Abstract
Purpose
This systematic review aimed to identify baseline patient demographic and controlled ovarian stimulation characteristics associated with a suboptimal response to GnRHa triggering, and available options for prevention and management of suboptimal response.
Methods
PubMed, Google Scholar, Medline, and the Cochrane Library were searched for keywords related to GnRHa triggering, and peer-reviewed articles from January 2000 to September 2021 included.
Results
Thirty-seven studies were included in the review. A suboptimal response to GnRHa triggering was more likely following long-term or recent oral contraceptive use and with a low or high body mass index. Low basal serum follicle-stimulating hormone (FSH), luteinizing hormone (LH), and estradiol serum levels were correlated with suboptimal oocyte yield, as was a low serum LH level on the day of triggering. A prolonged stimulation period and increased gonadotropin requirements were correlated with suboptimal response to triggering. Post-trigger LH < 15 IU/L best correlated with an increased risk for empty follicle syndrome and a lower oocyte retrieval rate. Retriggering with hCG may be considered in patients with suboptimal response according to post-trigger LH, as in cases of failed aspiration.
Conclusion
Pre-treatment assessment of patient characteristics, with pre- and post-triggering assessment of clinical and endocrine cycle characteristics, may identify cases at risk for suboptimal response to GnRHa triggering and optimize its utilization.
Keywords: In vitro fertilization (IVF), GnRH agonist (GnRHa), Ovulation triggering, Luteinizing hormone (LH), Review
Introduction
The utilization of gonadotropin-releasing hormone agonist (GnRHa) triggering in in vitro fertilization (IVF) cycles was first introduced in the early 1990s [1]. Although an off-label use of GnRHa, it was shortly reinforced as a potential agent for the induction of the preovulatory luteinizing hormone (LH) surge, with the advantage of ovarian hyperstimulation syndrome (OHSS) prevention [2]. Numerous randomized controlled trials have since assessed the efficacy of GnRHa compared with human chorionic gonadotropin (hCG) triggering in IVF. GnRH agonist triggering has been demonstrated to be as effective as hCG for follicular maturation, with comparable live birth rates achieved in donor-recipient cycles [3]. The risk for severe OHSS was also nearly eliminated with the use of the GnRHa trigger instead of hCG. However, in fresh autologous cycles, the efficacy of GnRHa triggering is probably hampered by a resulting suboptimal luteal phase, leading to significantly reduced ongoing pregnancy and live birth rates [3]. As controlled ovarian stimulation (COS) is becoming increasingly personalized, patient selection and identification of treatment characteristics associated with successful response are of major importance when assigning the safest and most effective triggering option.
The efficacy of GnRH agonist triggering can be assessed in several ways. A complete lack of oocytes (empty follicle syndrome) is an important outcome of interest. It has been described to occur in 0.5–3.4% of IVF cycles following GnRHa triggering [4–7]. Alternative measures of success include suboptimal oocyte yield [4], oocyte recovery rate, and oocyte maturation rate [4, 8, 9]. With constant increase in its popularity and widespread use, intensive research on the subject of GnRHa triggering has recently highlighted a combination of demographic, clinical, and endocrine characteristics at different stages of the treatment process, which may affect the efficacy of the GnRHa trigger.
The objective of our review was to systematically assess the current literature regarding the efficacy of GnRHa triggering in IVF cycles. We aimed to review current strategies to predict a suboptimal response to triggering based on baseline patient characteristics, laboratory assessment, and cycle dynamics, and to review available options for the management of cases with suspected suboptimal response.
Materials and methods
Protocol and registration
The protocol of the present study was registered at the PROSPERO registry (http://www.crd.york.ac.uk/PROSPERO), an international database for the prospective registration of systematic reviews in health and social care (PROSPERO ID: CRD42021228275).
This study was exempt from Institutional Review Board approval, as it was a systematic review. The study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10].
Search strategy
We conducted a systematic search of the literature published in the main databases—Medline/PubMed, Google Scholar, Embase, and Cochrane Library, from January 2000 until September 2021. We included all original English, peer-reviewed articles, irrespective of study design. Case reports and conference abstracts were excluded. The search strategy included keywords related to GnRH triggering, so that results were required to include a combination of two words—one from a first predefined list and another from a second. The first list of words included gonadotropin-releasing agonist, gonadotropin-releasing analog, GnRH agonist, GnRHa, GnRH-a, leuprolide, leuprolide acetate, triptorelin, buserelin, and nafarelin. The second list of words included trigger, triggering, oocyte maturation, oocyte maturity, final oocyte maturation, follicular maturation, ovulation triggering, oocyte recovery rate, oocyte maturity rate, and empty follicle syndrome. References were hand-searched to identify additional studies not covered by the literature search.
All citations were screened for eligibility by two authors (authors H.G.H. and A.W.) in a non-blinded fashion. Any discrepancies were resolved by discussion and, when needed, a consensus was reached with the help of third author (A.R.).
For the purpose of this study, we included all studies, in which the efficacy of GnRHa triggering in IVF was assessed. All types of GnRH agonists were eligible for inclusion.
Data collection
The following data were extracted from the selected studies: study design, number of participants, study population, baseline follicle-stimulating hormone (FSH) and LH levels, recent oral contraceptive use, patient age and BMI, stimulation parameters (stimulation duration and total gonadotropin dose number of days of antagonist administration), serum LH, FSH estradiol and progesterone (P) levels on trigger day, time interval between last GnRH antagonist dose and GnRHa trigger, time interval between GnRHa trigger and oocyte retrieval, post-trigger LH and P levels, lack of oocytes (empty follicle syndrome), number of metaphase II (MII) oocytes, suboptimal oocyte yield, oocyte recovery rate, and oocyte maturation rate.
Quality assessment
The level of evidence of the included studies was assessed using the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system [11].
Results
Search results
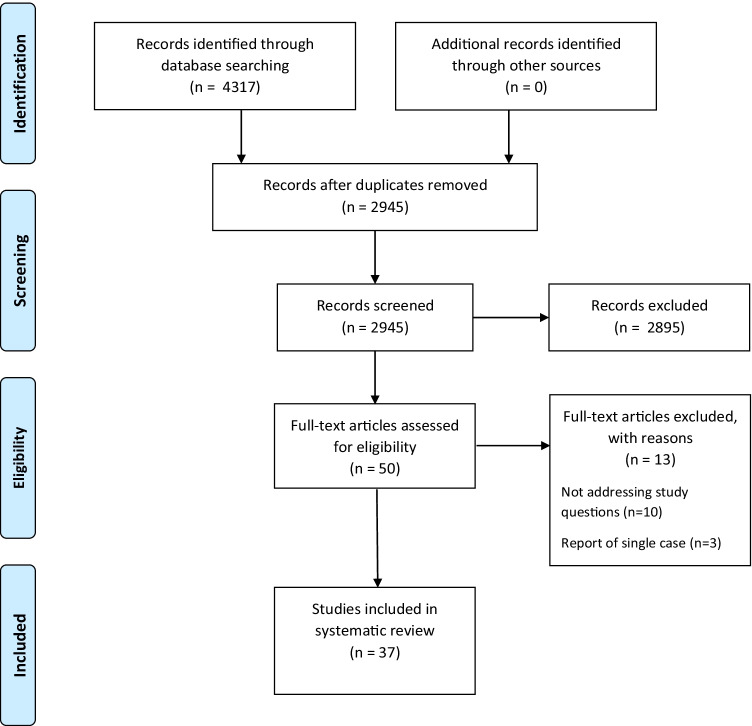
Our systematic search generated 4317 results, of which 2945 remained after removal of duplicates, and screened according to title and abstract. The full texts of 48 studies were reviewed, including a review of all reference lists for additional suitable records. A total of 37 studies were selected to be included in the review. The review of reference lists did not yield any additional suitable records for inclusion. A PRISMA flow chart diagram for our search process is presented in Fig. 1. A summary of the selected studies is presented in Table 1.
