Abstract
Purpose
To report the case of a young woman diagnosed with Turner syndrome (TS) who achieved a live birth using her own oocytes that had been vitrified for fertility preservation.
Methods
A 25-year-old woman with mosaic (45,X/46,XX) TS was referred for fertility preservation (FP) counseling. Serum anti-Müllerian hormone (AMH) level was normal (6.4 µg/L). In view of the unpredictable rate of follicle loss in TS individuals, she requested FP and underwent two cycles of ovarian stimulation (OS) for oocyte cryopreservation (OoC) using a GnRH antagonist protocol and recombinant follicle stimulating hormone (rFSH), 200–250 IU daily for 8 resp. 12 days.
Results
In total, 29 metaphase II oocytes (MII) were vitrified after OS. After conceiving spontaneously and achieving a live birth, she returned to the clinic five years after OoC with a desire for pregnancy using in vitro fertilization (IVF) of her cryopreserved oocytes and preimplantation genetic testing (PGT-A). All 29 MII oocytes were thawed; 23 oocytes survived (79.3%) and were inseminated with partner sperm using intracytoplasmic sperm injection (ICSI). Thirteen oocytes were fertilized resulting in three good quality blastocysts which were vitrified after trophectoderm biopsy for PGT-A using array-CGH. Two blastocysts were found to be euploid. One was thawed and transferred to the uterus using a HRT priming protocol. An uneventful pregnancy occurred. The patient delivered a healthy baby girl weighing 3490 g at 40 weeks of gestation.
Conclusions
We report the first live birth achieved using cryopreserved oocytes in a woman diagnosed with mosaic TS. Cryopreservation of oocytes after ovarian stimulation is a realistic option for FP in selected post menarche individuals with mosaic TS. Whether PGT-A may reduce the risk of pregnancy loss in TS has to be confirmed by further studies.
Keywords: Turner syndrome, Fertility preservation, Oocyte vitrification, PGT-A
Introduction
Turner syndrome (TS) is one of the most frequent sex chromosome disorders with an estimated prevalence of 1 in 2,500 live births and is the most diagnosed chromosomal aberration in female conceptuses [1, 2]. The clinical manifestations vary and may include short stature, webbed neck, unusual facies, broad chest with widely spaced nipples, cardiovascular and renal abnormalities, and autoimmune disorders such as primary hypothyroidism [3, 4]. The syndrome is characterized by a complete or partial loss of one X chromosome, and approximately half of the TS individuals have a mosaic complement, most commonly 45X/46XX [3]. The diagnosis of TS can be incidental, following a non-invasive prenatal test (NIPT) or an amniocentesis, or made postnatally. Most women with TS are infertile due to an accelerated rate of germ cell loss. Although the ovarian reserve in 90% of women with TS will be depleted before adulthood [5], fecundity in women with Turner syndrome mosaicism or a structural X chromosomal aberration is often less severely impacted [6]. Because of the high risk of premature ovarian failure (POF), timely counseling about reproductive options should allow young individuals with TS and their parents to make informed decisions regarding fertility preservation (FP) and future pregnancy [2]. Nevertheless, women with TS should only pursue pregnancy after evaluation of their cardiovascular risk [7]. Indeed, women with TS, particularly those with a bicuspid aortic valve, coarctation of the aorta, and/or hypertension, have an increased risk of aortic dissection or rupture during pregnancy or in the immediate postpartum period [8, 9], and the risk of death may be as high as two percent [6]. Moreover, women with TS, including those with mosaic karyotypes, have a higher risk of pregnancy loss and obstetric complications, irrespective of whether the pregnancy is achieved with the woman’s own or with donated oocytes [10]. In view of this, clinical guidelines have been developed to optimize care for women with TS who desire pregnancy [11].
To enhance the probability of own genetic offspring, cryopreservation of oocytes [12] or ovarian tissue [13] has been proposed, although pregnancies after FP have not been reported in women with TS. In view of the paucity of data on follicle health in women with TS, there is no consensus of the optimal timing of ovarian stimulation (OS) for oocyte vitrification, although it has been recommended that OS in young girls with TS should be postponed until AMH levels start to decline [14]. We here report the first live birth after FP using vitrification of oocytes in a young woman with mosaic TS.
