Abstract
Purpose
The use of fertility preservation (FP) techniques has significantly increased in recent years in the assigned female at birth (AFAB) transgender population. Oocyte cryopreservation is the established method for FP, but ovarian tissue cryopreservation may be considered an alternative option, especially during gender-affirming surgery (GAS). The slow freezing (SF) cryopreservation technique is the standard method for human ovarian tissue, but recently, several studies have shown good results with the vitrification (VT) technique. The objective of this study was to compare the effectiveness of VT and SF techniques in ovarian tissue from AFAB transgender people.
Methods
This was a prospective study including 18 AFAB transgender people after GAS. Ovarian tissue pieces from each ovary were cryopreserved by SF and VT and compared with fresh tissue. Study by light microscopy (LM) assessed follicular morphology and density. The percentage of surviving and degenerated follicles was studied with the tissue viability test. Oocytes, granulosa cells and stroma were analysed separately by transmission electron microscopy.
Results
The VT technique preserves follicle and stromal tissue as well as the SF method, but with some differences. Evaluation by LM showed better follicle preservation with VT, but the ultrastructural study showed the presence of minor damage with both techniques compared to fresh tissue.
Conclusion
Both cryopreservation techniques are accurate for maintaining the follicular population and stromal tissue. Further studies are needed to determine the impact of VT on ovarian tissue and the subsequent follicular activation mechanisms in AFAB ovarian tissue.
Keywords: Transgender people, Fertility preservation, Ovarian tissue cryopreservation, Vitrification
Introduction
Gender-incongruent persons require the initiation of safe and effective hormone treatment to develop the physical characteristics of their affirmed gender [1]. Assigned female at birth (AFAB) transgender people initiate testosterone therapy to achieve the secondary characteristics of the affirmed gender and maintain sex hormone levels within the normal male range.
The use of fertility preservation (FP) techniques has significantly increased in recent years in this population, because the reproductive wishes of transgender people have intensified, reaching up to 58 % at present [2]. Oocyte and embryo cryopreservations are the established methods for FP, but these treatments have certain drawbacks, especially if gender-affirming hormone treatment (GAHT) has already been started. There is a need to temporarily discontinue hormonal treatment, and an ovarian stimulation process increases oestrogen levels which can lead to unwanted physical changes [3]. In cases in which the cessation of hormonal treatment is not desired, ovarian tissue cryopreservation (OTC) may be considered an alternative option to preserve fertility, especially during gender-affirming surgery (GAS) in which the ovaries are removed [4].
OTC is an acceptable fertility preservation technique and is no longer considered experimental in the USA since 2019, according to the American Society for Reproductive Medicine [5]. More than 130 children have been born from this procedure worldwide although it is still considered an experimental FP technique by other European societies [6]. All the pregnancies published to date except two have been achieved in cisgender women with oncological or benign diseases through the slow freezing (SF) cryopreservation technique, which has been the standard cryopreservation method for human ovarian tissue since the end of the 1990s [4, 7, 8]. The vitrification (VT) method began in animal model studies and has been performed in different species (rabbits, monkeys, dogs, cows, rodents and sheeps) [9, 10]. Several studies in humans have compared SF protocols with different VT procedures, with conflicting results especially regarding aspects such as improvement in the survival follicle rate or the integrity of the ovarian stroma. At present, the best method of OTC remains to be determined.
In contrast to the tissue used in studies on ovarian cryopreservation in cisgender women with oncological disease or in the context of a benign gynaecological surgery, several changes mainly of the stromal and connective tissue have been described due to exposure to testosterone therapy in ovarian tissue removed during GAS (laparoscopic hystero-oophorectomy) from AFAB transgender people [11–13].
OTC has shown robust results for SF procedures, but various centres worldwide have started to test VT protocols [14]. Neither of these methods has been assessed in ovarian tissue from AFAB transgender people to determine which best preserves their fertility options. Therefore, the aim of this study was to compare the effectiveness of VT and SF cryopreservation techniques in ovarian tissue from AFAB transgender people, analysing morphological and viability parameters by histological means using light and electron microscopy and fluorescent viability markers.
Materials and methods
Study design, size, duration and settings
This was a prospective study including 18 AFAB transgender people aged between 20 and 40 years, receiving testosterone therapy and who were recruited from May 2011 to January 2016 before GAS.
Participants and materials
The study population was enrolled when GAS surgery consisting of hysterectomy and bilateral adnexectomy by laparoscopy was scheduled. Clinical follow-up was carried out in the Endocrinology Department at the Hospital Clinic of Barcelona, Spain. None of the AFAB transgender people used any contraceptives before testosterone therapy or during follow-up. All had had regular menstrual cycles before GAHT. The exclusion criteria were as follows: endocrine pathology, including type 1 diabetes, uncontrolled thyroid disease (hypothyroidism or hyperthyroidism), congenital adrenal hyperplasia and different sexual development. AFAB transgender people started testosterone therapy with intramuscular testosterone undecanoate (Reandron® 1000 mg every 2 to 3 months) which was maintained for a period of 27.3 ± 4.5 months.
Physical and biochemical studies
Weight and height measurements were performed in all AFAB transgender people at recruitment, and a blood sample was taken to determine serum hormone levels. The body mass index (BMI) was calculated, and the main biochemical parameters were measured in serum in the Core Laboratory of Hospital Clínic using standard methods, and the results were expressed according to male reference ranges. Total testosterone (TT) was measured by a chemiluminometric immunoassay (Testosteron II, Elecsys; Roche, Mannheim, Germany, limit of quantification (LOQ) 12 ng/dl, interassay coefficient of variation (CV) < 5%) and was within the normal range of 275.0–850.0 ng/dl. Sex hormone binding globulin was measured using a chemiluminometric immunoassay run on the Atellica analyzer (Siemens Healthcare Diagnostics, Tarrytown, NY, LOQ 1.11 nmol/l, interassay CV < 10%), being within the normal range of 25.0–96.0 nmol/l. Androstenedione was measured by immunoradiometric assay (Cisbio assays, Codolet, France, LOQ 10 ng/dl, interassay CV < 10%) showing a normal range of 50.0–250.0 ng/dl. Follicle-stimulating hormone (FSH), luteinizing hormone (LH) and oestradiol (E2) were measured using chemiluminometric immunoassays in an Atellica Immunochemistry analyzer (Siemens Healthineers, Tarrytown, NY, USA) with an interassay CV of < 10% and an LOQ of 0.55 mUI/ml for FSH, an interassay CV of < 5% and an LOQ of 1.44 mUI/ml for LH and, finally, an interassay CV of < 5% and an LOQ of 18.8 pg/ml for E2. Normal FSH and LH levels were between 1.7–8.0 U/l and 1.5–7.5 U/l, respectively, while normal E2 values were between 10.0 and 41.0 pg/ml. Anti-Müllerian hormone (AMH) was determined in serum by chemoluminescence immunoassay with paramagnetic particles for quantitative determination (AMH B13127 Beckman Coulter kit and in the ACCES2 device, LOQ 0.02 ng/ml, interassay CV <5%), and the results were expressed in ng/ml, with normal female values being between 1.0 and 4.5. AMH determination was only performed in 7 AFAB transgender people, due to difficulties in availability.
Histopathological study
Ovarian tissue processing
After hystero-oophorectomy surgery, both ovaries were transported to the laboratory. Part of the tissue was prepared for histological study, and the remaining tissue was immediately processed. A scalpel was used for dissection of the medulla since the cortex was 1–2 mm thick, and it was cut into squares of approximately 5 × 5 × 10 mm.