Fig. 1.
PRISMA flow diagram of search results
Table 1.
Assessing the efficacy of the GnRH agonist trigger—characteristics of the included studies
| Study | N | Population | Design | Parameter examined | Quality of evidencea |
|---|---|---|---|---|---|
| Abbara et al. [23] | 151 | Oocyte donors | Retrospective cohort study | Post-trigger endocrine characteristics | Low |
| Aflatoonian et al. [34] | 120 | PCOS | RCT | Repeat GnRH-a administration | Moderate |
| Asada et al. [46] | 8 | PCOS, unexplained infertility, male factor | Case series | HCG administration after failed retrieval | Very low |
| Blazquez et al. [15] | 12483 | Oocyte donors | Retrospective cohort study |
Incidence of empty follicle syndrome HCG administration after failed retrieval |
Low |
| Castillo et al. [7] | 3467 |
Oocyte donors IVF patients < 35 |
Retrospective cohort study | Incidence of empty follicle syndrome | Low |
| Chang et al. [17] | 1878 | High responders | Retrospective cohort study |
Background, cycle base line, stimulation, and post-trigger characteristics HCG administration after failed retrieval |
Low |
| Chen et al. [24] | 91 | High risk of OHSS—PCOS or previous OHSS/high response | Prospective cohort study | Post-trigger characteristics | Low |
| Christopoulos et al. [14] | 322 | High risk of OHSS—E2/follicles | Case series |
Incidence of empty follicle syndrome HCG administration after failed retrieval |
Very low |
| Cozzolino et al. [45] | 359 | Oocyte donors | Prospective cohort study | HCG/dual triggering after failed retrieval | Low |
| Deepika et al. [35] | 125 | PCOS | RCT | Repeat GnRH-a triggering | Moderate |
| Deepika et al. [13] | 271 | PCOS | Retrospective cohort study |
Incidence of empty follicle syndrome Cycle base line, stimulation, and post-trigger characteristics HCG/repeat GnRH-a triggering after failed retrieval |
Low |
| Dunne et al. [44] | 97 | High risk of OHSS—E2/follicles, previous OHSS, PCOS, and oocyte donors | Retrospective cohort study | Post-trigger endocrine characteristics | Low |
| Fauser et al. [32] | 32 | 18–39 Y/O, ovulatory, normal BMI | RCT | GnRH-a type for triggering | Low |
| Griffin et al. [43] | 102 | Age < 40, peak E2 < 4000 pg/mL, previous OHSS/cancellation d/t OHSS risk, >13 follicles > 11 mm | Retrospective cohort study | Dual trigger in high responders | Low |
| Gülekli et al. [29] | 21 | PCOS, male factor, tubal | Case-control study | GnRH-a dose for triggering | Very low |
| Gunnala et al. [41] | 10427 | Fresh cycles for which a sliding scale of HCG was used according to E2 levels | Retrospective cohort study | Dual trigger with partial HCG dose | Low |
| Hershkop et al. [33] | 220 | ICSI cycles | Retrospective cohort study | Trigger to ovum pick up interval | Low |
| Honnma et al. [47] | 2 | PCOS | Case series | HCG triggering after failed retrieval | Very low |
| Horowitz et al. [8] | 53 | Normogonadotropic | Retrospective cohort study | Trigger to ovum pick up interval | Low |
| Itskovitz-Eldor et al. [9] | 8 | High risk of OHSS—E2/follicles | Case series | Maturation rate | Very low |
| Kummer et al. [26] | 316 | High risk of OHSS—follicles, previous OHSS | Retrospective cohort study | Peak E2 levels | Low |
| Kummer et al. [6] | 508 | High risk of OHSS—follicles, previous OHSS | Retrospective cohort study |
Incidence of empty follicle syndrome Stimulation and post-trigger characteristics |
Low |
| Lainas et al. [12] | 113 | High risk of OHSS—follicles | Prospective cohort study | Body mass index | Low |
| Lainas et al. [27] | 131 | High risk of OHSS—follicles | Retrospective cohort study | GnRH-a dose for triggering | Low |
| Lu et al. [16] | 8970 | Not limited | Retrospective cohort study |
Background, cycle base line, stimulation, and post-trigger characteristics Dual trigger in suboptimal responders |
Low |
| Maslow et al. [25] | 959 | Planned oocyte cryopreservation, baseline LH > 2.5 IU/L | Retrospective cohort study | Peak E2 levels | Low |
| Meyer et al. [18] | 500 | Not limited | Retrospective cohort study | Background and stimulation characteristics | Low |
| O’Neill et al. [5] | 114 | High responders | Retrospective cohort study | Effect in PCOS patients | Low |
| Orvieto et al. [21] | 34 | High risk of OHSS, with E2 > 2000 pg/mL | Retrospective cohort study | Stimulation protocol | Low |
| Orvieto et al. [22] | 32 | E2 > 10000 pmol/L, >15 follicles > 10 mm, or planned freeze all | Retrospective cohort study | Stimulation protocol | Low |
| Pabuccu et al. [30] | 77 | High risk of OHSS—E2, follicles | Retrospective cohort study | GnRH-a dose for triggering | Low |
| Pereira et al. [42] | 156 | Prior fertilization rate < 40% in fresh ICSI cycle with HCG trigger | Retrospective cohort study | Dual trigger with partial HCG dose | Low |
| Popovic-Todorovic et al. [4] | 3334 | Not limited | Retrospective cohort study | Background, cycle base line, and stimulation characteristics | Low |
| Shapiro et al. [19] | 252 | High risk of OHSS—E2, follicles, history | Retrospective cohort study | Post-trigger endocrine characteristics | Low |
| Şükür et al. [31] | 137 | High risk of OHSS—E2 | Retrospective cohort study | GnRH-a type for triggering | Low |
| Vuong et al. [28] | 165 | Oocyte donors | RCT | GnRH-a dose for triggering | Moderate |
| Zarcos et al. [20] | 40 | Oocyte donors | RCT | GnRH-a dose for triggering | Low |
RCT, randomized clinical trial; PCOS, polycystic ovary syndrome; OHSS, ovarian hyperstimulation syndrome; E2, estradiol; Y/O, years old
aAccording to the Grading of Recommendation, Assessment, Development and Evaluation (GRADE) system
What are the means available to assess the efficacy of GnRH agonist triggering?
To assess the effect of GnRHa triggering on oocyte yield, and best express the extent of optimal utilization of potential follicles, two measures have been studied: oocyte recovery rate and suboptimal oocyte yield. Oocyte recovery rate was defined as the ratio between the total number of oocytes collected and the number of follicles with a mean diameter of ≥10–11 mm on the day of the trigger [4, 8, 12], and suboptimal oocyte recovery rate was defined as an oocyte yield below the <10th percentile of oocyte yield [4]. Following retrieval, oocyte maturation rate was defined as the ratio between the number of MII oocytes and the number of oocytes retrieved [4, 8, 9, 12]. Another “endpoint” outcome assessed in many studies was the incidence of empty follicle syndrome, which is a complete lack of oocytes aspirated from follicles. This has been demonstrated to occur in about 1.2% of patients (range 0.5 to 3.4%) triggered with GnRHa [4–7, 13–15] (Table 2).
