Abstract
Purpose
To determine if the inhibition of the interaction between the Hippo effector YAP or its transcriptional co-activator TAZ with the TEAD family of transcription factors is critical for the cumulus expansion–related events induced by the EGF network in cumulus-oocyte complexes (COCs).
Methods
We performed a series of experiments using immature bovine COCs subjected to an IVM protocol for up 24 h in which cumulus expansion was stimulated with EGF recombinant protein or FSH.
Results
The main results indicated that EGFR activity stimulation in bovine cumulus cells (CC) increases mRNA levels encoding the classic YAP/TAZ-TEAD target gene CTGF. To determine if important genes for cumulus expansion are transcriptional targets of YAP/TAZ-TEAD interaction in CC, COCs were then subjected to IVM in the presence of FSH with or without distinct concentrations of Verteporfin (VP; a small molecule inhibitor that interferes with YAP/TAZ binding to TEADs). COCs were then collected at 6, 12, 18, and 24 h for total RNA extraction and RT-qPCR analyses. This experiment indicated that VP inhibits in a time- and concentration-dependent manner distinct cumulus expansion and oocyte maturation–related genes, by regulating EGFR and CTGF expression in CC.
Conclusions
Taken together, the results presented herein represent considerable insight into the functional relevance of a completely novel signaling pathway underlying cumulus expansion and oocyte maturation in monovulatory species. YAP/TAZ or CTGF may represent potential targets to improve the efficiency of IVM systems, not only for monovulatory species of agricultural importance as the cow, but for human embryo production.
Keywords: Cow, Ovary, Oocyte in vitro maturation, CTGF, Verteporfin, EGF receptor
Introduction
In several species, cumulus cells (CC) expansion is an important marker for oocyte maturation, and is critical for its fertilization, subsequent cleavage, and blastocyst development. Defective cumulus expansion can result in reduced ovulation rates and, consequently, affects fertility [1, 2]. In vivo, the proper functioning of cumulus cells to support for the acquisition of oocyte competence in the preovulatory period is, however, highly dependent on mural granulosa cells (GC) [3]. The main trigger of the preovulatory cascade is LH, which activates a cascade of signaling events promoting ovulation of a mature egg [4, 5]. Although LH directly stimulates mural GC, its effects on CC and oocytes are probably indirect, as both cell types express few or no LH receptors and fail to respond when directly stimulated by LH [6].
The ovulatory process in mammals is, therefore, initiated by LH surge that acts upon its receptors on mural GC. This leads to activation of proteolytic sheddase enzymes (ADAMs) that cause the release of the membrane-bound proteins epiregulin (EREG) and amphiregulin (AREG), members of the epidermal growth factor (EGF) family. These proteins then activate the EGF receptor (EGFR) on mural GC, thereby stimulating the expression of prostaglandin endoperoxide synthase 2 (PTGS2) as well as increasing the transcriptional activity of the AREG and EREG genes, creating a positive feedback loop (autocrine action). The EGF signaling network in mural GC is then transmitted to CC (paracrine action) [5, 7], modulating the expression of important genes related to gap junctions closure and production of an extensive extracellular matrix by CC and downregulation of the meiotic inhibitory signaling network that ultimately will lead to cumulus expansion and oocyte meiotic maturation [5, 8]. It was actually demonstrated that sustained activity of the EGFR is essential for LH-induced cumulus expansion and oocyte maturation [9].
