Abstract
Radon exposure is the second leading cause of lung cancer, after smoking. In upper northern Thailand (UNT), lung cancer incidence was frequently reported by Thailand National Cancer Institute. Besides smoking, radon exposure may also influence the high lung cancer incidence in this region. Indoor radon concentrations were measured in 192 houses in eight provinces of UNT. Indoor radon concentrations ranged from 11 to 405 Bq m−3 and estimated annual effective dose ranged from 0.44 to 12.18 mSv y−1. There were significant differences in indoor radon concentrations between the houses of lung cancer cases and healthy controls (p = 0.033). We estimated that 26% of lung cancer deaths in males and 28% in females were attributable to indoor radon exposure in this region. Other factors influencing indoor radon levels included house characteristics and ventilation. The open window-to-wall ratio was negatively associated with indoor radon levels (B = −0.69, 95% CI −1.37, −0.02) while the bedroom location in the house and building material showed no association. Indoor radon hence induced the fractal proportion of lung cancer deaths in UNT.
Subject terms: Risk factors, Lung cancer, Natural hazards
Introduction
Lung cancer is the leading cause of cancer deaths worldwide. In 2020, there were an estimated 1.8 million lung cancer deaths, accounting for 18% of all cancer deaths globally1. In Thailand, lung cancer was a main cause of death with 23,713 cases in 2020, contributing to 12.4% of all cancer deaths2. The problem is especially severe in northern Thailand where lung cancer incidence and mortality were twice as high as other areas of the country3. Recently, lung cancer incidence in this region has declined, likely as a result of decreased tobacco smoking, the major risk for development of lung cancer4. However, lung cancer continues to have significantly higher incidence in northern Thai men and women compared to all other regions, and it is still one of the most causes of cancer death in upper northern Thailand (UNT)5.
There are many known risk factors causing lung cancer, particularly, tobacco smoking. In 2004, the International Agency for Research on Cancer (IARC), reported that more than 80% of lung cancer patients were related to tobacco smoking, both voluntary and involuntary6. However, more than 25% of lung cancer patients were non-smokers, particularly for women7. Lung cancer among non-smokers remains among the top-ten causes of cancer-related death in the world8. In northern Thailand, the prevalence of daily tobacco smoking has continually decreased to 18.4% in 20095,9. Concurrently, the types of lung cancer found in UNT have also shifted, with a decline incidence in squamous and small cell lung carcinomas, which are the types most linked to smoking, and increases in adenocarcinomas, which are more weakly linked to smoking but more strongly linked to environmental factors4,10,11. Therefore, other environmental factors might play a crucial role in lung cancer development, such as radon gas, air pollution, household smoke, asbestos and occupational risk factors12–14.
After smoking, radon is the second most important cause of lung cancer, excluding the genetic and other natural related biological factors. Radon and its progenies are the most important contributors to human exposure to high natural radiation15–18 and approximately 10–20% of lung cancer worldwide was a result of radon exposure19. Radon (222Rn) is a radioactive gas resulting from radium decay (226Ra), itself a decay product of uranium (238U), which is naturally found on the earth's crust. Radon gas is inert, odorless, tasteless, invisible and can readily emanate and be concentrated in enclosed areas where it is trapped 17,19. Most inhaled radon is rapidly exhaled, but inhaled progenies as solid particles are able to readily deposit on the walls of the bronchial epithelium, where it delivers most of the radiation dose. As these progenies emit alpha particles over the short term, these particles can interact with biological molecules in the lung, leading to DNA damage, mutations and ultimately development of cancer17,19–21. In 1988, radon has been classified as a known human carcinogen (Group1) by the IARC22. In the last several decades, many studies have found the association between lung cancer and long-term exposures to residential radon23–25. The induction period of lung cancer attributable to radon exposure in humans is between 5 and 25 years26. The high dose and long-term exposure to radon in UNT was a crucial factor that enhanced lung cancer development14. Radon is a linear non-threshold carcinogen that can induce the risk of lung cancer without minimal value of concentration17. Additionally, the general population study suggests that chronic low dose exposure to radon can cause lung cancer development, for every 100 Bq m−3 increase in indoor radon concentration, the risk of lung cancer is estimated to increase by 8–33%23,24,26,27. The WHO recommended average annual reference level of indoor radon is currently 100 Bq m−3 and it also varies by countries19. To elucidate the potential contribution of radon exposure on the high incidence of lung cancer in UNT, the case–control study was conducted to evaluate the relationship between radon exposure and lung cancer incidence in UNT where the research data are scarce.
Results and discussion
The demographic characteristics of participants are comparable and shown in Table S1 (Supplementary Table S1). The indoor radon concentration of 192 participant bedrooms in the eight provinces of UNT is presented in Table 1. This ranged from 11 to 405 Bq m−3, with an arithmetic mean of 105 ± 74 Bq m−3 and geometric mean of 80 Bq m−3, which is higher than the global average of 39 Bq m−319 and the domestic mean of 16 Bq m−3 in Thailand28. The arithmetic mean was slightly higher than the WHO reference level and lower than the EPA action level of 148 Bq m−3. The mean indoor radon concentration showed significant differences (p < 0.001) between the provinces of UNT. The highest indoor radon concentration was found in Phrae province, with a arithmetic mean ± SD (range) of 168 ± 69 (54–286) Bq m−3, follow by Phayao, Chiang Rai, Chiang Mai, Nan, Mae Hong Son, Lampang and Lamphun provinces with the mean ± SD (range) of 167 ± 52 (64–219), 139 ± 77 (31–242), 110 ± 87 (16–405), 90 ± 55 (25–207), 84 ± 55 (35–241), 78 ± 54 (32–216) and 75 ± 60 (11–193) Bq m−3, respectively.
Table 1.