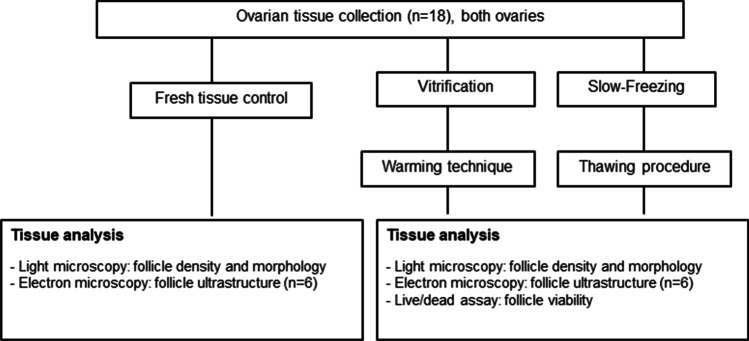
A minimum of seven pieces of ovarian tissue from each ovary were studied. In all cases, three pieces were cryopreserved by the SF method and three by VT. Between two and six fresh pieces were analysed as a control. The remaining cortical pieces were cryopreserved for other research purposes, according to the patients’ choice. All the samples from any of the patients were processed for each cryopreservation procedure at the same time and were warmed/thawed and analysed in parallel (Fig. 1).
Fig 1.
Study flow chart. Follicle morphology, staging, ultrastructure and viability measurements from warmed and thawed ovarian tissue were assessed (n = 18)
The mean area studied in the ovarian samples by light microscopy was very similar in both ovaries: 103.1 ± 44.1 mm2 in the right ovary (RO) versus 93.27 ± 38.1 mm2 in the left ovary (LO). A single ovarian tissue piece (25–30 mm2 approximately) was assessed by transmission electron microscopy and the tissue viability study (LIVE/DEAD Cell Viability Assays®) per ovary and per patient.
Slow freezing technique/thawing procedure
SF was performed according to a protocol described elsewhere with modifications [15]. The ovarian tissue slices were frozen using a 1.5M PrOH (Fluka Chemika, Switzerland) + 0.2M sucrose (Merck, Darmstadt, Germany) solution in 1.8 ml cryovials (Nunc, Denmark). The cryovials were kept rolling in an ice bath for 30 min at 4°C. Then the samples were placed into a computerized programmable freezer (Planner K10, Planner Ltd., UK).
The cooling programme started from 0 to −9°C at a rate of 2°C/min, and ice nucleation (seeding) was manually induced. After a 10-min holding period, the temperature was lowered to −40°C at 0.3°C/min and from −40 to −140°C at a −10°C/min. Finally, the cryovials were placed into liquid nitrogen for storage.
For the thawing procedure, the cryovials were immersed in a 37°C water bath for 2 min, and the ovarian slices were then equilibrated sequentially in 1.0M PrOH + 0.2M sucrose + 20% serum substitute (5 min); 0.5M PrOH + 0.2M sucrose + 20% serum substitute (5 min); 0.2M sucrose + 20% serum substitute (10 min); and PBS + 20% serum substitute (10 min) at room temperature.
Vitrification/warming procedure
The VT of ovarian tissue pieces was performed according to the protocol described by Kagawa with some modifications [16].
Cortex fragments were incubated in an equilibration solution composed of 7.5% ethylene glycol (Merck, Darmstadt) and 7.5% dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) in a buffer media with 20% human serum albumin (Grifols, Spain), for 25 min at room temperature followed by a 15-min incubation in a vitrification solution composed of 20% ethylene glycol, 20% DMSO and 0.5M sucrose. The samples were transferred to precooled 1.8 ml cryogenic vials and placed directly into liquid nitrogen.
For warming, the cryovials were immersed in a 37°C water bath for 2 min, and the ovarian fragments were transferred to a 1M sucrose solution for 5 min and to a solution of 0.5M sucrose for 5 min. Finally, the tissue pieces were washed in buffer media.
Light microscopy evaluation
After the thawing and warming procedures had been performed, the tissue pieces were fixed in 10% formalin. All samples were routinely paraffin embedded, and 3-μm sections were made. The sections were stained with haematoxylin–eosin using standard techniques and were examined by a gynaecological pathologist to perform the ovarian follicle count of each sample.
The developmental stages of the follicles were evaluated as defined by Gougeon in 1996. We defined primordial follicles as those of 30–60 μm in diameter and containing a single layer of flat granulosa cells (GC) around the oocyte. Primary follicles were those presenting a well-defined layer of cuboidal cells and > 60 μm in diameter. Secondary follicles were defined as those presenting two or more layers of cuboidal cells and 0.12–0.2 mm in diameter, and early-antral follicles had more than two layers of cubical GC surrounding the oocyte in the presence of a follicular antrum and 0.12–2 mm in diameter.
The number of primordial follicles per millimetre square of tissue surface was assessed in the samples of both ovaries (left and right), and the follicular density of the processed pieces was also evaluated. The follicular morphology was also assessed considering the parameters previously described by Isachenko [17]: (1) normal follicle being a spherical follicle surrounded by GCs, homogeneous oocyte cytoplasm and slightly granulated nucleus, the centre of which showed objective and spherical dense chromatin and (2) degenerated follicle showing partially or totally disorganized GCs and/or an altered oocyte cytoplasm with pyknotic nuclei.
Follicles were classified on a section containing the nucleus to avoid double counting. The follicular density in fresh tissue and after VT and SF procedures was measured, and the quality of primordial follicles (expressed as percentage of normal follicles) was also investigated.
Tissue viability study (LIVE/DEAD Cell Viability Assays®)
As previously described by Hreinsson et al. 2003, one tissue piece from each ovary and each cryopreservation technique was digested with 1.5 mg/ml collagenase type II (Invitrogen, NY) in pre-equilibrated a-MEM (Invitrogen Inc.) with 10% human serum at 37°C and 5% CO2 in a humidified incubator. After 1 h, the presence of isolated follicles was checked. If there were follicles floating in the medium, all the medium and tissue were filtered through a 100-μm filter (Gelman, Pall Life Sciences). The pieces on the filter were collected for further digestion, and the flow through medium was centrifuged at 100 g for 5 min. The pellet with a small amount of medium was checked under an inverted microscope for follicle identification. Follicles were stored in culture medium in the incubator, and this procedure was repeated. After 2±3 h digestion, a clear view of the follicles in the stroma was possible, and they were stained along with fully isolated follicles. At this point, the medium containing the tissue was centrifuged at 100 g for 5 min, and the pellet together with the individual follicles was resuspended in live/dead working solution (LIVE/DEAD Viability/Cytotoxicity Kit®) (L-3224), Molecular Probes, Eugene, OR). The working solution was PBS containing 2 mmol/l calcein AM which is converted to calcein by intracellular esterases and produces an intense green fluorescence in live cells and 4 mmol/l ethidium homodimer-1 (EthD-1), which enters cells with damaged membranes and undergoes a 40-fold enhancement of fluorescence upon binding to nucleic acids, producing bright red fluorescence in dead cells. EthD-1 is excluded by the intact plasma membrane of live cells. After 30-min incubation at room temperature, the cell suspension was centrifuged again (100 g for 1 min), and most of the supernatant was removed. Partly and fully isolated follicles were mounted onto glass slides. After the cover slides were mounted, the viability of the follicles was assessed under a fluorescence microscope. The follicles were classified depending on the staining: viable follicles (green colour) and degenerated follicles (bright red colour). The percentage of surviving and degenerated follicles was studied depending on the cryopreservation technique used.
Transmission electron microscopy analysis
Ovarian tissue samples were collected, placed and fixed (glutaraldehyde 2.5% in 0.1M phosphate buffer). The samples were cut into 1 mm3 fragments and kept for 2–4 h at 4 °C. Washings were carried out with 0.1 M phosphate buffer (3 × 10 min at 4 °C) and post fixation with 1% osmium tetraoxide (with 0.8 % potassium ferricyanide) in 0.1M phosphate buffer for 1–2 h at 4 °C. New washes were carried out with distilled water (3 × 10 min at 4 ×C), and afterwards, the samples were dehydrated with ketones of different duration depending on the percentage of solvent used (with 50 % solvent, 1 wash was carried out for 10 min at 4 °C). After these steps, the material was included, usually with Spurr and later the infiltration was carried out, varying the time according to the type of tissue to be infiltrated and the size of the sample. Subsequently, the tissue blocks were made and polymerized at 60 °C for 48 h. The sample obtained was then prepared for the study by transmission electron microscopy (TEM).