Table 2.
Incidence of empty follicle syndrome in cycles triggered with GnRH agonists
For the purpose of the review, we will refer to “suboptimal response” to triggering, as any negative endpoint, including suboptimal oocyte recovery and empty follicle syndrome as discussed above, or an LH level following triggering below the anticipated level, as discussed further below. We used this term to address different studies, which employed variable outcome measures, all aimed to express an unsatisfactory result.
In summary—the outcomes available to assess triggering efficacy are oocyte recovery rate, suboptimal oocyte yield, empty follicle syndrome and, for the purpose of the review, suboptimal response to triggering.
What are the background patient characteristics associated with a suboptimal response to GnRH agonist triggering?
Several background patient characteristics have been studied regarding their effect on GnRHa triggering. Patient age was found non-associated with triggering efficacy by some [16, 17], while others have noted a higher average age in the group of suboptimal responders [4, 18, 19]. Similarly, BMI was not found correlated with triggering efficacy by some [4, 15, 18], while others have noted a correlation between the two. Lu and colleagues found a lower average BMI among women with an adequate response to triggering (LH > 15 mIU/mL following triggering) [16], in line with Lainas et al., who noted a comparable retrieval rate but higher number of MII oocytes when patient BMI was lower than 25 kg/m2 [12]. In contrast, Chang et al. noted that the failure rate, defined by laboratory parameters (post-trigger serum LH level <15 mIU/m and serum P <3 ng/m) or failed retrieval, was twice as high when patient BMI was <22 kg/m2 [17]. However, one must note that the total number of failures with triggering in the latter study (38/1878 cycles) further sub-analyzed according to BMI was inadequate for conclusion.
Recent oral contraceptive use was more common in suboptimal responders to triggering [4, 18], although not in cases of empty follicle syndrome [15]. Menstrual regularity was inconclusively tied to triggering efficacy [4, 18]. One study found that suboptimal responders were more likely to be oocyte donors [18], but another found otherwise [19]. Finally, in a study by O’Neill and colleagues, the response to GnRHa triggering in polycystic ovary syndrome (PCOS) patients and controls was equally effective, according to both post-triggering laboratory parameters and oocyte maturation rate [5].
In summary—a high or low BMI and recent or prolonged oral contraceptive pill use have been found correlated to a suboptimal response to triggering, while PCOS has not.
Quality of evidence: low.
What are the pre-treatment characteristics (upon cycle initiation) associated with a suboptimal response to GnRH agonist triggering?
A number of laboratory parameters at initiation of stimulation have been investigated in regard to triggering efficacy. Lower baseline LH and FSH [4, 16–18] and estradiol levels [4, 17] were noted as correlated with inadequate response to triggering. In a study by Popovic-Todorovic et al., the risk for an inadequate oocyte yield following triggering was stratified according to basal LH—patients with immeasurable levels (<0.1 IU/L) had a 45.2% risk of suboptimal response, while a basal LH of up to 5 IU/L was associated with 13.6% risk [4]. This linear correlation between basal LH and triggering failure was reaffirmed by Chang et al. and Meyer et al. [17, 18]. Lu et al. further noted basal LH to be the single most valuable marker for identifying suboptimal responders [16]. Notably, in a study by Deepika et al. comparing PCOS patients triggered with GnRHa with empty follicle syndrome (n = 9) to normal responders (n = 262), no correlation was found with basal FSH, antral follicle count, and anti-Mullerian hormone level [13]. However, basal serum LH levels were not included in the assessment and the numbers were too low to allow for sufficient power to draw firm conclusions.
In summary—low basal serum LH levels have been found correlated to a suboptimal response to triggering.
Quality of evidence: low.
Does the type of stimulation protocol affect GnRHa triggering efficacy?
GnRHa triggering would seem applicable to four main stimulation protocols: classic antagonist cycles, stimulation cycles without any form of pituitary suppression, cycles with progesterone primed pituitary suppression (PPOS), and cycles in which GnRHa is stopped at an early stage of stimulation (ultrashort/stop protocol). Thirty-five of the 37 studies included used GnRH antagonists for pituitary suppression, while one study did not specify [20], and one used PPOS (medroxyprogesterone acetate or utrogestan) [16]. While a direct comparison of GnRHa trigger efficacy between antagonist and PPOS treatment cycles is not available, it appears that suboptimal response characteristics are comparable for both protocols. Notably, two studies included ultrashort GnRHa administration with subsequent GnRH antagonist administration [21, 22], and both proved effective for GnRHa triggering with similar clinical outcomes as compared to dual (hCG and GnRHa) triggering.
In summary—stimulation protocol type does not seem to influence the efficacy of GnRHa triggering.
Quality of evidence: low.
Which stimulation variables and trigger day characteristics are associated with a suboptimal response to GnRH agonist triggering?
The duration of stimulation and cumulative dose of gonadotropins administered have repeatedly been found to be correlated with triggering efficacy, so that a longer stimulation with higher doses of gonadotropins is more likely associated with a suboptimal response [4, 13, 15–19]. Chang et al. noted a failure rate of more than 3.5-fold among patients treated with >3800 IU of gonadotropins as compared with those treated with <3800 IU—5.0% versus 1.4% [17].
LH and FSH levels on the morning before triggering were found to be correlated with triggering success [18, 23], so that lower levels of both were associated with an inadequate response to triggering. Meyer et al. reported that patients with an undetectable serum LH level on the day of trigger had a 25% chance of a suboptimal LH surge [18]. In their study, limiting the use of the GnRHa trigger alone to patients with a trigger day serum LH >0.5 mIU/mL would have reduced the rate of suboptimal response from 5.2 to 0.2%. However, this conclusion was not supported by all [4, 16, 24].
With regard to peak serum estradiol levels, results are conflicting. Some have noted an inverse relation between the two, so that lower estradiol levels are associated with a higher risk of suboptimal response to triggering [4, 6, 16], although in a small cohort investigating empty follicle syndrome occurrence in PCOS patients, the opposite was found [13]. In contrast, several authors have failed to demonstrate a significant association between peak estradiol and triggering outcome [17–19, 25, 26]. Finally, no definitive correlation to follicle size on triggering was noted [17, 19], although an inverse relationship may exist between the number of intermediate follicles and empty follicle syndrome in PCOS patients [13].
In summary—a longer stimulation with higher gonadotropin requirements has been correlated to suboptimal response to triggering. A low baseline LH level is correlated to suboptimal response to triggering, while peak estradiol levels are not.
Quality of evidence: low.
Is there an optimal GnRHa dose for triggering?
Five studies have directly evaluated different GnRHa doses for triggering—four compared different doses of triptorelin (0.1 to 0.4 mg) [20, 27–29], and an additional study compared two doses of leuprolide (1 and 2 mg) [30]. A small comparison of fresh cycles triggered with 0.1 mg (n = 7) and 0.2 mg (n = 14) triptorelin did demonstrate less immature oocytes with the 0.1 mg dose but comparable oocyte recovery rate and number of mature oocytes in both groups [29]. Overall, most studies, with more adequate sample size, did not find a correlation between GnRHa dose and oocyte maturation rates and doses of triptorelin of 0.1–0.4 mg appear to be equally effective. To further support this conclusion, in a comparison of suboptimal responders (post-trigger LH < 15 mIU/mL) to adequate responders, no difference was found in the rate of patients triggered with 2 or 4 mg leuprolide [18].