Arithmetic and geometric means of indoor radon concentrations in eight provinces of upper northern Thailand (UNT).
| Provinces | Houses(n) | Rn concentration (Bq m−3) | |||
|---|---|---|---|---|---|
| Mean (SD) | Geomean | Min | Max | ||
| UNT | 192 | 105 (74) | 80 | 11 | 405 |
| Phrae | 16 | 168 (69) | 152 | 54 | 286 |
| Phayao | 7 | 167 (52) | 157 | 64 | 219 |
| Chiang Rai | 25 | 139 (77) | 112 | 31 | 242 |
| Chiang Mai | 46 | 110 (87) | 84 | 16 | 405 |
| Nan | 10 | 90 (55) | 75 | 25 | 207 |
| Mae Hong Son | 17 | 84 (55) | 71 | 35 | 241 |
| Lampang | 32 | 78 (54) | 65 | 32 | 216 |
| Lamphun | 39 | 75 (60) | 53 | 11 | 193 |
Of 192 surveyed houses, 41% and 30% had radon concentration higher than the WHO and EPA recommended levels (Table S1) which may be associated with the higher incidence and mortality of lung cancer in UNT compared to other regions of Thailand.
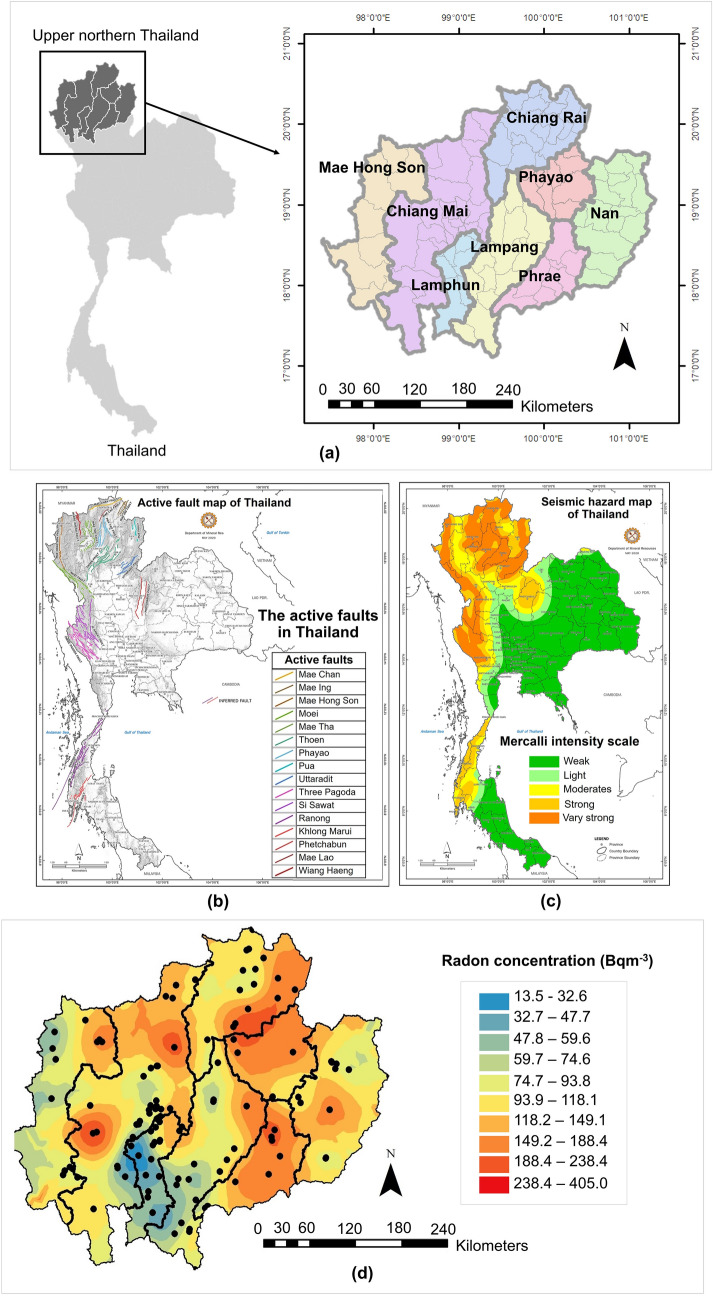
The distribution of indoor radon concentrations and measurement locations in UNT is shown in Fig. 1. To estimate the indoor radon value for all eight provinces, the geostatistical Kriging interpolation was used to create a radon distribution map. As the UNT region is located in different radon potential basin areas of granite, there is abundant uranium and its decay products around this area29,30. Reportedly, granitic gneiss has high frequency ratios for radon levels31,32.
Figure 1.
Study area, sample locations and distribution of indoor radon concentrations. (a) Study sites of eight provinces in upper northern Thailand, (b) Active fault zones in Thailand, (c) Seismic hazard map of Thailand, (d) Distribution of indoor radon concentration, with sampling points indicated in black dots using ArcMap software, Geostatistical wizard, Kriging method. Active fault zones in Thailand map and Seismic hazard map of Thailand obtained from the Department of Mineral Resources, Thailand (http://webeng.dmr.go.th/Show_Detail.aspx?DetailId=97).
UNT is also located in the area of nine active fault zones (Fig. 1b,c). Faults and fractures can preferentially release the radon gas to the surface33,34 and can enhance radon concentration by fault and seismic activity35–38. Therefore, the presence of active fault zones may contribute to high radon concentrations in the UNT region relative to the rest of the country.
When comparing the mean indoor radon concentration in this study with others conducted in UNT (Table 2), we found more than 80% of houses had indoor radon concentration higher than the global average (39 Bq m−3). Moreover, the concentrations found in this study were also higher than the mean value of 16 Bq m−3 for Thailand28.
Table 2.
