Abstract
Objectives
To determine the near-term risk of stroke following a retinal artery occlusion (RAO).
Methods
The risk of stroke was assessed in two manners; with a self-controlled case series (SCCS) and a propensity score (PS) matched cohort study using a US medical claims database. The date of RAO diagnosis was assigned as the index date. In the SCCS, incidence of stroke was compared in 30- and 7-day periods pre- and post-index date. In PS analysis, matched cohorts were created from patients with RAO or hip fracture. Cox proportional hazard regression assessed the hazard for stroke. Patients were censored at 1 year, upon leaving the insurance plan or if they had a qualifying event for the comparison group.
Results
The SCCS included 16,193 patients with RAO. The incidence rate ratio (IRR) of new stroke in the month after RAO was increased compared to all periods >2 months before and all months after the index date (IRRs: 1.68–6.40, p < 0.012). Risk was increased in the week immediately following the index date compared to most weeks starting 2 weeks prior to and all weeks immediately after the index date (IRRs: 1.93–29.00, p < 0.026). The PS study analysed 18,213 propensity-matched patients with RAO vs. hip fracture. The HR for having a stroke after RAO compared to a hip fracture was elevated in all analyses (All RAO HR: 2.97, 95% CI: 2.71–3.26, p < 0.001; CRAO HR: 3.24, 95% CI: 2.83–3.70, p < 0.001; BRAO HR: 2.76, 95% CI: 2.43–3.13, p < 0.001).
Conclusions
The highest risk for stroke occurs in the days following a CRAO or BRAO, supporting guidelines suggesting immediate referral to a stroke centre upon diagnosis.
Subject terms: Epidemiology, Retinal diseases
Introduction
Retinal arterial occlusions (RAO) are a rare but devastating cause of painless vision loss [1–4]. Over the last decade, significant evidence has been accrued to strongly associate central retinal artery occlusions (CRAOs) and strokes or cerebrovascular accidents (CVA), largely due to the shared athero-embolic aetiology [4–17]. This led the American Academy of Ophthalmology (AAO) to adopt the guidelines proposed by the American Heart Association, which recommend immediate complete work-up for any patient with an RAO or amaurosis fugax including transfer to a stoke centre [18, 19]. This represents a significant shift in management as surveys have found that <40% of ophthalmologists send patients for prompt work-up in the setting of an acute RAO [20, 21]. This is in stark contrast to neurologists who report referring at a rate of 73% for RAO [20].
While the exact cause of the low rate of ophthalmic referral is unclear, a few possibilities may be related to some of the unresolved questions that naturally arise from these studies. First, while some literature exists to link branch retinal artery occlusions (BRAOs) to strokes [13–17, 22], the data are not as extensive, as acknowledged in the AAO practice pattern report [18]. Similarly, but may be not as obvious, an issue is that the majority of studies to date have focused on RAO that have been evaluated in the inpatient setting [6–13]. One inference from using inpatients is that these are likely sicker patients that may have other neurologic symptoms that prompted the hospital visit and testing, making it more likely to diagnose concurrent strokes. In this light, it may seem reasonable to forgo emergency referral for a patient presenting to an outpatient setting with a CRAO or asymptomatic BRAO.
The following study aims to help fill in these gaps by providing data on a large national cohort that assessed the risk of stroke after RAO’s together and both CRAO and BRAO individually. This was done in two parts: first a self-controlled case series (SCCS) was performed, followed by a propensity score-matched cohort study using data from a predominately outpatient-based medical claims database.
Methods
Dataset
Optum’s Clinformatics Data Mart Database contains the de-identified medical claims of all beneficiaries obtained from a large private insurance network throughout the United States and was used for this study. Included within the database are all outpatient medical claims (office visits and associated diagnoses) and demographic data for each beneficiary during their enrolment in the insurance plan. The subset of data available for this study included all patients in the database from January 1, 2004 to December 31, 2016. The University of Pennsylvania’s Institutional Review Board declared this study exempt from review due to the de-identified nature of the data.
RAO cohort
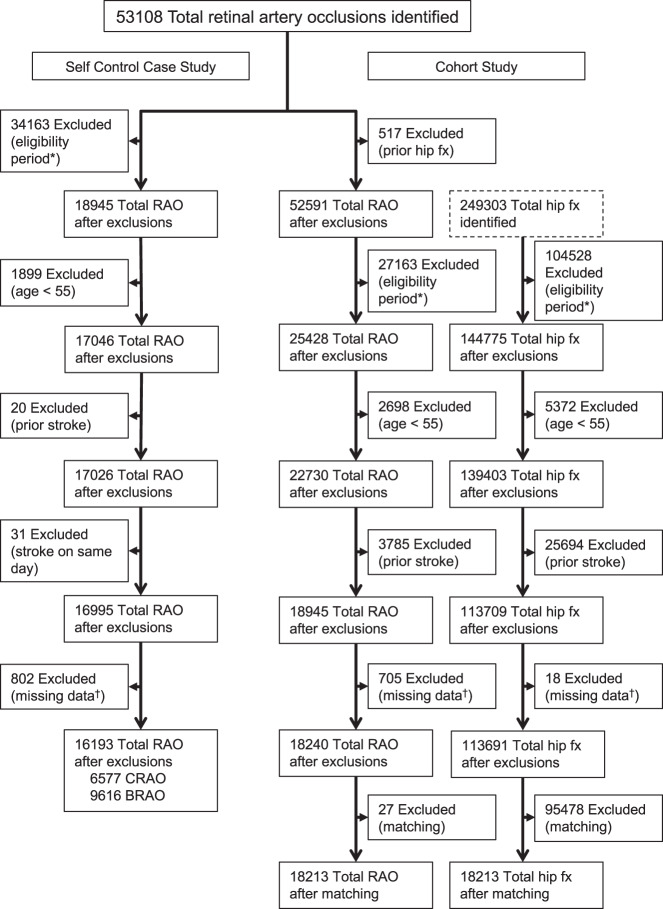
For both the SCCS and the cohort study, similar inclusion and exclusion criteria were used to define RAO patients (Fig. 1). All patients with a new ICD9 or ICD10 diagnosis of a CRAO or BRAO were included. The index date was assigned as the date corresponding to the date of the first RAO diagnosis. All patients were required to be 55 or older and have at least 2 years in the dataset prior to the index date. The database has some, but limited information on in-hospital care. Due to this, those patients that were diagnosed with a RAO during a hospital stay were additionally required to have had a stay of <3 days and to not be discharged with a concurrent diagnosis of stroke. For sub-analyses that differentiated between CRAO and BRAOs, those patients who had both a CRAO and BRAO diagnosis codes on the same day were categorized as CRAOs. For those with an unspecified RAO code and a CRAO or BRAO code, the CRAO and BRAO codes were used. (Please see Online Supplementary Appendix 1 for all ICD9, ICD10 and DRG codes used in this study.)