In summary—different GnRHa doses are not correlated to triggering efficacy.
Quality of evidence: low.
Is there a preferred GnRHa type for triggering?
Our systematic search yielded two studies evaluating the type of GnRHa used for triggering [31, 32]. In both studies, patients were administered 0.2 mg of triptorelin or 0.5–1 mg of leuprolide and were compared to controls triggered with hCG. No difference in the number of MII oocytes retrieved was noted between the triptorelin and leuprolide groups.
In summary—different GnRHa types are not correlated to triggering efficacy.
Quality of evidence: low.
Is there an ideal time interval between GnRH antagonist administration and GnRHa triggering, and between triggering and oocyte retrieval?
As GnRH antagonists occupy the pituitary GnRH receptors by competitive inhibition, it has become common practice to maintain a minimum time interval between the last antagonist dose and the agonist trigger so that the antagonist molecules can be displaced by the GnRHa and an effective trigger can occur. Horowitz and colleagues investigated the effect of the interval between the last antagonist dose and agonist trigger on treatment outcomes, in four study groups which varied from >2.5 to <7 h [8]. They did not find a correlation between the interval, oocyte recovery rate and MII oocyte rate.
In a study by Hershkop et al., patients triggered with GnRHa were compared based on the lag time between triggering and oocyte aspiration, which varied from 34 to 41 h [33]. While the authors did note a slight difference in the number of MII oocytes aspirated between the two intermediate interval groups in favor of a longer interval, overall, no difference was demonstrated in the proportion of MII oocytes and the number of cycles with >70% MII oocytes.
In summary—different time intervals between GnRH antagonist and GnRHa administration, and GnRHa and oocyte retrieval are not correlated to triggering efficacy.
Quality of evidence: low.
Does a repeat dose of GnRHa trigger improve outcomes?
Two randomized controlled trials have explored the efficacy of administering a repeat dose of GnRHa trigger, in an attempt to mimic the amplitude and duration of the gonadotropin surge, and consequently affect oocyte maturation. Both randomized PCOS patients to receive 0.2 mg triptorelin 35 h prior to oocyte retrieval, versus 0.2 mg triptorelin and an additional 0.1 mg dose 12 h later. In the first, the results of 100 patients randomized were analyzed [34]. Despite a trend for more MII oocytes with repeat trigger, no differences were noted in maturity rate. In the second, the results of 100 cycles analyzed did demonstrate a significantly lower maturity rate with a single dose of GnRHa administration (OR 0.47, 95% CI 0.38–0.57, p < 0.001) [35].
In summary—the evidence regarding the efficacy of repeat GnRHa triggering is conflicting.
Quality of evidence: moderate.
Does dual triggering (GnRHa and hCG) offer an advantage over triggering with GnRHa?
While the quality of evidence is currently low, dual triggering with both GnRHa and full dose hCG has been demonstrated by several authors to be associated with improved maturation and fertilization rates [36–38], and a higher rate of clinical pregnancies and live births [39]. Recently, the addition of a reduced dose of hCG to the GnRHa trigger has been suggested as a means of improving the efficacy of the GnRHa trigger. Lu et al. examined 229 suboptimal responders to trigger (LH < 15), of whom 198 were triggered with GnRHa and three different doses of hCG—1000, 2000, and 5000 IU, and 31 only with GnRHa alone [16]. The oocyte retrieval rate in the dual trigger group was significantly higher than the GnRHa group, and no difference was noted between the different doses of hCG. Notably, two cases of severe OHSS were noted with dual trigger with 5000 IU of hCG. The concept of incorporating a partial dose of hCG with GnRHa trigger is further supported by additional studies—Shapiro et al. noted improved implantation and pregnancy rates with dual triggering, with an hCG dose adjusted according to the patient’s BMI [40]. Only one case of clinically significant OHSS was noted in the group of 182 who received dual trigger. In addition, a sliding scale hCG trigger was investigated in a cohort of 10,427 cycles, triggered with a dose of 3300–10000 IU of hCG or with a dual trigger of GnRHa and 1500 IU hCG, according to serum estradiol levels [41]. The authors found a higher rate of mature oocytes with dual trigger as compared to low dose hCG, although no differences in pregnancy and live birth rates were noted. Four patients (0.03%) developed severe OHSS, who were triggered with 4000–5000 IU of hCG. Pereira et al. retrospectively compared 156 patients with <40% fertilization rate in a prior ICSI cycle with standard hCG trigger who underwent another ICSI cycle with a combined 2 mg leuprolide and 1500 IU hCG ovulatory trigger [42]. Significantly more mature oocytes were retrieved in the dual trigger group compared with the hCG trigger group. The fertilization, clinical pregnancy, and live birth rates were all improved in the dual trigger group. It was suggested that combined GnRHa and hCG trigger in ICSI cycles may increase oocyte maturity, thereby increasing fertilization and improving ICSI cycle outcomes in patients with a history of poor fertilization after standard hCG trigger alone. Finally, in a report by Griffin and colleagues, high responders at risk for OHSS were triggered with either GnRHa alone or GnRHa with 1000 IU hCG [43]. Live birth, clinical pregnancy, and implantation rates were significantly higher in the dual trigger group, with only one case of mild OHSS in this group.
In summary—dual trigger with a full or partial dose of hCG improves laboratory and clinical outcomes but has been associated with case reports of OHSS.
Quality of evidence: low.
Which post-trigger endocrine characteristics predict a suboptimal response to trigger?
One of the most studied variables in correlation with GnRHa triggering efficacy is post-triggering serum LH level. The correlation between the two has become a natural assumption by many, so that many studies investigating GnRHa triggering efficacy have used post-triggering LH level as a reflection of the extent of response to triggering [6, 16–19, 24].
Two studies to date have failed to demonstrate an association between post-triggering serum LH levels and oocyte maturation. In the first by Abbara et al., LH rise following triggering was not associated with the number of mature oocytes in general, except for patients with lowest and highest LH levels, 4 h following triggering [23]. Similarly, in a cohort of 97 patients, Dunne and colleagues did not find LH level or post-trigger rise as predictive of oocyte maturation [44].
In contrast, several studies have established a clear correlation between post-triggering LH, commonly assessed 12 h following administration, and treatment outcomes. One study found a lower LH level post-triggering in patients with empty follicle syndrome [13], while another found that in patients with a LH <15 IU/L, the incidence of empty follicle syndrome was as high as 18.8%, as compared with no cases when LH was >15 IU/L, p < 0.01 [6]. Three independent groups demonstrated a higher oocyte yield when LH post-triggering was >15 IU/L [16, 24] or >52 IU/L [19]. Two studies demonstrated an increase in the total number of oocytes and MII oocytes retrieved when LH post-trigger was >15IU/L, although the oocyte maturation rate was similar [6, 16]. Overall, a LH <15 IU/L post-trigger was demonstrated in 2.7% (range 2.0–5.4%) of patients triggered with GnRHa (Table 3).
Table 3.