Arithmetic means indoor radon concentrations previously reported in upper northern Thailand (UNT).
| Area | Detector | Study design | Indoor Radon (Bq m−3) | References | |
|---|---|---|---|---|---|
| Arithmetic mean (SD), case/control | |||||
| n | Type | ||||
| Saraphi, Chiang Mai | 50 | CR-39 | Survey | 21 (6) | Wanabongse et al.39 |
| Chiang Mai | 33 / 23 | Ionization chamber | Case–Control | 20 (15), 20.1 / 20.2 (p > 0.05) | Boonyaprapa et al.40 |
| Chiang Mai | 35/33 | CR-39 | Case–Control | 57 (7) | Autsavapromporn et al.30 |
| Doi Saket, Chiang Mai | 30 | CR-39 | Survey | 53 (15) | Thumvijit et al.29 |
| Lampang | 786 | Activated charcoal | Survey | 32 (21) | Tansurat et al.41 |
| Thailand | 16 (1.2)g | IAEA28 | |||
| Global | 39 | WHO19 | |||
| Upper northern Thailand | 77 / 78 | CR-39 | Case–Control | 105 (74), 109 / 102 (p = 0.033) | This study |
g = geometric mean.
The indoor radon concentration can also vary as a result of other factors. Table 3 shows indoor radon concentrations and open window-to-wall ratios (ventilation) according to location of the bedroom, construction material of the ground and walls, and air conditioner use. This study found no significant differences between indoor radon concentration in the first and second floor of the bedroom location (p > 0.05). There was also no significant difference in indoor radon concentration regarding the walls or ground constructed with wood or concrete, which were the major materials of houses in UNT42,43.Many studies showed that different building materials contribute less than 20 Bq m−3 difference thus it does not enhance indoor radon concentrations44. In contrast, we found significant differences in open window-to-wall ratios’ ventilation in the houses.
Table 3.
Arithmetic means indoor radon concentrations and open window-to-wall ratios depending on location, house construction materials, and air conditioner use.
| House characteristics | Radon concentration | Open window-to-wall ratio | |||
|---|---|---|---|---|---|
| (Bq m−3) | p-value | (%) | p-value | ||
| Bedroom location | On 1st floor (79) | 109 ± 82 | 0.42 | 25 ± 16 | 0.051 |
| On 2nd floor (66) | 103 ± 78 | 29 ± 15 | |||
| Wall construction material | Cement (65) | 112 ± 79 | 0.12 | 23 ± 15 | 0.004** |
| Wood (80) | 97 ± 75 | 30 ± 16 | |||
| Ground construction material | Cement (71) | 113 ± 80 | 0.12 | 23 ± 14 | 0.005** |
| Wood (71) | 95 ± 73 | 31 ± 16 | |||
| Air conditioning | Yes (26) | 146 ± 96 | 0.009** | 14 ± 12 | < 0.001** |
| No (111) | 99 ± 77 | 29 ± 15 | |||
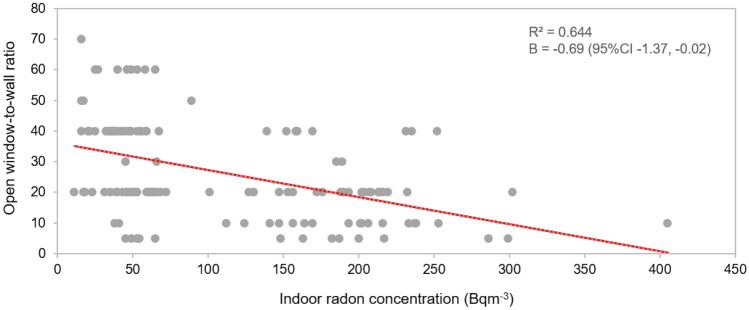
The presence of an air conditioning in the room was associated with having significantly higher indoor radon concentrations. Generally, rooms with the air conditioning are likely better sealed to reduce outdoor air exchange and help control indoor air humidity and temperature. Consequently, this allows radon gas to accumulate and increase45,46. This means that the house characteristics might have not much influenced indoor radon concentration but air ventilation was more impactful. Figure 2 shows the association between open window-to-wall ratios and indoor radon concentrations. By adjusting with wall and ground materials, air conditioner use, geographical location of provinces and season of measurement. Every 10% increase in the open window-to-wall ratio was associated with a 6.9 Bq m−3 (B = −0.69, 95% CI −1.37, −0.02) decrease in indoor radon concentration. Thus, ventilation seems to be a factor with a greater influence on indoor radon concentrations than materials used in construction of the house.
Figure 2.
The association between open window-to-wall ratios and indoor radon concentrations in participant bedrooms.
Table 4 shows the average indoor radon concentration in the bedrooms of lung cancer cases compared to the healthy controls. By using the Wilcoxon paired test, the average concentration in case houses (109 ± 82 Bq m−3) was significantly higher (p = 0.033) than those of the control houses (103 ± 79 Bq m−3). As a result, radon may be a significant risk factor for development of lung cancer in UNT.
Table 4.
Indoor radon concentrations (Bq m−3) and AED (annual effective dose) of lung cancer cases and healthy controls.
| All | Lung cancer case | Healthy controls | P-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
|
Indoor radon (Bq m−3) |
155 | 106 | 80 | 77 | 109 | 82 | 78 | 103 | 79 | 0.033* |
| AED (mSv y−1) | 155 | 4.27 | 3.22 | 77 | 4.29 | 3.30 | 78 | 4.16 | 3.16 | 0.032* |
In order to estimate the inhalation exposure of indoor radon, the annual effective dose (AED) was estimated. Participants spent between 12 to 24 h day−1 (average of 16.45 h day−1) indoors at home, which correlates to an indoor occupancy factor (T) of 0.69 (Table S1). This average, T, was used to estimate AED, which ranged between 0.44 and 12.18 mSv y−1 and an average of 4.27 mSv y−1, which is approximately 3 times higher than the global average AED of 1.3 mSv y−115. This value is also higher than the previously reported measurements in Pa Miang, Chiang Mai province, where it ranged between 0.9—3.8 mSv y−129. The AED of lung cancer cases was significantly higher than those of healthy controls (p = 0.032), with AED values of case and controls at 4.28 ± 3.0 and 4.11 ± 3.0 mSv y−1, respectively. This finding again suggests a role of radon exposure and the development of lung cancer in this region of Thailand.
