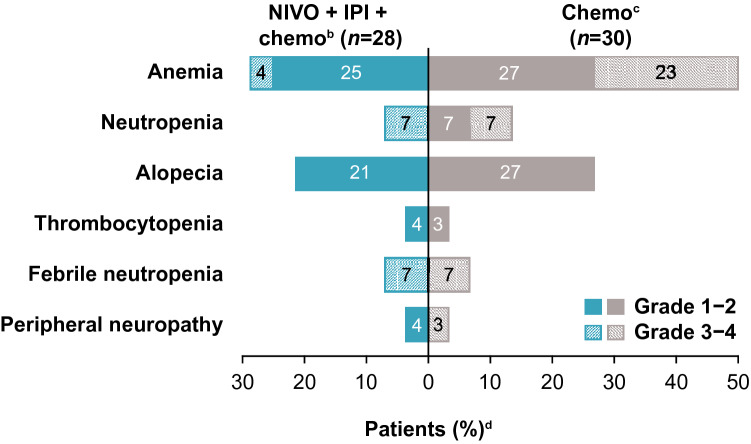
Treatment-related select AEs, defined as AEs with potential immunologic cause, with nivolumab plus ipilimumab combined with chemotherapy were reported by organ system as follows: skin (75%), endocrine (32%), hepatic (21%), gastrointestinal (18%), pulmonary (11%), and renal (7%) (Table 6); most events were grade 1–2. Of TRAEs typically associated with chemotherapy, anemia was the most common event in both the nivolumab plus ipilimumab combined with chemotherapy and chemotherapy arms (grade 1–2, 25% and 27%; grade 3–4, 4% and 23%, respectively), followed by alopecia (grade 1–2, 21% and 27%, respectively; no grade 3–4 events were reported for either arm) (Fig. 3).
Table 6.
Treatment-related select adverse eventsa in the nivolumab plus ipilimumab combined with chemotherapy arm of the Asian subpopulation of CheckMate 9LA
| Patients, n (%) | NIVO + IPI + chemob (n = 28) | |
|---|---|---|
| Any grade | Grade 3–4 | |
| Skin | 21 (75) | 5 (18) |
| Endocrine | 9 (32) | 1 (4) |
| Hepatic | 6 (21) | 0 |
| Gastrointestinal | 5 (18) | 0 |
| Pulmonary | 3 (11) | 0 |
| Renal | 2 (7) | 1 (4) |
Chemo chemotherapy, IPI ipilimumab, n number of patients, NIVO nivolumab
aSelect adverse events are those with potential immunologic etiology that require frequent monitoring/intervention; includes events reported between first dose and 30 days after last dose of study drug
bNivolumab plus ipilimumab combined with chemotherapy (2 cycles)
Fig. 3.
TRAEs typically associated with chemotherapya in the Asian subpopulation of CheckMate 9LA. aIncludes events reported between first dose and 30 days after last dose of study drug; bNivolumab plus ipilimumab combined with chemotherapy (2 cycles); cChemotherapy alone (4 cycles, with optional pemetrexed maintenance for nonsquamous histology); dData labels not shown for values < 1%. Chemo chemotherapy, IPI ipilimumab, n number of patients, NIVO nivolumab, TRAE treatment-related adverse event
Discussion
Among Asian patients with advanced NSCLC in CheckMate 9LA, first-line treatment with nivolumab plus ipilimumab combined with 2 cycles of chemotherapy provided clinically meaningful OS benefit and improved PFS and ORR versus chemotherapy alone, consistent with the results observed in the all randomized patient population [median OS, 15.6 versus 10.9 months (HR 0.66; 95% CI 0.55–0.80) at a minimum follow-up of 12.7 months; median PFS, 6.7 versus 5.0 months (HR 0.68, 95% CI 0.57–0.82); ORR, 38% versus 25%] [16]. While the Asian subpopulation comprised 8% of the total all randomized patient population, baseline characteristics were generally similar between Asian patients and the all randomized population. Notably, there was a slightly higher proportion of men among Asian patients versus the all randomized population (84% versus 70%) [16], which is reflective of the higher prevalence of NSCLC reported in men versus women in Asian countries [26]. Interestingly, the survival benefit provided by nivolumab plus ipilimumab combined with chemotherapy versus chemotherapy alone was achieved despite most patients in the chemotherapy arm receiving subsequent immunotherapy. While there may be numerical differences in efficacy results between the smaller Asian subpopulation and the all randomized patient population, the benefits of nivolumab plus ipilimumab combined with chemotherapy versus chemotherapy alone in key subgroups, including those based on tumor histology or tumor PD-L1 expression, are encouraging.