Fig. 1. Numbers excluded at each step and final inclusion counts for both the self-controlled case series and the cohort analyses.
*Denotes those patients that did not have enough time in the insurance plan for inclusion. †Denotes patients excluded for missing gender or having a diagnosis of an ‘unspecified’ retinal artery obstruction that did not delineate between a central or branch occlusion.
Self-controlled case series (SCCS)
In this analysis, every RAO patient acts as their own control with time frames before and after the diagnosis date compared to each other. The main outcome was an occurrence of the first stroke for each patient (if one occurred at all) and the time period that it occurred in. For example, if a person had a stroke in the month prior to index and another 3 months after index, only the first stroke was counted. Due to the vagueness of ICD diagnosis coding, it is indeterminable whether a second ICD code used at a date after the first stoke diagnosis is a follow up representing the first diagnosis or is indeed a new second stroke. Therefore, later instances of stroke codes were not excluded or censored from the analysis in anyway. A stroke was defined as having a new ICD9 or ICD10 code for an ischaemic or haemorrhagic stroke. After all new occurrences of stroke were identified, incidence rate ratios were created from comparing the rate of strokes 6 months before and after the index date to the month (30 days) immediately following the index date. Sub-analyses were run for individuals specifically with CRAOs and BRAOs as well as for numerous weeks (7 days) around the index date.
Propensity score-matched cohort analysis
For this study, additional criterion was mandated on the RAO patients in that they were excluded for any previous diagnosis of a stroke. A matched cohort was created from patients who were diagnosed with hip fractures. Since RAO patients are typically older and have a certain level of infirmity, hip fracture patients were chosen as a comparison group since they too tend to be older and have a certain level of infirmity. To account for the possible limited anticoagulation after hip fracture surgery, the index date for these patients was considered the date 30 days after the date the patient was discharged from the hospital. Similar to the RAO cohort, these patients had to be over 55, have at least 2 years in the database prior to the index date and have no previous history of stroke.
All patients in both cohorts were then assigned a propensity score for likelihood of having a RAO created from numerous demographic and clinical variables. Included in this were age, race, sex, year of the index date and clinical variables such as history of atrial fibrillation/flutter, congestive heart failure, previous myocardial infarction, arrhythmia, hypertension, diabetes mellitus, transient ischaemic attacks, chronic liver disease, chronic pulmonary disease, peripheral vascular disease, kidney disease (modelled as no disease, chronic kidney disease or end-stage renal disease) or any malignancy. Patients were then matched 1:1 in a nearest-neighbour matching algorithm.
A cox proportional hazards regression was then run to assess the hazard of developing a new stroke (ischaemic or haemorrhagic). Variables were included and controlled for in the final model only if a standard mean difference of >0.10 was seen between the cohorts after matching. Given the significant role age plays in the occurrence of RAOs, age was included in all final models regardless of standard mean difference findings. Patients were censored for an occurrence of an event defining the other cohort (IE a hip fracture in the RAO cohort), 1 year of observation or the end of eligibility in the plan was reached. Sub-analyses were again run for CRAO and BRAOs as distinct groups.
Results
Self-controlled case series (SCCS)
After inclusion and exclusion criteria, 16193 RAO (6577 CRAO, 9616 BRAO) were included in the study (Fig. 1). Table 1 shows the baseline characteristics and comorbidities of these patients. On average, the RAO group was 74.6 years old (SD ±8.4) (CRAO mean age 75.4 (SD 8.3); BRAO 74.1 (SD 8.4). They were 71.9% white (CRAO 70.9%, BRAO 72.5%) and 50.6% female (CRAO 51.0%, BRAO 50.4%). Medical comorbidities include congestive heart failure in 23.3% (CRAO 25.8%, BRAO 21.7%), myocardial infarction in 17.5% (CRAO 18.6%, BRAO 16.7%) any arrhythmia in 35.9% (CRAO 38.1%, BRAO 34.3%), atrial flutter/fibrillation in 19.2% (CRAO 20.8%, BRAO 18.1%), hypertension in 86.8% (CRAO 88.0%, BRAO 86.1%), diabetes in 40.3% (CRAO 41.7%, BRAO 39.4%), transient ischaemic attack in 14.2% (CRAO 15.0%, BRAO 13.6%), chronic liver disease in 1.0% (CRAO 1.0%, BRAO 1.0%), chronic pulmonary disease in 40.1% (CRAO 40.7%, BRAO 39.8%), peripheral vascular disease in 36.0% (CRAO 37.8%, BRAO 34.8%), any malignancy history in 22.1% (CRAO 37.8%, BRAO 34.8%), end-stage renal disease in 29.1% (CRAO 31.0%, BRAO 27.8%). There were a total of 394 strokes during the periods assessed before or after the index date for all RAOs (CRAO 187, BRAO 207).