The core Hippo pathway consists of a kinase cascade that ultimately regulates the activity of the transcriptional activators Yes-associated protein 1 (YAP) and transcriptional co-activator with PDZ-binding motif (TAZ). When these Hippo effectors are accumulated in the nucleus, they form complexes with numerous transcription factors, notably those of the TEAD (TEA domain) family of transcription factors, resulting in the modulation of the transcriptional activity of target genes in a cell type– and context-specific manner [10–13]. It has been recently presented data showing that YAP is expressed in bovine mural preovulatory GC and, most importantly, its interaction with TEADs is critical for the preovulatory EGF-like cascade induced by LH in mural GC [14]. Briefly, we demonstrated that YAP-TEADs interaction inhibition in mural GC affects basal and stimulated EGFR expression/activity in vitro and that intrafollicular injection of Verteporfin (a small molecule that interferes with YAP/TAZ binding to TEADs) affects GnRH-induced ovulation in vivo. Taken together, these findings brought us to hypothesize that the interaction of YAP and/or TAZ with TEADs expressed in bovine CC also exerts an important role in the signaling that leads to cumulus expansion and consequently oocyte maturation, at least in part, regulating EGFR expression in CC.
Although a study in rodents reported a functional interaction between YAP and EGF signaling in cumulus cells of mouse cumulus-oocyte complexes (COCs) subjected to an in vitro maturation protocol (IVM) [15], to our knowledge, if YAP and/or TAZ interacts with EGF network to regulate expansion-related events in monovulatory species, as the cow, remains unknown. Therefore, the main objective of the present study was to determine if the inhibition of the interaction between YAP/TAZ and TEADs is critical for the EGF signaling–related cumulus expansion events in bovine COCs in vitro. For this, we employed herein an IVM protocol [16] in which cumulus expansion was induced with EGF recombinant protein or with FSH.
Materials and methods
All chemicals used in the present study were purchased from Sigma Chemicals Company (San Luis, MO, EUA), unless otherwise indicated in the text.
Immunohistochemistry (IHC)
For IHC analyses, 3–8 mm follicles were isolated from bovine ovaries collected from adult cows, irrespective of stage of the estrous cycle, at a local abattoir. Following dissection, follicles were fixed in formaldehyde 10% solution for 24 h, rinsed, and dehydrated in alcohol until paraffin embedded. Serial sections were prepared (at a thickness of 3 µm) followed by deparaffinization, rehydration, sodium citrate heat-mediated antigen retrieval, peroxidase block, and protein blocking (10% goat for 30 min) and then slides were probed with primary antibody against total YAP (1:250, No. 14074; Cell Signaling, Whitby, ON, Canada) or total TAZ (1:300, No. 4883S; Cell Signaling, Whitby, ON, Canada) overnight at 4 °C. YAP and TAZ detection was then performed with the Vectastain Elite ABC HRP Kit (VECTPK6101, Vector Laboratories, Burlingame, CA, USA) and stained with DAB substrate kit (VECTSK4100, Vector Laboratories, Burlingame, CA, USA). Slides were then counterstained with hematoxylin and dehydrated with graded alcohols prior to mounting. Negative controls consisted of slides for which the primary antibodies were omitted. Photomicrographs were taken using a Carl Zeiss Axio Imager M1 microscope (Carl Zeiss Canada Ltd., Toronto, ON, Canada) at × 1000 magnification and using the Zen 2012 blue edition software (Carl Zeiss, Oberkochen, Germany).
Bovine COC collection and in vitro maturation (IVM)
Bovine ovaries were obtained from a local abattoir and transported to the laboratory in saline solution (0.9% NaCl; 30 °C) containing 100 IU/mL penicillin and 50 μg/mL streptomycin sulfate. COCs from 3 to 8 mm diameter follicles were aspirated with a vacuum pump (vacuum rate of 15 mL of water/minute) and selected as previously described [17] under a stereomicroscope. After selection, only grade 1 COCs were randomly distributed to four-well culture dishes (Nunc®, Roskilde, Zealand, DNK), containing 200 μL of basic maturation medium composed by Medium 199 (1 ×) containing Earle’s salts, L-glutamine, 2.2 mg/mL sodium bicarbonate, and 25 mM Hepes (Gibco Labs, Grand Island, NY, USA), supplemented with 0.2 mM pyruvic acid, 0.4% (v/v) bovine serum albumin (BSA), 100 IU/mL penicilin, and 50 μg/mL streptomycin sulfate. The experimental treatments varied according to the cumulus expansion stimulation treatment employed herein and with the aim of each experiment as described in the section “Results.” In summary, in a first model of cumulus expansion induction, COCs were first pretreated or not with the pharmacological inhibitor Tyrphostin AG 1478 (Sigma-Aldrich Co. Oakville, ON, Canada) and cumulus expansion was stimulated with recombinant human EGF (10 ng/mL; R&D Systems Inc., Minneapolis, MN, USA). In a second model of cumulus expansion induction, COCs were first pretreated with AG 1478 and then cumulus expansion was stimulated with FSH (500 ng/mL Bioniche, Belleville, ON, CAN); or COCs were pretreated or not with Verteporfin (VP; Sigma-Aldrich Co. Oakville, ON, Canada) and then challenged with FSH. In all series of cultures, COCs were cultured in an incubator at 39 °C in a saturated humidity atmosphere containing 5% CO2 and 95% air, for 6, 12, 18, or 24 h according to the objective of each series of experiments.