Chemotherapy for NSCLC is associated with interethnic differences in survival outcomes [23]; however, data directly comparing the efficacy of immunotherapy between Asian and global populations are limited. Some studies have shown clinical benefits of first-line immunotherapy combinations in both all randomized patients and the Asian subpopulation [27–29]. In a subanalysis of the phase 3 CheckMate 227 study in Asian patients with tumor PD-L1 expression ≥ 1% or < 1%, first-line treatment with nivolumab plus ipilimumab combination therapy demonstrated durable long-term survival and clinical benefits versus chemotherapy (median OS not reached versus 22.9 months; 2-year OS rate, 53% versus 45%, respectively), consistent with OS improvements observed in the all randomized population [29]. Subanalyses of the KEYNOTE-407 study in East Asian patients and the KEYNOTE-189 study in Japanese patients also favored first-line combination immunotherapy with pembrolizumab plus chemotherapy over chemotherapy in Asian/Japanese patients with metastatic squamous or nonsquamous NSCLC, respectively, consistent with findings in the all randomized population of each study [2, 3, 27, 28]. On the other hand, in the IMpower132 trial, atezolizumab plus chemotherapy as first-line treatment for nonsquamous NSCLC showed apparent OS improvement versus chemotherapy in the Japanese subpopulation [median OS, 30.8 versus 22.2 months; HR 0.63 (95% CI 0.36–1.14)], but OS difference in the global population was not statistically significant between the treatment arms (median OS, 17.5 versus 13.6 months; p = 0.1546) [30, 31]. These slight differences in outcomes between the Asian and global populations may potentially be due to underlying variance in genetic or disease characteristics as well as variations in clinical practice [23].
The safety profile of nivolumab plus ipilimumab combined with chemotherapy in Asian patients was generally consistent with that for the all randomized population of CheckMate 9LA [16], with no new safety signals. Frequencies of grade 3–4 TRAEs were higher in Asian patients versus the all randomized population in both the nivolumab plus ipilimumab combined with chemotherapy (57% versus 47%) and chemotherapy alone arms (60% versus 38%), which could be attributed to the small sample size of the Asian subpopulation. However, differences between the two treatment arms were consistent between the Asian subpopulation and the all randomized population. We also observed numerically higher rates of some select TRAEs (skin, endocrine, hepatic, and pulmonary) with nivolumab plus ipilimumab combined with chemotherapy among Asian patients than in the all randomized population, which could also be attributed to the small sample size of the Asian subpopulation.
As ethnicity was not a stratification factor in CheckMate 9LA [16], unknown confounding factors may not have been accounted for in this post hoc analysis. Additionally, the small sample size for this subpopulation analysis limits the extent of the conclusions that can be drawn. However, the benefits with nivolumab plus ipilimumab combined with chemotherapy in Asian patients were consistent across efficacy outcomes, similar to those in the all randomized patient population. Studies in larger populations of Asian patients to further evaluate the efficacy and safety of nivolumab plus ipilimumab combined with chemotherapy are warranted.
In conclusion, these CheckMate 9LA results in the Asian subpopulation support the use of nivolumab plus ipilimumab combined with a limited course of chemotherapy as first-line treatment for Asian patients with advanced NSCLC.