Incidence of suboptimal response to trigger—LH < 15 IU/mL following triggering
Post-triggering P level has also been studied. While one study did not demonstrate a lower P level in suboptimal responders [6], three studies noted a significantly higher P level in optimal responders [13, 17, 23]. Abbara et al. found the rise in P level following triggering to be the strongest laboratory predictor of the number of mature oocytes retrieved [23].
In summary—a low post-trigger LH is correlated to suboptimal response to triggering, with a common cutoff of 15 IU/L. Low progesterone is also correlated to suboptimal response to triggering.
Quality of evidence: low.
Can a suboptimal response to the GnRH agonist trigger be corrected?
Retriggering based on LH rise
Chang et al. described 22 cycles retriggered with hCG based on suboptimal LH rise following triggering (<15 IU/mL) [17]. These patients subsequently had an average 17 oocytes retrieved, and half delivered. Kummer et al. described five cases of lack of a LH rise, of which two were retriggered—retriggering with hCG yielded 15 oocytes in one case, while retriggering with GnRHa was unsuccessful the other [6].
Self-monitoring for the presence of LH secretion in urine post-triggering, to identify cases of lack of response, has been suggested by some [28, 45]. This approach potentially offers a non-expensive and accessible screening tool, to select patients who require further assessment with serum LH measurement, in case of a negative urine test. In a prospective study of 359 oocyte donors, post-triggering assessment of serum LH was required in only three patients with a negative urine LH test 12 h following triggering [45]. Two subsequently received dual triggering with GnRHa and hCG and underwent successful oocyte retrieval. In an additional report [28], only one of 165 patients required serum LH evaluation due to a negative urine test 4 h following triggering. Serum LH was found satisfactory, so that no additional triggering was administered and the patient underwent successful oocyte retrieval.
Retriggering following failed retrieval
Blazquez et al. described 17 cases of failed retrieval, who were retriggered [15]. Four cases with a LH level < 8 IU/L on retrieval day were retriggered with GnRHa, one successfully, and 13 cases with a LH > 8 IU/L on retrieval day were retriggered with hCG, 12 for whom oocytes were retrieved. Chang et al. reported 16 cycles in which oocyte retrievals were attempted but aborted after aspiration of several follicles without successful recovery of oocytes [17]. These patients were then retriggered with the use of hCG and underwent a second retrieval attempt 36 h later. In all cases, oocytes were successfully recovered. Notably, aspirating more than seven follicles prior to abandoning retrieval (and retriggering) was associated with less optimal outcomes [17]. Similarly, others have described successful retrieval after retriggering with hCG after initial failed trigger [13, 14, 46, 47].
In summary—retriggering with hCG following insufficient LH rise post-triggering or failed aspiration may be beneficial.
Quality of evidence: low.
Discussion
Since its initial introduction in IVF, the use of GnRHa for oocyte maturation triggering has become a popular choice in a variety of clinical indications. Its widespread use most probably stems from its comparable efficacy to hCG and its improved safety profile, as it virtually eliminates the risk for severe OHSS [48]. However, it is possible that not all patients are equally responsive to GnRHa triggering in terms of oocyte yield and maturation. Therefore, early identification of variables associated with suboptimal response can facilitate the decision-making process by physicians, and potentially optimize treatment results. Currently, there is no agreement on a protocol to determine the adequacy of the GnRHa trigger.
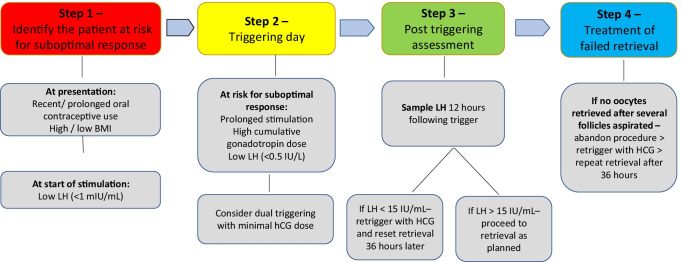
The objective of our systematic review was to identify and summarize, in a step-by-step manner, clinical and laboratory parameters that may affect the efficacy of GnRHa triggering and to suggest potential treatment strategies in cases with suboptimal response. We identified 37 studies eligible for inclusion and described their findings above. The majority of studies available were of low quality, as further discussed. However, based on the existent data to date, we propose the following four-step strategy for optimizing the efficacy of GnRHa triggering (Fig. 2).
Fig. 2.
Proposed four-step strategy for optimizing the efficacy of GnRHa triggering
Identification of the patient at risk for suboptimal response to triggering
Expect possible suboptimal response to GnRHa triggering in patients with recent/long-term oral contraceptive use [4, 18], and patients with high [12, 16] or low BMI [17].
Low serum LH levels at beginning of stimulation have been repeatedly and significantly associated with suboptimal response and a higher risk of failed retrieval [4, 16–18]. It is suggested that a LH cutoff level of <1 mIU/mL can define the patient at high risk for suboptimal response to GnRHa triggering.
Triggering
On the day of triggering—suspect a higher risk for suboptimal response in cycles with prolonged stimulation and high cumulative gonadotropin dose administered [4, 13, 15–19]. Patients with low or undetectable LH levels on the day of the trigger are also at risk for a suboptimal response [18, 23]. It is suggested that an LH cutoff level of 0.5 IU/L can define the patient at high risk for suboptimal response to GnRHa triggering.
There is paucity of data comparing the efficacy of different GnRHa types for triggering ovulation. In addition, except for triptorelin, dose-finding studies for the GnRHa preparations that are commonly used for triggering (leuprolide and buserelin) rarely exist. There appears to be no advantage for any of the GnRHa types [31, 32] and different doses [20, 27–30]. Doses of 0.1–0.4 mg of triptorelin or 1–2 mg of leuprolide appear to be equally effective.
In normogonadotropic patients, a GnRH agonist trigger can successfully induce an effective LH surge and oocyte maturation and release, irrespective of the time interval between the last antagonist dose and the agonist trigger [8].
There is insufficient data regarding the optimal interval from GnRHa triggering to oocyte retrieval. A single retrospective study has found an interval of 34–41 h to be equally effective [33].
There is insufficient data to support retrigger with a second dose of GnRHa 12 h following the initial trigger.
Post-triggering assessment
Past research has evaluated the expected serum concentration of LH and P following GnRHa triggering [17, 23, 32]. LH levels have been demonstrated to rapidly rise following GnRHa administration, peak at 4 h and return to baseline on the day of oocyte retrieval [32]. The increase in serum P levels, on the other hand, was found to be less pronounced and relatively stable from triggering to oocyte retrieval [32].
Blood sampling for serum LH and P levels 12 h after triggering has been suggested as an effective clinical tool to assess the response to the trigger. Due to a large diurnal variability of timing the triggering according to the scheduled time of oocyte retrieval, blood sampling 12 h post-trigger is not always feasible. In order to compensate for that, an exponential decay model to estimate LH values at 12 h post-trigger has been suggested [19], but is not widely used. It is our recommendation to assess serum LH levels 12 h following triggering, although earlier assessment may be considered in select cases, as LH peak occurs earlier.
The role of post-trigger P levels in the assessment of GnRHa trigger efficacy awaits further clarification. No clear threshold for adequate P level following triggering was demonstrated [13, 17, 23]. Progesterone levels reflect the degree of luteinization of the follicles in response to the GnRHa trigger but may be subjected to variability by the time interval from the trigger to blood sampling and from the number of corpora lutea present, a fact which makes the results difficult for interpretation. The higher the number of follicles, the greater the serum P level [17]. Therefore, unless used for research purposes, routine post-trigger blood sampling for P cannot be recommended.