Oocytes, GC and stroma were analysed separately. In the first two structures (oocytes and GC), the nuclear content, the integrity of the membrane, the density of the cytoplasm, the cytoplasmic organelles and the intracellular contact were evaluated. The nuclear content and the integrity of the extracellular matrix in the stromal cells were also assessed.
The structural changes were evaluated using a score system, with which the value 2 is assigned to the normality of the assessed aspects, the value 1 is given when the observed changes are minimal (slight alterations of the assessed structure) and the value 0 is assigned when the changes shown are severe (according to the criteria established by Hreinsson et al.) [18].
Statistical analysis
The statistical analyses were performed with IMB SPSS Statistics 23.0 (IBM Corp., New York, USA). Normality was tested using the Kolmogorov-Smirnov test. The ANOVA test was used to compare follicular density between samples, and the Pearson chi-square test was used to assess differences in the proportions of viable follicles in light microscopy and after the tissue viability study. The non-parametric Kruskal-Wallis test was used to analyse differences in the scores between groups after assessment by TEM. Correlation analysis between clinical features, hormone levels and the follicular population of the samples was performed using Spearman’s test. The level of statistical significance was set at p < 0.05.
Ethical approval
This clinical study was conducted according to the Declaration of Helsinki for Medical Research Involving Human Subjects [19]. The study protocol was approved by the Ethics Committee of the Hospital Clinic of Barcelona (registry number 2011/6272) in 2011. All subjects provided written, informed consent to participate in the study.
Results
Clinical data
An overview of the characteristics of the AFAB transgender cohort including hormonal status is shown in Table 1.
Table 1.
Clinical and hormonal characteristics of assigned female at birth transgender people (N = 18) before gender affirmation surgery.
| Clinical features | ||
|---|---|---|
| Parameter | Mean (range) | Reference values |
| Age, years | 26.6 ± 5.5 (20–40) | NA |
| Weight, kg | 59.6 ± 5.2 (45–65) | NA |
| Height, m | 1.6 ± 0.1 (1.5–1.9) | NA |
| BMI, kg/m2 | 22.5 ± 2.6 (16.8–25.0) | 20.0–25.0 |
| FSH, U/l | 5.6 ± 2.4 (1.2–9.2) | 1.7–8.0 |
| LH, U/l | 3.5 ± 2.6 (0.1–9.5) | 1.5–7.5 |
| Oestradiol, pg/ml | 45.5 ± 7.8 (34.0–59.0) | 10.0–41.0 |
| Total testosterone, ng/ml | 698.5 ± 309.3 (309.0–1224.0) | 275.0–850.0 |
| SHBG, nmol/l | 32.5 ± 15.3 (15.7–64.2) | 25.0–96.0 |
| Androstenedione, ng/dl | 260.8 ± 58.1 (171.0–310.0) | 50.0–250.0 |
| AMH, ng/ml* | 2.2 ± 0.5 (1.4–3.1) | 1.0–4.5 |
Acronyms: BMI body mass index, FSH follicle-stimulating hormone, LH, luteinizing hormone, TT total testosterone, SHBG sex hormone binding globulin, NA not applicable
*n = 18; anti-Müllerian hormone (AMH) was available for 7 people
The mean age of the study population at the time of surgery was 26.6 ± 5.5 years (range 20–40). None of the AFAB transgender people started androgens on their own before testosterone therapy, and all received Reandron® 1000 mg every 2 to 3 months.
All the AFAB transgender people became amenorrheic months after starting hormone therapy. None presented vaginal bleeding or spotting after the first 6–8 months of testosterone therapy. At the time of recruitment, when GAS was scheduled, the mean GAHT time was 27.3 ± 4.5 months, and all the participants showed serum testosterone levels within the male physiological range (mean 698.5 ± 309.3 ng/dl) and complete virilization. Fourteen AFAB transgender people (77.8 %) showed serum FSH levels within female pre-menopausal reference ranges (4.5–10.0 U/l) compared to 4 AFAB transgender people presenting suppressed serum levels (minimum value 1.2 U/l). Nine AFAB transgender people (50%) showed suppressed serum LH levels, with a minimum value of 0.1 U/l in one person. AMH determination was performed in a total of 7 AFAB transgender people, with a mean of 2.2 ± 0.5 ng/ml (range 1.4–3.1).
Histological results
Light microscopy
Primordial follicles in fresh tissue samples from the left (LO) and right ovaries (RO) of each person were assessed. More than 2000 follicles were studied, and significant variability was found in the different tissue samples. The mean value was 254.62 primordial follicles per sample (range between 8 and 1508). An average of 137.54 follicles in the RO (range 5–970) was found versus 120.15 follicles in the LO (range 1–538 follicles per fragment). Therefore, a heterogeneous distribution of the follicles within the ovarian cortex was observed, but the mean number of primordial follicles remained similar in each ovary (p > 0, 05). The follicular density in fresh samples was obtained, with a mean of 1.07 fols/mm2 (range 0.16–2.71). This value was similar both in the RO (mean of 1.0 fols/mm2) and in the LO (1.3 fols/mm2) (p = 0.09).
No correlation was found between the different hormonal parameters or patient age and the primordial follicular count.
One tissue sample from each ovary and patient was assessed after SF-thawing and VT-warming (Table 2). The overall evaluation of the thawed/warmed samples showed a greater proportion of grade 1 follicles in the VT-warmed (51.9 %) versus the SF-thawed pieces (43.2 %), but the difference was not statistically significant (p = 0.7). No differences were found between the follicular morphology in both cryopreserved tissue samples compared to the control ones (fresh tissue) that showed 59.2 % of grade 1 follicles and 40.8% of grade 2 ones (p = 0.08). The follicular density remained stable in both cryopreserved tissue samples, and comparison between the two techniques showed no statistically significant differences (p = 0.22). Fig. 2 shows follicular population data from ovaries in fresh pieces, SF-thawed tissue and VT-warmed tissue.
Table 2.
Description of different histological parameters in fresh tissue and after slow freezing and vitrification in the study population (N = 18).
| SF-thawed tissue | VT-warmed tissue | P value | |
|---|---|---|---|
| LM study* | |||
| Grade 1 follicles | 48.64 ± 45.03 (43.2%) | 49.0 ± 41.9 (51.9%) | P > 005 |
| Grade 2 follicles | 72.36 ± 62.4 (56.8%) | 45.29 ± 28.22 (48.1%) | P > 005 |
| Follicular density | 1.29 ±1.08 | 1.2 ± 0.8 | P > 005 |
| Tissue viability study^ | |||
| Viable follicles | 230 (66.9%) | 248 (71.3%) | P > 005 |
| Non-viable follicles | 114 (33.1%) | 100 (28.7%) | P > 005 |
Acronyms: LM light microscopy, SF slow-freezing, VT vitrification
*Data in LM study is shown as mean follicles ± standard deviation (percentage)
^Data in tissue viability study is shown as follicle number (percentage)
Fig 2.
Haematoxylin–eosin stained ovarian tissue sections showing primordial follicles (marked with black arrows) in a fresh sample (200×), b thawed ovarian piece (200×), and c warmed tissue (200×).
No statistically significant differences were found according to the ovary analysed in the processed tissue samples (thawed and warmed) according to the proportion of grade 1 or 2 follicles or follicular density.
Tissue viability study
The viability study was carried out using the LIVE/DEAD Cell Viability Assays® with fluorescent markers. One tissue piece from each patient and cryopreservation technique was assessed, and all the samples were processed using the aforementioned substances carrying out the subsequent incubation and centrifugation, after which a supernatant was obtained in which the follicles were assessed and identified under a fluorescence microscope. Follicles that exclusively exhibited esterase activity (green colour) were considered viable in the test, while those that stained red were considered non-viable (Fig. 3). The comparison between the two cryopreservation techniques showed no differences in relation to the viability parameters (p = 0.10), although there was a trend towards better results with the VT-warmed procedure compared to SF-thawed technique.