Methods
Patient history
The patient was a 24-year-old woman who had been diagnosed with TS mosaicism at the age of 11 years. Turner syndrome had been suspected due to growth retardation during childhood and was confirmed by cytogenetic analysis, with fluorescence in situ hybridization showing a 45,X[14]/46,XX[86] karyotype in lymphocytes and a 45,X[36]/46,XX[64] karyotype in cells from a buccal swab. The patient had shown spontaneous pubertal development and menarche at the age of 13 with regular menstrual cycles of 28 days. No structural cardiac or renal anomalies had been detected, except for recurrent pyelonephritis. Because of her short stature, growth hormone had been administered from the age of 11 until 15 years. At the age of 24 years, the patient was referred to our center to discuss future reproductive options. She did not have a partner at that time. She was referred to a cardiologist for pre-pregnancy work-up. Peripheral blood pressure was normal (124/82 mmHg on the right arm and 131/87 mmHg on the left arm). Aortic diameters were assessed using magnetic resonance angiography (MRA) and were normal, with an aortic index of 18.2 mm/m2. The patient provided her written informed consent for this case report.
Biophysical profile, hormone analysis, and pelvic imaging
The patient’s height, weight, and body mass index (BMI) were 160 cm, 70 kg, and 27.3 kg/m2. The baseline ovarian hormonal profile was as follows: anti-Müllerian hormone (AMH) 6.4 µg/L (reference range 1.7–8.2 µg/L, AMH Gen II ELISA kit, Beckman Coulter, Brea, CA), FSH 3.4 IU/L, LH 3.4 IU/L, and estradiol 33 ng/L (Cobas 6000®, Roche, Basel, Switzerland). A pelvic ultrasound scan showed a normal sized anteverted uterus and ovaries with an antral follicle count of 37.
Ovarian stimulation, oocyte retrieval, and vitrification
The potential future reproductive options and strategies were discussed with the patient, including the option of a conservative approach with follow-up of ovarian reserve markers and, alternatively, the option of fertility preservation using OoC. In view of the favorable functional ovarian reserve parameters, suggesting normal ovarian response after ovarian stimulation, the patient preferred to embark on a procedure for OoC rather than adopting an approach of regular follow-up of serum AMH levels. Ovarian stimulation was performed with recombinant follicle-stimulating hormone (rFSH, Puregon®, 200 IU daily, Organon). A GnRH antagonist ganirelix acetate (Orgalutran®, injection 250 µg, Organon) was added daily from the sixth day of stimulation. Oocyte maturation was triggered with GnRH agonist triptorelin (Gonapeptyl®, injection 0.2 mg, Ferring) when 3 follicles had a mean diameter of ≥ 20 mm. The patient underwent transvaginal oocyte retrieval under local anesthesia 36 h later. Denuded mature oocytes were vitrified within 2 h after oocyte retrieval with the High Security Vitrification® device (CryoBiosystems, France) using the Irvine Scientific Vitrification Freeze Kit (Irvine Scientific, USA) following manufacturer’s protocol with minor modifications [15]. A second ovarian stimulation cycle was performed 2 months later, on the patient’s request, to improve the total oocyte yield. This second round of ovarian stimulation was performed with recombinant follicle-stimulating hormone (rFSH, Puregon®, 250 IU daily, Organon) in a GnRH antagonist protocol with GnRH agonist ovulation trigger.
Oocyte warming was performed using the Irvine Scientific Vitrification Freeze Kit [15], and intact oocytes were inseminated within 2 h using intracytoplasmic sperm injection (ICSI). Embryos were cultured in individual 25 µl droplets of sequential media formulations (Fert™, Cleav™, Blast ™ medium, Origio) and scored according to the grading system developed by Gardner and Schoolcraft [16]. Artificially hatched blastocysts of sufficient quality were subjected to trophectoderm biopsy for PGT-A using array-CGH (aCGH; Bluegnome 24 Sure°) on days 5 or 6 of development [17] and subsequently vitrified [18].
Results
The ovarian stimulation cycle parameters are detailed in Table 1. In total, 29 metaphase II oocytes (MII) were vitrified after two rounds of ovarian stimulation.
Table 1.