The association between lung cancer and indoor radon, using indoor radon concentration less than 40 Bq m−3 as a reference level and adjusted by age, gender, smoking status, education and occupation was performed (Table S2). An association between radon exposure and lung cancer was restricted to males (OR = 4.60, 95% CI 1.00–21.09) and smokers (OR = 4.59, 95% CI 1.12–18.83) with indoor radon level 40–100 Bq m−3 only but not in overall groups (OR = 2.55, 95% CI 0.89–7.31 and OR = 1.79, 95% CI 0.66–4.87 for radon exposure at 40–100 Bq m−3 and more than 100 Bq m−3, respectively). Moreover, there was no association between higher exposure to indoor radon concentration. Therefore, the significant association found may be a result of chance47. Hence, EPA model16 and BEIR VI model17 were employed to estimate the number of lung cancer deaths attributable to indoor radon exposure in UNT. According to the Ministry of Public Health, Thailand, in 2015–2019, there were 10,164 lung cancer deaths in UNT, 6,115 males and 4,049 females. Table 5 shows the different exposures probably responsible for smoking, indoor radon exposure, the combination of smoking and indoor radon exposure for registered lung cancer deaths in UNT. Indoor radon exposure in UNT accounted for 26% and 28% of lung cancer deaths in males and females, respectively. Among these eight provinces, the highest lung cancer deaths attributable to indoor radon exposure was in Phrae province, at 37% of all lung cancer deaths, and the lowest was Lampang province (19%). The estimated number of lung cancer deaths due to radon exposure in male and female non-smokers was higher than those in smokers (Table 6). Since the sub-multiplicative interaction of smoking and radon were considered in the excess relative risk (ERR) calculation of the BEIR VI model that considered radon might be more influential in relative terms in non-smokers than in smokers16,17. These findings were consistent with other studies in several countries48–51. However, in our study approximately 96% of male lung cancer were smokers while 52% were female smokers (Table S3). Based on our study results, smoking is linked to a higher proportional risk of lung cancer death than radon exposure in males, but lower in females due to the higher male smokers than in females.
Table 5.
The estimates of lung cancer deaths attributable to indoor radon exposure from 2015—2019 in the eight provinces of upper northern Thailand (UNT).
| Number of lung cancer deaths attributable to: | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Only smoking | Smoking and radon | Only radon | Others | Radon | ||||||
| n | n | (%) | n | (%) | n | (%) | n | (%) | n | (%) | |
| UNT | 10,164 | 2580 | 25 | 571 | 6 | 2193 | 22 | 4820 | 47 | 2764 | 27 |
| Male | 6115 | 2093 | 34 | 475 | 8 | 1127 | 18 | 2420 | 40 | 1602 | 26 |
| Female | 4049 | 537 | 13 | 111 | 3 | 1017 | 25 | 2384 | 59 | 1128 | 28 |
| Phrae | 835 | 190 | 23 | 69 | 8 | 244 | 29 | 332 | 40 | 313 | 37 |
| Male | 548 | 168 | 31 | 62 | 11 | 136 | 25 | 182 | 33 | 198 | 36 |
| Female | 287 | 26 | 9 | 11 | 4 | 102 | 36 | 148 | 52 | 113 | 39 |
| Phayao | 998 | 343 | 34 | 116 | 12 | 219 | 22 | 320 | 32 | 335 | 34 |
| Male | 584 | 314 | 54 | 106 | 18 | 67 | 11 | 97 | 17 | 173 | 30 |
| Female | 414 | 30 | 7 | 16 | 4 | 142 | 34 | 226 | 55 | 158 | 38 |
| Chiang Rai | 1911 | 568 | 30 | 158 | 8 | 427 | 22 | 758 | 40 | 585 | 31 |
| Male | 1111 | 468 | 42 | 132 | 12 | 186 | 17 | 325 | 29 | 318 | 29 |
| Female | 800 | 132 | 17 | 36 | 5 | 225 | 28 | 407 | 51 | 261 | 33 |
| Chiang Mai | 2769 | 581 | 21 | 139 | 5 | 675 | 24 | 1374 | 50 | 814 | 29 |
| Male | 1572 | 417 | 27 | 102 | 6 | 353 | 22 | 700 | 45 | 455 | 29 |
| Female | 1197 | 165 | 14 | 38 | 3 | 319 | 27 | 675 | 56 | 357 | 30 |
| Nan | 869 | 153 | 18 | 29 | 3 | 190 | 22 | 497 | 57 | 219 | 25 |
| Male | 530 | 134 | 25 | 25 | 5 | 103 | 19 | 268 | 51 | 128 | 24 |
| Female | 339 | 40 | 12 | 4 | 1 | 86 | 25 | 209 | 62 | 90 | 27 |
| Mae Hong Son | 287 | 77 | 27 | 15 | 5 | 56 | 20 | 139 | 48 | 71 | 25 |
| Male | 183 | 50 | 27 | 10 | 5 | 36 | 20 | 87 | 48 | 46 | 25 |
| Female | 104 | 30 | 29 | 4 | 4 | 22 | 21 | 48 | 46 | 26 | 25 |
| Lampang | 1571 | 706 | 45 | 111 | 7 | 186 | 12 | 568 | 36 | 297 | 19 |
| Male | 1004 | 591 | 59 | 92 | 9 | 78 | 8 | 243 | 24 | 170 | 17 |
| Female | 567 | 117 | 21 | 19 | 3 | 108 | 19 | 323 | 57 | 127 | 22 |
| Lamphun | 924 | 142 | 15 | 24 | 3 | 195 | 21 | 563 | 61 | 219 | 24 |
| Male | 583 | 130 | 22 | 22 | 4 | 112 | 19 | 319 | 55 | 134 | 23 |
| Female | 341 | 27 | 8 | 4 | 1 | 79 | 23 | 231 | 68 | 83 | 24 |
Table 6.