Validation of EGF signaling–related induced cumulus expansion models
We employed herein EGF and FSH, two known treatments for in vitro induced cumulus expansion studies. To confirm that the cumulus expansion induced by these treatments in bovine COCs is EGFR-signaling dependent, a total of 15 COCs per replicate were randomly divided into three groups (n = 5 COCs/group in five independent replicates) for each experiment: EGF or FSH-challenged. Briefly, while subjected to the IVM-like conditions described above, COCs were pretreated with 6 μM of AG 1478 (a selective inhibitor of EGFR activity) or with equivalent amount of vehicle (Control; Dimethyl Sulfoxide: Methanol—DMSO: MeOH) for 1 h and then challenged with EGF or FSH for 24 h. Cumulus expansion was then assessed in the three groups of the EGF model (Control; EGF; and EGF + AG 1478) and the three groups of the FSH model (Control; FSH; and FSH + AG 1478) in each COC at 0 and 24 h post-treatments.
Cumulus expansion assessment
Cumulus expansion assessment was performed by measuring COC area (μm2). Briefly, COCs from each treatment group were photographed using an inverted microscope (Leica DMI 4000B; Leica Microsystems, Wetzlar, HE, GER). Images of the same COC were captured through Leica Application Suite (LAS, Version 3.8) software at 0 and 24 h of cultures. Total surface area expressed in pixels of each COC appearing on the 2-dimensional image was measured with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Effect of Verteporfin on FSH-induced cumulus expansion in vitro
To investigate the physiological importance of YAP/TAZ-TEAD interaction for in vitro induced cumulus expansion, we decided to employ the FSH-induced model in the presence or not of the pharmacological inhibitor Verteporfin (VP). While this in vitro model has been published several times [18–20] and it is closer (if not similar) to IVM protocols employed commercially for bovine embryo production, VP is the best described molecule that interferes with YAP/TAZ binding to TEADs [21]. So briefly, COCs were randomly divided into four groups (n = 5 COCs/group in five independent replicates) and were challenged with FSH (500 ng/mL) without or with 1 h of pretreatment with different concentrations (0.1, 0.3, 0.5 μM) of VP. COCs were then collected at 6, 12, 18, and 24 h post-FSH for total RNA extraction and RT-qPCR analyses.
