Abstract
Diabetic retinopathy (DR) is the most common microvascular complication of diabetes mellitus (DM) and the leading cause of blindness in patients with DM. In the pathogenesis of DR, chronic hyperglycemia leads to biochemical and structural alterations in retinal blood vessels’ wall, resulting in hyperpermeability and non-perfusion. Since vascular endothelial growth factor (VEGF) has been found to play a significant role in the pathogenesis of DR, this review sheds light on the effect of intravitreal anti-VEGF agents on retinal non-perfusion in patients with DR. Based on the existing literature, anti-VEGF agents have been shown to improve DR severity, although they cannot reverse retinal ischemia. The results of the published studies are controversial and differ based on the location of retinal non-perfusion, as well as the imaging modality used to assess retinal non-perfusion. In cases of macular non-perfusion, most of studies showed no change in both fundus fluorescein angiography (FFA) and optical coherence tomography (OCTA) in patients with DR treated with intravitreal anti-VEGF agents, while few studies reported worsening of non-perfusion with enlargement of foveal avascular zone (FAZ). Regarding peripheral ischemia, studies using wide-field-FFA demonstrated an improvement or stability in non-perfusion areas after anti-VEGF treatment. However, the use of wide-field-OCTA revealed no signs of re-perfusion of retinal vessels post anti-VEGF treatment. Further prospective studies with long follow-up and large sample size are still needed to draw solid conclusions.
Subject terms: Medical research, Health care
摘要
糖尿病视网膜病变 (DR) 是糖尿病 (DM) 最常见的微血管并发症, 也是DM患者致盲的主要原因。在DR的发病机制中, 慢性高血糖会引起视网膜血管壁的生化和结构发生改变, 并导致高渗和无灌注。由于血管内皮生长因子 (VEGF) 在DR的发病机制中起着重要作用, 本文综述了玻璃体内抗VEGF药物对DR患者视网膜非灌注区的影响。根据现有文献, 抗VEGF药物已被证明可以改善DR的严重程度, 但仍不能逆转视网膜缺血。目前已发表的研究结果存在争议, 并根据视网膜无灌注的位置以及用于评估视网膜无灌注的成像方式而有所不同。在黄斑区无灌注的病例中, 大多数研究报道了玻璃体内抗VEGF药物治疗后的DR患者, 其眼底荧光素血管造影(FFA)和光学相干断层成像(OCTA)均无变化, 而少数研究则报道了无灌注区域的加重伴随中央凹无血管区(FAZ)的增大。关于外周缺血, 使用广角FFA的研究表明, 抗VEGF治疗后的无灌注区有一定的改善或稳定。然而, 在抗VEGF治疗后, 使用广角OCTA并未发现视网膜血管有再灌注的迹象。未来还需要进一步的长期随访和大样本的前瞻性研究才能得出可靠的结论。
Introduction
Diabetes mellitus (DM) is a growing global epidemic and the leading cause of blindness in adults between the ages of 20 and 64 years; the disease affects more than 400 million people worldwide and is estimated to reach over 600 million individuals by 2040 [1, 2]. Diabetic retinopathy (DR) is considered the most common microvascular complication of DM. Early stages of DR (non-proliferative DR or NPDR) are characterized by microaneurysms, dot and blot hemorrhages, exudates, and intraretinal microvascular abnormalities, whereas the later stages (proliferative DR or PDR) are characterized by retinal neovascularization and its complications [3, 4]. Diabetic macular edema (DMO) may occur at any stage of DR, affecting ~20% of patients with type 1 DM and 14–25% of those with type 2 DM during a 10-year follow-up [5, 6]. In the pathogenesis of DR, hyperglycemia promotes biochemical and consequent structural changes in the retinal blood vessel wall, leading to retinal vascular hyperpermeability [2, 7, 8], while non-perfusion can also occur [8]. The latter event triggers a cascade of molecular processes, with vascular endothelial growth factor (VEGF) upregulation being the most prominent; [2] increased VEGF levels, in addition to vascular hyperpermeability, angiogenesis, and inflammatory response, may promote leukocytes’ recruitment within the retina, worsening retinal vessel closure and capillary drop-out [9].
Retinal ischemia ensues when the retinal circulation is insufficient to meet the metabolic demands of the retina and may be caused by general circulatory failure or, more commonly, by local circulatory failure [10]. It can be classified as macular, defined as an enlargement of the foveal avascular zone (FAZ), or peripheral, involving capillary non-perfusion in the retinal periphery [11]. In fact, the cause of the different regional distribution of ischemia remains elusive, while it is also unknown why ischemia occurs in some patients but not others and how it may affect visual function [11].
Vascular endothelial growth factor is required for the natural maintenance of the vasculature. However, since there is an association between VEGF upregulation and ischemia, it could be hypothesized that anti-VEGF treatment might improve retinal ischemia. Previous studies have shown that anti-VEGF agents offer improvement of DR severity, as shown on color fundus photographs [12–14], while reports on retinal re-perfusion after anti-VEGF treatment in DR patients are still controversial [15, 16]. On the other hand, animal studies have shown possible harmful cellular effects following VEGF inhibition [17, 18], raising concern regarding potential risks of anti-VEGF treatment in patients with retinal ischemia [19]. The purpose of this review is to scrutinize the current literature about the effect of anti-VEGF treatment on retinal non-perfusion in patients with DR.
Pathophysiology of diabetic retinopathy
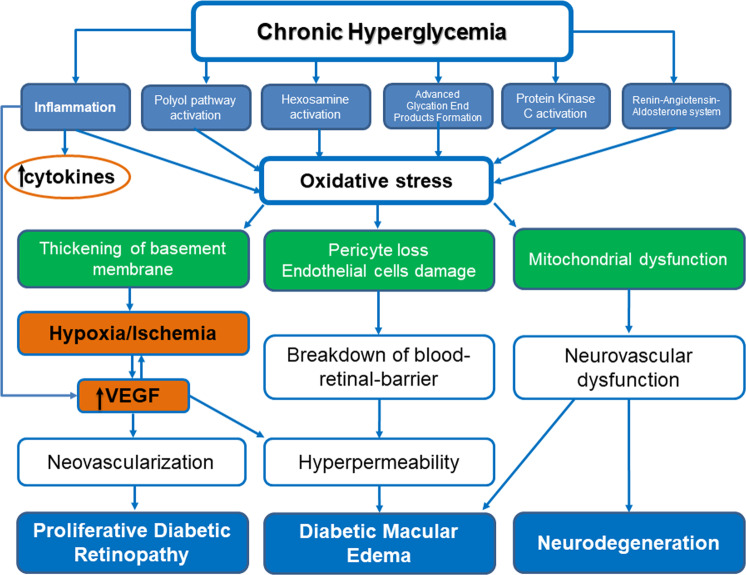
The pathophysiology of DR is not entirely clear, and several pathways are implicated, as it is shown in Fig. 1. Chronic hyperglycemia triggers the aberrant regulation of five main interconnected metabolic pathways, including the polyol and hexosamine pathway, advanced glycation end (AGE) product formation and accumulation, protein kinase C (PKC) activation, and the renin-angiotensin-aldosterone system [7, 20]. These biochemical changes induce retinal blood vessel structural alterations in patients with DM. Specifically, excess glucose is metabolized via the polyol pathway mediated through aldose reductase, producing sorbitol, which is cell-impermeable and accumulates inside retinal cells, enhancing tissue damage due to osmotic stress [7, 8, 20]. Moreover, the increase in glycolytic flux due to chronic hyperglycemia may exert endothelial cell damage and pericyte loss through the activation of multiple isoforms of PKC, leading to blood-retinal-barrier (BRB) disruption, hyperpermeability, and DMO [20–23]. Additionally, the thickening of capillaries’ basement membrane due to glycosylation leads to vessel occlusion and ischemia, which in turn causes upregulation of VEGF [20, 24]. The role of VEGF in promoting angiogenesis, breakdown of the BRB, and vascular hyperpermeability has been well-described in the development and progression of both PDR and DMO [20]; therefore, the VEGF pathway is the most attractive therapeutic target for the treatment of DMO and PDR available so far [25–27].
Fig. 1. Pathophysiology of diabetic retinopathy and diabetic macular oedema development.
Chronic hyperglycemia triggers a cascade of biochemical and structural changes in retinal vessels, leading to diabetic retinopathy, diabetic macular oedema and neurodegeneration.
