Abstract
Purpose
Chronic endometritis (CE) is diagnosed via endometrial biopsy and staining for plasma cells. A threshold plasma cell count that identifies CE and predicts pregnancy outcomes has not been established, and the prevalence of plasma cells in the general infertile population is unknown. The purpose of this study was to determine the prevalence of plasma cells in the general infertile population and whether a threshold exists which predicts live birth.
Methods
Endometrial samples were obtained prospectively from 80 women undergoing IVF, embedded in paraffin, and stained for plasma cells using mouse mono-clonal antibody for CD138. Slides were reviewed at 20× magnification and 10 random images captured. Three reviewers graded each image for plasma cells. Participants underwent single, euploid, and frozen blastocyst transfer.
Results
Forty-nine percent of samples had ≥1 plasma cell across 10 HPFs, 11% had ≥5 cells across 10 HPFs, and 4% had ≥10 cells across 10 HPFs. There was no difference in prevalence between those who did and did not achieve live birth. Using thresholds of 1, 5, and 10 plasma cells per 10 HPFs, there were no differences in implantation, clinical pregnancy, clinical pregnancy loss, or live birth rates between patients with and without CE.
Conclusion
Endometrial plasma cells are present in half the general infertile population and do not predict implantation, clinical pregnancy, clinical pregnancy loss, or live birth rates at low levels.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10815-021-02374-z.
Keywords: Plasma cells, Endometritis, Infertility, Pregnancy, Fertilization in vitro
Introduction
Implantation failure and early pregnancy loss after transfer of a euploid embryo pose ongoing challenges for reproductive specialists. Endometrial-embryonic asynchrony, maternal-fetal immune incompatibility via HLA-KIR mismatch, the maternal microbiome, and maternal chronic endometritis (CE) have all been suggested as possible mechanisms for poor outcomes [1–4]. CE is an inflammation of the endometrium characterized by stromal edema, increased stromal density, and influx of polymorphonuclear cells, which may alter endometrial receptivity [5]. Most commonly, CE is diagnosed via endometrial biopsy and histologic analysis for the presence of plasma cells [6]. Plasma cells may be identified with hematoxylin and eosin (H&E) staining or alternatively, by performing immunocytochemistry to detect Syndecan-1 (or CD138), a surface proteoglycan specific to plasma cells [7].
A consistent diagnostic threshold plasma cell count within the endometrium that can be used to identify CE has not yet been established. Multiple thresholds have been used in the literature, including one plasma cell per 10 high-power fields (HPFs), five plasma cells dispersed over 10 HPFs, and 10 plasma cells per 10 HPFs [8–11]. These thresholds have been selected arbitrarily without reference to any known population standard. The difficulty in selecting a threshold for clinical use is compounded by the fact that plasma cells are not evenly distributed; they may be concentrated in patches or diffusely spread throughout the stroma [6]. In addition, previous studies suggest that plasma cells may be present in the general population at baseline, thus limiting the clinical value of their detection [12]. The threshold of plasma cells which prognosticates altered clinical outcomes remains unknown [13].
A number of studies that have assessed the association of CE with impaired reproductive outcomes have demonstrated inferior pregnancy rates in women with CE compared to controls. These studies are limited by lack of endometrial biopsy in their control groups and are often limited to women who already have a history of poor reproductive outcomes. Less is known about the predictive value of a CE diagnosis in the general infertile population. The purpose of this study was therefore twofold: to characterize the prevalence of plasma cells on endometrial biopsy in an infertile population of patients undergoing IVF with single, euploid blastocyst transfer and to compare reproductive outcomes in women with undiagnosed, untreated chronic endometritis using multiple thresholds for plasma cell counts to those with biopsy confirmed normal endometrium.
Materials and methods
Study population and design
This was a prospective, multi-center, blinded, non-selection study conducted between August 2018 and December 2019 in which endometrial biopsy samples were obtained and analyzed for the presence of plasma cells. The goal of the larger study was to determine the value of PGT-A in predicting pregnancy outcomes after embryo transfer; thus, women with a good chance of obtaining an embryo for transfer (age 18-44 years undergoing their first IVF cycle) were included. The subset of women in the larger study who underwent single euploid frozen embryo transfer was included in the present analysis. Patients were excluded if they had a known history of endometrial insufficiency (defined as an endometrial lining of <6mm in the late follicular phase), recurrent pregnancy loss (defined as two or more euploid losses or three or more losses with unknown karyotype), recurrent implantation failure (defined as three or more unsuccessful euploid embryo transfers), or low markers of ovarian reserve (day 3 follicle stimulating hormone >12 mIU/mL, basal antral follicle count <8 follicles) [14].