Total RNA extraction and real-time qPCR
For mRNA abundance assessment, COCs were collected at the end of each treatment, and cumulus cells were removed from COCs, recovered, and immediately stored at − 80 °C. Total RNA was then extracted using PureLink™ RNA Mini Kit (Thermo Fisher Scientific, Waltham, MA, EUA) according to the manufacturer’s instructions and was quantified at 260 nm wavelength using a spectrophotometer (NanoDrop1000, Thermo Scientific, Wilmington, DE, USA). Total RNA (50 ng) was reverse transcribed (RT) using the iScript™ cDNA Synthesis Kit (Bio-Rad, Des Plaines, IL, USA) at 25 °C for 5 min and 46 °C for 30 min. The reaction was ended by incubation at 95 °C for 5 min. Real-time qPCR was performed using CFX384™ Real-Time System (Bio-Rad Laboratories, Hercules, CA, USA) using the GoTaq® DNA Polymerase (Promega, Madison, WI, USA) and specific primers (Table 1). After an initial denaturation step at 95 °C for 3 min, 40 cycles at 95 °C for 10 s were carried out, followed by 1 min at 60 °C to amplify each transcript. The reaction was performed in duplicate. To quantify relative gene expression, data were normalized to a calibrator sample using the Pfaffl ΔΔCt method with correction for amplification efficiency [22]. Briefly, the Ct of target gene amplification was normalized to the expression level of a housekeeping gene histone H2AFZ according to the ratio, R = ECt housekeeping /ECt target, where E is the amplification efficiency for each primer pair. Alternative analyzes were also performed with a different housekeeping gene (RPS18) to confirm our results and the stability of H2AFZ on our distinct experiments. Primers not published previously were designed based on sequences from GenBank, using Primer-BLAST platform and their respective amplicons were sequenced to confirm specificity.
Table 1.
Primers used for real-time PCRs in cumulus cells
| Gene | Sequence 5′ → 3′ | Reference/accession number |
|---|---|---|
| ADAM17 | F: TGGGATGTGAAGATGTTGCTAGA | (7) |
| R: ATCCAAGTGTTCCCATATCAAAATC | ||
| CTGF | F: AGCTGAGCGAGTTGTGTACC | NM_174030.2 |
| R: TCCGAAAATGTAGGGGGCAC | ||
| EGFR | F: ACCACCCATCCTGCCTGTATCAA | [23] |
| R: TGCCCAAACGGACAACATTCTTCC | ||
| EREG | F: ACTGCACAGCATTAGTTCAAACTGA | (7) |
| R: TGTCCATGCAAACAGTAGCCATT | ||
| FSHR | F: AGCCCCTTGTCACAACTCTATGTC | NM_174061.1 |
| R: GTTCCTCACCGTGAGGTAGATGT | ||
| HAS2 | F: GCATGTCACCCAGTTGGTCT | NM_174079.3 |
| R: TGGGTCAAGCATGGTGTCTG | ||
| H2AFZ | F: GAGGAGCTGAACAAGCTGTTG | [24] |
| R: TTGTGGTGGCTCTCAGTCTTC | ||
| PLAT | F: GGGGAAGCACAACCACTG | NM_174146.3 |
| R: AGCTGATCAGGATCCCCC | ||
| PTGS2 | F: TGCTGAGTTTAACACGCTCTACCA | (7) |
| R: TGAGACCATGTTCCAGTAAGACAGA | ||
| PTX3 | F: CCTCAGCTATCGGTCCATAA | NM_001076259.2 |
| R: ATTGAAGCCTGTGAGGTCTGC | ||
| RPS18 | F: CCTTCCGCGAGGATCCATTG | NM_001033614.2 |
| R: CGCTCCCAAGATCCAACTAC |
F, forward primer; R, reverse primer
Statistical analysis
All experiments were performed on five independent replicates, with each replicate using COCs aspirated from ovaries collected at a local abattoir at different times. Analysis of data was performed with JMP software (SAS Institute, Cary, NC). Data that did not follow a normal distribution (Shapiro–Wilk test) were transformed to logarithms. Treatment as main effect and culture replicate as a random variable in the F-test. Where the F test was significant (P < 0.05), treatments were compared to controls with the Tukey–Kramer HSD test or t-tests. Data are presented as means ± SEM.
Results
YAP and TAZ are expressed in cumulus cells of immature bovine COCs
We first decided to perform IHC assessment to confirm the presence and to determine the subcellular localization of YAP and TAZ in COCs located in antral follicles from the same size range from which we aspirate COCs for the expansion-related experiments performed herein. Our results confirmed that both YAP and TAZ are expressed in bovine COCs (Fig. 1). Positive staining for both total YAP and total TAZ was detected in the cytoplasm and nuclei of bovine cumulus. In addition, stronger staining for both Hippo effectors was detected in the nuclei of CC, but only staining for TAZ was detected in the bovine oocyte. The specificity of YAP and TAZ antibodies was tested by western blotting which detected a single band of the expected molecular weight for each Hippo effector (not shown).