The estimates of lung cancer deaths attributable to indoor radon exposure in 2015—2019 for eight provinces of upper northern Thailand (UNT) by gender and smoking status, according to the EAC models.
| Provinces | Number of lung cancer deaths attributable to indoor radon exposure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Male | Female | ||||||||||
| Smoker | Non-smoker | Smoker | Non-smoker | Smoker | Non-smoker | |||||||
| n | % | n | % | n | % | n | % | n | % | n | % | |
| UNT | 405 | 18 | 2479 | 31 | 418 | 18 | 1224 | 32 | 63 | 17 | 1101 | 30 |
| Phrae | 42 | 27 | 286 | 42 | 49 | 27 | 157 | 43 | 5 | 14 | 111 | 42 |
| Phayao | 65 | 25 | 300 | 41 | 60 | 25 | 140 | 41 | 11 | 25 | 149 | 40 |
| Chiang Rai | 95 | 22 | 530 | 36 | 93 | 22 | 250 | 36 | 15 | 9 | 259 | 36 |
| Chiang Mai | 134 | 19 | 684 | 33 | 124 | 20 | 316 | 34 | 25 | 19 | 342 | 32 |
| Nan | 26 | 16 | 195 | 28 | 26 | 16 | 102 | 28 | 4 | 8 | 86 | 27 |
| Mae Hong Son | 11 | 16 | 63 | 29 | 12 | 17 | 33 | 29 | 2 | 5 | 26 | 28 |
| Lampang | 214 | 14 | 306 | 25 | 46 | 13 | 162 | 24 | 7 | 14 | 130 | 25 |
| Lamphun | 30 | 14 | 183 | 26 | 32 | 14 | 94 | 26 | 4 | 14 | 79 | 26 |
Previous studies have estimated that about 4–29% of all lung cancer deaths were attributable to radon exposure, depending on radon concentration and the employed model52,53. Table 7 shows the comparison percentage of lung cancer deaths attributable to radon with previous studies calculated using the exposure-age concentration (EAC) model. The percentage varied depending on indoor radon concentrations and smoking status in males and females in each population.
Table 7.
Comparison percentage of lung cancer deaths attributable to indoor radon in previous studies using the exposure-age-concentration model (EAC) of BEIR VI.
| Country | Average indoor radon (Bqm−3) |
Lung cancer deaths attributable to indoor radon (%) | References | ||
|---|---|---|---|---|---|
| Male | Female | Total | |||
| USA | 46 | 14.1 | 15.3 | 13.9 | BEIR VI16 |
| Canada | 43 | 13.6 | Peterson et al.46 | ||
| France | 89 | 13 | Catelinois et al.44 | ||
| Portugal | 81 | 27 | 34 | Veloso et al.52 | |
| South Korea | 62 | 19.5 | 28.2 | Lee et al.47 | |
| Thailand | 16 | 9.4 | Gaskin et al.49 | ||
| UNT Thailand | 105 | 26 | 28 | 27 | This study |
According to our estimation, approximately 553 lung cancer deaths every year were attributable to indoor radon exposure in UNT between 2015 and 2019. This result is high relative to the total lung cancer deaths in Thailand estimated to be attributable to radon, which was 1,660 cases in 201253. Thus, approximately one third of lung cancer deaths attributable to indoor radon exposure in Thailand was in UNT. The higher attributable risk in this study is due to the higher indoor radon concentration measured in UNT than the national average. However, these values were in a worldwide range between 3 and 40% of all lung cancer deaths due to indoor radon exposure52,53. These values tend to increase in high radon countries.
Our study found that there was significantly higher smoking behavior among lung cancer cases (80%) than healthy controls (52%) (p > 0.001) (Table S3). Smoking is the primary causal development of lung cancer worldwide11. Reportedly, smoking has a synergistic effect with high radon concentration to increase lung cancer risk by up to 25 times17,23. However, in northern Thailand, smoking prevalence was the lowest (11.3%) than those of the other part and the country mean (15.2%)9 while lung cancer incidence was the highest prevalence3,5. Further, the attributable risk of only smoking and smoking with radon of lung cancer in this region of Thailand also needs to be elucidated. Greater than 40% of houses in this study had radon levels higher than the recommended activity level of WHO as 100 Bq m−3.
The higher indoor radon value in lung cancer cases compared to those of healthy controls in this study suggests that radon may be a risk factor for development of lung cancer in UNT. This may be synergistic in effect with other factors such as smoking to increase the high incidence and mortality of lung cancer in this area. In UNT, open biomass burning, primarily for agricultural purposes, also results in high ambient air pollution, which may further contribute to the increased risk of lung cancer in this region14,54–56.
Conclusion
Lung cancer is one of the major health burdens in UNT. High levels of residential radon can increase the risk of lung cancer in the general population, and these levels are influenced by different geological and topographic characteristics, along with house ventilation. In eight provinces of UNT, the measured indoor radon concentration ranged from 11–405 Bq m−3, corresponding to an annual effective dose of 0.44–12.18 mSv y−1. The mean, which exceeded the global mean, and greater than 41% of houses in this study had higher indoor radon concentrations than the WHO recommended level (100 Bq m−3). The finding of higher indoor radon concentrations in the houses of lung cancer patients compared to those of healthy controls suggests a contribution of indoor radon to lung cancer in this region. The EAC model of BEIR VI estimated that 27% of all lung cancer deaths were attributable to residential radon exposure or approximately 553 lung cancer deaths per year. Indoor radon may be responsible for a substantial proportion of lung cancer deaths in UNT, and an effective strategy to prevent and mitigate indoor radon exposure is needed to reduce the high lung cancer mortality in UNT.
Methods
Study design
This study was conducted in eight provinces of UNT. The study process included field measurement and data collection from participants. The primary lung cancer patients were enrolled from hospitals while the healthy controls were enrolled at the same communities of lung cancer cases who had no history of lung cancer in family members. All participants must have lived in UNT at least 5 years. All participants were informed about the study information, including risk or any inconveniences that may have occurred from the study. Informed consent was obtained from all participants prior to enrollment. All experiment protocols and ethical clearance were approved from the Human Experimentation Committee, Research Institute for Health Sciences (Study code: Project No. 1/59, approved on 10 May 2016) and the Research Ethics Committee, Faculty of Medicine, Chiang Mai University (Study code: NONE-2558-03633, approved on 20 July 2016).