IVF protocol
Patients underwent routine clinical and laboratory care with respect to their pre-treatment screening, controlled ovarian hyperstimulation cycle, handling of the gametes in the embryology lab, embryo transfer, and pregnancy monitoring. Controlled ovarian hyperstimulation cycles were managed by the patients’ physicians using either a GnRH antagonist, long GnRH agonist, or GnRH microflare protocol with subsequent human chorionic gonadotropin (hCG) and/or GnRH agonist trigger for final oocyte maturation. Vaginal oocyte retrieval (VOR) was conducted under ultrasound guidance, 36 h after administration of the trigger shot. All mature MII oocytes were inseminated via intracytoplasmic sperm injection (ICSI), underwent laser-assisted hatching, and were grown in sequential culture media until the expanded blastocyst stage. Trophectoderm (TE) biopsy occurred on day 5, 6, or 7 and embryos were subsequently vitrified. All patients underwent single embryo transfer in a subsequent frozen embryo transfer cycle using either a synthetic preparation with oral estrogen and intramuscular progesterone or a natural cycle protocol, with embryo selection based on morphology.
Tissue processing
Endometrial biopsy samples were obtained at the time of VOR and embedded in paraffin. Paraffin blocks were then cut in 5-μm sections and mounted on glass slides. Slides were stained using a mouse mono-clonal antibody for human CD138 (B-A38, Cell Marque) using a standard protocol. Briefly, slides were warmed at 65°C for 30 min to remove paraffin and rehydrated in a series of ethanol solutions of descending concentrations and phosphate-buffered saline solution (PBS). Slides were immersed in 3% hydrogen peroxidase and 70% ethanol solution to block endogenous peroxidase for 10 min and placed in citrate buffer for 20 min at 100°C for antigen retrieval. The tissue was blocked with goat serum to prevent nonspecific binding and then stained overnight at 4°C with a 1:25 dilution of mouse mono-clonal antibody against Syndecan-1 (CD-138). Slides were washed with PBS Tween, stained with 1:200 goat anti-mouse (Alexa Flour 488) antibody for 1 h, and again washed with PBS Tween. Propidium iodide (1:10) was added for 10 min to stain cell nuclei and the slides were washed a final time before coverslips were applied. A sample of human tonsil known to contain plasma cells was stained using the same protocol for a positive control, with an additional sample without primary antibody serving as a negative control.
Image acquisition and determination of plasma cell count
All slides were examined at 20× magnification, and 10 images of randomly selected high-power fields (HPF) were captured. Three independent observers reviewed the images for each slide and recorded number of plasma cells per HPF. Plasma cells were identified based on CD138 staining of the cell membrane and an eccentrically located nucleus. For each image, the average number of plasma cells of the three observers was used when there was a discrepancy among observers. Inter-observer variability of plasma cell count was determined for each slide.
Data analysis
Baseline patient characteristics and plasma cell counts were compared between patients who did and did not achieve live birth. Additionally, pregnancy outcomes including implantation rate, clinical pregnancy rate, clinical pregnancy loss rate, and live birth rate were compared in patients using three different commonly used thresholds for plasma cell count to be defined as CE: ≥ 1 plasma cell per 10 HPFs, ≥ 5 plasma cells in 10 HPFs, and ≥ 10 plasma cells in 10 HPFs (or 1 plasma cell per HPF). Previous literature suggests that the presence of CE decreases live birth rates by 50% [15]. Using a threshold of ≥1 plasma cell per 10 HPFs, we determined that a sample size of 40 women with CE and 40 without CE would be needed to detect a 50% difference in live birth rates with 80% power and a type I error probability of 0.05.
Implantation was defined as positive serum beta HCG after embryo transfer. Clinical pregnancy was defined as a gestational sac on ultrasound after a positive pregnancy test. Clinical pregnancy loss was defined as pregnancy loss within the first trimester. Live birth was defined as delivery of a liveborn infant.