Cycle characteristics
| Cycle 1 | Cycle 2 | |
|---|---|---|
| Protocol | Antagonist | Antagonist |
| Total gonadotropin stimulation dose | 2400 IU rFSH | 2000 IU rFSH |
| Duration of ovarian stimulation (days) | 12 | 8 |
| Peak E2 levels on the day of trigger (ng/L) | 1455 | 2065 |
| Total number of cumulus-oocyte complexes retrieved | 11 | 27 |
| Number of mature oocytes vitrified | 8 | 21 |
Five years later, at the age of 29 years, the patient returned to our clinic with her partner. She had been trying to conceive for more than 12 months. She had regular menstrual cycles of 28 days. Hormone analysis did not show any endocrine disturbance. The baseline hormonal ovarian profile on cycle day 3 was as follows: FSH 6.6 IU/L, LH 5.6 IU/L, estradiol 78 ng/L, and progesterone 1.09 µg/L. Analysis of the serum AMH level showed a considerable decline to 2.86 µg/L (i.e., minus 56% in 4 years). The partner was in good health. Sperm analysis was normal with a concentration of 2.5 106/ml and motility of 92% (A + B + C) after capacitation.
In view of the increased incidence of early pregnancy loss and chromosomal abnormalities in offspring of women with TS using autologous oocytes, the patient was advised to consider intracytoplasmic sperm injection (ICSI) with pre-implantation genetic testing for aneuploidy (PGT-A). To avoid the side effects of ovarian stimulation, the patient decided to utilize her cryopreserved oocytes instead of collecting fresh oocytes for IVF/ICSI and PGT-A.
All 29 MII oocytes were warmed; 23 oocytes survived (79.3%) and were inseminated using ICSI. Thirteen oocytes were fertilized normally resulting in 11 good quality cleavage stage embryos (quality A + B). Three good quality blastocysts developed and were vitrified after trophectoderm biopsy for PGT-A. Two blastocysts were found euploid; the third one was not transferable because of a segmental 19-Mb deletion (del11q23.3-11qter). One embryo was warmed and transferred into the uterus using a HRT priming protocol [19], but no pregnancy ensued. A few weeks after this first transfer, the patient became pregnant spontaneously. A chorionic villus sampling (CVS) was performed and showed a normal male fetal karyotype. Thoracic echocardiography was performed in each trimester of pregnancy as well as post-partum and was reassuring. The patient delivered a healthy baby boy at 38 weeks of gestation.
One year later, the patient returned to the clinic because of a renewed desire for children. The second euploid embryo was thawed and transferred to the uterus using a HRT priming protocol. An uneventful pregnancy occurred. The patient delivered a healthy baby girl weighing 3490 g at 40 weeks of gestation.
Discussion
We here report the first live birth after oocyte cryopreservation (OoC) in a woman with TS. Although the pregnancy was achieved in a woman with mosaic TS who had previously conceived spontaneously and who had not developed ovarian insufficiency, this case can be considered a proof-of-principle and illustrates that OoC to anticipate ovarian insufficiency is a feasible option in post-menarche individuals with mosaic TS.
On average, 15 mature oocytes per ovarian stimulation cycle were obtained for cryopreservation in the case reported here. This favorable ovarian response is comparable to a mean number of 9.2 oocytes per ovarian stimulation cycle reported in women with TS by Vergier et al. [12] and 13 oocytes for cryopreservation after one cycle in a TS patient reported by Kavoussi et al. [20]. The oocyte survival rate after warming for our patient was almost 80%. Reported survival rates after oocyte warming range between 94% in oocyte donation cycles [15] and 82% in cycles for elective FP [21]. It has been shown that, at least in young patients (≤ 36 years), the overall health status of the patient may be reflected by the oocytes’ cryopreservation efficiency, with oocytes from patients cryopreserving their oocytes for non-medical reasons showing a significantly better survival rate compared to those of their age-matched peers with an oncologic diagnosis (91% vs. 81% respectively) [21]. Specific oocyte vitrification/warming results in women with TS have not yet been reported, but our data support the feasibility of OoC in TS patients.
The fertilization rate after ICSI was only 57% despite a normal sperm sample after capacitation, which could have contributed to the relatively low euploid embryo utilization rate of 7% per vitrified oocyte. A useable cleavage stage embryo rate per vitrified oocyte of as high as 47% was obtained in oocyte donor cycles [15] and 22–29% after (elective) FP [21], although these data are not comparable with those of our case, because blastulation rates or genetic constitution were not considered in these manuscripts. If live birth per vitrified oocyte is considered a more objective parameter, our TS patient showed a 3% success rate, where 3–4% live birth per vitrified oocyte was obtained in a large population of (elective) FP patients [21] and 2% ongoing pregnancy rate per fresh oocyte after PGT-A in a large series of mosaic TS patients undergoing ovarian stimulation cycles [22]. These data support the realistic FP potential of oocytes after ovarian stimulation in women with TS.