Data sharing
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients and their families, as well as the clinical study teams, for making this study possible. The PD-L1 IHC 28-8 pharmDx assay was developed in collaboration with Dako, an Agilent Technologies company (Santa Clara, CA, USA). Professional medical writing assistance was provided by Brooke Middlebrook, CMPP, of Evidence Scientific Solutions (Philadelphia, Pennsylvania), and was funded by Bristol Myers Squibb.
Author contributions
TJ, AO, and SL contributed to the conceptualization and design of the study. Patient treatment and data collection were performed by HS, SI, YC, KK, YS, YN, MT, HK, HZ, MM, KM, TH, YM, MS, and KH. Data analysis was performed by TJ, AL, AO, and SL. All authors contributed to the interpretation of results and preparation of the manuscript. All authors have read and approved the final manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions.
Declarations
Conflict of interest
TJ received honoraria from MSD, Bristol Myers Squibb, AstraZeneca, Pfizer, Roche, Novartis, and Amgen; current affiliation: Peter MacCallum Cancer Centre, Dept Medical Oncology, Level 7 VCCC, 305 Grattan St, Melbourne 3000, Australia. HS served an advisory role at Bristol Myers Squibb and Ono Pharmaceutical Co.; and received honoraria from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd; current affiliation: Ageo Central General Hospital, 1-10-10 Kashiwaza, Ageo-shi, Saitama 362-8588, Japan. SI received research funding from AstraZeneca and Chugai Pharmaceutical Co. Ltd.; and honoraria from Bristol Myers Squibb, Ono, Taiho, AstraZeneca, Chugai Pharmaceutical Co. Ltd, Eli Lilly, Pfizer, and Boehringer Ingelheim. KK received research funding and honoraria from Bristol Myers Squibb and Ono Pharmaceutical Co. Ltd. YS received honoraria from Chugai Pharmaceutical Co. Ltd., MSD, Ono Pharmaceutical Co. Ltd., Novartis, Taiho Pharmaceutical Co. Ltd., AstraZeneca, Bristol Myers Squibb, Nippon Kayaku Co. Ltd., and Pfizer. YN received research funding from Takeda Pharmaceutical Co. Ltd. and Bristol Myers Squibb; and honoraria from Ono Pharmaceutical Co. Ltd., Bristol Myers Squibb, Boehringer Ingelheim, AstraZeneca, and Chugai Pharmaceutical Co. Ltd.; current affiliation: Kitasato University School of Medicine, 1-15-1, Kitazato, Minami-ku, Sagamihara-shi, Kanagawa 252-0374, Japan. MT received honoraria from Chugai Pharmaceutical Co. Ltd., AstraZeneca K.K., Bristol Myers Squibb, Novartis Pharma K.K., and Ono Pharmaceutical Co. Ltd. HK received research funding from Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., and Eli Lilly; and honoraria from Bristol Myers Squibb, and Ono Pharmaceutical Co. Ltd. MM received honoraria from Ono Pharmaceutical Co. Ltd., and Bristol Myers Squibb. YM received research funding from AstraZeneca, Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., and Boehringer Ingelheim. MS received research funding from MSD, Bristol Myers Squibb, Chugai Pharmaceutical Co. Ltd., and AstraZeneca; and honoraria from MSD, Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., Chugai Pharmaceutical Co. Ltd., AstraZeneca, Eli Lilly, and Taiho Pharmaceutical Co. Ltd. KH received research funding from MSD, AstraZeneca, Chugai Pharmaceutical Co. Ltd., Eli Lilly, and Bristol Myers Squibb; and honoraria from Pfizer, AstraZeneca, Chugai Pharmaceutical Co. Ltd., Eli Lilly, Takeda, MSD, Bristol Myers Squibb, Ono Pharmaceutical Co. Ltd., NipponKayaku, Taiho Pharmaceutical Co. Ltd., and Boehringer Ingelheim. AL and AO are employees and stock owners of Bristol Myers Squibb. SL served advisory roles at AstraZeneca, Pfizer, Boehringer Ingelheim, Hutchison MedPharma, Yuhan Corporation, Menarini, InventisBio, and Roche; received honoraria from AstraZeneca, Roche, Hansoh, and Hengrui Therapeutics; and received research funding from AstraZeneca, Hutchison, Bristol Myers Squibb, Hengrui Therapeutics, Beigene, Roche, and Hansoh. YC, KM, HZ, and TH declare no conflicts of interest.