Student’s t test and Wilcoxon rank-sum were used for normally and non-normally distributed continuous variables, respectively. Chi-squared test and Fisher’s exact test (if expected counts <5) were used for categorical variables. All statistical analyses were performed by using SAS (SAS 9.4, SAS Institute Inc, Cary, NC, USA). Statistical significance was defined by the two-sided test with a p value < 0.05.
Ethical approval
Institutional Review Board approval was obtained for the larger study, including endometrial biopsy collection (Protocol RMA-2018-01) and registered with clinicaltrials.gov (NCT03604107). Written informed consent for endometrial biopsy was obtained for all participants prior to enrollment.
Results
Baseline patient characteristics
A total of 80 patients who had endometrial biopsies performed prior to single, euploid embryo transfer were included. Baseline demographics including age, BMI, markers of ovarian reserve, blastocyst quality (“fair” (4-6BC, CB) and “good” (4-6AA, AB, BA, BB)), endometrial thickness at time of transfer, and plasma cell counts in patients with and without live birth are listed in Table 1. The mean patient age in each group was 33.9 ± 3.9 years and 34.7 ± 3.9 years, in the live birth and no live birth groups, respectively. There were no significant differences between groups with regard to BMI, AMH, Day 3 FSH, or indication for IVF (Table 1). Both groups transferred predominantly good quality blastocysts. The mean endometrial thickness at embryo transfer was 10.3 ± 3.0 mm and 9.8 ± 1.8 mm, in the live birth and no live birth groups, respectively, and did not significantly differ (Table 1). Baseline characteristics were additionally compared between patients with and without at least one plasma cell across 10 HPFs (Supplementary Table I). Patient characteristics were similar between groups; however, patients in the group with at least one plasma cell were more likely to have ovulatory dysfunction as an indication for IVF (p=0.01).
Table 1.
Comparison of patient age, markers of ovarian reserve, and embryo transfer cycle characteristics between patients with and without live birth
| Live birth (n= 39) |
No live birth (n= 41) |
p value | |
|---|---|---|---|
| Patient age years (mean, SD) | 33.9 (3.9) | 34.7 (3.9) | 0.37 |
| BMI (mean, SD) | 26.1 (4.6) | 25.9 (4.4) | 0.83 |
| Ovarian reserve (mean, SD) | |||
| AMH (ng/mL) | 4.3 (3.2) | 4.0 (2.4) | 0.95 |
| Day 3 FSH (mIU/mL) | 7.0 (1.9) | 7.3 (1.8) | 0.51 |
| Indication for IVF (%) | |||
| Male factor | 23% | 32% | 0.41 |
| Tubal factor | 6% | 20% | 0.05 |
| Ovulatory dysfunction | 28% | 20% | 0.38 |
| Unexplained | 28% | 17% | 0.24 |
| DOR | 15% | 12% | 0.83 |
| Blastocyst quality (%) | |||
| Fair | 8% | 7% | 0.95 |
| Good | 92% | 93% | 0.95 |
| Endometrial thickness at transfer, mm mean (SD) | 10.3 (3.0) | 9.8 (1.8) | 0.89 |
| No plasma cells identified (%) | 44% | 59% | 0.18 |