Table 1.
SCCS stroke cohort demographics and medical history.
| All RAO (N = 16,193) | CRAO (N = 6577) | BRAO (N = 9616) | |
|---|---|---|---|
|
Age mean (SD) |
74.6 (8.4) | 75.4 (8.3) | 74.1 (8.4) |
| Race | |||
| White | 11,638 (71.9%) | 4662 (70.9%) | 6976 (72.5%) |
| Asian | 408 (2.5%) | 143 (2.2%) | 265 (2.8%) |
| Black | 1475 (9.1%) | 669 (10.2%) | 806 (8.4%) |
| Hispanic | 1228 (7.6%) | 512 (7.8%) | 716 (7.4%) |
| Unknown | 1444 (8.9%) | 591 (9.0%) | 853 (8.9%) |
| Gender (female) | 8201 (50.6%) | 3354 (51.0%) | 4847 (50.4%) |
| Cong. heart failure | 3780 (23.3%) | 1698 (25.8%) | 2082 (21.7%) |
| Myocardial infarction | 2828 (17.5%) | 1225 (18.6%) | 1603 (16.7%) |
| Arrhythmia | 5807 (35.9%) | 2506 (38.1%) | 3301 (34.3%) |
| Atrial fib/flutter | 3110 (19.2%) | 1365 (20.8%) | 1745 (18.1%) |
| Hypertension | 14,063 (86.8%) | 5788 (88.0%) | 8275 (86.1%) |
| Diabetes mellitus | 6525 (40.3%) | 2741 (41.7%) | 3784 (39.4%) |
| Transient ischaemic attack | 2298 (14.2%) | 988 (15.0%) | 1310 (13.6%) |
| Chronic liver disease | 163 (1.0%) | 69 (1.0%) | 94 (1.0%) |
| Chronic pulmonary disease | 6501 (40.1%) | 2674 (40.7%) | 3827 (39.8%) |
| Peripheral vascular disease | 5837 (36.0%) | 2487 (37.8%) | 3350 (34.8%) |
| Any malignancy | 3576 (22.1%) | 1500 (22.8%) | 2076 (21.6%) |
| Chronic kidney disease | |||
| – No | 9388 (58.0%) | 3603 (54.8%) | 5785 (60.2%) |
| – Any chronic kidney disease | 2093 (12.9%) | 933 (14.2%) | 1160 (12.1%) |
| – End-stage renal disease | 4712 (29.1%) | 2041 (31.0%) | 2671 (27.8%) |
| Stroke (during study periods only) | 1756 (10.8%) | 787 (12.0%) | 969 (10.1%) |
Figure 2 shows the number of strokes for each month 6 months before and after the index date. In the month following the index date, 64 strokes were recorded, the second-highest amount behind the month prior to the index date (72). Figure 3 shows the number of strokes that occurred in select weeks before and after the index date. Once again, the week immediately following the index date had the second-highest number of recorded strokes at 29, only behind the week immediately preceding the index date (31). Table 2 shows the incidence rate ratios of stroke for each time point assessed around the RAO index date in both monthly and weekly increments. For the monthly comparisons, the IRR was higher for the month immediately following the index date compared to months 6 through 3 prior to the index date (IRR range: 1.68–3.37, p ≤ 0.011) and all months after the index date (IRR range: 2.21–6.40, p < 0.001). The IRR for the week after the index date was higher than each of the 16 weeks assessed (select weeks from week 2 through week 22 post-index date) after the index date (IRR ranges: 2.07–29.00, p ≤ 0.025). It was also higher for 13 of the 16 weeks assessed prior to the index date (IRR range: 1.93–9.67, p ≤ 0.038) for all comparisons. The weeks prior to index that were not significantly associated with a higher IRR for the first week post-index were weeks −1, −2 and −6 (IRR range: 0.94–1.38, p < 0.152).
Fig. 2.
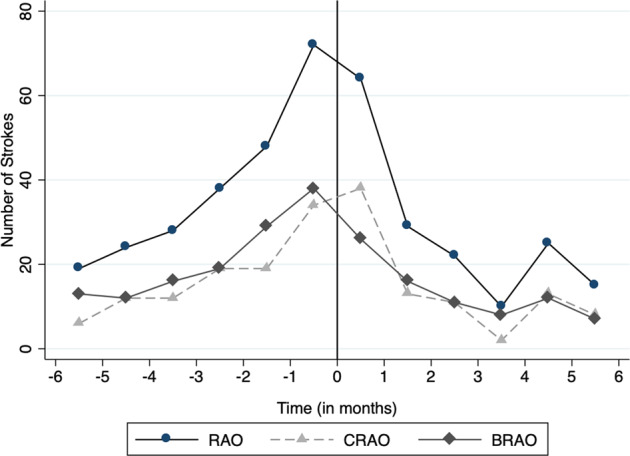
Number of strokes in each month before and after the index date.