Additionally, hyperglycemia induces a local, chronic, low-grade inflammation with consequent release of cytokines, such as tumor necrosis factor-alpha (TNF-a), interleukin-6 (IL-6), IL-8, intercellular adhesion molecule (ICAM)−1, monocyte chemotactic protein (MCP)−1, and VEGF [8, 28–30]. Finally, other biochemical pathways and molecules have been also associated with DR pathophysiology, such as angiopoietin-2 (Ang-2), tyrosine-protein kinase receptor complex, and the kallikrein-kinin system [31–33]. Of note, both pathways have attracted increased interest as potential treatment targets for DR and DMO. Specifically, the Ang-tyrosine kinase with immunoglobulin-like domains (Tie) signaling pathway has been implicated in vascular homeostasis, controlling vessel permeability, inflammation, and the angiogenic response [31, 32]. The activation of Tie2 signaling with angiopoietin 1 (Ang-1) promotes vascular stability and inhibits vascular permeability, enhancing pericyte recruitment [31, 32]. Moreover, Ang-2 competitively binds to Tie2, inhibiting Ang-1 signaling and leading to vascular destabilization and disruption of the blood-retinal-barrier [31, 32]. In addition, recent evidence suggests that kinins play a primary role in the development of DR, enhancing vascular permeability, leukocytes infiltration, and other inflammatory mechanisms [33].
Recent evidence has supported that DR is not only a microvascular disease, but also a neurodegenerative disorder, since both structural and functional neuronal changes occur simultaneously or even before vascular alterations. Of note, functional abnormalities in contrast sensitivity, multifocal electroretinography, and microperimetry have been reported before DR changes on fundoscopy [8, 34]. In vitro studies have shown that high glucose exposure is associated with increased mitochondrial fragmentation, mitochondrial dysfunction, and retinal neurons apoptosis, contributing to glucotoxicity and neurovascular degeneration [20, 34, 35]. Recent studies have used the term “neurovascular unit”, which includes microvascular and neural elements, namely retinal ganglion cells, bipolar cells, horizontal cells, astrocytes, Müller cells, microglia, pericytes, and endothelial microvascular cells, suggesting that glial, neural, and microvascular dysfunction are interdependent and essential for the development of DR [34, 36].
Imaging of non-perfusion areas in patients with diabetes mellitus
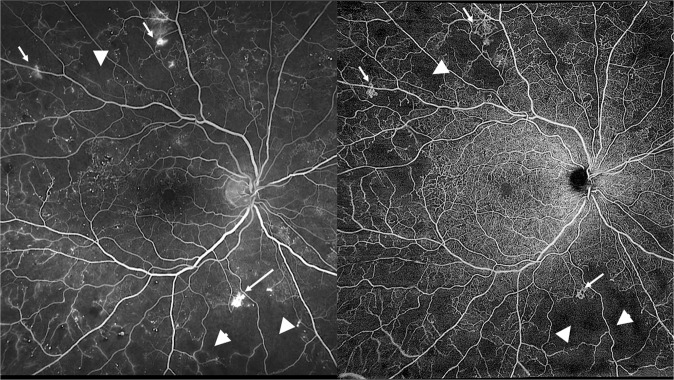
Retinal imaging is important in the diagnosis, management, and follow-up of DR, as well as in its classification as NPDR or PDR [37]. Fundus fluorescein angiography (FFA) is the gold standard imaging technique to evaluate hypofluorescent non-perfusion areas in patients with DM, as well as to depict an enlargement of the foveal avascular zone (FAZ) area as a marker of macular ischemia. FFA can also detect increased vascular permeability and neovascularization as areas of hyperfluorescent leakage of the dye. Traditional FFA normally covers only a 20–50° field of the fundus [38]. Advances in retinal imaging with the introduction of wide field (WF-FFA) and ultra-wide-field fluorescein angiography (UW-FFA) allow the visualization of the entire posterior segment, extending beyond the equator, which could not be previously captured. Specifically, UW-FFA can visualize up to 200° of the retina in a single image, offering the possibility to evaluate peripheral retinal perfusion and vascular pathology in DR (Fig. 2) [39–41].
Fig. 2. Patient with proliferative diabetic retinopathy.
Wide-field fundus fluorescein angiography (left) and wide-field optical coherence tomography angiography (right), showing non-perfusion areas (white triangles) and neovascularization (white arrows).
On the other hand, optical coherence tomography angiography (OCTA) is a recent non-invasive imaging modality that allow a detailed examination of each retinal layer and the retinal microvasculature. OCTA provides a quantitative assessment of non-perfusion areas, as well as FAZ measurement without obscuration by any dye leakage. The vessel density (VD) in the foveal and parafoveal areas can be also assessed [37, 39, 42–45]. Studies have shown that the microvascular changes of DR, such as microaneurysms, neovascularization, and capillary non-perfusion can be well depicted with OCTA [42, 46]. Specifically, in patients with DM, a decrease in VD along with an increase in FAZ area can be found in both the superficial (SCP) and the deep capillary plexus (DCP) [38, 42, 46]. Τhe need to visualize the peripheral retina has led to the development of ultra-wide OCTA (UW-OCTA) [44], which gives more detailed images of the fundus, up to 100o (Fig. 2). UW-OCTA is also able to detect pathologies, such as peripheral non-perfusion areas or NVEs, which cannot be captured by traditional FFA or OCTA [44, 45].
Methods
A comprehensive systematic literature review in the PubMed engine search was performed, using the algorithm [(diabetic OR diabetes OR “diabetic retinopathy”) AND (ischemia OR ischemic OR perfusion OR foveal avascular zone) AND (ranibizumab OR aflibercept OR bevacizumab OR anti-VEGF)] for articles in the English language published up to 1 September 2020. All abstracts derived using this algorithm were reviewed, while the references’ lists of the selected papers were examined to find additional articles. Reviews or meta-analyses, as well as pre-clinical studies were excluded, while eligible articles included case reports, case series, and clinical studies.
In total, 287 abstracts were identified and 52 were found relevant to our study. Out of 52 full-text articles, 2 were not written in English (one was in German and one was in Japanese), 3 articles were reviews, and 10 did not report parameters of ischemia. Therefore, 37 eligible articles were finally included in the review.
Results
Retinal non-perfusion in diabetic retinopathy
As mentioned above, in the pathophysiology of DR, biochemical and structural changes on retinal blood vessels lead to capillary dropout and consequent areas of retinal non-perfusion, which in turn may result in the development of neovascularization [10, 11, 20]. Of note, the location of retinal capillary non-perfusion on FFA has been related to the presence of retinal neovascularization in eyes with PDR with eyes presenting neovascularization on the disc having the largest area of retinal non-perfusion, while the threshold of non-perfusion required for conversion from NPDR to PDR remains unclear [46]. It has been shown that increased hyperpermeability, endothelial cell proliferation, and cell migration in PDR are largely mediated by VEGF [47], while VEGF injection has been also reported to be sufficient to cause capillary occlusion with ischemia, thereby creating non-perfusion areas [48]. Since VEGF has been considered the main molecule in the pathogenesis of retinal non-perfusion in patients with DM, it is hypothesized that intravitreal anti-VEGF treatment may improve retinal ischemia [12–16].
Effect of anti-VEGF treatment on retinal non-perfusion in patients with DR
Table 1 summarizes the characteristics and findings of the existing literature, included in the review, which examined the effect of anti-VEGF treatment on retinal non-perfusion in patients with DR.
Table 1.