| One to nine plasma cells per 10 HPFs (%) | 51% | 39% | 0.27 |
| ≥10 plasma cell per 10 HPFs (%) | 5% | 2% | 0.61 |
CD138 positive cell distribution in endometrial sections
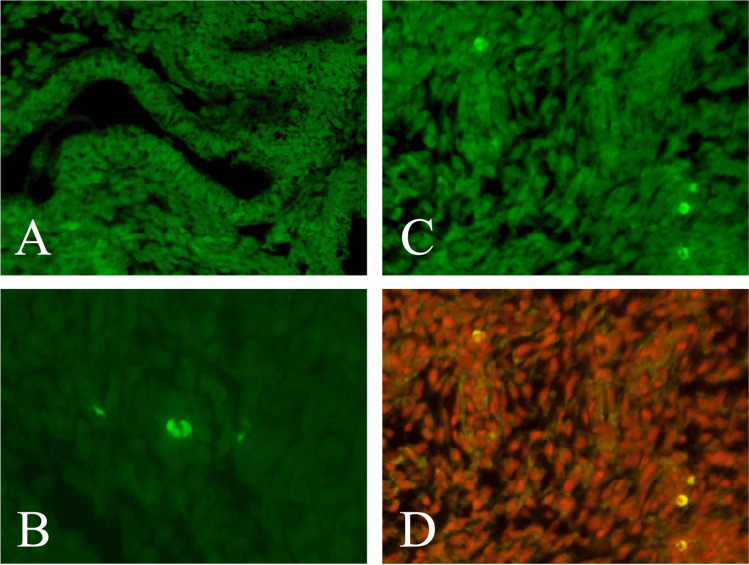
Figure 1 depicts four sections of tissue representing negative and positive controls as well as endometrium with plasma cells, with and without propidium iodide overlay. Of the 80 biopsies, 39 had at least one plasma cell present across 10 HPFs, while the other 41 had no plasma cells in their sample. Plasma cells were heterogeneously distributed in the endometrium, often as a single isolated cell across all 10 HPFs and other times occurring in clusters and in multiple HPFs. Of the 39 patients who had plasma cells present in the endometrium, 18 women had plasma cells present in only one HPF, eight women had plasma cells in two HPFs, three patients had plasma cells in three HPFs, four patients had plasma cells in four HPFs, four patients had plasma cells in five HPFs, one patient had plasma cells in six HPFs, and one patient had plasma cells present in eight HPFs (Figure 2). Inter-observer variability for plasma cell count on all HPFs examined was 0.706, which was considered substantial agreement.
Fig. 1.
Four sections of tissue: a negative control, b positive control, c endometrium staining positive for CD138, d endometrium staining positive for CD138 with propidium iodide (PI) overlay
Fig. 2.
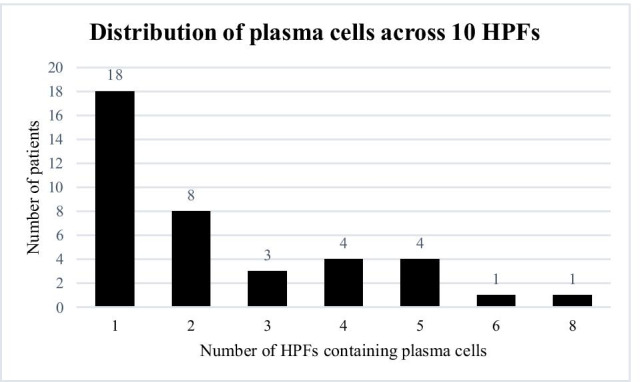
Distribution of plasma cells across 10 HPFs for each biopsy sample. The x axis represents the number of HPFs out of 10 which had at least one plasma cell and the y axis describes the number of patients in each category
CD138 positive cell presence in the live birth and no live birth groups
Comparing women with live birth to those without live birth, 44% of women with live birth had no plasma cells identified across 10 HPFs compared to 59% without live birth (Table 1), leaving approximately half of patients in each group with at least one plasma cell across 10 HPFs. Only two patients who achieved live birth and one who did not achieve live birth had 10 plasma cells per 10 HPF examined. There were no significant differences between groups with regard to prevalence of plasma cells.
The impact of CE diagnosis on cycle outcome
Cycle outcomes for each of three commonly used thresholds for CE are presented in Table 2. Forty-one patients had no plasma cells identified in any of the sections compared to 39 patients with at least 1 plasma cell in 10 HPFs. Using this threshold, 49% of patients were classified as having CE. There were no significant differences in implantation rate, clinical pregnancy rate, clinical pregnancy loss rate, and live birth rate between those classified as having CE and those who were not using this threshold. The predictive value of a CE diagnosis resulting in no live birth was 44% (n=17/39).
Table 2.