It remains to be determined whether post-trigger assessment should be reserved for patients suspected to be at risk for suboptimal response or should be universally applied. Although patient friendly and potentially cost saving, insufficient data exists to suggest performing post-trigger urinary LH testing and subsequent serum testing as indicated. A post-trigger serum LH level >15 IU/mL should be used as the threshold to define an adequate response and in most of the limited studies available lower levels have been correlated with inferior retrieval results [6, 13, 16, 24]. In the event of an LH < 15 IU/mL, retriggering with hCG seems reasonable, with subsequent retrieval differed to 36 h following retriggering [17]. The increased risk for OHSS associated with hCG triggering should be taken into consideration before the final decision is made. The minimal effective dose of hCG for this indication has yet to be determined.
Treatment of patients with failed retrieval
In light of the limited number of studies investigating retriggering following failed retrieval with hCG (52 patients in all of the aforementioned studies) as compared to GnRHa (7 patients), and relatively higher success with hCG retrigger as described, retriggering with hCG may be considered. Concerns regarding the risk of OHSS seem justified, as suboptimal responders to triggering may display similar peak estradiol levels during stimulation [4, 6, 16], and GnRHa triggering is commonly applied to reduce the risk for OHSS. Indeed, the authors identified one case of severe OHSS following retriggering with hCG which has been so far described [14]. Yet, in freeze-all cycles, the risk for severe OHSS is further reduced since no embryo transfer is performed. The minimal effective dose of hCG that should be given for retriggering has yet to be determined, with special attention to the resulting risk for OHSS. Partial aspiration from one ovary (prior to abandoning the procedure) may also reduce the risk for severe OHSS [49].
Identification of the patient at risk for suboptimal response to triggering
A suboptimal LH surge has been observed under a variety of clinical situations described in our study such as previous oral contraceptive use, prolonged ovarian stimulation with increased gonadotropin requirements, deviation from optimal BMI indices, and decreased basal and trigger day serum LH levels. Common to all of the above conditions in the event of a suboptimal trigger is the lack of an adequate response of the pituitary gonadotrophs to the GnRHa bolus which results in failure to elicit an effective endogenous LH surge. In addition, although not addressed in past studies, patients with “induced” hypogonadotropic hypogonadism such as those with recent long-acting use of a GnRH agonist products or continuous hormonal treatment for endometriosis should be also considered at risk for suboptimal response to GnRHa triggering. The pathophysiology of suboptimal response to the GnRHa trigger in normogonadotropic patients is currently unknown. Evaluation of pituitary function during COS is limited by the administration of exogenous gonadotropins and high serum level of ovarian steroids. Dynamic testing of pituitary function, for example, by means of the GnRH test [50], is not possible during COS, and performing the GnRH test during pre-treatment evaluation is most probably cumbersome and not cost effective. Honnma et al. reported on two patients with PCOS with multifollicular development who had a normal response to a GnRH test at the pre-treatment phase but empty follicles during oocyte retrieval [47]. Retriggering with hCG resulted in a successful retrieval of oocytes in both cases, suggesting that different conditions may prevail during COS and that normal pituitary response to a GnRH test does not ensure subsequent optimal response to a GnRHa trigger. More research should be directed to developing methods for the assessment of pituitary function during COS.
Our study is not without limitations, the major of which being the lack of high-quality randomized controlled trials addressing the review questions. Most studies included were retrospective cohorts, with several case series. This, unfortunately, precluded us from conducting a meta-analysis and certainly limits the validity of our conclusions. In addition, many studies compared different variables associated with suboptimal response, and presented them as averages [6, 16, 24]. While this did allow for our understanding of the influence of these variables on cycle outcomes, it did not allow the authors to establish clear cutoff values on which to base specific recommendations (for example, which duration of stimulation or gonadotropin consumption should raise suspicion of potential suboptimal response to triggering). Notably, further information was not available for all outcomes investigated, included the important differentiation between genuine empty follicle syndrome and false cases, such as those following improper administration of medication. The incidence of genuine empty follicle syndrome is probably lower than the overall incidence depicted, and needs to be weighed against the low but clinically significant risk for ovarian hyperstimulation syndrome.
The strength of the current review is that it provides clinicians with a comprehensive step by step approach to identify patients at risk for a suboptimal response to GnRHa triggering, and strategies for prevention or correction once a suboptimal response has been identified. With the increased utilization of the GnRHa trigger that we are currently witnessing, increasing physicians’ awareness and providing them with a simplified tool for optimizing outcomes in cycles with GnRHa triggering is of utmost importance.
In conclusion, the utilization of GnRH agonists for triggering final oocyte maturation in IVF cycles is effective and safe. It may, however, be associated with suboptimal results in some patients and under certain clinical circumstances. Early identification of patients at-risk, proper post-trigger assessment and individualized management with hCG retrigger, may improve cycle outcome. By employing this approach, our patients can benefit from OHSS risk reduction in most cases, with a carefully selected small subgroup of patients who require dual or repeat hCG triggering. Considering the effective vitrification techniques available today [51], even in the event of hCG administration, the risk for severe OHSS is significantly reduced with the freeze-all approach, without risking cycle cancelation and the need for repeat unplanned stimulated cycles. Yet, the majority of our findings and the application of our proposed model remain to be proven in a prospective, preferably randomized setting. We strongly encourage future standardization of trigger efficacy assessment in future studies, adhering to select outcome measures such as oocyte recovery and maturity rate.
Acknowledgements
The authors thank Ruth Suhami from Tel Aviv University for her valuable assistance in performing the systematic literature search.
Author contribution
All authors contributed to conception and design, acquisition of data, analysis and interpretation of the data, drafting of the article, and final approval of the version to be published.
Data availability
As per request from corresponding author.