Characteristics and outcomes in the included studies, showing the effect of anti-vascular endothelial growth factors on retinal non-perfusion in patients with diabetic retinopathy.
| Study | Design | Number of eyes | Treatment | Follow-up | VA outcome | Imaging modality | Characterization of outcome | Outcome in non-perfusion | Location of examined ischemia |
|---|---|---|---|---|---|---|---|---|---|
| Neubauer et al. [49] | Prospective case series | 19 eyes with DME and NPDR | Bevacizumab 1.25 mg single injection | 1 month | Significant improvement in VA from 0.87 ± 0.37 to 0.13 ± 0.28 logMAR | UW-FFA |
Neutral (macula) Positive (peripheral) |
No significant change in FAZ area Significant decrease in peripheral retinal ischemia |
Macular and peripheral ischemia |
| Kook et al. [50] | Prospective case series | 126 eyes with diffuse DME and NPDR (86) or PDR (40) | Bevacizumab 1.25 mg repeated injections | 12 months | Significant improvement in VA from 40.3 ± 20.9 to 45.4 ± 18.5 ETDRS letters | FFA | Neutral | No significant change in macular ischemia | Macular ischemia |
| Lee and Koh (2009) [64] | Case report | 1 eye with PDR and vitreous hemorrhage | Bevacizumab 2.5 mg single injection and PPV/endolaser | – | VA stable to 20/400 | FFA | Negative | FAZ area increase by 24.5% | Macular ischemia |
| Michaelides et al. [51] | Prospective, randomized, controlled study |
40 in anti-VEGF group and 40 in laser group 80 eyes with DME |
Bevacizumab 1.25 mg (3 intravitreal 6-weekly injections) vs laser | 4 months | Improvement in VA from 55.7 ± 9.7 to 61.3 ± 10.4 in the bevacizumab group at month 12 | FFA | Neutral | No worsening of macular ischemia in either group (anti-VEGF or laser) | Macular ischemia |
| Goel et al. [71] | Case Report | 1 eye with diffuse DME and NPDR | Bevacizumab 1.25 mg single injection | 6 months | Decrease in VA from 20/80 to 20/200 | FFA | Negative | FAZ increased from 0.69 to 1.26 mm | Macular ischemia |
| Erol et al. [65] | Prospective case series | 29 eyes with DME | Bevacizumab 1.25 mg (3 injections) | 3 months | No significant change in VA | FFA | Negative | Significant increase in FAZ area by 13% after 3 anti-VEGF injections | Macular ischemia |
| Feucht et al. [72] | Retrospective case series | 28 eyes with DME and NPDR | Bevacizumab 1.25 mg single injection | 6–8 weeks | Not reported | FFA | Negative | FAZ area increase by 19.7% | Macular ischemia |
| Comyn et al. [52] | Prospective, randomized | 33 eyes with DME and no macular ischemia (22 Ran and 11 laser) | Ranibizumab 0.5 mg (3 monthly injections) vs laser every 12 weeks, as required | 48 weeks | Increase in VA from 70.4 ± 4.9 to 76.4 ± 8.5 in ranibizumab group, while decrease from 63.8 ± 5.7 to 62.9 ± 10.6 to laser group | FFA | Neutral | Slight increase in FAZ area, but no significant | Macular ischemia |
| Campochiaro et al. [73] | Retrospective controlled |
666 with DME 213 ran 0.3 225 ran 0.5 228 sham |
Ranibizumab 0.3 mg vs 0.5 mg vs sham | 24 months | Not reported | FFA | Neutral |
Less worsening with anti-VEGF, but no difference Stable percentage with no progression to macular ischemia in ranibizumab group |
Macular ischemia |
| Ghasemi Falavarjani et al. [66] | Prospective, non-comparative case series | 13 eyes with DME |
Bevacizumab 1.25 mgRanibizumab 0.5 mg Aflibercept 2 mg (1 injection) |
1 month | No significant change in VA | OCTA 3×3 | Neutral | No significant change in FAZ area and VD after single injection | Macular ischemia |
| Levin et al. [74] | Retrospective case series | 16 eyes with DME and NPDR (1) or PDR (15) | At least one injection (Bevacizumab 3 or Ranibizumab 12 or Aflibercept 1) | 5 months | Not reported | UW-FFA | Positive | Re-perfusion of areas of non-perfusion in 12 out of 16 eyes (75%) | Peripheral ischemia AND/OR macular ischemia |
| Douvali et al. [53] | Retrospective study |
49 patients with DME 32 non-ischemic 17 ischemic |
Ranibizumab 0.5 mg (3 injections + PRN) | 6 months | Significant improvement in VA in non-ischemic group-No change in ischemic group | FFA | Neutral | No difference in ischemic region in eyes with ischemia at baseline | Macular ischemia |
| Gill et al. [83] | Retrospective | 11 eyes with DME |
Aflibercept 2 mg (6 eyes-1 injection) Bevacizumab 1.25 mg (2 eyes-2 injections) Ranibizumab 0.5 mg (3 eyes-2 injections) |
3 months | Not reported | OCTA 3 × 3 | Positive | Significant decrease in FAZ area | Macular ischemia |
| Michalska-Malecka and Heinke Knudsen [76] | Retrospective case reports | 3 eyes with DME and DR | Aflibercept 2 mg (3 monthly injections and 2 bimonthly) | 1 month after last injection | Not reported | OCTA 3 × 3 | Neutral |
No re-perfusion noted - No change in VD |
Macular ischemia |
| Chandra et al. [54] | Case report | 1 eyes with DME and PDR | Ranibizumab 0.5 mg (3 monthly injections) | 1 month after last injection | Improvement in VA from 6/12 to 6/9 | WF-FFA | Positive | Regression of neovascularization-Improvement in retinal perfusion in previous ischemic areas | Peripheral ischemia |
| Moon et al. [67] | Retrospective case series | 67 eyes with DME and NPDR (37 eyes) or PDR (30 eyes) |
At least 3 injections of Bevacizumab (64) Aflibercept (2) Ranibizumab (1) |
12 months | No significant difference in VA | OCTA 3 × 3 | Neutral | No significant difference in FAZ area and VD in SCP, but significant increase in DCP VD, while no difference in DCP FAZ area | Macular ischemia |
| Karst et al. [75] | Prospective, randomized | 240 eyes with DME |
Ranibizumab 0.5 mg (3 injections and PRN)−83 eyes Ran + laser 83 Laser alone 74 |
36 months | Not reported | Neutral | No change in FAZ area, no difference in capillary loss in 3 groups | Macular ischemia | |
| Gupta et al. [77] | Case report | 1 eye with PDR without DME | Ranibizumab 0.3 mg (1 injection) | 12 months | Not reported | UW-FFA | Positive | Regression of neovascularization-No progression of retinal non-perfusion | Peripheral ischemia |
| Bonnin et al. [16] | Retrospective case series | 18 with DME and NPDR (15 eyes) or PDR (3 eyes) |
3 monthly injections of Ranibizumab (13) Aflibercept (5) |
3 months | Significant improvement in VA from 0.53 ± 0.28 at baseline to 0.26 ± 0.18 logMAR at month 3 | UW-FFA | Neutral |
No re-perfusion noted one month after 3 injections DRSS score improved at least one level in 61% of eyes Regression of neovascularization in PDR eyes |
Peripheral ischemia |
| Busch et al. [55] | Retrospective case series | 23 eyes with DME and NPDR (14 eyes) or PDR (9 eyes) | Aflibercept 2 mg (1–3 injections and PRN) | 8.5 ± 5.6 months | Significant improvement in VA from 0.28 ± 0.23 to 0.15 ± 0.22 logMAR | OCTA 3 × 3 | Neutral | No significant change in FAZ area in both SCP and DCP | Macular ischemia |
| Wykoff et al. [81] | Retrospective controlled | 466 eyes with DME | Aflibercept 2 mg 2q4 (130) vs 2q8 (126) vs laser (129) | 100 weeks | Not reported | WF-FFA | Positive | Improvement in retinal perfusion in aflibercept more than laser | Macular and peripheral ischemia |
| Sorour et al. [78] | Retrospective case series | 46 eyes with DME and 9 eyes with PDR |
Bevacizumab 45.7% Aflibercept 42.4% Ranibizumab 11.9% |
1–3 months | Not reported | OCTA 3 × 3 and 6 × 6 | Neutral | No significant change in VD in both SCP and DCP | Macular ischemia |
| Filek et al. [82] | Prospective study | 30 eyes with DME | Ranibizumab 0.5 mg (3 monthly injections and PRN) | 24 months | Not reported | UW-FFA (Optos) | Positive | The mean perfused ratio was significantly improved | Peripheral /macular ischemia |
| Babiuch et al. [68] | Prospective study | 20 eyes with DME | Aflibercept 2 mg monthly until resolution of fluid and then bimontlhy | 6 months | No significant change in VA | OCTA 3 × 3 | Neutral |
No change in FAZ area No change in VD |
Macular ischemia |
| Hsieh et al. [56] | Retrospective study | 50 eyes DME | Ranibizumab 0.5 mg (3 injections) | 3 months | Significant improvement in VA from 0.73 ± 0.39 to 0.56 ± 0.39 logMAR | OCTA 3 × 3 | Positive | Significant decrease in FAZ area and increase in VD | Macular ischemia |
| Couturier et al. [57] | Prospective case series | 7 eyes with NPDR and 3 eyes with PDR | Ranibizumab 0.5 mg (8 eyes) or Aflibercept 2 mg (2 eyes) (3 monthly injections) | 3 months |
Significant improvement in VA from 64.40 ± 9.39 to 73.50 ± 8.47 ETDRS letters |
UW-FFA OCTA 3 × 3 WF-OCTA |