Fig 3.
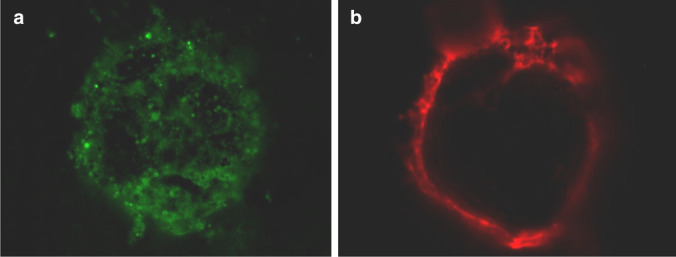
Follicles after LIVE/DEAD assay kit staining: viable follicle (a, green) and atretic follicle (b, red)
Transmission electron microscopy study
Ovarian tissue samples from a total of 6 AFAB transgender people were evaluated, including fresh ovarian and VT and SF cryopreservation samples. A total of 22 follicles were assessed, comprising 22 oocytes and 132 GC and their surrounding stroma.
Seven oocytes and 46 GC from fresh tissue were studied, while eight oocytes and 51 GC from VT-warmed samples and seven oocytes and 35 GC from SF-thawed samples were evaluated. Stromal cells and extracellular matrix were also studied in all the processed samples.
The following items were assessed in each of the structures mentioned above: (1) nuclear content (oocytes, GC and stromal cells); (2) membrane integrity (oocytes and GC); (3) density of the cytoplasm (oocytes and GC); (4) cytoplasmic organelles (oocytes and GC); (5) intracellular contact (oocytes and GC); and (6) integrity of the extracellular matrix (stromal cells). Structural changes were evaluated using a scoring system (0–2), as mentioned previously.
The ultrastructure of the oocytes did not vary among groups (p = 0.19). The number of oocytes containing pyknotic nuclei in VT-warmed or SF-thawed follicles did not differ (p = 0.9), but it was of note that some follicles in both techniques showed the presence of a non-rounded nucleus, with a wavy irregular shape compared to control ones (Fig. 4 a, b and c). The cytoplasm was well organized in all cases, containing easily visible microtubules, with no differences on comparing the two techniques (p = 0.8). The presence of vacuoles in some oocytes was observed, both in VT-warmed and in SF-thawed oocytes.
Fig 4.
Transmission electron microscopy images of follicles within pieces of non-frozen (control) AFAB transgender ovarian tissue (a) (bar 10 μm), SF-thawed tissue (b) (bar 10 μm) and tissue cryopreserved using VT-warming protocol (c) (bar 5 μm). The oocyte (Ooc) is surrounded by one layer of flattened granulosa cells (GC) that are attached to the basement membrane (red asterisk). The magnification of the images shows the oocyte nuclei with euchromatin (N). SF-thawed and VT-warmed follicles show an irregular shaped nucleus membrane (black arrows) compared to the nucleus membrane from fresh follicle that appear regular and intact (red arrow).
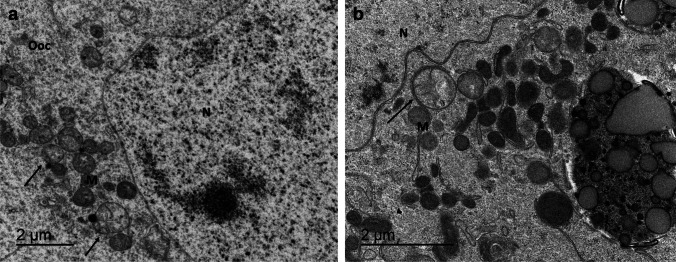
Abundant mitochondria with uniform crests were observed in all the oocytes studied, although a few dilated or even oedematous mitochondria were observed in various oocytes, both in VT and SF compared to fresh oocytes (Fig. 5a, b). Organelles such as the Golgi apparatus and lysosomes were correctly preserved in most oocytes. The oocyte cell membrane was well preserved in VT-warmed as well as in SF-thawed follicles compared to fresh follicles, but it should be noted that in some VT-warmed follicles, the oocyte membrane and intercellular space were slightly increased (Fig. 6), without reaching statistical significance (p = 0.08).
Fig 5.
A high magnification of the transmission electron microscopy images of the oocytes (Ooc) of SF-thawed follicle (a) (bar 2 μm) and VT-warmed one (b) (bar 2 μm) shows abundant mitochondria (M) with uniform cristae grouped around the nucleus (N). The presence of some oedematous mitochondria can be distinguished, marked by black arrows
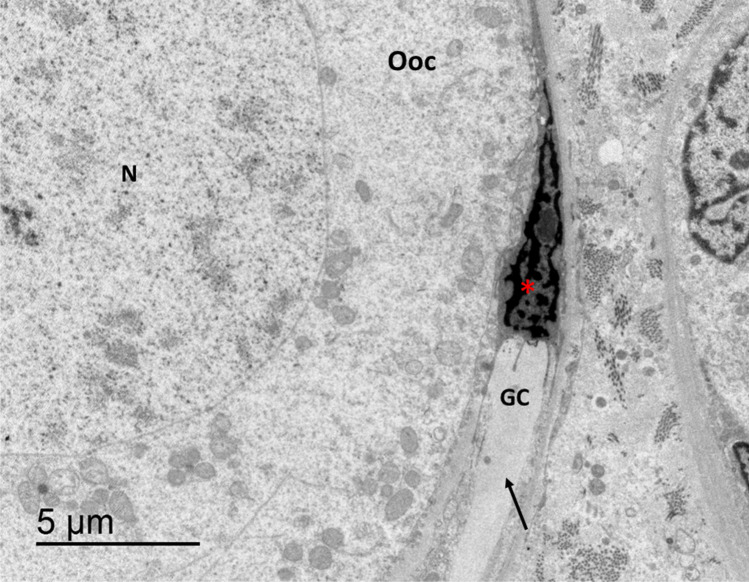
Fig 6.
Detail of VT-warmed follicle with a granulosa cell (GC) and an oocyte (Ooc) (bar 2 μm). The granulosa cell shows a well-preserved nucleus (N) and is attached to thin basement membrane of the oocyte (black arrow) but shows a partially detached and expanded intercellular space, marked with red asterisks.
Granulosa cells surrounding the oocyte were evaluated carefully. Most were well preserved, and close adhesion between oocyte and GC was observed. Junction areas were clearly visible, and in most cases, the nucleus appeared as a homogeneous structure with partial densities and a dense aggregation of heterochromatin adhered to the nuclear membrane. Few atretic GC were observed in SF-thawed follicles, with pyknotic nuclei and granular cytoplasm (Fig. 7).
Fig 7.
Detail of SF-thawed follicle with a granulosa cell (GC) and an oocyte (Ooc), with a well-preserved nucleus with homogenous euchromatin (N) (bar 5 μm). The granulosa cell shows clear signs of apoptosis: pyknotic nucleus, marked with a red asterisk, and granular and condensed cytoplasm, marked with a black arrow.
On the other hand, alterations in the cell cytoplasm were observed in some VT-warmed GC, highlighting vacuolization in the endoplasmic reticulum, an early sign of necrosis. In addition, lipid vacuoles were also observed in these GC, which were not observed in SF or fresh follicles (Fig. 8).
Fig 8.
High magnification of the transmission electron microscopy image of a VT-warmed follicle that shows an oocyte (Ooc) and a granulosa cell (GC) attached to the thin basement membrane (black arrow) (bar 2 μm). A detailed observation of the granulosa cell shows a well-preserved nucleus (N), but a vacuolization in the endoplasmic reticulum can be observed forming empty spaces (red asterisks) and also a big lipid vacuole (yellow asterisk).