In women with TS who have structural aberrations involving the X chromosome and in mosaic cases, ovarian reserve may persist for a variable period post menarche [23]. However, there is no reliable genotype–phenotype correlation [24, 25]; no study has thus far reported a link between the level of TS mosaicism and biomarkers of ovarian reserve (AMH and AFC), and accelerated follicle depletion is still likely to occur in women with TS mosaicism. Fertility preservation in young individuals with TS is considered controversial for several reasons. First, in subset of women with TS, pregnancy may be contraindicated because of an increased cardiovascular risk. Secondly, there is a paucity of data supporting the efficiency of the approach in terms of reproductive outcomes, as no live births have so far been reported following utilization of cryopreserved ovarian tissue or cryopreserved autologous oocytes in TS individuals. Nevertheless, it has been recommended to consider and discuss FP from an early age onwards post menarche, depending on the psychosocial maturity of the adolescent with TS [14, 26]. Indeed, ovarian stimulation followed by oocyte retrieval and cryopreservation of mature oocytes has not only been reported in adults with TS [14], but also in girls with mosaic TS as young as 13 years [27, 28]. Serial assessment of serum AMH levels has been advocated to determine the optimal time of intervention, which is when a plateau of serum AMH is reached and before AMH starts to decline [28]. The frequency of AMH monitoring may vary from every 2 to 3 months [14] to a more conservative approach with longer intervals of 2 to 3 years [29]. Although there is no consensus regarding the optimal frequency of follow-up of ovarian reserve markers in girls and women with TS mosaicism, we advised our patient not to postpone ovarian stimulation for OoC; the above-average AMH serum levels were considered to predict favorable ovarian response, which would more likely yield a high enough number of oocytes to result in euploid blastocysts after PGT-A. In a series of 256 embryos tested for aneuploidy in mosaic TS women, 28.9% of blastocysts tested were euploid, 9.4% mosaic, and 61.3% were aneuploid [22]. Nevertheless, in a recent cytogenetic analysis of small follicles in TS patients, most oocytes within primordial/primary follicles of mosaic TS patients had a normal karyotype, and aneuploidy was largely confined to the granulosa cells of follicles [2]. Nevertheless, there is currently little knowledge about the exact role of PGT-A as a tool to reduce the incidence of pregnancy loss and to increase the chances of motherhood using autologous oocytes in women diagnosed with TS [22]. Indeed, the risk of pregnancy loss in women with TS who use donor oocytes is still increased compared to the general oocyte donor population [30], which seems to point towards other factors such as prolonged hypoestrogenism or intrinsic hypoplasia of the uterus.
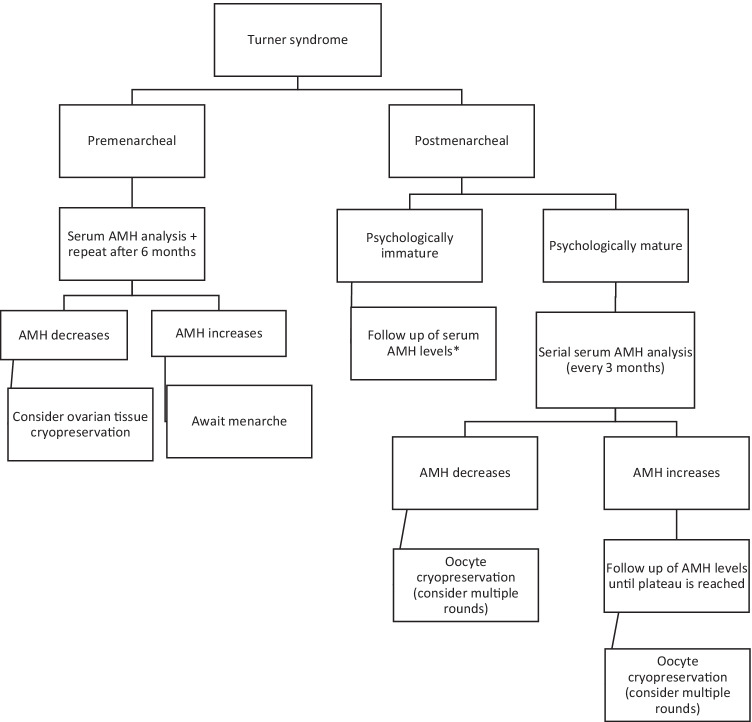
Spontaneous pregnancies occur in 2–5% of women with TS, mostly in women with mosaic karyotypes [31, 32]. The patient reported here also had a live birth after spontaneous conception and an uneventful pregnancy before she had a pregnancy using her vitrified oocytes. The use of autologous cryopreserved oocytes in a woman with TS and premature ovarian failure would have illustrated more convincingly the importance of oocyte cryopreservation for FP in women with TS. Our patient may be considered someone at the more favorable end of the reproductive spectrum of women with TS. Therefore, one could argue that oocyte cryopreservation in this patient with mosaic TS may be categorized as an intervention for planned OoC rather than medical fertility preservation. On