Neutral |
No change in retinal capillary density in FAZ No change in peripheral ischemia No re-perfusion was detected |
Macular and peripheral ischemia |
| Sugimoto et al. [58] | Prospective study | 25 eyes with DME and NPDR | Aflibercept 2 mg (3 monthly injections) | 1 week after last injection | Significant improvement in VA from 0.45 ± 0.35 to 0.40 ± 0.38 logMAR | UW-FFA | Positive | Significant decrease of the mean ischemic index | Peripheral ischemia |
| Pereira et al. [59] | Prospective case series | 5 eyes with DME and previously treated PDR | Bevacizumab 1.25 mg (6 injections) | 6 months | Significant improvement in VA from 20/180 to 20/74 |
FFA OCTA 3 × 3 and 4.5 × 4.5 |
Negative |
No changes in macular perfusion status FAZ area increase, but not significantly in FFA, while significant increase in OCTA |
Macular ischemia |
| Wykoff et al. [15] | Prospective, randomized | 40 eyes with PDR and without DME | Aflibercept 2 mg monthly (20 eyes) vs quarterly (20 eyes) | 12 months | No significant improvement in VA | UW-FFA | Neutral |
2-step DRSS score improvement in both groups Significant regression of neovascularization Significant increase of ischemic index in the whole cohort Significant increase of retinal non-perfusion in quarterly group and stable non-perfusion in monthly group Localized re-perfusion in some patients |
Peripheral and macular ischemia (total) |
| Conti et al. [69] | Prospective study | 19 eyes with DME and NPDR (15) or PDR (4) | Aflibercept 2 mg monthly until resolution of fluid and then bimonthly | 12 months | No significant improvement in VA | OCTA 6 × 6 | Neutral | No change in FAZ area No change in VD | Macular ischemia |
| Figueiredo et al. [60] | Prospective case series | 14 eyes with DME and NPDR (11 eyes) or PDR (3 eyes) | Aflibercept 2 mg monthly for 6 months and then bimonthly | 12 months | Significant improvement in VA at month 6 (6.6 ± 5.9 letters), but not at month 12 (5.0 ± 7.4 letters) | UW-FFA | Neutral |
Stability in panretinal ischemic index Significant reduction in panretinal leakage index |
Peripheral and macular ischemia (total) |
| Dastiridou et al. [79] | Prospective case series | 20 eyes with DME | Aflibercept 2 mg (3 injections) | 3 months | Not reported | OCTA 6 × 6 | Neutral |
No change in FAZ in SCP Decrease in FAZ in DCP Significant decrease in central VD, but stable VD in parafoveal area |
Macular ischemia |
| Elnahry et al. [61] | Prospective, non-comparative case series | 40 eyes with DME and NPDR (31 eyes) or PDR (9 eyes) | Bevacizumab 1,25 mg (3 monthly injections) | 3 months | Significant improvement in VA from 0.68 ± 0.34 to 0.47 ± 0.25 logMAR | OCTA 6 × 6 | Negative | Significant increase in FAZ area by 8.1% and significant decrease in VD in both SCP and DCP | Macular ischemia |
| Lee et al. [63] | Prospective study | 25 eyes with DME | Ranibizumab 0.5 mg (6 monthly injections) | 6 months | Significant improvement in VA from 67.6 ± 3.29 to 76.4 ± 1.61 letters | FFA | Neutral | No significant change in the perifoveal non-perfused area | Macular ischemia |
| Barash et al. [80] | Retrospective case series | 9 PDR and 5 DME with NPDR | Bevacizumab 1.25 mg or Aflibercept 2 mg single injection | Immediately after injection | Not reported | OCTA 3 × 3 | Negative | SCP VD dropped by 7.8% while DCP VD dropped by 3.5% immediately after injection | Macular ischemia |
| Mirshahi et al. [70] | Prospective case series | 23 eyes with DME | Bevacizumab 1.25 mg | 1 month after injection | No significant improvement in VA 0.46 ± 0.25 to 0.45 ± 0.25 logMAR | OCTA 3 × 3 | Neutral |
No significant changes in the capillary non-perfusion area, FAZ area, FD-300, or in the VD of the foveal and parafoveal SCP and DCP VD in choriocapillaris significantly increased |
Macular ischemia |
| Statler et al. [62] | Prospective | 16 eyes with persistent DME | Aflibercept 2.0 mg every 4 weeks | 24 months | Significant improvement in VA by 5.5 letters | OCTA 3 × 3 | Negative | Enlargement of FAZ and decrease in VD in superficial and deep capillary plexus | Macular ischemia |
DCP deep capillary plexus, DME diabetic macular edema, FAZ foveal avascular zone, FFA fundus fluorescein angiography, NPDR non-proliferative diabetic retinopathy, OCTA optical coherence tomography angiography, PDR proliferative diabetic retinopathy, PPV pars plana vitrectomy, SCP superficial capillary plexus, UW ultra-wide-field, VA visual acuity, VD vessel density, WF wide–field.
Evolution of visual acuity after anti-VEGF treatment in patients with DR or DMO
Based on the results of the included studies, anti-VEGF agents led to a statistically significant improvement [16, 49–63] or stabilization [15, 64–70] in visual acuity in all the studies, except for one case report, in which a decrease in visual acuity from 20/80 to 20/200 was noticed after one 1.25 mg bevacizumab injection in one eye with NPDR and diffuse DMO [71]. However, not all studies reported results for visual acuity [72–83].
Evolution of DR severity after anti-VEGF treatment
Anti-VEGF treatment offers improvement in DR severity, as confirmed on fundus photography [12–14]. Recent studies, using UW-FFA, have shown similar results. Specifically, Bonnin et al. examined 18 eyes with NPDR (15 eyes) or PDR (3 eyes) and co-existent DMO treated with 3 monthly ranibizumab or aflibercept injections and found at least one-level DRSS score improvement in 61% of eyes at the 3-month follow-up [16]. Accordingly, Wykoff et al., in a prospective randomized study with a follow-up of 12 months, including 40 eyes with PDR treated with 2 mg aflibercept either monthly or quarterly, showed a 2-step DRSS score improvement in both groups (monthly and quarterly) [15].
Furthermore, the pivotal phase III clinical trials RISE and RIDE included patients with DMO, who received monthly intravitreal injections of 0.3 mg ranibizumab, 0.5 mg ranibizumab or sham for 24 months, while during months 24–36 patients in the sham group were allowed to crossover to active treatment with 0.5 mg ranibizumab [84, 85]. A post hoc analysis of the RISE and RIDE trials evaluated the DR outcomes through month 36 by baseline DR severity, showing that 35.7–8.5% of ranibizumab-injected eyes presented an improvement in their retinopathy compared to 4–7% in the sham group. Interestingly, ranibizumab treatment resulted in DR improvements in all three baseline DR severity subsets examined, with the greatest benefits in DR improvement occurring in patients with baseline moderately severe to severe NPDR (DR levels 47/53) [86]. In addition, in patients with baseline severe NPDR, ranibizumab reduced the probability of patients experiencing a new proliferative event at month 36 by three times compared with sham treatment (12.4% and 11.9% vs. 35.2% for ranibizumab 0.3 mg, ranibizumab 0.5 mg, and sham, respectively) [86].
Evolution of macular non-perfusion in patients with DR after anti-VEGF treatment
Regarding the effect of anti-VEGF injections on macular ischemia in patients with DR, the existing literature reports conflicting data. Some studies have shown no change in macular ischemia on FFA after anti-VEGF treatment in patients with DMO and DR [49, 50] or with DMO alone [51, 52, 66, 73, 80] independent of the anti-VEGF molecule used, namely ranibizumab, aflibercept, or bevacizumab. Interestingly, a retrospective post hoc analysis of the prospective RISE/RIDE studies, including 666 DMO patients treated with intravitreal ranibizumab or sham, showed that the percentage of patients who exhibited increase in posterior retinal non-perfusion from baseline to month 24 increased over time in all groups, but at a significantly faster rate in the sham group at every time point between months 3 and 24 (9.6% in the 0.5 mg ranibizumab group and 18.5% in the sham group, p < 0.001), suggesting that monthly anti-VEGF injections might slow the progression of macular ischemia in DMO patients [73]. Moreover, initiation of ranibizumab in the sham group at month 24 was followed by reduction in the percentage of patients exhibiting an increase in posterior retinal non-perfusion from baseline at months 30 and 36 [73]. Nevertheless, there are also studies, reporting a worsening of macular non-perfusion after anti-VEGF treatment in patients with DMO and DR [59, 65, 72] or PDR without DMO [64] with an enlargement of the FAZ area seen on FFA.