The morphology of the stroma, composed of collagen fibres and spindle-shaped fibroblast-like, was well-preserved and very similar among the three processed samples (p= 0.4). A higher magnification evaluation showed that the stromal cell nuclei exhibited a normal morphology, and the presence of some mitochondria was observed. Stromal collagen fibre disruption was not observed in either transverse or longitudinal sections in any of the samples studied.
Discussion
The present study is the first to evaluate and compare two cryopreservation techniques in ovarian tissue from AFAB transgender people. The results indicate that VT preserves follicle and stroma morphology as well as the SF method, but with some differences. While evaluation by light microscopy showed better follicle preservation with VT and similar follicular density, the ultrastructural study exhibited the presence of minor changes with both techniques, mainly in the preservation of GC.
OTC is a FP technique recommended in cisgender female patients undergoing gonadotoxic treatment in whom oocyte/embryo cryopreservation is not feasible, or for patient preference. According to the European Society of Human Reproduction and Embryology guideline on FP, OTC can be proposed as an experimental option when ovaries are removed during GAS in transgender men [4] and is no longer considered experimental and can be used in prepubertal patients or when there is not time for ovarian stimulation according to the American Society for Reproductive Medicine [5]. At present, GAHT is one of the indications for FP according to various organizations and recent literature since testosterone therapy may adversely affect fertility and GAS may involve the removal of both ovaries [1, 20, 21]. Different studies have evaluated the wishes of up to 47% of transgender individuals to have children with whom they are genetically related [22]. In a previous study, Wierckx et al. reported that up to 54% of transgender men manifested a desire for children, and 37.5% would have considered freezing germ cells had it been offered [23].
Several studies have reported that many transgender individuals are eager to initiate GAHT, with the number of AFAB transgender young adults and adolescents referred to clinics increasing, and some even choose to start GAHT during adolescence [2, 24]. Due to the young age of these AFAB transgender people, they may not yet be concerned about a future with biological children; however, it is likely that some may develop a desire to have biological children later in life [25, 26].
Given that oocyte cryopreservation requires discontinuation of testosterone therapy for varying lengths of time before starting ovarian stimulation (OS) (ranging from 1 to 12 months according to previous studies), almost all transgender men will experience menses, usually within 3 months of stopping testosterone, and this can cause gender dysphoria [27–32]. Moreover, in these cases, the increase of oestrogen levels during OS can cause mental distress and adversely affected the view of their bodies [33, 34]. In addition, this process can be an expensive medical procedure in some countries, and cost could be a major barrier preventing AFAB transgender people from pursuing FP. OTC, however, can be performed at the same time as GAS without the need for OS or gynaecologic examination, but no publication has evaluated OTC in the transgender population [24].
Previous reports performing systematic comparisons of the two cryopreservation procedures have been carried out in human ovarian tissue from cisgender females with different and contradictory results [35–38]. Only two studies have been performed with ovarian tissue from AFAB transgender people after GAS (n total = 20) to test VT protocols [39, 40]. In these two studies, comparison between the two available cryopreservation techniques was not made, but only the use of VT as a technique for the preservation of ovarian tissue was validated.
All other studies have compared the two techniques with ovarian tissue from cisgender women of reproductive age with an oncological disease or benign gynaecological pathology. According to our results, the study of follicular morphology by light microscopy showed that 51.9 % of the VT-warmed follicles were considered normal follicles, compared to 43.2 % of the thawed follicles. No statistical differences were found, although a clear trend towards better preservation was observed with the VT method. Li et al. [41] also described similar results with both cryopreservation techniques, although a higher percentage of follicles in both groups had a normal appearance (80.3 % in VT group after warming vs. 72.6 % in SF group after thawing). Other publications comparing the follicular morphology between cryopreservation techniques reported similar follicular normality rate [–, 17, 42, 43], while some groups reported clearly worse results after the VT procedure [35, 44–47]. On the other hand, histomorphometric analysis in our study showed that follicular densities were comparable between fresh, warmed and thawed tissues, in agreement with previous studies [48], while other authors reported a significant decrease in the follicular population after cryopreservation techniques and mainly after the VT procedure [44, 45]. In addition, two recent studies have reported in vitro and xenotransplantation data confirming worse results of follicular density after the VT procedure [47, 49]. Of note is the great heterogeneity in the preservation protocols applied and/or the devices used to cryopreserve the tissue, to which the ovarian components may be particularly sensitive to [43, 50, 51]. Regarding this issue, Fabbri et al. [50] studied ovarian tissue from six cisgender women with oncological diseases and compared 4 cryopreservation techniques (2 SF procedures and 2 VT protocol with 5- or 10-min incubation in each vitrification solution) and concluded that samples showed cryodamage of small entity with both techniques and different protocols compared to fresh samples, but no differences were found among the four techniques. In our study, the protocols used were previously published by Fabbri and Kagawa, who established groups with experience in ovarian tissue preservation [40, 50, 52, 53], with slight differences being found in our samples.
Little data is available regarding the ultrastructural study of the oocytes, GC and stroma. The same study described above showed no differences in TEM between protocols, although warmed samples showed oocyte nuclei with slightly thickened chromatin and irregular shapes [50]. In agreement with this study, our results showed good preservation of the follicles with both cryopreservation techniques, although GC showed poor preservation with VT and SF compared to fresh cells. In some VT-warmed follicles, the oocyte membrane and intercellular space were slightly increased, but important and significant alterations were not observed in the ultrastructural assessment, and no follicle destruction by ice formation was noted, contrary to what was described by Lee et al. [47]. This latter study assessed primordial follicles in three ovarian samples from cisgender women who underwent benign ovarian surgery, and according to their results, many of the VT-warmed follicles and stroma were deformed and destroyed by ice crystal formation. Follicles from the SF and control group were intact and showed no morphological deformation. On the contrary, Keros et al. [36] compared 4 cryopreservation techniques (2 SF procedures with different cryoprotectants and 2 VT protocols with 5 or 10 min of incubation in each vitrification solution) and concluded that the ovarian stroma was better preserved by VT than SF, whereas more necrotic cells and empty areas were found in the stromal tissue after the SF technique. Slightly different results regarding preservation of the GC and stromal tissue were observed in our study compared to this last study. These differences may be due to the ovarian tissue samples, since in our study the pieces were obtained from AFAB transgender people who present differential characteristics with respect to the ovarian tissue of cisgender females.
Initial publications in the AFAB cohort reported that ovaries removed during GAS showed polycystic ovary morphology (PCOM) features [54–57] according to Stein-Leventhal criteria [58]. In the last decade, five groups have studied ovarian morphology in an AFAB cohort after initiation of GAHT. According to the studies by Grynberg et al. and Loverro et al. [59, 60], the morphology and histology of the majority of ovarian specimens (79–82%) resembled that of PCOS. However, other recent studies revealed a normal follicular distribution compared with controls [11–13]. Two of the studies found a higher proportion of atretic follicles within the AFAB group compared with age and BMI-matched controls or with published literature [11, 13]. Among the histological changes described, a thicker ovarian cortex, more hyperplastic collagen, ovarian stromal hyperplasia and stromal luteinization were of note.
Based on the data currently available and given that a normal follicular distribution has been observed but stromal changes have been described in AFAB people with testosterone therapy, both VT and SF seem to be good techniques to preserve oocytes and stromal tissue, with similar results in ovarian tissue samples from cisgender women and AFAB transgender people. On the other hand, both procedures showed minor changes in the preservation of GC compared to fresh samples, perhaps due to the differential characteristics of ovarian tissue from the AFAB cohort with GAHT.