Fig. 3.
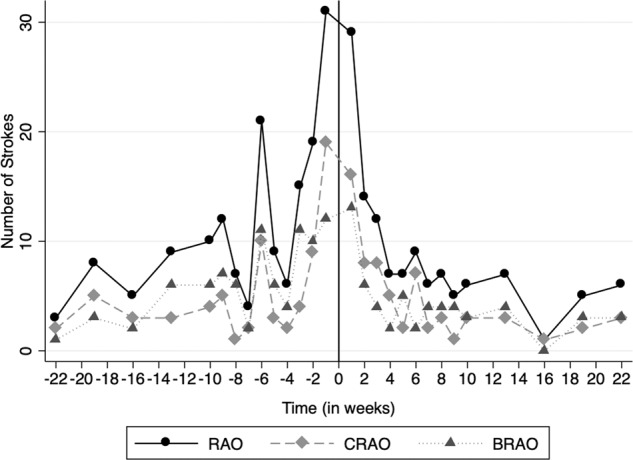
Number of strokes in each week before and after the index date.
Table 2.
Incidence of CVA across various time intervals before and after the index date of RAO.
| All RAO | CRAO | BRAO | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CVA (N) | IRR (95% CI) | p | CVA (N) | IRR (95% CI) | p | CVA (N) | IRR (95% CI) | p | ||
| 30-Day comparisons | ||||||||||
| 1st month (days 1–30) post-index date vs. | −6 month | 19 | 3.37 (2.02–5.62) | <0.001 | 6 | 6.33 (2.68–14.98) | <0.001 | 13 | 2.00 (1.03–3.89) | 0.041 |
| −5 month | 24 | 2.68 (1.67–4.26) | <0.001 | 12 | 3.17 (1.66–6.06) | <0.001 | 12 | 2.17 (1.09–4.29) | 0.027 | |
| −4 month | 28 | 2.29 (1.47–3.56) | <0.001 | 12 | 3.17 (1.66–6.06) | <0.001 | 16 | 1.63 (0.87–3.03) | 0.127 | |
| −3 month | 38 | 1.68 (1.13–2.52) | 0.011 | 19 | 2.00 (1.15–3.47) | 0.014 | 19 | 1.37 (0.76–2.47) | 0.299 | |
| −2 month | 48 | 1.33 (0.92–1.94) | 0.132 | 19 | 2.00 (1.15–3.47) | 0.014 | 29 | 0.90 (0.53–1.52) | 0.686 | |
| −1 month | 72 | 0.89 (0.64–1.25) | 0.493 | 34 | 1.12 (0.70–1.78) | 0.638 | 38 | 0.68 (0.42–1.13) | 0.136 | |
| 1–30 days | 64 | REF | 38 | REF | 26 | REF | ||||
| 2 month | 29 | 2.21 (1.42–3.42) | <0.001 | 13 | 2.92 (1.56–5.49) | <0.001 | 16 | 1.63 (0.87–3.03) | 0.127 | |
| 3 month | 22 | 2.91 (1.79–4.72) | <0.001 | 11 | 3.46 (1.77–6.76) | <0.001 | 11 | 2.36 (1.17–4.78) | 0.017 | |
| 4 month | 10 | 6.40 (3.29–12.46) | <0.001 | 2 | 19.00 (4.58–78.76) | <0.001 | 8 | 3.25 (1.47–7.18) | 0.004 | |
| 5 month | 25 | 2.56 (1.61–4.06) | <0.001 | 13 | 2.92 (1.56–5.49) | <0.001 | 12 | 2.17 (1.09–4.29) | 0.027 | |
| 6 month | 15 | 4.27 (2.43–7.49) | <0.001 | 8 | 4.75 (2.22–10.18) | <0.001 | 7 | 3.71 (1.61–8.56) | 0.002 | |
| 7-Day comparisons | ||||||||||
| 1st week (days 1–7) post-index date vs. | −22 week | 3 | 9.67 (2.95–31.73) | <0.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 1 | 13.00 (1.70–99.38) | 0.013 |
| −19 week | 8 | 3.63 (1.66–7.93) | 0.001 | 5 | 3.20 (1.17–8.74) | 0.023 | 3 | 4.33 (1.24–15.21) | 0.022 | |
| −16 week | 5 | 5.80 (2.25–14.98) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 2 | 6.50 (1.47–28.80) | 0.014 | |
| −13 week | 9 | 3.22 (1.52–6.81) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 6 | 2.17 (0.82–5.70) | 0.117 | |
| −10 week | 10 | 2.90 (1.41–5.95) | 0.004 | 4 | 4.00 (1.34–11.97) | 0.013 | 6 | 2.17 (0.82–5.70) | 0.117 | |
| −9 week | 12 | 2.42 (1.23–4.74) | 0.01 | 5 | 3.20 (1.17–8.74) | 0.023 | 7 | 1.86 (0.74–4.66) | 0.187 | |
| −8 week | 7 | 4.14 (1.82–9.46) | <0.001 | 1 | 16.00 (2.12–120.65) | 0.007 | 6 | 2.17 (0.82–5.70) | 0.117 | |
| −7 week | 4 | 7.25 (2.55–20.62) | <0.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 2 | 6.50 (1.47–28.80) | 0.014 | |
| −6 week | 21 | 1.38 (0.79 – 2.42) | 0.260 | 10 | 1.60 (0.73–3.53) | 0.0.244 | 11 | 1.18 (0.53–2.64) | 0.683 | |
| −5 week | 9 | 3.22 (1.52–6.81) | 0.002 | 3 | 5.33 (1.56–18.30) | 0.008 | 6 | 2.17 (0.82–5.70) | 0.117 | |
| −4 week | 6 | 4.83 (2.01–11.64) | <0.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| −3 week | 15 | 1.93 (1.04–3.61) | 0.038 | 4 | 4.00 (1.34–11.97) | 0.013 | 11 | 1.18 (0.53–2.64) | 0.683 | |
| −2 week | 19 | 1.53 (0.86–2.72) | 0.152 | 9 | 1.78 (0.79–4.02) | 0.167 | 10 | 1.30 (0.57–2.97) | 0.533 | |
| −1 week | 31 | 0.94 (0.56–1.55) | 0.796 | 19 | 0.84 (0.43–1.64) | 0.613 | 12 | 1.08 (0.49–2.37) | 0.842 | |
| 1–7 days | 29 | REF | 16 | REF | 13 | REF | ||||