Study area
This study area is located in UNT that covers approximately 82,500 km2 and comprises 8 provinces including Chiang Mai, Chiang Rai, Lamphun, Lampang, Phayao, Nan, Phrae and Mae Hong Son province (Fig. 1a). UNT consists of basins of the 4 main rivers namely Ping, Wang, Yom, and Nan and 9 active fault zones. There are also basins surrounded by the mountains57,58.
Data collection
From September 2018 to December 2020, seventy-seven lung cancer cases and 78 healthy controls matched by sex and age (± 5 years) who lived within a 5 km radius of lung cancer cases were enrolled into this study with the inclusion criteria as Supplementary Table S4. All participants were interviewed by questionnaire about individual data, history of smoking, occupation, lifestyle, and house characteristics (construction materials, bedroom location and ventilation). Ventilation in the bedroom was estimated using an open window-to-wall ratio that refers to the percentage of the open area of the window or vent in the wall to the gross wall area of the room (Fig. S2).
Indoor radon measurements
Participants' houses were located on a map of UNT which was 50 × 50 km gridded. The empty grids where no participant houses installed the radon detectors, additional, 37 new houses were enrolled to the extra heathy control. 192 houses (houses of the 77 lung cancer cases and those of the 115 healthy controls) underwent indoor radon measurement (Fig. S1). The indoor radon concentration was determined using a closed alpha-track detector that contained electrically conducting plastic film of allyl diglycol carbonate (CR-39/PADC) using the Radtrak system manufactured by Radonova Laboratory AB, (Uppsala, Sweden). From February 2019 to February 2021, a total of 192 CR-39 detectors were placed in the bedrooms of all participants, installing the units away from windows, doors, electric devices or heat sources, and at least 20 cm away from the wall and 1 m above the floor. The detectors were installed for 3 months to measure indoor radon concentration. Then, the detectors were individually packed in ziplock plastic bags and placed in the large bag, shipped and measured by the Radonova Laboratory AB. On the film, the alpha particles make small tracks which are enlarged with chemical etching and later counted in a microscope using a state-of-the-art image scanner to determine the radon concentration by ISO 17,025 accredited system with an uncertainty of 6% at 200 Bq m−3 (source: https://radonovalaboratories.com).
Statistical analysis and health risk assessments
Radon gas can be inhaled through the respiratory tract and interact with biological molecules in the lung leading to lung cancer by damaging DNA, which is a potential health risk. Thus, to evaluate the exposure doses received for indoor radon inhalation, the annual effective dose (AED) in the unit of.
mSv y−1 was estimated using following the equation:
| 1 |
where CRn is the radon concentration (Bq m−3), F is the equilibrium factor of radon and its daughters which is equal to 0.4 for indoors17, T is the occupancy time and D is the dose conversion coefficient, FD can merge into the dose conversion factor equal to 6.7 × 10–6 mSv/Bq h m−3 for indoor radon59.
The number of lung cancer deaths attributable to radon exposure in this region was estimated using Eq. (2)
| 2 |
where Nr,a is the lung cancer deaths attributable to r radon exposure at attained age a, ERR is the excess relative risk at attained age a and exposure r, and N is the number of lung cancer deaths at attained age a.
The excess relative risk (ERR) can be calculated following the exposure-age concentration (EAC) model from BEIR VI17 and some parameters were received from EPA model16 that giving as follows the Eq. (3) by assumed that all individuals in the same provinces were equally exposed and the concentrations that exposed were unchanged over their lifetime.
| 3 |
where β is the exposure–response parameter or risk coefficient that equal to 6.9 × 10–3 for attained age greater than 75 years old, w is the exposure windows, w5-14, w15-24 and w25+ define the exposure rate incurred between 5-14y, 15–24 y and more than 25 y before the current age, respectively.
Supplementary Information
Acknowledgements
The authors would like to acknowledge research funding support from Chiang Mai University (T.P., CMU 2558-2559) and the Royal Golden Jubilee (RGJ) PhD. Scholarship (K.S., PHD/0169/2559), Thailand Research Fund (TRF). We are grateful to the Environment and Health Research Unit, Research Institute for Health Sciences, Chiang Mai University, for laboratory and field research support. We also gratefully acknowledge the medical and nursing staff at the Maharaj Nakorn Chiang Mai Hospital, the Chiang Mai University Hospital; and the Lampang Cancer Hospital for their support with participant enrollment. We are grateful to Dr. Voravit Suwanvanichkij, Research Institute for Health Sciences, Chiang Mai University for kindly making valuable suggestions on English usage of this manuscript. We deeply respect and appreciate the time and effort of all participants and their family members for their kind cooperation that made this study possible.
Author contributions
K.S., T.P. and W.N. designed the study and prepared the manuscript writing, S.T. did fact checking and contributed to manuscript writing, C.P., C.L., D.P., and D.F. advised on participants’ recruitment, T.P., C.P.,S.C., R.W., and N.A. advised on research methodology.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Tippawan Prapamontol, Email: tippawan.prapamontol@cmu.ac.th.
Shinji Tokonami, Email: tippawan.prapamontol@cmu.ac.th.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-022-09122-y.
References
- 1.Sung H, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.International Agency for Research on Cancer. Thailand Source: Globocan 2020, https://gco.iarc.fr/today/data/factsheets/populations/764-thailand-fact-sheets.pdf. (2021).
- 3.Imsamran, W. et al. Cancer in Thailand Vol. VIII, 2010–2012. National Cancer Institute, Thailand (2015).