Otherwise, OTC as an option for FP remains in the developmental phase, while the method to mature the immature follicles is being perfected [61]. Some authors [62, 63] have reported that during the OTC, cumulus-oocyte complexes could be found, and in vitro maturation (IVM) could be possible in AFAB transgender people who had not stopped GAHT prior to oophorectomy. Spindle analysis performed in these studies found a normal chromosomal pattern in 87% of the metaphase II oocytes obtained. However, achievement of metaphase II does not equate the ability for successful fertilization, and, to date, successful fertilization and embryo implantation following IVM of an immature oocyte from a AFAB population has not been reported [61, 64]. It is necessary to increase our knowledge regarding OTC and the subsequent in vitro culture of ovarian tissue and IVM of oocytes in order to activate, mature and use primordial follicle pools in patients who are not eligible for transplantation, as the transgender people [63]. Nowadays, efficient use of the cryopreserved cortical follicular reserve remains a challenge in this population, and techniques of in vitro growth combined with IVM are currently being developed in non-human and human primates [65].
An important strength of this study is that it is the first to compare SF and VT cryopreservation techniques in AFAB transgender people on GAHT, including hormonal assessment and histomorphometric study by light microscopy and TEM and also with viability investigation of the follicles. Histological study describing normal follicular population and density along with the ultrastructural description of the ovarian cells is the most adequate to assess the cryopreservation techniques. In addition, we discuss the differences found with both procedures in AFAB transgender people. According to our results, both cryopreservation techniques preserve follicles and stroma correctly, but with some differences. While VT showed better follicle preservation according to light microscopy study, the ultrastructural study exhibited minor cryoinjury levels with both techniques compared to the fresh samples. More studies are needed to confirm these results.
Our study also has several limitations. Only ovarian tissue samples from six AFAB transgender people were evaluated by TEM, but the assessment of the follicles and surrounding stroma was comprehensive and from fresh and processed samples by both techniques. Moreover, the absence of a control group is another limitation. The same ovarian tissue from AFAB transgender people has been considered as a control group, and some pieces were evaluated in fresh, as a control. In our hospital, only a few pieces of ovarian tissue can be harvested from oncological patients, and some samples can be obtained from cisgender fertile women with ovarian masses. However, Pavone et al. [66] showed that the tissue surrounding an ovarian malignancy is not an appropriate control group as follicle density in the cortex is decreased, and therefore, we do not consider this group suitable as a control. On the other hand, the lack of functional tests that would allow determining whether the damage to follicles and stroma was reversible or not is another limitation. Further investigations such as xeno-transplantation might give information about viability of the follicles within the tissue.
In conclusion, this study shows that OTC by VT and SF cryopreservation techniques is effective to maintain the follicular population and the stromal tissue. Further studies are needed to determine the impact of VT on ovarian tissue and evaluate the subsequent follicular activation mechanisms with a view to the use of this tissue for FP in the AFAB transgender population.
Author contribution
All authors contributed to the study conception and design. Material preparation was performed by Josep Maria Calafell, Adela Saco and Aina Borrás. Data collection and analysis were performed by Aina Borrás, Inés Agustí, Sara Peralta and Gemms Casals. The original draft preparation was performed by Aina Borrás. The study supervision was performed by Dolors Manau, Francesc Fabregues and Francisco Carmona. All authors commented on previous versions of the manuscript and approved the final version of article submitted.
Funding
This study was supported by the grant “Premi Fi de Residencia Emili Letang 2011” from the Hospital Clinic of Barcelona and the grant “Fundación Dexeus Salud de la Mujer” in 2013–2015.
Declarations
It was conducted according to the Declaration of Helsinki for Medical Research involving Human Subjects [19]. The study protocol was approved by the Ethics Committee of the Hospital Clinic of Barcelona (registry number 2011/6272) in 2011. All subjects provided written, informed consent to participate in the study.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hembree WC, Cohen-Kettenis PT, Gooren L, Hannema SE, Meyer WJ, Murad MH, Rosenthal SM, Safer JD, Tangpricha V, T'Sjoen GG. Endocrine treatment of gender-dysphoric/ gender-incongruent persons: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2017;102(11):3869–3903. doi: 10.1210/jc.2017-01658. [DOI] [PubMed] [Google Scholar]
- 2.Auer MK, Fuss J, Nieder TO, Briken P, Biedermann SV, Stalla GK, Beckmann MW, Hildebrandt T. Desire to have children among transgender people in Germany: a cross-sectional multi-center study. J Sex Med. 2018;15(5):757–767. doi: 10.1016/j.jsxm.2018.03.083. [DOI] [PubMed] [Google Scholar]
- 3.Neblett MF, Hipp HS. Fertility considerations in transgender persons. Endocrinol Metab Clin N Am. 2019;48(2):391–402. doi: 10.1016/j.ecl.2019.02.003. [DOI] [PubMed] [Google Scholar]
- 4.ESHRE Guideline Group on Female Fertility Preservation. Anderson RA, Amant F, Braat D, D’Angelo A, Chuva de Sousa Lopes SM, Demeestere I, Dwek S, Frith L, Lambertini M, Maslin C, Moura-Ramos M, Nogueira D, Rodriguez-Wallberg K, Vermeulen N. ESHRE guideline: female fertility preservation. Hum Reprod Open. 2020;2020(4):hoaa052. doi: 10.1093/hropen/hoaa052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Practice Committee of the American Society for Reproductive Medicine Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: a committee opinion. Fertil Steril. 2019;112:1022–1033. doi: 10.1016/j.fertnstert.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 6.Donnez J, Dolmans MM. Fertility preservation in women. N Engl J Med. 2017;377(17):1657–1665. doi: 10.1056/nejmra1614676. [DOI] [PubMed] [Google Scholar]
- 7.Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, Morimoto Y, Kawamura K. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. doi: 10.1093/humrep/deu353. [DOI] [PubMed] [Google Scholar]
- 8.Nakano MS, Simoes RS, Baracat MCP, Lobel A, Shiroma ME, Igami DZ, Serafini PC, Soares JM, Jr, Baracat EC. Live birth rate after ovarian tissue cryopreservation followed by autotransplantation in cancer patients: a systematic review. Gynecological and Reproductive. Endocrinol Metab. 2020;1(2):89–94. [Google Scholar]
- 9.Rodriguez-Wallberg KA, Oktay K. Recent advances in oocyte and ovarian tissue cryopreservation and transplantation. Best Pract Res Clin Obstet Gynaecol. 2012;26(3):391–405. doi: 10.1016/j.bpobgyn.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devi L, Goel S. Fertility preservation through gonadal cryopreservation. Reprod Med Biol. 2016;15(4):235–251. doi: 10.1007/s12522-016-0240-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ikeda K, Baba T, Noguchi H, Nagasawa K, Endo T, Kiya T, Saito T. Excessive androgen exposure in female-to-male transsexual persons of reproductive age induces hyperplasia of the ovarian cortex and stroma but not polycystic ovary morphology. Hum Reprod. 2013;28(2):453–461. doi: 10.1093/humrep/des385. [DOI] [PubMed] [Google Scholar]