| 2 week | 14 | 2.07 (1.10–3.92) | 0.025 | 8 | 2.00 (0.86–4.67) | 0.109 | 6 | 2.17 (0.82–5.70) | 0.117 | |
| 3 week | 12 | 2.42 (1.23–4.73) | 0.01 | 8 | 2.00 (0.86–4.67) | 0.109 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| 4 week | 7 | 4.14 (1.82–9.46) | <0.001 | 5 | 3.20 (1.17–8.74) | 0.023 | 2 | 6.50 (1.47–28.80) | 0.014 | |
| 5 week | 7 | 4.14 (1.82–9.46) | <.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 5 | 2.60 (0.93–7.29) | 0.069 | |
| 6 week | 9 | 3.22 (1.52–6.81) | 0.002 | 7 | 2.29 (0.94–5.56) | 0.068 | 2 | 6.50 (1.47–28.80) | 0.014 | |
| 7 week | 6 | 4.83 (2.01–11.64) | <0.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| 8 week | 7 | 4.14 (1.82–9.46) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| 9 week | 5 | 5.80 (2.25–14.98) | <0.001 | 1 | 16.00 (2.12–120.65) | 0.007 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| 10 week | 6 | 4.83 (2.01–11.64) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 3 | 4.33 (1.24–15.21) | 0.022 | |
| 13 week | 7 | 4.14 (1.82–9.46) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 4 | 3.25 (1.06–9.97) | 0.039 | |
| 16 week | 1 | 29.0 (3.95–212.90) | <0.001 | 1 | 16.00 (2.12–120.65) | 0.007 | 0 | – | – | |
| 19 week | 5 | 5.80 (2.25–14.98) | <0.001 | 2 | 8.00 (1.84–34.80) | 0.006 | 3 | 4.33 (1.24–15.21) | 0.022 | |
| 22 week | 6 | 4.83 (2.01–11.64) | <0.001 | 3 | 5.33 (1.56–18.30) | 0.008 | 3 | 4.33 (1.24–15.21) | 0.022 | |
Sub-analyses performed on CRAO patients showed a similar pattern to the broader RAO analysis with the post-index month having a higher IRR compared to all other months, except the month preceding the index date (significant IRRs: 2.00–19.00, p ≤ 0.014 for all comparisons; −1 month IRR: 1.12, 95% CI: 0.70–1.78, p = 0.64). (See Table 2 for detailed results of CRAO and BRAO monthly and weekly sub-analyses). The BRAO monthly comparison also showed elevated risk in months −6, −5, 3, 4 and 5 (IRR range: 2.00–3.71, p ≤ 0.041 for all comparisons). Of the 28 weekly comparisons for the CRAO subset, again the week after the index date had a significantly higher IRR in all but 5 weeks. The BRAO analysis showed that the IRR was higher the week after index compared to 16 of the 24 individual week analyses (Table 2).
Propensity score-matched cohort study
After propensity score matching, 18213 RAO patients were matched with 18,213 hip fracture patients. The post-matching baseline characteristics can be seen in Table 3. Of the 18213 RAO patients, 7190 were categorized as CRAO and 11,023 as BRAO. After propensity score matching, no variables were found to have a standard mean difference between RAO and the hip fracture cohorts of >0.10 either in the primary analysis or the individual CRAO and BRAO sub-analyses. In the RAO group, 1807 (9.9%) new strokes occurred during the follow up period versus 606 (3.3%) in the hip fracture cohort. In the individual RAO sub-analyses, 902 (12.5%) and 905 (8.2%) strokes occurred in the CRAO and BRAO cohorts, respectively. After matching, the RAO cohort was found to have a 2.97 (95% CI: 2.71–3.26, p < 0.001) increased hazard ratio for having a stroke compared to the hip fracture patients (Table 4). Individually, CRAOs (HR = 3.24, 95% CI: 2.83–3.70, p < 0.001) and BRAOs (HR = 2.76, 95% CI: 2.43–3.13, p < 0.001) also had increased hazards for stroked compared to a matched cohort of hip fracture patients.
Table 3.