Cycle outcomes based on three different thresholds of plasma cell count for diagnosis of chronic endometritis
| Plasma cell count across 10 HPFs | |||
|---|---|---|---|
|
None n=41 (51%) |
≥1 n=39 (49%) |
p value | |
| Implantation rate | 29 (71%) | 32 (82%) | 0.23 |
| Clinical pregnancy rate | 24 (59%) | 28 (72%) | 0.21 |
| Clinical pregnancy loss rate | 7 (29%) | 6 (21%) | 0.56 |
| Live birth rate | 17 (42%) | 22 (56%) | 0.18 |
| Plasma cell count across 10 HPFs | |||
|
<5 n=71 (89%) |
≥5 n=9 (11%) |
p value | |
| Implantation rate | 53 (75%) | 8 (89%) | 0.34 |
| Clinical pregnancy rate | 46 (65%) | 6 (67%) | 0.91 |
| Clinical pregnancy loss rate | 10 (22%) | 3 (50%) | 0.13 |
| Live birth rate | 36 (51%) | 3 (33%) | 0.33 |
| Plasma cell count across 10 HPFs | |||
|
<10 n=77 (96%) |
≥10 n=3 (4%) |
p value | |
| Implantation rate | 58 (75%) | 3 (100%) | 0.33 |
| Clinical pregnancy rate | 49 (64%) | 3 (100%) | 0.19 |
| Clinical pregnancy loss rate | 12 (24%) | 1 (33%) | 0.93 |
| Live birth rate | 37 (48%) | 2 (67%) | 0.58 |
When examining a threshold of five plasma cells in 10 HPFs, nine of 80 patients (11%) of patients were classified as having CE. Using a threshold of 10 plasma cells per 10 HPFs, only three of 80 patients (4%) were classified as having CE. There were no significant differences between groups for any pregnancy outcome using these thresholds, although at these sample sizes, the study was underpowered to detect differences.
Discussion
We analyzed endometrial biopsies from 80 women undergoing IVF with euploid, single blastocyst transfer and determined that 49% had at least one plasma cell present in their endometrial biopsy sample, while only 11% had biopsies showing five plasma cells per 10 HPFs examined, and 4% had biopsies showing one plasma cell per HPF examined. If the most conservative threshold is used, our results suggest that almost half of women in the general infertile population carry a diagnosis of CE, whereas if the most rigorous threshold is used, CE is only found in a small percentage of patients. At all three thresholds, a diagnosis of CE did not carry a higher risk of implantation failure or clinical pregnancy loss.
The reported prevalence of CE in the infertile population varies tremendously in the literature. Kasius et al. examined endometrial biopsy samples from screening hysteroscopy of 606 patients prior to IVF and determined that 2.8% of patients displayed evidence of CE, or the presence of at least one plasma cell, based on traditional H&E staining and immunohistochemistry for the plasma cell marker CD138 [16]. This report differs substantially from that of Cicinelli et al., who used hysteroscopic evidence of hyperemia, mucosal edema, and micropolyps to diagnose chronic endometritis in a population of 2190 women referred for diagnostic hysteroscopy [17]. They diagnosed 438 (20%) women undergoing hysteroscopy with chronic endometritis and confirmed the diagnosis with both histologic review (88%) and evidence of a microorganism present (73%).
The discrepancy in the reported prevalence of chronic endometritis likely stems from both the method of diagnosis and the populations tested. The use of plasma cells to diagnose chronic endometritis originates from Hitschman and Adler in 1907, as plasma cells were found in endometrium of patients with inflammatory adnexal disease and postpartum infection and absent in normal endometrium [18]. More recently, the identification of Syndecan-1, a transmembrane protein on the plasma cell surface, has improved our ability to detect plasma cells in endometrial tissue [7]. Hysteroscopy and visualization of abundant polypoid tissue and hyperemia may be a more accurate diagnostic method but is time intensive and invasive. Thus, endometrial biopsy and histopathologic examination for plasma cells remains one of the most widely used clinical methods for diagnosis.
The concern with using plasma cells to diagnose CE is that they may be found in seemingly normal endometrium. Dumoulin et al. demonstrated that plasma cells are present in the endometrium of healthy postpartum women [19]. Similarly, McQueen et al. recently demonstrated that 31% of healthy controls have at least one plasma cell across 10 HPFs [12]. Our data suggests that half of women undergoing infertility treatment have plasma cells in their endometrium and that their presence at low levels does not predict pregnancy outcomes. Accordingly, we argue that a threshold of one plasma cell in 10 HPFs should not be used to diagnose CE. We did observe lower live birth rates in the women with ≥5 plasma cells in 10 HPFs, although the difference was not statistically significant. A larger sample size of women with ≥5 plasma cells may demonstrate that a threshold of plasma cells exists which alters pregnancy outcomes.