Ethical statement
This study was conducted according to the Declaration of Helsinki and the international standards of Good Clinical Practice. The study protocol and all amendments were approved by the independent ethics committee or institutional review board of each participating center. All patients provided written informed consent. BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, Alexandru A, Lupinacci L, de la Mora JE, Sakai H, Albert I, Vergnenegre A, Peters S, Syrigos K, Barlesi F, Reck M, Borghaei H, Brahmer JR, O'Byrne KJ, Geese WJ, Bhagavatheeswaran P, Rabindran SK, Kasinathan RS, Nathan FE, Ramalingam SS. Nivolumab plus ipilimumab in advanced non–small-cell lung cancer. N Engl J Med. 2019;381(21):2020–2031. doi: 10.1056/NEJMoa1910231. [DOI] [PubMed] [Google Scholar]
- 2.Paz-Ares L, Luft A, Vicente D, Tafreshi A, Gumus M, Mazieres J, Hermes B, Cay Senler F, Csoszi T, Fulop A, Rodriguez-Cid J, Wilson J, Sugawara S, Kato T, Lee KH, Cheng Y, Novello S, Halmos B, Li X, Lubiniecki GM, Piperdi B, Kowalski DM, Investigators K. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. doi: 10.1056/NEJMoa1810865. [DOI] [PubMed] [Google Scholar]
- 3.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC, Investigators K. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 4.Socinski MA, Jotte RM, Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodriguez-Abreu D, Moro-Sibilot D, Thomas CA, Barlesi F, Finley G, Kelsch C, Lee A, Coleman S, Deng Y, Shen Y, Kowanetz M, Lopez-Chavez A, Sandler A, Reck M, Group IMS Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288–2301. doi: 10.1056/NEJMoa1716948. [DOI] [PubMed] [Google Scholar]
- 5.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers S, Salman P, Borghaei H, Ramalingam SS, Brahmer J, Reck M, O'Byrne KJ, Geese WJ, Green G, Chang H, Szustakowski J, Bhagavatheeswaran P, Healey D, Fu Y, Nathan F, Paz-Ares L. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med. 2018;378(22):2093–2104. doi: 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doroshow DB, Sanmamed MF, Hastings K, Politi K, Rimm DL, Chen L, Melero I, Schalper KA, Herbst RS. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res. 2019;25(15):4592–4602. doi: 10.1158/1078-0432.CCR-18-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.OPDIVO (nivolumab) [summary of product characteristics] (2021) European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo#product-information-section. Accessed 23 June 2021
- 8.Ono Pharmaceutical Co. Ltd (2020) Combination therapy concerning Opdivo and Yervoy approved in Japan for first-line treatment of unresectable advanced or recurrent non-small cell lung cancer. Ono Pharmaceutical Co. Ltd. https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn201127_1.pdf. Accessed 23 June 2021
- 9.Brahmer JR, Drake CG, Wollner I, Powderly JD, Picus J, Sharfman WH, Stankevich E, Pons A, Salay TM, McMiller TL, Gilson MM, Wang C, Selby M, Taube JM, Anders R, Chen L, Korman AJ, Pardoll DM, Lowy I, Topalian SL. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–3175. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C, Thudium KB, Han M, Wang XT, Huang H, Feingersh D, Garcia C, Wu Y, Kuhne M, Srinivasan M, Singh S, Wong S, Garner N, Leblanc H, Bunch RT, Blanset D, Selby MJ, Korman AJ. In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res. 2014;2(9):846–856. doi: 10.1158/2326-6066.CIR-14-0040. [DOI] [PubMed] [Google Scholar]