Post-matching baseline characteristics of patients in the propensity score-matched cohort study.
| Hip Fx (N = 18,213) | RAO (N = 18,213) | SMD | |
|---|---|---|---|
|
Age mean (SD) |
74.24 (9.31) | 74.17 (8.58) | 0.009 |
| Race | 0.020 | ||
| White | 13,017 (71.5%) | 13,007 (71.4%) | |
| Asian | 434 (2.4%) | 445 (2.4%) | |
| Black | 1627 (8.9%) | 1669 (9.2%) | |
| Hispanic | 1318 (7.2%) | 1364 (7.5%) | |
| Unknown | 1817 (10.0%) | 1728 (9.5%) | |
| Gender (female) | 9191 (50.5%) | 9142 (50.2%) | 0.005 |
| Atrial fib/flutter | 3265 (17.9%) | 3253 (17.9%) | 0.002 |
| Cong. heart failure | 4146 (22.8%) | 4018 (22.1%) | 0.017 |
| Myocardial infarction | 2902 (15.9%) | 2853 (15.7%) | 0.007 |
| Arrhythmia | 6034 (33.1%) | 5950 (32.7%) | 0.010 |
| Hypertension | 15,561 (85.4%) | 15,597 (85.6%) | 0.006 |
| Diabetes mellitus | 7243 (39.8%) | 7188 (39.5%) | 0.006 |
| Transient ischaemic attack | 1546 (8.5%) | 1578 (8.7%) | 0.006 |
| Chronic liver disease | 203 (1.1%) | 195 (1.1%) | 0.004 |
| Chronic pulmonary disease | 7293 (40.0%) | 7144 (39.2%) | 0.017 |
| Peripheral vascular disease | 5945 (32.6%) | 5939 (32.6%) | 0.001 |
| Any malignancy | 4254 (23.4%) | 4122 (22.6%) | 0.017 |
| Chronic kidney disease | 0.053 | ||
| None | 10,936 (60.0%) | 10,964 (60.2%) | |
| CKD | 2055 (11.3%) | 2326 (12.8%) | |
| ESRD | 5222 (28.7%) | 4923 (27.0%) | |
| Stroke outcome | 606 (3.3%) | 1807 (9.9%) | 0.267 |
Table 4.
Hazard ratio for stroke in RAO compared to hip fracture after propensity score matching.
| N | CVA (N) | HR (95% CI) | p | ||
|---|---|---|---|---|---|
| ALL RAO | Hip fx | 18,213 | 606 | Reference | |
| All RAO | 18,213 | 1807 | 2.97 (2.71–3.26) | <0.001 | |
| CRAO | Hip fx | 7190 | 282 | Reference | |
| CRAO | 7190 | 902 | 3.24 (2.83–3.70) | <0.001 | |
| BRAO | Hip fx | 11,023 | 324 | Reference | |
| BRAO | 11,023 | 905 | 2.76 (2.43–3.13) | <0.001 | |
Discussion
Our dual analysis found that RAO is associated with an increased stroke risk, which was also true when CRAO and BRAO were analysed individually. Our time-to-event analysis (the cohort study) found that the hazard of stroke was considerably higher than a matched cohort of hip fracture patients. Furthermore, our SCCS analysis found that the stroke risk after an RAO is highest in the period of time immediately following the occlusion, whether assessed by month or even week. Previous literature has demonstrated a low rate of referral among ophthalmologists after a new RAO diagnosis [20, 21]. Our results further emphasize the importance of following the recent AAO guidelines recommending emergent referral for a stroke evaluation.
One of the surprising findings in our study was the large number of first strokes that occurred in the periods immediately preceding the date of RAO diagnosis in the SCCS model. One possible explanation for this is that patients have a ‘risk period’ in which a patient is at increased risk for any embolic phenomena, and it is only chance that determines which occurs first, the stroke or the RAO. Given this theory, it would be reasonable to argue that a patient who has a known history of recent stroke can be the exception to the emergent referral guidelines after an RAO diagnosis if it is discovered that stroke mitigation has already been actively addressed. Another possible explanation is a methodological one. In SCCS studies when an outcome (stroke) and exposure (RAO) are associated, it is not uncommon for a high number of outcomes to occur immediately prior to the exposure which is why this period is typically not recommend for the preceding period to form the primary analysis [23].
It is not clear why the referral rate for emergent care after an RAO is not higher amongst ophthalmologists. One possible explanation is potential belief that the existing data does not reflect what ophthalmologists see within a typical clinical setting. For one, little previous data focused on BRAOs, leaving unanswered what to do with a significant portion of the RAOs seen. Our study directly addresses this issue finding that while BRAOs have less risk for stroke than CRAOs, the risk is still elevated enough to warrant referral. In addition, most other studies have focused on CRAOs seen in emergency departments or as inpatients [6–13]. We believe this is an important contrast with our study, which is derived from a database that includes some inpatient data, but is predominately outpatient-based, more accurately reflecting the presentation of RAOs to the ophthalmologist’s clinic.
Another argument against immediate RAO referral is that it is uncommon for intervention to occur as a result of the emergency referral. However, a study from Lavin et al. directly refutes this, reporting that of CRAO patients sent for prompt systemic work-up, >30% had critical carotid disease, coincident stroke or hypertensive emergency and 20% had a simultaneous myocardial infarction or critical heart disease. Over 90% of their cohort received a change in their medical management and 25% underwent urgent surgical intervention as a result of prompt work-up [10].