- 12.De Roo C, Lierman S, Tilleman K, Peynshaert K, Braeckmans K, Caanen M, Lambalk CB, Weyers S, T'Sjoen G, Cornelissen R, De Sutter P. Ovarian tissue cryopreservation in female-to-male transgender people: insights into ovarian histology and physiology after prolonged androgen treatment. Reprod BioMed Online. 2017;34(6):557–566. doi: 10.1016/j.rbmo.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 13.Borrás A, Manau MD, Fabregues F, Casals G, Saco A, Halperin I, Mora M, Goday A, Barral Y, Carmona F. Endocrinological and ovarian histological investigations in assigned female at birth transgender people undergoing testosterone therapy. Reprod BioMed Online. 2021;43(2):289–297. doi: 10.1016/j.rbmo.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Rivas Leonel EC, Lucci CM, Amorim CA. Cryopreservation of human ovarian tissue: a review. Transfus Med Hemother. 2019;46(3):173–181. doi: 10.1159/000499054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fabbri R, Venturoli S, Errico AD, Iannascoli C, Gabusi E, Valeri B, Seracchioli R, Grigioni WF. Ovarian tissue banking and fertility preservation in cancer patients : histological and immunohistochemical evaluation. Gynecol Oncol. 2003;89:259–266. doi: 10.1016/S0090-8258(02)00098-7. [DOI] [PubMed] [Google Scholar]
- 16.Kagawa N, Silber S, Kuwayama M. Successful vitrification of bovine and human ovarian tissue. Reprod BioMed Online. 2009;18(4):568–577. doi: 10.1016/S1472-6483(10)60136-8. [DOI] [PubMed] [Google Scholar]
- 17.Isachenko V, Lapidus I, Isachenko E, Krivokharchenko A, Kreienberg R, Woriedh M, Bader M, Weiss JM. Human ovarian tissue vitrification versus conventional freezing: morphological, endocrinological, and molecular biological evaluation. Reproduction. 2009. 10.1530/REP-09-0039. [DOI] [PubMed]
- 18.Hreinsson J, Zhang P, Swahn ML, Hultenby K, Hovatta O. Cryopreservation of follicles in human ovarian cortical tissue. Comparison of serum and human serum albumin in the cryoprotectant solutions. Hum Reprod. 2003;18(11):2420–2428. doi: 10.1093/humrep/deg439. [DOI] [PubMed] [Google Scholar]
- 19.World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191–4. 10.1001/jama.2013.281053. [DOI] [PubMed]
- 20.Ethics Committee of the American Society for Reproductive Medicine Access to fertility services by transgender persons: an ethics committee opinion. Fertil Steril. 2015;104(5):1111–1115. doi: 10.1016/j.fertnstert.2015.08.021. [DOI] [PubMed] [Google Scholar]
- 21.Shumer DE, Nokoff NJ, Spack NP. Advances in the care of transgender children and adolescents. Adv Pediatr Infect Dis. 2016;63(1):79–102. doi: 10.1016/j.yapd.2016.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tornello SL, Bos H. Parenting intentions among transgender individuals. LGBT Health. 2017;4(2):115–120. doi: 10.1089/lgbt.2016.0153. [DOI] [PubMed] [Google Scholar]
- 23.Wierckx K, Van Caenegem E, Pennings G, Elaut E, Dedecker D, Van de Peer F, Weyers S, De Sutter P, T'Sjoen G. Reproductive wish in transsexual men. Hum Reprod. 2012;27(2):483–487. doi: 10.1093/humrep/der406. [DOI] [PubMed] [Google Scholar]
- 24.Baram S, Myers SA, Yee S, Librach CL. Fertility preservation for transgender adolescents and young adults: a systematic review. Hum Reprod Update. 2019;25(6):694–716. doi: 10.1093/humupd/dmz026. [DOI] [PubMed] [Google Scholar]
- 25.Wierckx K, Stuyver I, Weyers S, Hamada A, Agarwal A, De Sutter P, T'Sjoen G. Sperm freezing in transsexual women. Arch Sex Behav. 2012;41(5):1069–1071. doi: 10.1007/s10508-012-0012-x. [DOI] [PubMed] [Google Scholar]
- 26.Stöbel-Richter Y, Beutel ME, Finck C, Brähler E. The 'wish to have a child', childlessness and infertility in Germany. Hum Reprod. 2005;20(10):2850–2857. doi: 10.1093/humrep/dei121. [DOI] [PubMed] [Google Scholar]
- 27.De Roo C, Tilleman K, T'Sjoen G, De Sutter P. Fertility options in transgender people. Int Rev Psychiatry. 2016;28(1):112–119. doi: 10.3109/09540261.2015.1084275. [DOI] [PubMed] [Google Scholar]
- 28.Light AD, Obedin-Maliver J, Sevelius JM, Kerns JL. Transgender men who experienced pregnancy after female-to-male gender transitioning. Obstet Gynecol. 2014;124(6):1120–1127. doi: 10.1097/AOG.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 29.Adeleye AJ, Cedars MI, Smith J, Mok-Lin E. Ovarian stimulation for fertility preservation or family building in a cohort of transgender men. J Assist Reprod Genet. 2019;36(10):2155–2161. doi: 10.1007/s10815-019-01558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amir H, Oren A, Klochendler Frishman E, Sapir O, Shufaro Y, Segev Becker A, Azem F, Ben-Haroush A. Oocyte retrieval outcomes among adolescent transgender males. J Assist Reprod Genet. 2020;37(7):1737–1744. doi: 10.1007/s10815-020-01815-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amir H, Yaish I, Samara N, Hasson J, Groutz A, Azem F. Ovarian stimulation outcomes among transgender men compared with fertile cisgender women. J Assist Reprod Genet. 2020;37(10):2463–2472. doi: 10.1007/s10815-020-01902-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leung A, Sakkas D, Pang S, Thornton K, Resetkova N. ART outcomes in female to male transgender patients: a new frontier in reproductive medicine. Fertil Steril. 2018;109(3):e35. doi: 10.1016/j.fertnstert.2018.02.068. [DOI] [PubMed] [Google Scholar]
- 33.Mattawanon N, Spencer JB, Schirmer DA, Tangpricha V. Fertility preservation options in transgender people: a review. Rev Endocr Metab Disord. 2018;19(3):231–242. doi: 10.1007/s11154-018-9462-3. [DOI] [PubMed] [Google Scholar]
- 34.Armuand G, Dhejne C, Olofsson JI, Rodriguez-Wallberg KA. Transgender men’s experiences of fertility preservation: a qualitative study. Hum Reprod. 2017;32(2):383–390. doi: 10.1093/humrep/dew323. [DOI] [PubMed] [Google Scholar]
- 35.Gandolfi F, Paffoni A, Papasso Brambilla E, Bonetti S, Brevini TAL, Ragni G. Efficiency of equilibrium cooling and vitrification procedures for the cryopreservation of ovarian tissue: comparative analysis between human and animal models. Fertil Steril. 2006;85(SUPPL. 1):1150–1156. doi: 10.1016/j.fertnstert.2005.08.062. [DOI] [PubMed] [Google Scholar]
- 36.Keros V, Xella S, Hultenby K, Pettersson K, Sheikhi M, Volpe A, Hreinsson J, Hovatta O. Vitrification versus controlled-rate freezing in cryopreservation of human ovarian tissue. Hum Reprod. 2009;24(7):1670–1683. doi: 10.1093/humrep/dep079. [DOI] [PubMed] [Google Scholar]
- 37.Shah JS, Sabouni R, Cayton Vaught KC, Owen CM, Albertini DF, Segars JH. Biomechanics and mechanical signaling in the ovary: a systematic review. J Assist Reprod Genet. 2018;35(7):1135–1148. doi: 10.1007/s10815-018-1180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Q, Xie Y, Wang Y, Li S. Vitrification versus slow freezing for human ovarian tissue cryopreservation: a systematic review and meta-anlaysis. Sci Rep. 2017;7(1):1–9. doi: 10.1038/s41598-017-09005-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shams Mofarahe Z, Ghaffari Novin M, Jafarabadi M, Salehnia M, Noroozian M, Ghorbanmehr N. Effect of human ovarian tissue vitrification/warming on the expression of genes related to folliculogenesis. Iran Biomed J. 2015;19(4):220–225. doi: 10.7508/ibj.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fabbri R, Vicenti R, Macciocca M, Pasquinelli G, Paradisi R, Battaglia C, Martino NA, Venturoli S. Good preservation of stromal cells and no apoptosis in human ovarian tissue after vitrification. Biomed Res Int. 2014;2014:673537. doi: 10.1155/2014/673537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bin Li Y, Zhou CQ, Yang QF, Wang Q, Dong Y. Modified vitrification method for cryopreservation of human ovarian tissues. Chin Med J. 2007;120(2):110–114. doi: 10.1097/00029330-200701020-00007. [DOI] [PubMed] [Google Scholar]