Fig. 1.
Localization of YAP and TAZ in cumulus cells of immature bovine COCs. Immunohistochemistry (IHC) analysis was used to determine the cellular and subcellular localization of total YAP and total TAZ protein levels in cumulus-oocyte complexes (COCs) located in 3–8 mm bovine ovarian follicles (CC, cumulus cell; MGC, mural granulosa cells; TC, theca cells; SC, stromal cells). Representative IHC images of staining for YAP and TAZ (10 and 20 × , respectively) and their antibody control (NEG) showing negative staining of total YAP and total TAZ in MGC and TC
EGFR activity stimulation in bovine cumulus cells increases mRNA levels encoding the classic YAP/TAZ-TEAD target gene CTGF
To validate the positive effect of EGF and FSH on cumulus expansion in our culture systems, and, most importantly, to confirm that the cumulus expansion induced by these treatments is EGFR-signaling dependent, we first pretreated COCs with or without AG 1478, a selective inhibitor of EGFR activity, and then challenged them with EGF (Figs. 2A and 3A) or FSH (Figs. 2B and 3B). In such models, COCs clearly responded to EGF and FSH as both treatments significantly induced cumulus expansion (P < 0.05, Fig. 2A and B, respectively) and increased mRNA levels for the classic EGFR-downstream target genes EREG and AREG (P < 0.05, Fig. 3A and B, respectively) in comparison to respective controls. In addition, when COCs were pretreated with AG 1478, neither EGF (P < 0.05, Fig. 2A) nor FSH (P < 0.05, Fig. 2B) were capable to induce full COC expansion or significant increase in mRNA levels for EREG or AREG in cumulus cells (P < 0.05, Fig. 3A and B, respectively) in comparison to EGF or FSH treatments alone. In this context, we then decided to assess the mRNA abundance for the connective tissue growth factor (CTGF), the most classic YAP/TAZ-TEAD target gene [11]. The results indicated that both EGF and FSH significantly increased mRNA levels for CTGF in cumulus cells in a time-dependent manner (P < 0.05, Fig. 3A and B, respectively) but both treatments failed to do so in all time points tested when the activity of the EGFR was inhibited with AG 1478.
Fig. 2.
Validation of the EGF signaling–related induced cumulus expansion models. COCs were randomly divided into groups (n = 5 COCs/group per replicate) and were pretreated without (Control and EGF groups) or with 6 μM of AG 1478 (a selective inhibitor of EGFR) for 1 h before adding 10 ng/mL of EGF (A) or pretreated without (Control and FSH groups) or with 6 μM of AG 1478 for 1 h before adding 500 ng/mL of FSH (B). Cumulus expansion was assessed by measuring COC area (µm2) in each COC at 0 and 24 h post-EGF (A) or post-FSH (B). Data represent the mean ± SEM of five independent replicates and asterisk (*) represents statistical difference in comparison to respective Control (P < 0.05)
Fig. 3.