- 4.Pongnikorn D, Daoprasert K, Waisri N, Laversanne M, Bray F. Cancer incidence in northern Thailand: Results from six population-based cancer registries 1993–2012. Int. J. Cancer. 2018;142:1767–1775. doi: 10.1002/ijc.31203. [DOI] [PubMed] [Google Scholar]
- 5.Virani, S. et al. National and Subnational Population-Based Incidence of Cancer in Thailand: Assessing Cancers with the Highest Burdens. Cancers (Basel)9, 1. 10.3390/cancers9080108 (2017). [DOI] [PMC free article] [PubMed]
- 6.IARC. Tobacco smoke and involuntary smoking. IARC Monogr. Eval. Carcinog. Risks Hum83, 1–1438 (2004). [PMC free article] [PubMed]
- 7.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 8.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 9.Health System Research Institute, T. Thai National Health Examination Survey, NHES V, <https://kb.hsri.or.th/dspace/handle/11228/5425> (2021).
- 10.Khuder SA. Effect of cigarette smoking on major histological types of lung cancer: A meta-analysis. Lung Cancer. 2001;31:139–148. doi: 10.1016/s0169-5002(00)00181-1. [DOI] [PubMed] [Google Scholar]
- 11.Pesch B, et al. Cigarette smoking and lung cancer–relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int. J. Cancer. 2012;131:1210–1219. doi: 10.1002/ijc.27339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibelin C, Couraud S. Somatic alterations in lung cancer: Do environmental factors matter? Lung Cancer. 2016;100:45–52. doi: 10.1016/j.lungcan.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 13.Shankar A, et al. Environmental and occupational determinants of lung cancer. Transl. Lung Cancer Res. 2019;8:S31–S49. doi: 10.21037/tlcr.2019.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiwatanadate P. Lung cancer related to environmental and occupational hazards and epidemiology in Chiang Mai, Thailand. Genes Environ. 2011;33:120–127. doi: 10.3123/jemsge.33.120. [DOI] [Google Scholar]
- 15.Charles M. UNSCEAR report 2000: sources and effects of ionizing radiation. United Nations Scientific Comittee on the Effects of Atomic Radiation. J. Radiol. Prot. 2001;21:83–86. doi: 10.1088/0952-4746/21/1/609. [DOI] [PubMed] [Google Scholar]
- 16.EPA, E. P. A. EPA Assessment of Risks from Radon in Homes. (Office of Radiation and Indoor air, 2003).
- 17.National Research Council Committee on Health Risks of Exposure to, R. in Health Effects of Exposure to Radon (BEIR VI) (National Academies Press 1999). [PubMed]
- 18.Mc Laughlin JP. Some characteristics and effects of natural radiation. Radiat. Prot. Dosimetry. 2015;167:2–7. doi: 10.1093/rpd/ncv206. [DOI] [PubMed] [Google Scholar]
- 19.World Health Organization. WHO handbook on indoor radon: A public health perspective. (World Health Organization, 2009). [PubMed]
- 20.IARC. Ionizing radiation, part 2: some internally deposited radionuclides. Views and expert opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Lyon, 14–21 June 2000. IARC Monogr Eval Carcinog Risks Hum78, 1–559 (2001). [PMC free article] [PubMed]
- 21.Harley NH, Chittaporn P, Heikkinen MS, Meyers OA, Robbins ES. Radon carcinogenesis: Risk data and cellular hits. Radiat Prot Dosimetry. 2008;130:107–109. doi: 10.1093/rpd/ncn123. [DOI] [PubMed] [Google Scholar]
- 22.International Agency for Research on Cancer Radon. IARC Monogr. Eval. Carcinog. Risks Hum. 1988;43:173–259. [PMC free article] [PubMed] [Google Scholar]
- 23.Darby S, et al. Radon in homes and risk of lung cancer: collaborative analysis of individual data from 13 European case-control studies. BMJ. 2005;330:21. doi: 10.1136/bmj.38308.477650.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krewski D, et al. A combined analysis of North American case-control studies of residential radon and lung cancer. J. Toxicol. Environ. Health A. 2006;69:533–597. doi: 10.1080/15287390500260945. [DOI] [PubMed] [Google Scholar]
- 25.Turner M, et al. Radon and Lung Cancer in the American Cancer Society Cohort. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsor. Am. Soc. Prev. Oncol. 2011;20:438–448. doi: 10.1158/1055-9965.EPI-10-1153. [DOI] [PubMed] [Google Scholar]
- 26.Al-Zoughool M, Krewski D. Health effects of radon: A review of the literature. Int. J. Radiat. Biol. 2009;85:57–69. doi: 10.1080/09553000802635054. [DOI] [PubMed] [Google Scholar]
- 27.Vineis P, et al. Air pollution and risk of lung cancer in a prospective study in Europe. Int. J. Cancer. 2006;119:169–174. doi: 10.1002/ijc.21801. [DOI] [PubMed] [Google Scholar]
- 28.International Agency for Research on Cancer. National and Regional Surveys of Radon Concentration in Dwelling. (IAEA, 2014).