Other studies have used OCTA, which is more reliable for assessing macular ischemia, providing quantification of the FAZ area and VD. Specifically, anti-VEGF agents were shown to provide no significant change in the FAZ area or the VD in both SCP and DCP, as depicted in OCTA, in patients with DMO alone [66, 70, 75, 79] or in those with DMO and DR [58, 67–69, 76–78]. It should be noted, however, that most of the above-mentioned studies had a short-term follow-up, ranging from 1–6 months post-treatment. Almost similar results were found in a post hoc analysis of the RESTORE study with a long-term 3-year follow-up, which demonstrated no significant increase in the FAZ area on OCTA after repeated ranibizumab injections over 36 months, while patients with moderate-to-severe capillary loss did not change significantly over the study period [75]. On the other hand, some authors have found significant enlargement of the FAZ area and a decrease in VD after an anti-VEGF treatment course, raising concerns regarding the risk of worsening of macular perfusion in DR eyes [15, 61, 62, 80]. Only one retrospective study with 50 DMO eyes mentioned a significant decrease in the FAZ area and an increase in VD after three injections of 0.5 mg intravitreal ranibizumab [56].
Evolution of peripheral non-perfusion in patients with DR after anti-VEGF treatment
Regarding peripheral ischemia, to date, studies have presented conflicting results, which are mainly based on the imaging modality used for the assessment of ischemia. In studies using UW-FFA, some authors reported no significant change in peripheral non-perfusion in patients with DR after anti-VEGF treatment and a follow-up ranging from 3 to 12 months [16, 57, 60]. It is worth noting that a prospective UW-FFA study, comparing patients with PDR treated with intravitreal 2.0 mg aflibercept either monthly or quarterly, showed stability in the amount of non-perfusion in patients receiving monthly aflibercept and not in those with lower dosage; therefore, the authors suggested that the dose regimen of an anti-VEGF agent may affect the perfusion status [15]. Other studies demonstrated positive results in terms of peripheral ischemia in patients with DR, reporting a significant decrease in the mean ischemic index on UW-FFA or improvement in retinal perfusion [49, 54, 58, 81]. Interestingly, Levin et al. found re-perfusion of previously non-perfused peripheral areas on UW-FFA in 75% of eyes with DMO and PDR treated with at least one intravitreal injection at a 5-month follow-up [74].
It should be mentioned, however, that WF-OCTA seems to be more precise and reliable in assessing non-perfusion in patients with DR. Couturier et al. [57] used both swept-source WF-OCTA (SS-WF-OCTA) and UW-FFA to prospectively study retinal capillary perfusion changes in patients with DR after treatment with 3 monthly injections of anti-VEGF agents. The authors found that none of the cases bearing non-perfusion areas at baseline experienced re-perfusion in the arterioles, venules, or capillaries on UW-FFA in the 3 months post-treatment, despite significant improvement in DRSS based on color fundus photography. The absence of re-perfusion of retinal capillaries became evident on OCTA, while additional non-perfusion areas were detected only on SS-WF-OCTA at both the baseline and the post-treatment assessment [57]. The latter study firmly concluded that anti-VEGF did not have a rescuing role in retinal non-perfusion [57].
Challenges in the interpretation of the results-limitations
Elevated VEGF levels may cause further DR progression by maintaining a pathologic loop between ischemia and further vascular occlusion [20]. In detail, retinal ischemia leads to increased intraocular VEGF levels, which in turn worsens and causes leukocyte adhesion and capillary clogging, thereby amplifying retinal ischemia in a vicious cycle [20]. Anti-VEGF treatment may interrupt this VEGF-induced feedback loop, slowing or halting disease progression [16].
As mentioned above, based on the existing literature, anti-VEGF treatment leads to improvement in the DR severity over time [12–16, 57], although retinal ischemia does not seem to improve over time [15, 16, 57]. The majority of studies showed no change in macular non-perfusion in patients with DR in response to anti-VEGF agents, while some authors found worsening of macular ischemia with enlargement of the FAZ area after anti-VEGF treatment. On the other hand, there is controversy in the existing literature regarding peripheral ischemia in DR patients after treatment, mainly dependent on the imaging modality used. In some studies, UW-FFA revealed no progression of retinal non-perfusion or improvement of retinal perfusion in the periphery [15, 16, 54]. However, more recent reports based on SS-WF-OCTA showed no re-perfusion in patients with DR undergoing intravitreal anti-VEGF injections [57].
Potential mechanisms explaining re-perfusion may entail restoration of the normal retinal architecture, remodeling of pericytes, and normalization of the basement membranes, allowing for the retinal microvasculature to regrow; [54] VEGF suppression may also reduce leukostasis and might allow the re-opening of the small vessels [9]. The difference in the pathophysiology between macular and peripheral ischemia may explain the opposite findings in the macula and the retinal periphery after anti-VEGF treatment. Macular ischemia could be attributed mainly to pericyte loss, hyperpermeability, and VEGF increase (according to a vasogenic theory), while peripheral ischemia is mainly caused by thickening of the basement membrane of the vessels’ walls, although the exact mechanism has not been clarified so far [87].
In eyes that do not re-perfuse, it can be hypothesized that the ischemic areas are either irreversibly infarcted or may require a higher or more frequent dose of VEGF inhibitors [15, 74]. Non-selective VEGF blockade may downregulate the normal functions of VEGF, disturbing the normal retinal and choriocapillaris circulation and resulting in further endothelial dysfunction [4, 18, 20, 88]. In addition, anti-VEGF may cause regression of NV and vasoconstriction, a decreased retinal blood flow, and a reduced capillary density due to inhibition of nitric oxide induced by VEGF; this effect is usually transient and regresses over time [51]. Additionally, DR has a complex pathophysiology, implicating different pathways besides the VEGF pathway, including those of inflammation, Ang-2 upregulation and glucotoxicity, which may also play a role in capillary bed closure, explaining why sustained anti-VEGF treatment can slow but not completely prevent or reverse retinal non-perfusion [73].
A challenge in the interpretation of the results in this review pertains to the fact that different methods have been used to measure the areas of non-perfusion in both the macula and periphery, and quantification of ischemic areas has been often performed manually. Since new technologies, such as wide-field imaging and OCTA, are still under development, several limitations may influence the correct interpretation of their results. OCTA seems to be superior to FFA in detecting capillary non-perfusion, however due to motion perception limits of OCTA devices, WF-FFA and UW-FFA remain the gold standard in the detection and quantification of retinal non-perfusion areas, while digitally reconstructed OCTA vessels’ images critically evaluated both in research and clinical settings. Specifically, the diagnosis of ischemic areas is based only on indirect, and sometimes subjective signs on FFA, such as the loss of perfused retinal arteriolar and venular branches, a pruned appearance of the adjacent vessels, or the darkening of choroidal fluorescence [57]. Due to these limitations, the described changes in non-perfusion areas on FFA could be potentially attributed to the change in the choroidal background after anti-VEGF treatment, which may appear brighter when the leakage from the overlying retinal vessels decreases. OCTA is not affected by choroidal fluorescence or dye leakage and depicts more precisely the areas of capillary non-perfusion [57]. Nevertheless, it should be taken into account that OCTA only detects vessels with flow above a certain velocity threshold, and it can be assumed that OCTA could miss portions of vessels with low blood flow [57].
The presence of intraretinal fluid may also alter the anatomy of the FAZ in eyes with DMO, and FAZ measurement might be inaccurate in such patients, due to capillaries displacement and masking effects. With the resolution of edema after treatment, the correct segmentation of the macular vascular networks is restored, especially in the deep vascular plexus. Therefore, the degree of vessel re-opening after anti-VEGF treatment is difficult to prove, and, so far, remains speculative [79].
It is also worthy to note that the majority of studies included in this review were retrospective, with relatively small sample sizes and limited follow-up, ranging from 1 to 6 months.