Effect of the selective EGFR inhibitor AG 1478 on mRNA levels encoding the classic YAP/TAZ-TEAD target gene CTGF in cumulus cells post-EGF or FSH. COCs were randomly divided into groups (n = 5 COCs/group per replicate) and were pretreated without (Control and EGF groups) or with 6 μM of AG 1478 (a selective inhibitor of EGFR) for 1 h before adding 10 ng/mL of EGF (A) or pretreated without (Control and FSH groups) or with 6 μM of AG 1478 for 1 h before adding 500 ng/mL of FSH (B) for 6, 12, 18, and 24 h. Messenger RNA abundance was measured in cumulus cells by real-time qPCR and normalized to the housekeeping genes H2AF2 and RPS18. Data represent the mean ± SEM of five independent replicates. Different letters show statistically significant differences between treatments in the same time point (P ˂0.05)
YAP/TAZ-TEAD interaction affects the expression of critical cumulus expansion–related genes
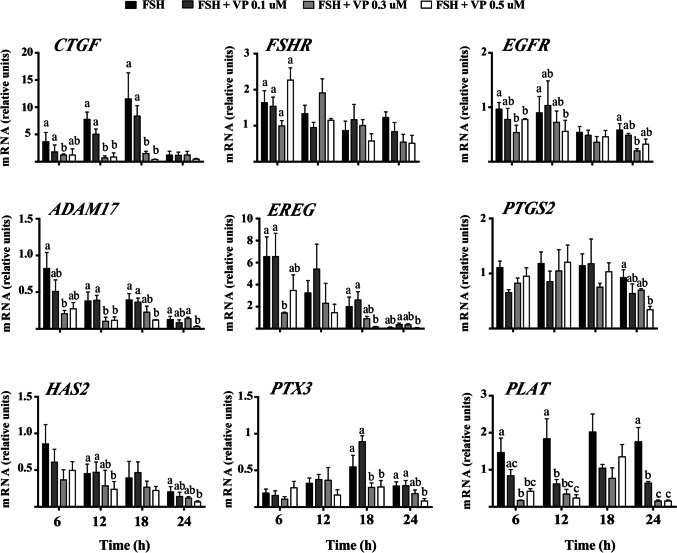
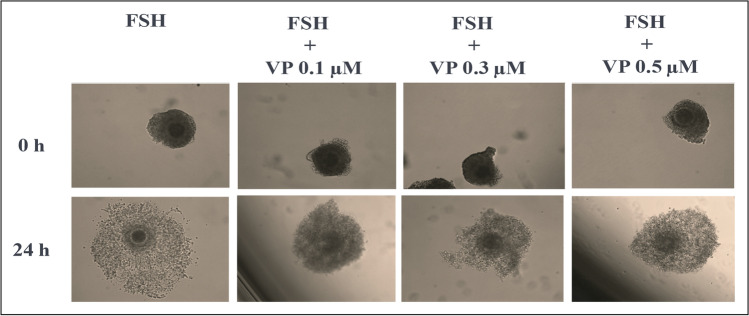
To start elucidating the physiological importance of YAP/TAZ-TEAD interaction to the induced cumulus expansion of immature bovine COCs in vitro, COCs were cultured in maturation medium containing FSH without or with pretreatment of distinct concentrations of VP for 6, 12, 18, and 24 h. The results confirmed the VP effect in inhibiting/disrupting YAP/TAZ-TEAD interaction, as CTGF levels were reduced in a dose-dependent manner (P < 0.05, Fig. 4) whereas FSHR did not change significantly in comparison to respective controls at each time point evaluated (P > 0.05, Fig. 4). Most importantly, this experiment clearly indicated that VP inhibits in a time- and concentration-dependent manner distinct cumulus expansion–related genes, including the essential gene for EGF signaling in cumulus cells, EGFR (P > 0.05, Fig. 4), and its downstream targets as ADAM17, EREG, and PTGS2 (P < 0.05, Fig. 4). In addition, VP treatments also reduced in a time- and concentration-dependent manner hyaluronan synthase 2 (HAS2), pentraxin-related protein 3 (PTX3), and the plasminogen activator tissue-type A (PLAT) mRNA levels (P < 0.05, Fig. 4), which are important markers for cumulus expansion, matrix remodeling, and oocyte maturation, respectively. To determine if these alterations in cumulus expansion–related genes would alter the FSH-induced cumulus expansion in vitro, images of COCs from each treatment were captured at 0 and 24 h of culture. The results indicated that while COCs treated with FSH alone presented full cumulus expansion 24 h post-treatment, in the presence of VP doses, COCs presented only a partial cumulus expansion in comparison to FSH alone (Fig. 5).
Fig. 4.