The second concern with the previous literature is the heterogeneity in populations tested. CE is thought to play a role in abnormal uterine bleeding, pelvic pain, as well as infertility. Sampling a broad spectrum of patients in the general population, as done by Cicinelli et al., may capture more patients who have evidence of CE but may not be relevant with regard to ability to achieve implantation and live birth. On the other end of the spectrum, studies of women with recurrent implantation failure and recurrent pregnancy loss demonstrate CE rates as high as 66% [20, 21]. Our study evaluated endometrial biopsy and assessment of plasma cells in the general infertile population, the most clinically relevant population for us, and one in which this modality is often used despite its limited prognostic value.
Finally, much of the previous literature suggests that for women with CE, treatment with antibiotics improves pregnancy outcomes. The concern with this conclusion is that if CE is detected in the course of clinical care, treatment is provided. No studies in the general infertile population compare those with CE who were untreated to those without to determine the predictive value of a CE diagnosis on pregnancy outcome. The studies that exist are also confounded by the fact that they contain patients transferring embryos of unknown ploidy status, limiting their ability to conclude that implantation failure is due to endometritis and not aneuploidy. Our study is strengthened by the fact that we analyzed our endometrial biopsy samples after embryo transfer, and no patients ever received treatment. Therefore, we were able to compare those with a diagnosis of CE who were untreated to those without and determined that there were no differences in pregnancy outcomes. Furthermore, all patients underwent single, euploid embryo transfer, eliminating the potential for ploidy status to affect their clinical outcome. At our institution, all embryo transfers are done in a subsequent cycle to the controlled ovarian hyperstimulation (COH) cycle. A weakness of our study is that endometrial biopsies were performed during the COH cycle and not during the transfer cycle. We cannot guarantee that the plasma cell count remains constant despite the varying physiologic milieus of proliferative and secretory endometrium. However, performing endometrial biopsy in the same cycle in which the embryo is transferred would likely disrupt the endometrium and potentially impact pregnancy outcomes. Another limitation of our study is that we only evaluated plasma cells on endometrial biopsy samples. We chose this marker because it is frequently used to diagnose CE both in our practice and in the existing literature. However, evaluating both plasma cells and other markers of inflammation, such as stromal changes, may have altered our results. Finally, our study was designed to evaluate the endometrial milieu of the general infertile population. Similar analysis of a poor prognosis subset of the population, including those with a history of recurrent pregnancy loss or recurrent implantation failure, may yield different results.
In summary, we determined that half of women undergoing infertility treatment have plasma cells present in their endometrium and that their presence at low levels does not prognosticate pregnancy outcomes. Future studies to determine the threshold of plasma cells which alters clinical outcomes are needed.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
All authors listed on this manuscript contributed meaningfully to the conception of the study, data acquisition, and writing of the final manuscript. All authors approved of the final manuscript prior to submission.
Data availability
The data underlying this article cannot be shared publicly in order to protect the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Code availability
Not applicable.
Declarations
Ethics approval
Institutional Review Board approval was obtained for the study from Western IRB (Protocol RMA-2018-01).
Consent to participate
Informed consent was obtained from all individuals participants included in the study.
Consent to publish
Patients signed informed consent regarding publishing their data.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alecsandru D, et al. Maternal KIR haplotype influences live birth rate after double embryo transfer in IVF cycles in patients with recurrent miscarriages and implantation failure. Hum Reprod. 2014;29(12):2637–2643. doi: 10.1093/humrep/deu251. [DOI] [PubMed] [Google Scholar]
- 2.Franasiak, J.M., et al., Investigating the impact of the timing of blastulation on implantation: management of embryo-endometrial synchrony improves outcomes. Hum Reprod Open 2018. 2018(4):hoy022 [DOI] [PMC free article] [PubMed]