a similar note, OoC has also been offered to women at risk of premature ovarian failure (POF) for other reasons, e.g., because of a family history of POF or because of severe endometriosis; these women often do not show any signs of incipient follicle depletion at the time of FP, which illustrates the thin line between medical FP and planned (elective) FP. Nevertheless, after proper counselling of the pros and cons of a conservative versus a more proactive approach of FP using OoC, including the potential beneficial impact on the psychosocial well-being of the individual, we would advocate to discuss the option of OoC to all young women with TS mosaicism because of the increased risk of accelerated follicle loss. Whether OoC should be considered in TS individuals in whom pregnancy is contra-indicated because of an unacceptably high cardiovascular risk and who may consider gestational surrogacy in the future is another matter of debate. In line with previous recommendations by others [11], we recommend OoC in TS individuals at a young age, including in those with favorable ovarian reserve markers. At best, OoC should be performed at a time when AMH levels have reached a plateau and before they start to decline, to ensure optimal ovarian response and maximize the number of available oocytes for the patient, especially when a PGT-A procedure is considered upon utilization of the oocytes (Fig. 1).
Fig. 1.
A proposed algorithmic approach to decision-making for fertility preservation in individuals with Turner Syndrome. For post menarcheal girls and adolescents, serial AMH analysis has been recommended, to identify a plateau phase at which optimal ovarian response can be expected, and to anticipate an incipient drop of serum AMH levels. *If serum AMH levels reach a plateau or start to decline before the adolescent with TS is psychologically mature to accept and undergo oocyte cryopreservation, then consider ovarian tissue cryopreservation
Conclusion
We here illustrate that oocyte cryopreservation is a viable option for young individuals with TS mosaicism who have experienced menarche and are psychologically mature enough to undertake the procedures involved. We recommend that all post menarche girls with TS be evaluated for ovarian reserve assessment and patients and families receive multidisciplinary counselling regarding oocyte cryopreservation as a realistic option for fertility preservation.
Declarations
Conflict of interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Eroğlu Filibeli B, Havare N, Erbak Yılmaz H, Yıldırım JG, Çatlı G, Dündar BN. Evaluation of Turner syndrome knowledge among physicians and parents. J Clin Res Pediatr Endocrinol. 2020;12:95–103. doi: 10.4274/jcrpe.galenos.2019.2019.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peek R, Schleedoorn M, Smeets D, van de Zande G, Groenman F, Braat D, et al. Ovarian follicles of young patients with Turner’s syndrome contain normal oocytes but monosomic, 45 X granulosa cells. Hum Reprod. 2019;34:1686–1696. doi: 10.1093/humrep/dez135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gravholt CH, Viuff MH, Brun S, Stochholm K, Andersen NH. Turner syndrome: mechanisms and management. Nat Rev Endocrinol. 2019;15:601–614. doi: 10.1038/s41574-019-0224-4. [DOI] [PubMed] [Google Scholar]
- 4.Schleedoorn MJ, van der Velden AAEM, Braat DDM, Peek R, Fleischer K. To freeze or not to freeze? An update on fertility preservation in females with Turner syndrome. Pediatr Endocrinol Rev. 2019;16:369–382. doi: 10.17458/per.vol16.2019.svb.tofreezeornot. [DOI] [PubMed] [Google Scholar]