- 42.Rahimi G, Isachenko V, Kreienberg R, Sauer H, Todorov P, Tawadros S, Mallmann P, Nawroth F, Isachenko E. Re-vascularisation in human ovarian tissue after conventional freezing or vitrification and xenotransplantation. Eur J Obstet Gynecol Reprod Biol. 2010;149(1):63–67. doi: 10.1016/j.ejogrb.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 43.Amorim CA, Dolmans MM, David A, Jaeger J, Vanacker J, Camboni A, Donnez J, Van Langendonckt A. Vitrification and xenografting of human ovarian tissue. Fertil Steril. 2012;98(5):1291–8.e1-2. doi: 10.1016/j.fertnstert.2012.07.1109. [DOI] [PubMed] [Google Scholar]
- 44.Isachenko V, Isachenko E, Reinsberg J, Montag M, van der Ven K, Dorn C, Roesing B, van der Ven H. Cryopreservation of human ovarian tissue: comparison of rapid and conventional freezing. Cryobiology. 2007;55(3):261–268. doi: 10.1016/j.cryobiol.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 45.Oktem O, Alper E, Balaban B, Palaoglu E, Peker K, Karakaya C, Urman B. Vitrified human ovaries have fewer primordial follicles and produce less antimüllerian hormone than slow-frozen ovaries. Fertil Steril. 2011;95(8):2661–4.e1. doi: 10.1016/j.fertnstert.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 46.Terren C, Fransolet M, Ancion M, Nisolle M, Munaut C. Slow freezing versus vitrification of mouse ovaries: from ex vivo analyses to successful pregnancies after auto-transplantation. Sci Rep. 2019;9(1):1–13. doi: 10.1038/s41598-019-56182-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S, Ryu KJ, Kim B, Kang D, Kim YY, Kim T. Comparison between slow freezing and vitrification for human ovarian tissue cryopreservation and xenotransplantation. Int J Mol Sci. 2019;20(13):3346. doi: 10.3390/ijms20133346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sanfilippo S, Canis M, Smitz J, Sion B, Darcha C, Janny L, Brugnon F. Vitrification of human ovarian tissue: a practical and relevant alternative to slow freezing. Reprod Biol Endocrinol. 2015;13(1):1–7. doi: 10.1186/s12958-015-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dolmans MM, Cordier F, Amorim CA, Donnez J, Vander LC. In vitro activation prior to transplantation of human ovarian tissue: is it truly effective? Front Endocrinol (Lausanne) 2019;10(August):1–9. doi: 10.3389/fendo.2019.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fabbri R, Vicenti R, Macciocca M, Martino NA, Dell'Aquila ME, Pasquinelli G, Morselli-Labate AM, Seracchioli R, Paradisi R. Morphological, ultrastructural and functional imaging of frozen/thawed and vitrified/warmed human ovarian tissue retrieved from oncological patients. Hum Reprod. 2016;31(8):1838–1849. doi: 10.1093/humrep/dew134. [DOI] [PubMed] [Google Scholar]
- 51.Herraiz S, Novella-Maestre E, Rodríguez B, Díaz C, Sánchez-Serrano M, Mirabet V, Pellicer A. Improving ovarian tissue cryopreservation for oncologic patients: slow freezing versus vitrification, effect of different procedures and devices. Fertil Steril. 2014;101(3):775–784. doi: 10.1016/j.fertnstert.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 52.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 53.Kikuchi I, Kagawa N, Silber S, Kuwayama M, Takehara Y, Aono F, Kumakiri J, Kato O, Takeda S. Oophorectomy for fertility preservation via reduced-port laparoscopic surgery. Surg Innov. 2013;20(3):219–224. doi: 10.1177/1553350612449074. [DOI] [PubMed] [Google Scholar]
- 54.Chadha S, Pache TD, Huikeshoven JM, Brinkmann AO, van der Kwast TH. Androgen receptor expression in human ovarian and uterine tissue of long-term androgen-treated transsexual women. Hum Pathol. 1994;25(11):1198–1204. doi: 10.1016/0046-8177(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 55.Dunaif A, Scully RE, Andersen RN, Chapin DS, Crowley WF. The effects of continuous androgen secretion on the hypothalamic-pituitary axis in woman: evidence from a luteinized thecoma of the ovary. J Clin Endocrinol Metab. 1984;59(3):389–393. doi: 10.1210/jcem-59-3-389. [DOI] [PubMed] [Google Scholar]
- 56.Pache TD, Chadha S, Gooren LJ, Hop WC, Jaarsma KW, Dommerholt HB, Fauser BC. Ovarian morphology in long-term androgen-treated female to male transsexuals. A human model for the study of polycystic ovarian syndrome? Histopathology. 1991;19(5):445–452. doi: 10.1111/j.1365-2559.1991.tb00235.x. [DOI] [PubMed] [Google Scholar]
- 57.Spinder T, Spijkstra JJ, van den Tweel JG, Burger CW, van Kessel H, Hompes PG, Gooren LJ. The effects of long term testosterone administration on pulsatile luteinizing hormone secretion and on ovarian histology in eugonadal female to male transsexual subjects. J Clin Endocrinol Metab. 1989;69(1):151–157. doi: 10.1210/jcem-69-1-151. [DOI] [PubMed] [Google Scholar]
- 58.Hughesdon PE. Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called "hyperthecosis". Obstet Gynecol Surv. 1982;37(2):59–77. doi: 10.1097/00006254-198202000-00001. [DOI] [PubMed] [Google Scholar]
- 59.Grynberg M, Fanchin R, Dubost G, Colau JC, Brémont-Weil C, Frydman R, Ayoubi JM. Histology of genital tract and breast tissue after long-term testosterone administration in a female-to-male transsexual population. Reprod BioMed Online. 2010;20(4):553–558. doi: 10.1016/j.rbmo.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Loverro G, Resta L, Dellino M, Edoardo DN, Cascarano MA, Loverro M, Mastrolia SA. Uterine and ovarian changes during testosterone administration in young female-to-male transsexuals. Taiwan J Obstet Gynecol. 2016;55(5):686–691. doi: 10.1016/j.tjog.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 61.Sterling J, Garcia MM. Fertility preservation options for transgender individuals. Transl Androl Urol. 2020;9(Suppl 2):S215–S226. doi: 10.21037/tau.2019.09.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lierman S, Tilleman K, Braeckmans K, Peynshaert K, Weyer S. Fertility preservation for trans men : frozen-thawed in vitro matured oocytes collected at the time of ovarian tissue processing exhibit normal meiotic spindles. J Assist Reprod Genet. 2017;34(11):1449–1456. doi: 10.1007/s10815-017-0976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De Roo C, Lierman S, Tilleman DS, P. In-vitro fragmentation of ovarian tissue activates primordial follicles through the Hippo pathway. Hum Reprod Open. 2020;2020(4):1–16. doi: 10.1093/hropen/hoaa048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology In vitro maturation: a committee opinion. Fertil Steril. 2013;99(3):663–666. doi: 10.1016/j.fertnstert.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 65.Telfer EE, Zelinsk MB. Ovarian follicle culture: advances and challenges for human and non-human primates. Fertil Steril. 2013;29(6):997–1003. doi: 10.1016/j.fertnstert.2013.03.043.Ovarian. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, Schink JC, Woodruff TK. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci. 2014;21(5):582–589. doi: 10.1177/1933719113506498. [DOI] [PMC free article] [PubMed] [Google Scholar]
Further reading
- 67.Committee Opinion No685 care for transgender adolescents. Obstet Gynecol. 2017;129(1):e11–e16. doi: 10.1097/AOG.0000000000001861. [DOI] [PubMed] [Google Scholar]
- 68.Klocke S, Bündgen N, Köster F, Eichenlaub-Ritter U, Griesinger G. Slow-freezing versus vitrification for human ovarian tissue cryopreservation. Arch Gynecol Obstet. 2015;291(2):419–426. doi: 10.1007/s00404-014-3390-6. [DOI] [PubMed] [Google Scholar]