- 11.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma P, Allison JP. Dissecting the mechanisms of immune checkpoint therapy. Nat Rev Immunol. 2020;20(2):75–76. doi: 10.1038/s41577-020-0275-8. [DOI] [PubMed] [Google Scholar]
- 13.Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086. doi: 10.1158/2159-8290.CD-18-0367. [DOI] [PubMed] [Google Scholar]
- 14.Das R, Verma R, Sznol M, Boddupalli CS, Gettinger SN, Kluger H, Callahan M, Wolchok JD, Halaban R, Dhodapkar MV, Dhodapkar KM. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol. 2015;194(3):950–959. doi: 10.4049/jimmunol.1401686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramalingam SS, Ciuleanu TE, Pluzanski A, Lee JS, Schenker M, Caro RM, Lee KH, Zurawski B, Audigier-Valette C, Provencio M, Linardou H, Kim SW, Borghaei H, Hellmann MD, O'Byrne KJ, Paz-Ares LG, Reck M, Nathan FE, Brahmer JR. Nivolumab + ipilimumab versus platinum-doublet chemotherapy as first-line treatment for advanced non-small cell lung cancer: three-year update from CheckMate 227 Part 1. J Clin Oncol. 2020;38(15_suppl):9500. doi: 10.1200/JCO.2020.38.15_suppl.9500. [DOI] [Google Scholar]
- 16.Paz-Ares L, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Salman P, Souquet PJ, De Marchi P, Martin C, Perol M, Scherpereel A, Lu S, John T, Carbone DP, Meadows-Shropshire S, Agrawal S, Oukessou A, Yan J, Reck M. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9LA): an international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):198–211. doi: 10.1016/S1470-2045(20)30641-0. [DOI] [PubMed] [Google Scholar]
- 17.Reck M, Ciuleanu TE, Cobo M, Schenker M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E, Juan-Vidal O, Alexandru A, Sakai H, Lingua A, Reyes F, Souquet PJ, De Marchi P, Martin C, Pérol M, Scherpereel A, Lu S, Paz-Ares L, Carbone DP, Memaj A, Marimuthu S, Zhang X, Tran P, John T. First-line nivolumab plus ipilimumab with two cycles of chemotherapy versus chemotherapy alone (four cycles) in advanced non-small-cell lung cancer: CheckMate 9LA 2-year update. ESMO Open. 2021;6(5):100273. doi: 10.1016/j.esmoop.2021.100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.OPDIVO (nivolumab) [package insert] (2020) Bristol Myers Squibb https://packageinserts.bms.com/pi/pi_opdivo.pdf. Accessed 23 June 2021
- 19.YERVOY (ipilimumab) [package insert] (2020) Bristol Myers Squibb https://packageinserts.bms.com/pi/pi_yervoy.pdf. Accessed 23 June 2021
- 20.Ono Pharmaceutical Co. Ltd (2020) Opdivo® (Nivolumab) intravenous infusion approved for first-line treatment of unresectable advanced or recurrent non-small cell lung cancer in South Korea. Ono Pharmaceutical Co. Ltd. https://www.ono-pharma.com/sites/default/files/en/news/press/sm_cn201217.pdf. Accessed 23 June 2021
- 21.Ono Pharmaceutical Co. Ltd (2020) Opdivo® (Nivolumab) intravenous infusion approved for first-line treatment of advanced or recurrent non-small cell lung cancer in combination therapy in Taiwan. https://www.ono-pharma.com/sites/default/files/en/news/press/enews20210226_2.pdf. Accessed 23 June 2021