- 5.Hreinsson JG, Otala M, Fridström M, Borgström B, Rasmussen C, Lundqvist M, et al. Follicles are found in the ovaries of adolescent girls with Turner’s syndrome. J Clin Endocrinol Metab. 2002;87:3618–3623. doi: 10.1210/jcem.87.8.8753. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs P, Dalton P, James R, et al. Turner syndrome: a cytogenetic and molecular study. Ann Hum Genet. 1997;61:471–548. doi: 10.1017/S0003480097006507. [DOI] [PubMed] [Google Scholar]
- 7.Silberbach M, Roos-Hesselink JW, Andersen NH, Braverman AC, Brown N, Collins RT, et al. Cardiovascular health in Turner syndrome: a scientific statement from the American Heart Association. Circ Genom Precis Med. 2018;11:e000048. doi: 10.1161/HCG.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 8.Karnis MF, Zimon AE, Lalwani SI, et al. Risk of death in pregnancy achieved through oocyte donation in patients with Turner syndrome: a national survey. Fertil Steril. 2003;80:498. doi: 10.1016/S0015-0282(03)00974-9. [DOI] [PubMed] [Google Scholar]
- 9.Grewal J, Valente AM, Egbe AC, et al. Cardiovascular outcomes of pregnancy in Turner syndrome. Heart. 2021;107:61–66. doi: 10.1136/heartjnl-2020-316719. [DOI] [PubMed] [Google Scholar]
- 10.Calanchini M, Aye CYL, Orchard E, Baker K, Child T, Fabbri A, et al. Fertility issues and pregnancy outcomes in Turner syndrome. Fertil Steril. 2020;114:144–154. doi: 10.1016/j.fertnstert.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin K, Sandberg DE, Sas TCJ, Silberbach M, Söderström-Anttila V, Stochholm K, van Alfen-van derVelden JA, Woelfle J, Backeljauw PF, International Turner syndrome consensus group Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. Eur J Endocrinol. 2017;177(3):G1–G70. doi: 10.1530/EJE-17-0430. [DOI] [PubMed] [Google Scholar]
- 12.Vergier J, Bottin P, Saias J, et al. Fertility preservation in Turner syndrome: karyotype does not predict ovarian response to stimulation. Clin Endocrinol (Oxf) 2019;91:646. doi: 10.1111/cen.14076. [DOI] [PubMed] [Google Scholar]
- 13.Mamsen LS, Charkiewicz K, Anderson RA, et al. Characterization of follicles in girls and young women with Turner syndrome who underwent ovarian tissue cryopreservation. Fertil Steril. 2019;111:1217. doi: 10.1016/j.fertnstert.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Oktay K, Bedoschi G, Berkowitz K, Bronson R, Kashani B, McGovern P, et al. Fertility preservation in women with Turner syndrome: a comprehensive review and practical guidelines. J Pediatr Adolesc Gynecol. 2016;29:409–416. doi: 10.1016/j.jpag.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Munck N, Santos-Ribeiro S, Stoop D, Van de Velde H, Verheyen G. Open versus closed oocyte vitrification in an oocyte donation programme: a prospective randomized sibling oocyte study. Hum Reprod. 2016;31:377–384. doi: 10.1093/humrep/dew029. [DOI] [PubMed] [Google Scholar]
- 16.Gardner DK, Schoolcraft WB. In-vitro culture of human blastocysts. In: Jansen R, Mortimer D, editors. Towards reproductive certainty: fertility and genetics beyond 1999. Carnforth: Parthenon Press; 1999. pp. 378–388. [Google Scholar]
- 17.De Vos A, Van Landuyt L, De Rycke M, Verdyck P, Verheyen G, Buysse A, et al. Multiple vitrification-warming and biopsy procedures on human embryos: clinical outcome and neonatal follow-up of children. Hum Reprod. 2020;35:2488–2496. doi: 10.1093/humrep/deaa236. [DOI] [PubMed] [Google Scholar]
- 18.Van Landuyt L, Polyzos NP, De Munck N, Blockeel C, Van de Velde H, Verheyen G. A prospective randomized controlled trial investigating the effect of artificial shrinkage (collapse) on the implantation potential of vitrified blastocysts. Hum Reprod. 2015;30:2509–2518. doi: 10.1093/humrep/dev218. [DOI] [PubMed] [Google Scholar]
- 19.Roelens C, Santos-Ribeiro S, Becu L, Mackens S, Van Landuyt L, Racca A, et al. Frozen-warmed blastocyst transfer after 6 or 7 days of progesterone administration: impact on live birth rate in hormone replacement therapy cycles. Fertil Steril. 2020;114:125–132. doi: 10.1016/j.fertnstert.2020.03.017. [DOI] [PubMed] [Google Scholar]