- 3.Franasiak JM, Scott RT., Jr Reproductive tract microbiome in assisted reproductive technologies. Fertil Steril. 2015;104(6):1364–1371. doi: 10.1016/j.fertnstert.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 4.McQueen DB, et al. Pregnancy outcomes in women with chronic endometritis and recurrent pregnancy loss. Fertil Steril. 2015;104(4):927–931. doi: 10.1016/j.fertnstert.2015.06.044. [DOI] [PubMed] [Google Scholar]
- 5.Greenwood SM, Moran JJ. Chronic endometritis: morphologic and clinical observations. Obstet Gynecol. 1981;58(2):176–184. [PubMed] [Google Scholar]
- 6.Kitaya K, Yasuo T. Immunohistochemistrical and clinicopathological characterization of chronic endometritis. Am J Reprod Immunol. 2011;66(5):410–415. doi: 10.1111/j.1600-0897.2011.01051.x. [DOI] [PubMed] [Google Scholar]
- 7.Bayer-Garner IB, Nickell JA, Korourian S. Routine syndecan-1 immunohistochemistry aids in the diagnosis of chronic endometritis. Arch Pathol Lab Med. 2004;128(9):1000–1003. doi: 10.5858/2004-128-1000-RSIAIT. [DOI] [PubMed] [Google Scholar]
- 8.Liu Y, et al. Comparison of the prevalence of chronic endometritis as determined by means of different diagnostic methods in women with and without reproductive failure. Fertil Steril. 2018;109(5):832–839. doi: 10.1016/j.fertnstert.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Zolghadri J, et al. The value of hysteroscopy in diagnosis of chronic endometritis in patients with unexplained recurrent spontaneous abortion. Eur J Obstet Gynecol Reprod Biol. 2011;155(2):217–220. doi: 10.1016/j.ejogrb.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Chen Y-Q, et al. Analysis of the diagnostic value of CD138 for chronic endometritis, the risk factors for the pathogenesis of chronic endometritis and the effect of chronic endometritis on pregnancy: a cohort study. BMC Women's Health. 2016;16(1):1–7. doi: 10.1186/s12905-016-0341-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouet P-E, et al. Chronic endometritis in women with recurrent pregnancy loss and recurrent implantation failure: prevalence and role of office hysteroscopy and immunohistochemistry in diagnosis. Fertil Steril. 2016;105(1):106–110. doi: 10.1016/j.fertnstert.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 12.McQueen, D.B., et al., Redefining chronic endometritis: the importance of endometrial stromal changes. Fertil Steril. 2021;116(3):855–861 [DOI] [PubMed]
- 13.Groth JV. Chronic endometritis and the plasma cell, fact versus fiction. Fertil Steril. 2018;109(5):788. doi: 10.1016/j.fertnstert.2018.02.116. [DOI] [PubMed] [Google Scholar]
- 14.Tiegs AW, et al. A multicenter, prospective, blinded, nonselection study evaluating the predictive value of an aneuploid diagnosis using a targeted next-generation sequencing–based preimplantation genetic testing for aneuploidy assay and impact of biopsy. Fertil Steril. 2021;115(3):627–637. doi: 10.1016/j.fertnstert.2020.07.052. [DOI] [PubMed] [Google Scholar]
- 15.Cicinelli E, et al. Prevalence of chronic endometritis in repeated unexplained implantation failure and the IVF success rate after antibiotic therapy. Hum Reprod. 2015;30(2):323–330. doi: 10.1093/humrep/deu292. [DOI] [PubMed] [Google Scholar]
- 16.Kasius JC, et al. The reliability of the histological diagnosis of endometritis in asymptomatic IVF cases: a multicenter observer study. Hum Reprod. 2012;27(1):153–8. doi: 10.1093/humrep/der341. [DOI] [PubMed] [Google Scholar]
- 17.Cicinelli E, et al. Chronic endometritis: correlation among hysteroscopic, histologic, and bacteriologic findings in a prospective trial with 2190 consecutive office hysteroscopies. Fertil Steril. 2008;89(3):677–84. doi: 10.1016/j.fertnstert.2007.03.074. [DOI] [PubMed] [Google Scholar]
- 18.Hitschmann F, Adler L. Die lehre von der endometritis. Ztschr F Geburtsh Gynäk. 1907;60:63–86. [Google Scholar]
- 19.Dumoulin, J. and P. Hughesdon, Chronic endometritis. BJOG 1951. 58(2): 222-235 [DOI] [PubMed]
- 20.Johnston-MacAnanny EB, et al. Chronic endometritis is a frequent finding in women with recurrent implantation failure after in vitro fertilization. Fertil Steril. 2010;93(2):437–441. doi: 10.1016/j.fertnstert.2008.12.131. [DOI] [PubMed] [Google Scholar]
- 21.Yang R, et al. The hysteroscopy and histological diagnosis and treatment value of chronic endometritis in recurrent implantation failure patients. Arch Gynecol Obstet. 2014;289(6):1363–1369. doi: 10.1007/s00404-013-3131-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly in order to protect the privacy of the individuals who participated in the study. The data will be shared on reasonable request to the corresponding author.
Not applicable.