Conclusions
In conclusion, anti-VEGF agents improve DR severity over time. Several studies have been performed to evaluate the effect of intravitreal anti-VEGF injections on retinal non-perfusion in patients with DR. Although new technologies, such as UW-FFA and OCTA, allow for more accurate visualization and quantification of ischemic areas, the results of published studies about the effect of anti-VEGF treatment on retinal ischemia remain controversial. The majority of studies, using either FFA or OCTA, showed no significant change in macular ischemia in patients with DR treated with anti-VEGF agents, while few studies reported a worsening of macular non-perfusion with enlargement of the FAZ area as assessed by FFA and OCTA. Regarding peripheral ischemia, the conclusions are ambiguous and dependent on the imaging modality used. In cases of UW-FFA, most studies have reported that peripheral ischemia either remains stable or may improve over time, while studies using WF-OCTA have noticed no change in peripheral non-perfusion and no signs of re-perfusion after anti-VEGF treatment, suggesting that anti-VEGF agents do not have a protective role in retinal ischemia. As the studies available thus far are limited by their retrospective design, a small sample size, and a relatively short follow-up, the impact of prolonged anti-VEGF treatment in retinal non-perfusion needs elucidation, based on the assumption that multiple pathways are implicated in the pathophysiology of DR and may play a role in retinal non-perfusion. Further prospective studies with long-term follow-ups and larger cohorts are needed to draw more solid conclusions.
Author contributions
IC conceived the idea of the review, collected data, extracted data, analyzed and interpreted data, and drafted the manuscript; ST critically revised the manuscript; MVC provided data and critically revised the manuscript; CA and ED collected data, extracted data, and drafted the manuscript; GT and PT critically revised the manuscript. All authors have read and approved the current version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas. 7th ed. Brussels, Belgium: International Diabetes Federation; 2015.
- 2.Das A. Diabetic retinopathy: battling the global epidemic. Invest Ophthalmol Vis Sci. 2016;57:6669–82. doi: 10.1167/iovs.16-21031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93:137–88. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 4.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. New Engl J Med. 2012;366:1227–39. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 5.Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35:556–64. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 7.Das A, McGuire PG, Rangasamy S. Diabetic macular edema: pathophysiology and novel therapeutic targets. Ophthalmology. 2015;122:1375–94. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 8.Romero-Aroca P, Baget-Bernaldiz M, Pareja-Rios A, Lopez-Galvez M, Navarro-Gil R, Verges R. Diabetic macular edema pathophysiology: vasogenic versus inflammatory. J Diabetes Res. 2016;2016:2156273. doi: 10.1155/2016/2156273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y, Shen J, Fortmann SD, Wang J, Vestweber D, Campochiaro PA. Reversible retinal vessel closure from VEGF-induced leukocyte plugging. JCI insight. 2017;2:e95530. doi: 10.1172/jci.insight.95530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Osborne NN, Casson RJ, Wood JP, Chidlow G, Graham M, Melena J. Retinal ischemia: mechanisms of damage and potential therapeutic strategies. Prog Retin Eye Res. 2004;23:91–147. doi: 10.1016/j.preteyeres.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 11.Sim DA, Keane PA, Rajendram R, Karampelas M, Selvam S, Powner MB, et al. Patterns of peripheral retinal and central macula ischemia in diabetic retinopathy as evaluated by ultra-widefield fluorescein angiography. Am J Ophthalmol. 2014;158:144–53. doi: 10.1016/j.ajo.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Mitchell P, McAllister I, Larsen M, Staurenghi G, Korobelnik JF, Boyer DS, et al. Evaluating the impact of intravitreal aflibercept on diabetic retinopathy progression in the VIVID-DME and VISTA-DME studies. Ophthalmol Retina. 2018;2:988–96. doi: 10.1016/j.oret.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Bressler SB, Odia I, Glassman AR, Danis RP, Grover S, Hampton GR, et al. Changes in diabetic retinopathy severity when treating diabetic macular edema with ranibizumab: DRCR.net Protocol I 5-Year Report. Retina. 2018;38:1896–904. doi: 10.1097/IAE.0000000000002302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bressler SB, Liu D, Glassman AR, Blodi BA, Castellarin AA, Jampol LM, et al. Change in diabetic retinopathy through 2 years: secondary analysis of a randomized clinical trial comparing aflibercept, bevacizumab, and ranibizumab. JAMA Ophthalmol. 2017;135:558–68. doi: 10.1001/jamaophthalmol.2017.0821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wykoff CC, Nittala MG, Zhou B, Fan W, Velaga SB, Lampen SIR, et al. Intravitreal aflibercept for retinal nonperfusion in proliferative diabetic retinopathy: outcomes from the randomized RECOVERY trial. Ophthalmol Retina. 2019;3:1076–86. doi: 10.1016/j.oret.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 16.Bonnin S, Dupas B, Lavia C, Erginay A, Dhundass M, Couturier A, et al. Anti-vascular endothelial growth factor therapy can improve diabetic retinopathy score without change in retinal perfusion. Retina. 2019;39:426–34. doi: 10.1097/IAE.0000000000002422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorrell MI, Aguilar E, Scheppke L, Barnett FH, Friedlander M. Combination angiostatic therapy completely inhibits ocular and tumor angiogenesis. Proc Natl Acad Sci USA. 2007;104:967–72. doi: 10.1073/pnas.0607542104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 19.Manousaridis K, Talks J. Macular ischaemia: a contraindication for anti-VEGF treatment in retinal vascular disease? Br J Ophthalmol. 2012;96:179–84. doi: 10.1136/bjophthalmol-2011-301087. [DOI] [PubMed] [Google Scholar]
- 20.Whitehead M, Wickremasinghe S, Osborne A, Van Wijngaarden P, Martin KR. Diabetic retinopathy: a complex pathophysiology requiring novel therapeutic strategies. Expert Opin Biol Ther. 2018;18:1257–70. doi: 10.1080/14712598.2018.1545836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gan J, Huang M, Lan G, Liu L, Xu F. High glucose induces the loss of retinal pericytes partly via NLRP3-caspase-1-GSDMD-mediated pyroptosis. Biomed Res Int. 2020;2020:4510628. doi: 10.1155/2020/4510628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banks WA. The blood-brain barrier interface in diabetes mellitus: dysfunctions, mechanisms and approaches to treatment. Curr Pharm Des. 2020;26:1438–47. doi: 10.2174/1381612826666200325110014. [DOI] [PubMed] [Google Scholar]
- 23.Eleftheriou CG, Ivanova E, Sagdullaev BT. Of neurons and pericytes: the neuro-vascular approach to diabetic retinopathy. Vis Neurosci. 2020;37:E005. doi: 10.1017/S0952523820000048. [DOI] [PubMed] [Google Scholar]
- 24.Roy S, Kim D. Retinal capillary basement membrane thickening: Role in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 2021;82:100903. [DOI] [PMC free article] [PubMed]
- 25.Haritoglou C, Maier M, Neubauer AS, Augustin AJ. Current concepts of pharmacotherapy of diabetic macular edema. Expert Opin Pharmacother. 2020;21:467–75. doi: 10.1080/14656566.2020.1713093. [DOI] [PubMed] [Google Scholar]
- 26.Gross JG, Glassman AR, Liu D, Sun JK, Antoszyk AN, Baker CW, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136:1138–48. doi: 10.1001/jamaophthalmol.2018.3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sivaprasad S, Prevost AT, Vasconcelos JC, Riddell A, Murphy C, Kelly J, et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet. 2017;389:2193–203. doi: 10.1016/S0140-6736(17)31193-5. [DOI] [PubMed] [Google Scholar]