Inhibition of YAP/TAZ-TEAD interaction affects the expression of critical FSH-induced cumulus expansion–related genes. COCs were randomly divided into groups (n = 5 COCs/group per replicate) and were pretreated or not for 1 h with different doses of Verteporfin (VP; 0.1, 0.3, and 0.5 μM), a small molecule inhibitor that interferes with YAP/TAZ binding to TEAD proteins, followed with FSH (500 ng/mL) treatment for 6, 12, 18, and 24 h. Messenger RNA abundance was measured in cumulus cells by real-time qPCR and normalized to the housekeeping genes H2AF2 and RPS18. Data represent the mean ± SEM of five independent replicates. Different letters show statistically significant differences between treatments in the same time point (P ˂0.05)
Fig. 5.
Effect of Verteporfin (VP) on FSH-induced cumulus expansion. COCs were randomly divided into groups (n = 5 COCs/group per replicate) and were pretreated or not for 1 h with different doses of Verteporfin (VP; 0.1, 0.3, and 0.5 μM) followed with FSH (500 ng/mL) for 24 h. Representative images of the effect of VP treatments on FSH-induced COCs expansion at 0 and 24 h of culture
Discussion
Hippo is an evolutionarily conserved signaling pathway with established roles in cell differentiation, proliferation, and apoptosis in a variety of tissues, particularly during embryogenesis [25, 26]. Although several studies (mainly using mice models) have reported some of the physiological roles of Hippo effectors in the ovarian physiology in the adult life [27–31], studies showing the importance of Hippo effectors to cumulus functions and to cumulus expansion–related events are extremely scarce. To the best of our knowledge, this is the first report in monovulatory species that indicates that YAP/TAZ-TEAD interaction is critical for the EGF signaling–related cumulus expansion events. Briefly, we performed a series of experiments using bovine cumulus-oocyte complexes (COCs) subjected to an IVM protocol for up 24 h in the presence or not of EGF or FSH with or without pretreatment with pharmacological inhibitors. Our main findings show that YAP and TAZ interaction with TEADs during IVM is mediated by EGFR-downstream signaling and it is critical for the expression of important cumulus expansion and oocyte maturation–related genes.
The expression and localization of the Hippo effectors YAP and TAZ proteins in bovine ovarian follicles were recently reported [28]. Using IHC and Western blotting analyses, these authors clearly indicated that both Hippo effectors are expressed in mural GC from 3 to 8 mm diameter follicles, and most importantly, intense staining for YAP and TAZ could be detected in the nuclei of these mural GC. These authors nevertheless did not show or mention the expression pattern for both Hippo effectors in bovine cumulus cells and oocytes. We therefore first sought to confirm if YAP and TAZ would be detected in CC from COCs located in antral follicles from the same size range from which we aspirated COCs for the IVM expansion–related experiments performed in the present study. Indeed, our IHC results indicated strong staining for both Hippo effectors in CC, particularly in the nuclei of these cells. The accumulation of the Hippo effectors YAP and/or TAZ in the cell nucleus normally results in formation of complexes, notably with the TEAD family of transcription factors, which can then modulate the expression of several target genes. A classic YAP/TAZ-TEAD target gene is CTGF. Interestingly, when we stimulated COCs with both EGF and FSH, there was a significant time-dependent increase in mRNA levels for CTGF which was completed abrogated in the presence of a selective EGFR activity inhibitor. Together, these findings strongly suggest an increase in YAP/TAZ-TEAD interaction following EGF signaling stimulation in bovine cumulus cells.