- 20.Kavoussi SK, Fisseha S, Smith YR, Smith GD, Christman GM, Gago LA. Oocyte cryopreservation in a woman with mosaic Turner syndrome: a case report. J Reprod Med. 2008;53:223–226. [PubMed] [Google Scholar]
- 21.Cobo A, García-Velasco J, Domingo J, Pellicer A, Remohí J. Elective and Onco-fertility preservation: factors related to IVF outcomes. Hum Reprod. 2018;1(33):2222–2231. doi: 10.1093/humrep/dey321. [DOI] [PubMed] [Google Scholar]
- 22.Giles J, Meseguer M, Mercader A, Rubio C, Alegre L, Vidal C, Trabalon M, Bosch E. Preimplantation genetic testing for aneuploidy in patients with partial X monosomy using their own oocytes: is this a suitable indication? Fertil Steril. 2020;114:346–353. doi: 10.1016/j.fertnstert.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Purushothaman R, Lazareva O, Oktay K, Ten S. Markers of ovarian reserve in young girls with Turner’s syndrome. Fertil Steril. 2010;94:1557–1559. doi: 10.1016/j.fertnstert.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 24.Miguel-Neto J, Carvalho AB, Marques-de-Faria AP, Guerra-Júnior G, Maciel-Guerra AT. New approach to phenotypic variability and karyotype-phenotype correlation in Turner syndrome. J Pediatr Endocrinol Metab. 2016;29:475–479. doi: 10.1515/jpem-2015-0346. [DOI] [PubMed] [Google Scholar]
- 25.Nadesapillai S, van der Velden J, Smeets D, van de Zande G, Braat D, Fleischer K, et al. Why are some patients with 45, X Turner syndrome fertile? A young girl with classical 45, X Turner syndrome and a cryptic mosaicism in the ovary. Fertil Steril. 2021;115:1280–1287. doi: 10.1016/j.fertnstert.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 26.Nadesapillai S, van der Velden J, Braat D, Peek R, Fleischer K. The challenge of defining predictive parameters for fertility preservation counseling in young females with Turner syndrome. Acta Obstet Gynecol Scand. 2021;100:1155–1156. doi: 10.1111/aogs.14094. [DOI] [PubMed] [Google Scholar]
- 27.Oktay K, Bedoschi G. Oocyte cryopreservation for fertility preservation in postpubertal female children at risk for premature ovarian failure due to accelerated follicle loss in Turner syndrome or cancer treatments. J Pediatr Adolesc Gynaecol. 2014;27:342–346. doi: 10.1016/j.jpag.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oktay K, Rodriguez-Wallberg KA, Sahin G. Fertility preservation by ovarian stimulation and oocyte cryopreservation in a 14-year-old adolescent with Turner syndrome mosaicism and impending premature ovarian failure. Fertil Steril. 2010;94(2):753.e715–753.e759. doi: 10.1016/j.fertnstert.2010.01.044. [DOI] [PubMed] [Google Scholar]
- 29.Nawroth F, Schüring AN, von Wolff M. The indication for fertility preservation in women with Turner syndrome should not only be based on the ovarian reserve but also on the genotype and expected future health status. Acta Obstet Gynecol Scand. 2020;99:1579–1583. doi: 10.1111/aogs.13984. [DOI] [PubMed] [Google Scholar]
- 30.Bryman I, Sylven L, Berntorp K, et al. Pregnancy rate and outcome in Swedish women with Turner syndrome. Fertil Steril. 2011;95:2507–2510. doi: 10.1016/j.fertnstert.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 31.Hadnott TN, Gould HN, Gharib AM, Bondy CA. Outcomes of spontaneous and assisted pregnancies in Turner syndrome: the U.S. National Institutes of Health experience. Fertil Steril. 2011;95:2251–2256. doi: 10.1016/j.fertnstert.2011.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lucaccioni L, Wong SC, Smyth A, et al. Turner syndrome–issues to consider for transition to adulthood. Br Med Bull. 2015;113:45–58. doi: 10.1093/bmb/ldu038. [DOI] [PubMed] [Google Scholar]