While our CRAO results confirm those of previous studies, the rate of stroke in our population was lower than has been reported previously CRAO patients [6]. We believe that this is an effect of using predominately outpatient data compared to predominantly inpatient data in prior work, and this distinction is important. Hospitalized patients with RAO are more likely to have simultaneous neurologic deficits and also to receive complete neuro-imaging, both of which make a diagnosis of stroke more likely. The discrepancy in incidence seen in our study is also likely being further exacerbated by our exclusion of patients who were diagnosed with simultaneous (same day) CVA and RAO from our analysis, suggesting that our associations are in fact an underestimate of this association. Regardless, both studies agree that the 30-day risk following the RAO is a heightened risk period for arterio-thrombolic events.
The results of our study need to be understood within the context of the study design. While we are unable to verify the diagnosis codes with chart level data, many of the ICD codes used within this study have been validated previously [24–27]. RAO codes specifically, however, have not been validated. Including patients who do not have an RAO in the RAO cohorts would be a misclassification bias. This would potentially bias to the null and means that the risk associations would actually be underestimates of the true risk. Next, although propensity scores are an excellent way to balance known confounders between comparison groups, they do not rule out the possibility that unmeasured confounding still existed within the study. Finding confirmatory results in the SCCS, however, makes this possibility less likely. In addition, this data was collected from a single insurer, and may not generalize to other groups of patients using other insurers or in uninsured populations. Last, we chose control group for the cohort study from patients who were discharged from the hospital after having a hip fracture. It is possible that patients who were recently hospitalized had better control of comorbid conditions (e.g. hypertension) after being discharged than non-hospitalized patients, however it is also possible that this difference would be ameliorated as RAO patients were worked up for other CVA risk factors. It also would not explain the increased risk seen in the SCCS analysis.
Last, it is important to note that due to the vagueness of ICD coding for strokes, it was not possible for us to determine if a second ICD code for a stroke in the SCCS analysis represented a follow up visit for the initial stroke or the occurrence of a new stroke. This mandated us to consider only the first instance of stroke in this analysis. However, the impact of this on the SCCS results would mean that when a person had a stroke in the months prior to the RAO and had a second (or third) stroke shortly after the RAO would not have a stroke associated in those time frames. This suggests that our findings may underestimate the true association between RAOs and CVA.
The analysis here confirms that both CRAO and BRAO are a significant risk factor for CVA in the US and that the risk is highest immediately after RAO. The current guidelines for prompt evaluation of patients with RAO [18, 19] should be followed.
Summary
What was known before
CRAO is associated with elevated stroke risk.
Ophthalmologists traditionally seek outpatient rather than emergent work-up for acute retinal artery occlusion.
What this study adds
Outpatients with branch, in addition to CRAOs, have significantly elevated near-term risk of stroke.
There is a ‘risk period’ for stroke and retinal artery occlusion. Either event may occur first.
Supplementary information
Funding
National Institutes of Health K23 Award (1K23EY025729-01) and University of Pennsylvania Core Grant for Vision Research (2P30EY001583). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additional funding was provided by Research to Prevent Blindness, Karen & Herbert Lotman Fund for Macular Vision Research Foundation and the Paul and Evanina Mackall Foundation. None of the funding organizations had any role in the design or conduction of the study.
Author contributions
Research design: Scoles, VanderBeek. Data collection: McGeehan, VanderBeek. Data analysis and interpretation: Scoles, McGeehan, VanderBeek. Obtaining funding: VanderBeek. Manuscript preparation: Scoles, McGeehan, VanderBeek. Brian VanderBeek had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41433-021-01546-6.
References
- 1.Brown GC. Systemic associations of retinal arterial obstructive disease. Int Ophthalmol Clin. 1991;31:1–14. doi: 10.1097/00004397-199103130-00003. [DOI] [PubMed] [Google Scholar]
- 2.Park SJ, Choi NK, Seo KH, Park KH, Woo SJ. Nationwide incidence of clinically diagnosed central retinal artery occlusion in Korea, 2008 to 2011. Ophthalmology. 2014;121:1933–8. doi: 10.1016/j.ophtha.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Leavitt JA, Larson TA, Hodge DO, Gullerud RE. The incidence of central retinal artery occlusion in Olmsted County, Minnesota. Am J Ophthalmol. 2011;152:820–3. doi: 10.1016/j.ajo.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hayreh SS, Podhajsky PA, Zimmerman MB. Retinal artery occlusion: associated systemic and ophthalmic abnormalities. Ophthalmology. 2009;116:1928–36. doi: 10.1016/j.ophtha.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Recchia FM, Brown GC. Systemic disorders associated with retinal vascular occlusion. Curr Opin Ophthalmol. 