- 29.Thumvijit T, et al. Identifying indoor radon sources in Pa Miang, Chiang Mai, Thailand. Sci. Rep. 2020;10:17723. doi: 10.1038/s41598-020-74721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Autsavapromporn N, et al. Short telomere length as a biomarker risk of lung cancer development induced by high radon levels: A pilot study. Int. J. Environ. Res. Public Health. 2018;15:1. doi: 10.3390/ijerph15102152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cho B-W. Spatial relationships between radon and topographical, geological, and geochemical factors and their relevance in all of South Korea. Environ. Earth Sci. 2015;74:5155–5168. doi: 10.1007/s12665-015-4526-0. [DOI] [Google Scholar]
- 32.Kim Y, Chang BU, Park HM, Kim CK, Tokonami S. National radon survey in Korea. Radiat. Prot. Dosimet. 2011;146:6–10. doi: 10.1093/rpd/ncr094. [DOI] [PubMed] [Google Scholar]
- 33.Neri M, Giammanco S, Ferrera E, Patanè G, Zanon V. Spatial distribution of soil radon as a tool to recognize active faulting on an active volcano: the example of Mt Etna (Italy) J Environ Radioact. 2011;102:863–870. doi: 10.1016/j.jenvrad.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Chen Z, et al. Radon emission from soil gases in the active fault zones in the Capital of China and its environmental effects. Sci. Rep. 2018;8:16772–16772. doi: 10.1038/s41598-018-35262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ciotoli G, Lombardi S, Annunziatellis A. Geostatistical analysis of soil gas data in a high seismic intermontane basin: Fucino Plain, central Italy. J. Geophys. Res. 2007;112:1. [Google Scholar]
- 36.Padilla GD, et al. Soil gas radon emissions and volcanic activity at El Hierro (Canary Islands): The 2011–2012 submarine eruption. Geochem. Geophys. Geosyst. 2013;14:432–447. doi: 10.1029/2012GC004375. [DOI] [Google Scholar]
- 37.Neri M, et al. Soil radon measurements as a potential tracer of tectonic and volcanic activity. Sci. Rep. 2016;6:24581. doi: 10.1038/srep24581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang Y, et al. Correlations between the radon concentrations in soil gas and the activity of the Anninghe and the Zemuhe faults in Sichuan, southwestern of China. Appl. Geochem. 2018;89:23–33. doi: 10.1016/j.apgeochem.2017.11.006. [DOI] [Google Scholar]
- 39.Wanabongse P, Tokonami S, Bovornkitti S. Current studies on radon gas in Thailand. Int. Congr. Ser. 2005;1276:208–209. doi: 10.1016/j.ics.2004.11.009. [DOI] [Google Scholar]
- 40.Boonyaprapa SW, Cheepsattayakorn P, Saeung A, Sola S, Bovornkitt B, S. Residential radon exposure and lung cancer: A survey in Chiang Mai Province. J. Health Syst. Res. 2008;2:460–463. [Google Scholar]
- 41.Tansurus BS, Polong A, Bovornkiti P, S. A Survey of Indoor Radon in Lampang Province. Siriraj Hosp. Gaz. 1998;50:311–318. [Google Scholar]
- 42.Ashok GV, Nagaiah N, Shiva Prasad NG. Indoor radon concentration and its possible dependence on ventilation rate and flooring type. Radiat. Prot. Dosime. 2012;148:92–100. doi: 10.1093/rpd/ncq590. [DOI] [PubMed] [Google Scholar]
- 43.Bräuner EV, Rasmussen TV, Gunnarsen L. Variation in residential radon levels in new Danish homes. Indoor Air. 2013;23:311–317. doi: 10.1111/ina.12021. [DOI] [PubMed] [Google Scholar]
- 44.Bundesamt für Strahlenschutz. Radon in building materials, https://www.bfs.de/EN/topics/ion/environment/radon/occurrence/building-materials.html
- 45.Andersen CE, Bergsøe NC, Majborn B, Ulbak K. Radon and Natural Ventilation in Newer Danish Single-Family Houses. Indoor Air. 1997;7:278–286. doi: 10.1111/j.1600-0668.1997.00007.x. [DOI] [Google Scholar]
- 46.Akbari K, Mahmoudi J, Ghanbari M. Influence of indoor air conditions on radon concentration in a detached house. J. Environ. Radioact. 2013;116:166–173. doi: 10.1016/j.jenvrad.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 47.Pisa FE, et al. Residential radon and risk of lung cancer in an Italian alpine area. Arch. Environ. Health. 2001;56:208–215. doi: 10.1080/00039890109604444. [DOI] [PubMed] [Google Scholar]
- 48.Catelinois O, et al. Lung cancer attributable to indoor radon exposure in france: Impact of the risk models and uncertainty analysis. Environ. Health Perspect. 2006;114:1361–1366. doi: 10.1289/ehp.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hänninen O, et al. Environmental burden of disease in Europe: Assessing nine risk factors in six countries. Environ. Health Perspect. 2014;122:439–446. doi: 10.1289/ehp.1206154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Peterson E, et al. Lung cancer risk from radon in Ontario, Canada: how many lung cancers can we prevent? Cancer Causes Control. 2013;24:2013–2020. doi: 10.1007/s10552-013-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lee HA, et al. Risks of lung cancer due to radon exposure among the regions of Korea. J. Kor. Med. Sci. 2015;30:542–548. doi: 10.3346/jkms.2015.30.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kim SH, Hwang WJ, Cho JS, Kang DR. Attributable risk of lung cancer deaths due to indoor radon exposure. Ann. Occup. Environ. Med. 2016;28:8. doi: 10.1186/s40557-016-0093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gaskin J, Coyle D, Whyte J, Krewksi D. Global estimate of lung cancer mortality attributable to residential radon. Environ. Health Perspect. 2018;126:057009–057009. doi: 10.1289/EHP2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Khamkaew C, et al. Investigation of Biomass Burning Chemical Components over Northern Southeast Asia during 7-SEAS/BASELInE 2014 Campaign. Aerosol. Air Qual. Res. 2016;16:2655–2670. doi: 10.4209/aaqr.2016.03.0105. [DOI] [Google Scholar]
- 55.Wiriya W, Prapamontol T, Chantara S. PM10-bound polycyclic aromatic hydrocarbons in Chiang Mai (Thailand): Seasonal variations, source identification, health risk assessment and their relationship to air-mass movement. Atmos. Res. 2013;124:109–122. doi: 10.1016/j.atmosres.2012.12.014. [DOI] [Google Scholar]
- 56.Kawichai S, et al. Seasonal variation and sources estimation of PM25bound pahs from the ambient air of Chiang Mai City: An all-year-round study in 2017. Chiang Mai J. Sci. 2020;47:958–972. [Google Scholar]
- 57.Department of Mineral Resources. Geology, Environment and Disasters, http://webeng.dmr.go.th/Show_Detail.aspx?DetailId=97.
- 58.Wood S. Geothermal systems of northern Thailand and their association with faults active during the Quaternary. Trans. Geotherm. Resourc. Council. 2014;38:607–615. [Google Scholar]
- 59.International Commission on Radiological Protection. Summary of ICRP Recommendations on Radon. (2018). http://www.icrpaedia.org/images/f/fd/ICRPRadonSummary.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.