- 28.Funatsu H, Noma H, Mimura T, Eguchi S, Hori S. Association of vitreous inflammatory factors with diabetic macular edema. Ophthalmology. 2009;116:73–79. doi: 10.1016/j.ophtha.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 29.Sfikakis PP, Markomichelakis N, Theodossiadis GP, Grigoropoulos V, Katsilambros N, Theodossiadis PG. Regression of sight-threatening macular edema in type 2 diabetes following treatment with the anti-tumor necrosis factor monoclonal antibody infliximab. Diabetes Care. 2005;28:445–7. doi: 10.2337/diacare.28.2.445. [DOI] [PubMed] [Google Scholar]
- 30.Sfikakis PP, Grigoropoulos V, Emfietzoglou I, Theodossiadis G, Tentolouris N, Delicha E, et al. Infliximab for diabetic macular edema refractory to laser photocoagulation: a randomized, double-blind, placebo-controlled, crossover, 32-week study. Diabetes Care. 2010;33:1523–8. doi: 10.2337/dc09-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Khan M, Aziz AA, Shafi NA, Abbas T, Khanani AM. Targeting angiopoietin in retinal vascular diseases: a literature review and summary of clinical trials involving faricimab. Cells. 2020;9:1869. doi: 10.3390/cells9081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saharinen P, Eklund L, Alitalo K. Therapeutic targeting of the angiopoietin-TIE pathway. Nat Rev Drug Discov. 2017;16:635–61. doi: 10.1038/nrd.2016.278. [DOI] [PubMed] [Google Scholar]
- 33.Abdulaal M, Haddad NM, Sun JK, Silva PS. The role of plasma kallikrein-kinin pathway in the development of diabetic retinopathy: pathophysiology and therapeutic approaches. Semin Ophthalmol. 2016;31:19–24. doi: 10.3109/08820538.2015.1114829. [DOI] [PubMed] [Google Scholar]
- 34.Simó R, Stitt AW, Gardner TW. Neurodegeneration in diabetic retinopathy: does it really matter? Diabetologia. 2018;61:1902–12. doi: 10.1007/s00125-018-4692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miller DJ, Cascio MA, Rosca MG. Diabetic retinopathy: the role of mitochondria in the neural retina and microvascular disease. Antioxidants. 2020;9:E905. doi: 10.3390/antiox9100905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gardner TW, Davila JR. The neurovascular unit and the pathophysiologic basis of diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2017;255:1–6. doi: 10.1007/s00417-016-3548-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cicinelli MV, Cavalleri M, Brambati M, Lattanzio R, Bandello F. New imaging systems in diabetic retinopathy. Acta Diabetol. 2019;56:981–94. doi: 10.1007/s00592-019-01373-y. [DOI] [PubMed] [Google Scholar]
- 38.Cole ED, Novais EA, Louzada RN, Waheed NK. Contemporary retinal imaging techniques in diabetic retinopathy: a review. Clin Exp Ophthalmol. 2016;44:289–99. doi: 10.1111/ceo.12711. [DOI] [PubMed] [Google Scholar]
- 39.Or C, Sabrosa AS, Sorour O, Arya M, Waheed N. Use of OCTA, FA, and ultra-widefield imaging in quantifying retinal ischemia: a review. Asia Pac J Ophthalmol. 2018;7:46–51. doi: 10.22608/APO.201812. [DOI] [PubMed] [Google Scholar]
- 40.Liu TYA, Arevalo JF. Wide-field imaging in proliferative diabetic retinopathy. Int J Retina Vitreous. 2019;5:20. doi: 10.1186/s40942-019-0170-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rabiolo A, Parravano M, Querques L, Cicinelli MV, Carnevali A, Sacconi R, et al. Ultra-wide-field fluorescein angiography in diabetic retinopathy: a narrative review. Clin Ophthalmol. 2017;11:803–7. doi: 10.2147/OPTH.S133637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tey KY, Teo K, Tan ACS, Devarajan K, Tan B, Tan J, et al. Optical coherence tomography angiography in diabetic retinopathy: a review of current applications. Eye Vis. 2019;6:37. doi: 10.1186/s40662-019-0160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran K, Pakzad-Vaezi K. Multimodal imaging of diabetic retinopathy. Curr Opin Ophthalmol. 2018;29:566–75. doi: 10.1097/ICU.0000000000000524. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Q, Rezaei KA, Saraf SS, Chu Z, Wang F, Wang RK. Ultra-wide optical coherence tomography angiography in diabetic retinopathy. Quant Imaging Med Surg 2018;8:743–53. [DOI] [PMC free article] [PubMed]
- 45.Pichi F, Smith SD, Abboud EB, Neri P, Woodstock E, Hay S, et al. Wide-field optical coherence tomography angiography for the detection of proliferative diabetic retinopathy. Graefes Arch Clin Exp Ophthalmol. 2020;258:1901–9. doi: 10.1007/s00417-020-04773-x. [DOI] [PubMed] [Google Scholar]
- 46.Nicholson L, Ramu J, Chan EW, Bainbridge JW, Hykin PG, Talks SJ, et al. Retinal nonperfusion characteristics on ultra-widefield angiography in eyes with severe nonproliferative diabetic retinopathy and proliferative diabetic retinopathy. JAMA Ophthalmol. 2019;137:626–31. doi: 10.1001/jamaophthalmol.2019.0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shibuya M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J Biochem Mol Biol. 2006;39:469–78. doi: 10.5483/bmbrep.2006.39.5.469. [DOI] [PubMed] [Google Scholar]
- 48.Tolentino MJ, Miller JW, Gragoudas ES, Jakobiec FA, Flynn E, Chatzistefanou K, et al. Intravitreous injections of vascular endothelial growth factor produce retinal ischemia and microangiopathy in an adult primate. Ophthalmology. 1996;103:1820–8. doi: 10.1016/s0161-6420(96)30420-x. [DOI] [PubMed] [Google Scholar]
- 49.Neubauer AS, Kook D, Haritoglou C, Priglinger SG, Kampik A, Ulbig M, et al. Bevacizumab and retinal ischemia. Ophthalmology. 2007;114:2096. doi: 10.1016/j.ophtha.2007.05.057. [DOI] [PubMed] [Google Scholar]
- 50.Kook D, Wolf A, Kreutzer T, Neubauer A, Strauss R, Ulbig M, et al. Long-term effect of intravitreal bevacizumab (avastin) in patients with chronic diffuse diabetic macular edema. Retina. 2008;28:1053–60. doi: 10.1097/IAE.0b013e318176de48. [DOI] [PubMed] [Google Scholar]
- 51.Michaelides M, Fraser-Bell S, Hamilton R, Kaines A, Egan C, Bunce C, et al. Macular perfusion determined by fundus fluorescein angiography at the 4-month time point in a prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (Bolt Study): report 1. Retina. 2010;30:781–6. doi: 10.1097/iae.0b013e3181d2f145. [DOI] [PubMed] [Google Scholar]
- 52.Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study) Am J Ophthalmol. 2014;157:960–70. doi: 10.1016/j.ajo.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 53.Douvali M, Chatziralli IP, Theodossiadis PG, Chatzistefanou KI, Giannakaki E, Rouvas AA. Effect of macular ischemia on intravitreal ranibizumab treatment for diabetic macular edema. Ophthalmologica. 2014;232:136–43. doi: 10.1159/000360909. [DOI] [PubMed] [Google Scholar]
- 54.Chandra S, Sheth J, Anantharaman G, Gopalakrishnan M. Ranibizumab-induced retinal reperfusion and regression of neovascularization in diabetic retinopathy: An angiographic illustration. Am J Ophthalmol Case Rep. 2018;9:41–44. doi: 10.1016/j.ajoc.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Busch C, Wakabayashi T, Sato T, Fukushima Y, Hara C, Shiraki N, et al. Retinal microvasculature and visual acuity after intravitreal aflibercept in diabetic macular edema: an optical coherence tomography angiography study. Sci Rep. 2019;9:1561. doi: 10.1038/s41598-018-38248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hsieh YT, Alam MN, Le D, Hsiao CC, Yang CH, Chao DL, et al. OCT angiography biomarkers for predicting visual outcomes after ranibizumab treatment for diabetic macular edema. Ophthalmol Retina. 2019;3:826–34. doi: 10.1016/j.oret.2019.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Couturier A, Rey PA, Erginay A, Lavia C, Bonnin S, Dupas B, et al. Widefield OCT-angiography and fluorescein angiography assessments of nonperfusion in diabetic retinopathy and edema treated with anti-vascular endothelial growth factor. Ophthalmology. 2019;126:1685–94. doi: 10.1016/j.ophtha.2019.06.022. [DOI] [PubMed] [Google Scholar]