2000;11:462–7. doi: 10.1097/00055735-200012000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Park SJ, Choi NK, Yang BR, Park KH, Lee J, Jung SY, et al. Risk and risk periods for stroke and acute myocardial infarction in patients with central retinal artery occlusion. Ophthalmology. 2015;122:2336–43. doi: 10.1016/j.ophtha.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 7.Callizo J, Feltgen N, Pantenburg S, Wolf A, Neubauer AS, Jurklies B, et al. Cardiovascular risk factors in central retinal artery occlusion: results of a prospective and standardized medical examination. Ophthalmology. 2015;122:1881–8. doi: 10.1016/j.ophtha.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 8.Chang YS, Jan RL, Weng SF, Wang JJ, Chio CC, Wei FT, et al. Retinal artery occlusion and the 3-year risk of stroke in Taiwan: a nationwide population-based study. Am J Ophthalmol. 2012;154:645–52. doi: 10.1016/j.ajo.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 9.Woo SC, Lip GY, Lip PL. Associations of retinal artery occlusion and retinal vein occlusion to mortality, stroke, and myocardial infarction: a systematic review. Eye. 2016;30:1031–8. doi: 10.1038/eye.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavin P, Patrylo M, Hollar M, Espaillat KB, Kirshner H, Schrag M. Stroke risk and risk factors in patients with central retinal artery occlusion. Am J Ophthalmol. 2018;196:96–100. doi: 10.1016/j.ajo.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 11.French DD, Margo CE, Greenberg PB. Ischemic stroke risk in medicare beneficiaries with central retinal artery occlusion: a Retrospective Cohort Study. Ophthalmol Ther. 2018;7:125–31. doi: 10.1007/s40123-018-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mir TA, Arham AZ, Fang W, Alqahtani F, Alkhouli M, Gallo J, et al. Acute vascular ischemic events in patients with central retinal artery occlusion in the United States: a Nationwide Study 2003–2014. Am J Ophthalmol. 2019;200:179–86. [DOI] [PMC free article] [PubMed]
- 13.Lee J, Kim SW, Lee SC, Kwon OW, Kim YD, Byeon SH. Co-occurrence of acute retinal artery occlusion and acute ischemic stroke: diffusion-weighted magnetic resonance imaging study. Am J Ophthalmol. 2014;157:1231–8. doi: 10.1016/j.ajo.2014.01.033. [DOI] [PubMed] [Google Scholar]
- 14.Lauda F, Neugebauer H, Reiber L, Juttler E. Acute silent brain infarction in monocular visual loss of ischemic origin. Cerebrovasc Dis. 2015;40:151–6. doi: 10.1159/000437274. [DOI] [PubMed] [Google Scholar]
- 15.Golsari A, Bittersohl D, Cheng B, Griem P, Beck C, Hassenstein A, et al. Silent brain infarctions and leukoaraiosis in patients with retinal ischemia: a Prospective Single-Center Observational Study. Stroke. 2017;48:1392–6. doi: 10.1161/STROKEAHA.117.016467. [DOI] [PubMed] [Google Scholar]
- 16.Rim TH, Han J, Choi YS, Hwang SS, Lee CS, Lee SC, et al. Retinal artery occlusion and the risk of stroke development: Twelve-Year Nationwide Cohort Study. Stroke. 2016;47:376–82. doi: 10.1161/STROKEAHA.115.010828. [DOI] [PubMed] [Google Scholar]
- 17.Hong JH, Sohn SI, Kwak J, Yoo J, Ahn SJ, Woo SJ, et al. Retinal artery occlusion and associated recurrent vascular risk with underlying etiologies. PloS ONE. 2017;12:e0177663. doi: 10.1371/journal.pone.0177663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flaxel CJ, Adelman RA, Bailey ST, Fawzi A, Lim JI, Vemulakonda GA, et al. Retinal and ophthalmic artery occlusions preferred practice pattern®. Ophthalmology. 2020;127:259–87. doi: 10.1016/j.ophtha.2019.09.028. [DOI] [PubMed] [Google Scholar]
- 19.Biousse V, Nahab F, Newman NJ. Management of acute retinal ischemia: follow the guidelines! Ophthalmology. 2018;125:1597–607. doi: 10.1016/j.ophtha.2018.03.054. [DOI] [PubMed] [Google Scholar]
- 20.Abel AS, Suresh S, Hussein HM, Carpenter AF, Montezuma SR, Lee MS. Practice patterns after acute embolic retinal artery occlusion. Asia Pac. Asia Pac J Ophthalmol. 2017;6:37–9. doi: 10.22608/APO.201690. [DOI] [PubMed] [Google Scholar]
- 21.Atkins EJ, Bruce BB, Newman NJ, Biousse V. Translation of clinical studies to clinical practice: survey on the treatment of central retinal artery occlusion. Am J Ophthalmol. 2009;148:172–3. doi: 10.1016/j.ajo.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 22.Cho KH, Kim CK, Woo SJ, Park KH, Park SJ. Cerebral small vessel disease in branch retinal artery occlusion. Invest Ophthalmol Vis Sci. 2016;57:5818–24. doi: 10.1167/iovs.16-20106. [DOI] [PubMed] [Google Scholar]
- 23.Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. Bmj. 2016;354:i4515. doi: 10.1136/bmj.i4515. [DOI] [PubMed] [Google Scholar]
- 24.Porter J, Mondor L, Kapral MK, Fang J, Hall RE. How reliable are administrative data for capturing stroke patients and their care. Cerebrovasc Dis Extra. 2016;6:96–106. doi: 10.1159/000449288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bearelly S, Mruthyunjaya P, Tzeng JP, Suner IJ, Shea AM, Lee JT, et al. Identification of patients with diabetic macular edema from claims data: a validation study. Arch Ophthalmol. 2008;126:986–9. doi: 10.1001/archopht.126.7.986. [DOI] [PubMed] [Google Scholar]
- 26.Muir KW, Gupta C, Gill P, Stein JD. Accuracy of international classification of diseases, ninth revision, clinical modification billing codes for common ophthalmic conditions. JAMA Ophthalmol. 2013;131:119–20. doi: 10.1001/jamaophthalmol.2013.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau M, Prenner JL, Brucker AJ, VanderBeek BL. Accuracy of billing codes used in the therapeutic care of diabetic retinopathy. JAMA Ophthalmol. 2017;135:791–4. doi: 10.1001/jamaophthalmol.2017.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.