Code availability
Not applicable.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gonen Y, Balakier H, Powell W, Casper RF. Use of gonadotropin-releasing hormone agonist to trigger follicular maturation for in vitro fertilization. J Clin Endocrinol Metab. 1990;71:918–922. doi: 10.1210/jcem-71-4-918. [DOI] [PubMed] [Google Scholar]
- 2.Itskovitz J, Boldes R, Levron J, Erlik Y, Kahana L, Brandes JM. Induction of preovulatory luteinizing hormone surge and prevention of ovarian hyperstimulation syndrome by gonadotropin-releasing hormone agonist. Fertil Steril. 1991;56:213–220. [PubMed] [Google Scholar]
- 3.Youssef MAFM, Van der Veen F, Al-Inany HG, Mochtar MH, Griesinger G, Nagi Mohesen M, et al. Gonadotropin-releasing hormone agonist versus HCG for oocyte triggering in antagonist-assisted reproductive technology. Cochrane Database Syst Rev. 2014;31(10). [DOI] [PMC free article] [PubMed]
- 4.Popovic-Todorovic B, Santos-Ribeiro S, Drakopoulos P, De Vos M, Racca A, Mackens S, et al. Predicting suboptimal oocyte yield following GnRH agonist trigger by measuring serum LH at the start of ovarian stimulation. Hum Reprod. 2019;34:2027–2035. doi: 10.1093/humrep/dez132. [DOI] [PubMed] [Google Scholar]
- 5.O’Neill KE, Senapati S, Dokras A. Use of gonadotropin-releasing hormone agonist trigger during in vitro fertilization is associated with similar endocrine profiles and oocyte measures in women with and without polycystic ovary syndrome. Fertil Steril. 2015;103:264–269. doi: 10.1016/j.fertnstert.2014.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kummer NE, Feinn RS, Griffin DW, Nulsen JC, Benadiva CA, Engmann LL. Predicting successful induction of oocyte maturation after gonadotropin-releasing hormone agonist (GnRHa) trigger. Hum Reprod. 2013;28:152–159. doi: 10.1093/humrep/des361. [DOI] [PubMed] [Google Scholar]
- 7.Castillo JC, Garcia-Velasco J, Humaidan P. Empty follicle syndrome after GnRHa triggering versus hCG triggering in COS. J Assist Reprod Genet. 2012;29:249–253. doi: 10.1007/s10815-011-9704-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Horowitz E, Mizrachi Y, Farhi J, Raziel A, Weissman A. Does the interval between the last GnRH antagonist dose and the GnRH agonist trigger affect oocyte recovery and maturation rates? Reprod BioMed Online. 2020;41:917–924. doi: 10.1016/j.rbmo.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Itskovitz-Eldor J, Kol S, Mannaerts B. Use of a single bolus of GnRH agonist triptorelin to trigger ovulation after GnRH antagonist ganirelix treatment in women undergoing ovarian stimulation for assisted reproduction, with special reference to the prevention of ovarian hyperstimulation syndr. Hum Reprod. 2000;15:1965–1968. doi: 10.1093/humrep/15.9.1965. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328:1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lainas GT, Lainas TG, Sfontouris IA, Venetis CA, Bosdou JK, Chatzimeletiou A, et al. Association between body mass index and oocyte maturation in patients triggered with GnRH agonist who are at high risk for severe ovarian hyperstimulation syndrome: an observational cohort study. Reprod BioMed Online. 2020;40:168–175. doi: 10.1016/j.rbmo.2019.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Deepika K, Sindhuma D, Kiran B, Ravishankar N, Gautham P, Kamini R. Empty follicle syndrome following GnRHa trigger in PCOS patients undergoing IVF cycles. J Reprod Infertil. 2018;19:16–25. [PMC free article] [PubMed] [Google Scholar]
- 14.Christopoulos G, Vlismas A, Barsoum-Derias E, El-Shawarby S, Trew G, Lavery S. Rescue hCG to treat empty follicle syndrome after the use of a GnRH agonist as oocyte maturation trigger: first report on fresh embryo transfer and clinical pregnancy. Hum Fertil. 2015;18:248–252. doi: 10.3109/14647273.2015.1071500. [DOI] [PubMed] [Google Scholar]
- 15.Blazquez A, Guillén JJ, Colomé C, Coll O, Vassena R, Vernaeve V. Empty follicle syndrome prevalence and management in oocyte donors. Hum Reprod. 2014;29:2221–2227. doi: 10.1093/humrep/deu203. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Hong Q, Sun L, Chen Q, Fu Y, Ai A, et al. Dual trigger for final oocyte maturation improves the oocyte retrieval rate of suboptimal responders to gonadotropin-releasing hormone agonist. Fertil Steril. 2016;106:1356–1362. doi: 10.1016/j.fertnstert.2016.07.1068. [DOI] [PubMed] [Google Scholar]
- 17.Chang FE, Beall SA, Cox JM, Richter KS, DeCherney AH, Levy MJ. Assessing the adequacy of gonadotropin-releasing hormone agonist leuprolide to trigger oocyte maturation and management of inadequate response. Fertil Steril. 2016;106:1093–1100.e3. doi: 10.1016/j.fertnstert.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 18.Meyer L, Murphy LA, Gumer A, Reichman DE, Rosenwaks Z, Cholst IN. Risk factors for a suboptimal response to gonadotropin-releasing hormone agonist trigger during in vitro fertilization cycles. Fertil Steril. 2015;104:637–642. doi: 10.1016/j.fertnstert.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 19.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Efficacy of induced luteinizing hormone surge after “trigger” with gonadotropin-releasing hormone agonist. Fertil Steril. 2011;95:826–828. doi: 10.1016/j.fertnstert.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Zarcos SM, Mejía PV, Stefani CD, Martin PS, Martin FS. Comparison of two different dosage of GnRH agonist as ovulation trigger in oocyte donors: a randomized controled trial. JBRA Assist Reprod. 2017;21:183–187. doi: 10.5935/1518-0557.20170036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orvieto R, Nahum R, Zohav E, Liberty G, Anteby EY, Meltcer S. GnRH-agonist ovulation trigger in patients undergoing controlled ovarian hyperstimulation for IVF with ultrashort flare GnRH-agonist combined with multidose GnRH-antagonist protocol. Gynecol Endocrinol. 2013;29:51–53. doi: 10.3109/09513590.2012.705376. [DOI] [PubMed] [Google Scholar]
- 22.Orvieto R, Nahum R, Frei J, Zandman O, Frenkel Y, Haas J. GnRH-agonist ovulation trigger in patients undergoing controlled ovarian hyperstimulation for IVF with stop GnRH-agonist combined with multidose GnRH-antagonist protocol. Gynecol Obstet Investig. 2021;86(5):427–31. [DOI] [PubMed]
- 23.Abbara A, Hunjan T, Ho VNA, Clarke SA, Comninos AN, Izzi-Engbeaya C, et al. Endocrine requirements for oocyte maturation following hCG, GnRH agonist, and kisspeptin during IVF treatment. Front Endocrinol (Lausanne) 2020;11:537205. doi: 10.3389/fendo.2020.537205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen S-L, Ye D-S, Chen X, Yang X-H, Zheng H-Y, Tang Y, et al. Circulating luteinizing hormone level after triggering oocyte maturation with GnRH agonist may predict oocyte yield in flexible GnRH antagonist protocol. Hum Reprod. 2012;27:1351–1356. doi: 10.1093/humrep/des049. [DOI] [PubMed] [Google Scholar]
- 25.Maslow BSL, Guarnaccia M, Stefanacci C, Ramirez L, Klein JU. The use of GnRH-agonist trigger for the final maturation of oocytes in normal and low responders undergoing planned oocyte cryopreservation. Hum Reprod. 2020;35:1054–1060. doi: 10.1093/humrep/deaa042. [DOI] [PubMed] [Google Scholar]
- 26.Kummer N, Benadiva C, Feinn R, Mann J, Nulsen J, Engmann L. Factors that predict the probability of a successful clinical outcome after induction of oocyte maturation with a gonadotropin-releasing hormone agonist. Fertil Steril. 2011;96:63–68. doi: 10.1016/j.fertnstert.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 27.Lainas GT, Lainas TG, Sfontouris IA, Chatzimeletiou K, Venetis CA, Bosdou JK, et al. Is oocyte maturation rate associated with triptorelin dose used for triggering final oocyte maturation in patients at high risk for severe ovarian hyperstimulation syndrome? Hum Reprod. 2019;34:1770–1777. doi: 10.1093/humrep/dez105. [DOI] [PubMed] [Google Scholar]