- 22.Nyberg K (2020) Combined first-line treatment with ipilimumab, nivolumab, limited chemotherapy prolongs survival in advanced NSCLC. https://dailynews.ascopubs.org/do/10.1200/ADN.20.200203/full/. Accessed 30 May 2020
- 23.Soo RA, Loh M, Mok TS, Ou SH, Cho BC, Yeo WL, Tenen DG, Soong R. Ethnic differences in survival outcome in patients with advanced stage non-small cell lung cancer: results of a meta-analysis of randomized controlled trials. J Thorac Oncol. 2011;6(6):1030–1038. doi: 10.1097/JTO.0b013e3182199c03. [DOI] [PubMed] [Google Scholar]
- 24.Zhou W, Christiani DC. East meets West: ethnic differences in epidemiology and clinical behaviors of lung cancer between East Asians and Caucasians. Chin J Cancer. 2011;30(5):287–292. doi: 10.5732/cjc.011.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang J, He X, Lv Q, Jing J, Shi H. Management of adverse events in cancer patients treated with PD-1/PD-L1 blockade: focus on Asian populations. Front Pharmacol. 2019;10:726. doi: 10.3389/fphar.2019.00726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.WHO (2021) World Health Organization cancer today. https://gco.iarc.fr/today/home. Accessed 25 Jan 2021
- 27.Horinouchi H, Nogami N, Saka H, Nishio M, Tokito T, Takahashi T, Kasahara K, Hattori Y, Ichihara E, Adachi N, Noguchi K, Souza F, Kurata T. Pembrolizumab plus pemetrexed-platinum for metastatic nonsquamous non-small-cell lung cancer: KEYNOTE-189 Japan Study. Cancer Sci. 2021;112(8):3255–3265. doi: 10.1111/cas.14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kato T, Lee S, Cheng Y, Lee GW, Lee K, Luft A, Trigo J, Hui R, Balint B, Robinson A, Okamoto I, Gerstner GJ, Paz-Ares L, Li X, Shentu Y, Piperdi B, Tafreshi A. 491O—carboplatin-paclitaxel/nab-paclitaxel with or without pembrolizumab in first-line metastatic squamous NSCLC: results from the KEYNOTE-407 east Asia subgroup. Ann Oncol. 2018;29:ix151. doi: 10.1093/annonc/mdy425.002. [DOI] [Google Scholar]
- 29.O'Byrne KJ, Lee KH, Kim SW, Park K, Nishio M, Sakai H, Ohe Y, Fukuhara T, Kang JH, Daga H, Yu CJ, Hotta K, Tanaka H, Takeda M, Yokoyama T, Nathan FE, Lee JS. 1274P First-line (1L) nivolumab (NIVO) plus ipilimumab (IPI) in Asian patients (pts) with advanced non-small cell lung cancer (aNSCLC) in CheckMate 227. Ann Oncol. 2020;31:S824. doi: 10.1016/j.annonc.2020.08.1588. [DOI] [Google Scholar]
- 30.Nishio M, Barlesi F, West H, Ball S, Bordoni R, Cobo M, Longeras PD, Goldschmidt J, Jr, Novello S, Orlandi F, Sanborn RE, Szalai Z, Ursol G, Mendus D, Wang L, Wen X, McCleland M, Hoang T, Phan S, Socinski MA. Atezolizumab plus chemotherapy for first-line treatment of nonsquamous NSCLC: results from the randomized phase 3 IMpower132 trial. J Thorac Oncol. 2021;16(4):653–664. doi: 10.1016/j.jtho.2020.11.025. [DOI] [PubMed] [Google Scholar]
- 31.Nishio M, Saito H, Goto K, Watanabe S, Sueoka-Aragane N, Okuma Y, Kasahara K, Chikamori K, Nakagawa Y, Kawakami T. IMpower132: atezolizumab plus platinum-based chemotherapy vs chemotherapy for advanced NSCLC in Japanese patients. Cancer Sci. 2021;112(4):1534–1544. doi: 10.1111/cas.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.