- 58.Sugimoto M, Ichio A, Mochida D, Tenma Y, Miyata R, Matsubara H, et al. Multiple effects of intravitreal aflibercept on microvascular regression in eyes with diabetic macular edema. Ophthalmol Retina. 2019;3:1067–75. doi: 10.1016/j.oret.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 59.Pereira F, Godoy BR, Maia M, Regatieri CV. Microperimetry and OCT angiography evaluation of patients with ischemic diabetic macular edema treated with monthly intravitreal bevacizumab: a pilot study. Int J Retin Vitreous. 2019;5:24. doi: 10.1186/s40942-019-0176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueiredo N, Srivastava SK, Singh RP, Babiuch A, Sharma S, Rachitskaya A, et al. Longitudinal panretinal leakage and ischemic indices in retinal vascular disease after aflibercept therapy: The PERMEATE Study. Ophthalmol Retina. 2020;4:154–63. doi: 10.1016/j.oret.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Elnahry AG, Abdel-Kader AA, Raafat KA, Elrakhawy K. Evaluation of changes in macular perfusion detected by optical coherence tomography angiography following 3 intravitreal monthly bevacizumab injections for diabetic macular edema in the IMPACT Study. J Ophthalmol. 2020;2020:5814165. doi: 10.1155/2020/5814165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Statler B, Conti TF, Conti FF, Silva FQ, Rachitskaya A, Yuan A, et al. Twenty-four-month OCTA assessment in diabetic patients undergoing fixed-interval intravitreal aflibercept therapy. Ophthalmic Surg Lasers Imaging Retina. 2020;51:448–55. doi: 10.3928/23258160-20200804-05. [DOI] [PubMed] [Google Scholar]
- 63.Lee SJ, Shin IC, Jeong IW, Choi CW, Yang YS. Prospective, single-center, six-month study of intravitreal ranibizumab for macular edema with nonproliferative diabetic retinopathy: effects on microaneurysm turnover and non-perfused retinal area. Clin Ophthalmol. 2020;14:1609–18. doi: 10.2147/OPTH.S248529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee SJ, Koh HJ. Enlargement of the foveal avascular zone in diabetic retinopathy after adjunctive intravitreal bevacizumab (avastin) with pars plana vitrectomy. J Ocul Pharm Ther. 2009;25:173–4. doi: 10.1089/jop.2008.0092. [DOI] [PubMed] [Google Scholar]
- 65.Erol N, Gursoy H, Kimyon S, Topbas S, Colak E. Vision, retinal thickness, and foveal avascular zone size after intravitreal bevacizumab for diabetic macular edema. Adv Ther. 2012;29:359–69. doi: 10.1007/s12325-012-0009-9. [DOI] [PubMed] [Google Scholar]
- 66.Ghasemi Falavarjani K, Iafe NA, Hubschman JP, Tsui I, Sadda SR, Sarraf D. Optical coherence tomography angiography analysis of the foveal avascular zone and macular vessel density after anti-VEGF therapy in eyes with diabetic macular edema and retinal vein occlusion. Invest Ophthalmol Vis Sci. 2017;58:30–34. doi: 10.1167/iovs.16-20579. [DOI] [PubMed] [Google Scholar]
- 67.Moon BG, Um T, Lee J, Yoon YH. Correlation between deep capillary plexus perfusion and long-term photoreceptor recovery after diabetic macular edema treatment. Ophthalmol Retina. 2018;2:235–43. doi: 10.1016/j.oret.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 68.Babiuch AS, Conti TF, Conti FF, Silva FQ, Rachitskaya A, Yuan A, et al. Diabetic macular edema treated with intravitreal aflibercept injection after treatment with other anti-VEGF agents (SWAP-TWO study): 6-month interim analysis. Int J Retina Vitreous. 2019;5:17. doi: 10.1186/s40942-019-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Conti FF, Song W, Rodrigues EB, Singh RP. Changes in retinal and choriocapillaris density in diabetic patients receiving anti-vascular endothelial growth factor treatment using optical coherence tomography angiography. Int J Retina Vitreous. 2019;5:41. doi: 10.1186/s40942-019-0192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mirshahi R, Falavarjani KG, Molaei S, Habibi A, Anvari P, Khorasani MA, et al. Macular microvascular changes after intravitreal bevacizumab injection in diabetic macular edema. Can J Ophthalmol. 2021;56:57–65. doi: 10.1016/j.jcjo.2020.07.004. [DOI] [PubMed] [Google Scholar]
- 71.Goel N, Kumar V, Ghosh B. Ischemic maculopathy following intravitreal bevacizumab for refractory diabetic macular edema. Int Ophthalmol. 2011;31:39–42. doi: 10.1007/s10792-010-9390-z. [DOI] [PubMed] [Google Scholar]
- 72.Feucht N, Schönbach EM, Lanzl I, Kotliar K, Lohmann CP, Maier M. Changes in the foveal microstructure after intravitreal bevacizumab application in patients with retinal vascular disease. Clin Ophthalmol. 2013;7:173–8. doi: 10.2147/OPTH.S37544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Campochiaro PA, Wykoff CC, Shapiro H, Rubio RG, Ehrlich JS. Neutralization of vascular endothelial growth factor slows progression of retinal nonperfusion in patients with diabetic macular edema. Ophthalmology. 2014;121:1783–9. doi: 10.1016/j.ophtha.2014.03.021. [DOI] [PubMed] [Google Scholar]
- 74.Levin AM, Rusu I, Orlin A, Gupta MP, Coombs P, D’Amico DJ, et al. Retinal reperfusion in diabetic retinopathy following treatment with anti-VEGF intravitreal injections. Clin Ophthalmol. 2017;11:193–200. doi: 10.2147/OPTH.S118807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Karst SG, Deak GG, Gerendas BS, Waldstein SM, Lammer J, Simader C, et al. Association of changes in macular perfusion with ranibizumab treatment for diabetic macular edema: a subanalysis of the RESTORE (Extension) study. JAMA Ophthalmol. 2018;136:315–21. [DOI] [PMC free article] [PubMed]
- 76.Michalska-Małecka K, Heinke, Knudsen A. Optical coherence tomography angiography in patients with diabetic retinopathy treated with anti-VEGF intravitreal injections: case report. Medicine. 2017;96:e8379. doi: 10.1097/MD.0000000000008379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gupta MP, Kiss S, Chan RVP. Reversal of retinal vascular leakage and arrest of progressive retinal nonperfusion with monthly anti-vascular endothelial growth factor therapy for proliferative diabetic retinopathy. Retina. 2018;38:e74–e75. doi: 10.1097/IAE.0000000000002261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sorour OA, Sabrosa AS, Yasin Alibhai A, Arya M, Ishibazawa A, Witkin AJ, et al. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int Ophthalmol. 2019;39:2361–71. doi: 10.1007/s10792-019-01076-x. [DOI] [PubMed] [Google Scholar]
- 79.Dastiridou A, Karathanou K, Riga P, Anagnostopoulou S, Balasubramanian S, Mataftsi A, et al. OCT angiography study of the macula in patients with diabetic macular edema treated with intravitreal aflibercept. Ocul Immunol Inflamm. 2020 (in press). [DOI] [PubMed]
- 80.Barash A, Chui TYP, Garcia P, Rosen RB. Acute macular and peripapillary angiographic changes with intravitreal injections. Retina. 2020;40:648–56. doi: 10.1097/IAE.0000000000002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wykoff CC, Shah C, Dhoot D, Coleman HR, Thompson D, Du W, et al. Longitudinal retinal perfusion status in eyes with diabetic macular edema receiving intravitreal aflibercept or laser in VISTA study. Ophthalmology. 2019;126:1171–80. doi: 10.1016/j.ophtha.2019.03.040. [DOI] [PubMed] [Google Scholar]
- 82.Filek R, Hooper P, Sheidow TG, Gonder J, Chakrabarti S, Hutnik CM. Two-year analysis of changes in the optic nerve and retina following anti-VEGF treatments in diabetic macular edema patients. Clin Ophthalmol. 2019;13:1087–96. doi: 10.2147/OPTH.S199758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gill A, Cole ED, Novais EA, Louzada RN, de Carlo T, Duker JS, et al. Visualization of changes in the foveal avascular zone in both observed and treated diabetic macular edema using optical coherence tomography angiography. Int J Retina Vitreous. 2017;3:19. doi: 10.1186/s40942-017-0074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119:789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 85.Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120:2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 86.Wykoff CC, Eichenbaum DA, Roth DB, Hill L, Fung AE, Haskova Z. Ranibizumab induces regression of diabetic retinopathy in most patients at high risk of progression to proliferative diabetic retinopathy. Ophthalmol Retina. 2018;2:997–1009. doi: 10.1016/j.oret.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 87.Takahashi K, Kishi S, Muraoka K, Shimizu K. Reperfusion of occluded capillary beds in diabetic retinopathy. Am J Ophthalmol. 1998;126:791–7. doi: 10.1016/s0002-9394(98)00242-6. [DOI] [PubMed] [Google Scholar]
- 88.Chatziralli I, Dimitriou E, Theodossiadis G, Kazantzis D, Theodossiadis P. Intravitreal ranibizumab alone or in combination with panretinal photocoagulation for the treatment of proliferative diabetic retinopathy with coexistent macular edema: long-term outcomes of a prospective study. Acta Diabetol. 2020;57:1219–25. doi: 10.1007/s00592-020-01548-y. [DOI] [PubMed] [Google Scholar]