- 28.Vuong TNL, Ho MT, Ha TD, Phung HT, Huynh GB, Humaidan P. Gonadotropin-releasing hormone agonist trigger in oocyte donors co-treated with a gonadotropin-releasing hormone antagonist: a dose-finding study. Fertil Steril. 2016;105:356–363. doi: 10.1016/j.fertnstert.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Gülekli B, Göde F, Sertkaya Z, Işık AZ. Gonadotropin-releasing hormone agonist triggering is effective, even at a low dose, for final oocyte maturation in ART cycles: case series. J Turkish Ger Gynecol Assoc. 2015;16:35–40. doi: 10.5152/jtgga.2015.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pabuccu E, Pabuccu R, Caglar G, Yilmaz B, Yarci A. Different gonadotropin releasing hormone agonist doses for the final oocyte maturation in high-responder patients undergoing in vitro fertilization/intra-cytoplasmic sperm injection. J Hum Reprod Sci. 2015;8:25–29. doi: 10.4103/0974-1208.153123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Şükür YE, Özmen B, Özdemir ED, Seval MM, Kalafat E, Sönmezer M, et al. Final oocyte maturation with two different GnRH agonists in antagonist co-treated cycles at risk of ovarian hyperstimulation syndrome. Reprod BioMed Online. 2017;34:5–10. doi: 10.1016/j.rbmo.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 32.Fauser BC, de Jong D, Olivennes F, Wramsby H, Tay C, Itskovitz-Eldor J, et al. Endocrine profiles after triggering of final oocyte maturation with GnRH agonist after cotreatment with the GnRH antagonist ganirelix during ovarian hyperstimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87:709–715. doi: 10.1210/jcem.87.2.8197. [DOI] [PubMed] [Google Scholar]
- 33.Hershkop E, Khakshooy A, Simons J, Weiss A, Geslevich J, Goldman S, et al. Ideal lag time from ovulation to oocyte aspiration using a GnRH agonist trigger. J Gynecol Obstet Hum Reprod. 2021;50:102055. doi: 10.1016/j.jogoh.2020.102055. [DOI] [PubMed] [Google Scholar]
- 34.Aflatoonian A, Haghighi F, Hoseini M, Haghdani S. Does the repeat dose of gonadotropin-releasing hormone agonist trigger in polycystic ovarian syndrome improve in vitro fertilization cycles outcome? A clinical trial study. Int J Reprod Biomed. 2020;18:485–490. doi: 10.18502/ijrm.v13i7.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deepika K, Baiju P, Gautham P, Suvarna R, Arveen V, Kamini R. Repeat dose of gonadotropin-releasing hormone agonist trigger in polycystic ovarian syndrome undergoing in vitro fertilization cycles provides a better cycle outcome - a proof-of-concept study. J Hum Reprod Sci. 2017;10:271–280. doi: 10.4103/jhrs.JHRS_102_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding N, Liu X, Jian Q, Liang Z, Wang F. Dual trigger of final oocyte maturation with a combination of GnRH agonist and hCG versus a hCG alone trigger in GnRH antagonist cycle for in vitro fertilization: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2017;218:92–98. doi: 10.1016/j.ejogrb.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, Wang Y, Mao X, Chen Q, Hong Q, Cai R, et al. Dual trigger of final oocyte maturation in poor ovarian responders undergoing IVF/ICSI cycles. Reprod BioMed Online. 2017;35:701–707. doi: 10.1016/j.rbmo.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Griffin D, Feinn R, Engmann L, Nulsen J, Budinetz T, Benadiva C. Dual trigger with gonadotropin-releasing hormone agonist and standard dose human chorionic gonadotropin to improve oocyte maturity rates. Fertil Steril. 2014;102:405–409. doi: 10.1016/j.fertnstert.2014.04.028. [DOI] [PubMed] [Google Scholar]
- 39.Lin MH, Shao-Ying Wu F, Kuo-Kuang Lee R, Li SH, Lin SY, Hwu YM. Dual trigger with combination of gonadotropin-releasing hormone agonist and human chorionic gonadotropin significantly improves the live-birth rate for normal responders in GnRH-antagonist cycles. Fertil Steril. 2013;100:1296–1302. doi: 10.1016/j.fertnstert.2013.07.1976. [DOI] [PubMed] [Google Scholar]
- 40.Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C. Comparison of “triggers” using leuprolide acetate alone or in combination with low-dose human chorionic gonadotropin. Fertil Steril. 2011;95:2715–2717. doi: 10.1016/j.fertnstert.2011.03.109. [DOI] [PubMed] [Google Scholar]
- 41.Gunnala V, Melnick A, Irani M, Reichman D, Schattman G, Davis O, et al. Sliding scale HCG trigger yields equivalent pregnancy outcomes and reduces ovarian hyperstimulation syndrome: analysis of 10,427 IVF-ICSI cycles. PLoS One. 2017;12:e0176019. doi: 10.1371/journal.pone.0176019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pereira N, Elias RT, Neri QV, Gerber RS, Lekovich JP, Palermo GD, et al. Adjuvant gonadotrophin-releasing hormone agonist trigger with human chorionic gonadotrophin to enhance ooplasmic maturity. Reprod BioMed Online. 2016;33:568–574. doi: 10.1016/j.rbmo.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 43.Griffin D, Benadiva C, Kummer N, Budinetz T, Nulsen J, Engmann L. Dual trigger of oocyte maturation with gonadotropin-releasing hormone agonist and low-dose human chorionic gonadotropin to optimize live birth rates in high responders. Fertil Steril. 2012;97:1316–1320. doi: 10.1016/j.fertnstert.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Dunne C, Shan A, Nakhuda G. Measurement of luteinizing hormone level after gonadotropin-releasing hormone agonist trigger is not useful for predicting oocyte maturity. J Obstet Gynaecol Canada JOGC = J d’obstetrique Gynecol du Canada JOGC. 2018;40:1618–1622. doi: 10.1016/j.jogc.2018.01.032. [DOI] [PubMed] [Google Scholar]
- 45.Cozzolino M, Matey S, Alvarez A, Toribio M, López V, Perona M, et al. Self-detection of the LH surge in urine after GnRH agonist trigger in IVF—how to minimize failure to retrieve oocytes. Front Endocrinol (Lausanne) 2020;11:221. doi: 10.3389/fendo.2020.00221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Asada Y, Itoi F, Honnma H, Takiguchi S, Fukunaga N, Hashiba Y, et al. Failure of GnRH agonist-triggered oocyte maturation: its cause and management. J Assist Reprod Genet. 2013;30(4):581–5. [DOI] [PMC free article] [PubMed]
- 47.Honnma H, Hashiba Y, Asada Y, Endo T. Failure of triggering oocyte maturation with a GnRH agonist in polycystic ovary syndrome: two case reports. Eur J Obstet Gynecol Reprod Biol. 2011;157:239–240. doi: 10.1016/j.ejogrb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Youssef MAF, Abdelmoty HI, Ahmed MAS, Elmohamady M. GnRH agonist for final oocyte maturation in GnRH antagonist co-treated IVF/ICSI treatment cycles: systematic review and meta-analysis. J Adv Res. 2015;6:341–349. doi: 10.1016/j.jare.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tomazevic T, Meden-Vrtovec H. Early timed follicular aspiration prevents severe ovarian hyperstimulation syndrome. J Assist Reprod Genet. 1996;13:282–286. doi: 10.1007/BF02070139. [DOI] [PubMed] [Google Scholar]
- 50.Schlaff WD. Dynamic testing in reproductive endocrinology. Fertil Steril. 1986;45:589–606. doi: 10.1016/s0015-0282(16)49328-3. [DOI] [PubMed] [Google Scholar]
- 51.Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update. 2017;23:139–155. doi: 10.1093/humupd/dmw038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As per request from corresponding author.
Not applicable.