One obvious question raised by the findings described above was the physiological importance of an increase in YAP/TAZ binding levels to TEADs during the induced cumulus expansion process in cattle. Although the in vitro induced cumulus expansion models employed herein do not mimic precisely the same LH-triggered cumulus expansion events observed in vivo, the stimulation of COCs (retrieved from bovine growing antral follicles: 3–8 mm) with supraphysiolgical doses of EGF or FSH is still considered a useful approach to assess, in vitro, the same machinery (markers and/or signaling pathways) that is normally involved with cumulus expansion in vivo. In addition, although cumulus expansion in vivo normally occurs in COCs from preovulatory large follicles (≥ 12 mm), we have observed that both YAP and TAZ expression seem to remain stable in bovine cumulus cells independently of the follicle size where the COCs are located (not shown). The latter observation, however, allowed us to hypothesize that our in vitro findings can be extrapolated to cumulus expansion process in vivo. To start elucidating the functional importance of YAP/TAZ-TEAD interaction to bovine cumulus expansion, we decided to subject COCs to an IVM protocol in the presence of FSH with or without distinct concentrations of VP, a pharmacological inhibitor that interferes with YAP/TAZ binding to TEADs. Due to our recent findings in which it was showed that YAP-TEADs interaction inhibition in bovine mural GC affects basal and stimulated EGFR expression/activity in vitro [14], we initially hypothesized that the same effect would be found in bovine cumulus cells. Indeed, the results showed herein clearly indicate that YAP/TAZ-TEAD interaction inhibition with VP affects the expression of critical cumulus expansion–related genes, at least in part, regulating the transcription of EGFR. VP treatment significantly decreased, in a dose-dependent manner, EGFR mRNA levels in cumulus cells from COCs cultured for 6 h and 12 h. Such decline in EGFR can explain, at least in part, the decrease of mRNA levels for ADAM17, EREG, and PLAT (from 6 h on) and PTGS2 (at 24 h), as for HAS2 and PTX3 mRNA levels (from 12 h on), once all these genes are classic targets of EGFR-downstream signaling [7, 8, 32–34]. Although we did not measure VP effects between time 0 and 6 h post-FSH, we assumed that, as we verified in our study with bovine mural granulosa cells, VP may downregulate basal and FSH-induced EGFR mRNA and protein levels in bovine cumulus from 1 h on post-treatment.
A recent study also tested the effects of VP on cumulus expansion events in mouse COCs subjected to IVM [15]. Intriguingly, mouse COCs pretreated with VP presented not only increased basal expression levels for cumulus expansion–related genes in a dose-dependent manner (PTGS2, HAS2, and PTX3) as well increased basal cumulus expansion in comparison to untreated controls [15]. These data, nevertheless, are the opposite of what we are demonstrating in the present study, in which we clearly show that VP inhibits in a dose-dependent manner critical genes for cumulus expansion. In addition, bovine COCs pretreated with VP herein failed to reach optimal expansion at 24 h post-FSH challenge. Although such discrepancies could be explained by eventual species-specific differences, intriguingly other functional studies in mice suggest that the interaction of YAP with TEADs may be important to allow and/or even modulate the preovulatory cascade that leads to cumulus expansion and ovulation [30]. These authors demonstrated that knockdown of YAP dramatically suppressed expression of EGFR in mouse mural granulosa cells. Moreover, there is also evidence in tumor cells that Hippo signaling effectors, YAP and TAZ, can modulate major EGFR-downstream signaling pathways by regulating the EGFR expression and activity [35, 36].
For many authors, the data with VP should be interpreted with some caution, since YAP-TEAD independent effects of the drug are reported in cancer cells [37]. In our model, however, the pharmacological effects of VP seem to be YAP/TAZ-TEAD specific, once CTGF was downregulated in cumulus cells in a dose-dependent manner following VP treatment, whereas mRNA levels for some genes as FSHR and YAP itself (not shown) were not affect by VP at any dose or time points tested. In terms of mechanism of action, we strongly believe that most of the results observed herein following VP treatment are consequence of its effect on EGFR. It has been shown in distinct human cell types that YAP positively regulates EGFR transcription through the interaction with an intact TEAD binding site at the EGFR promoter [38] and that TEAD1 is a critical regulator of EGFR transcription in vitro [39]. However, we cannot exclude the possibility that CTGF itself may directly contribute to the altered expression profile observed in cumulus cells herein, particularly at later time points assessed. CTGF knockout mice showed disrupted follicle development and decreased ovulation rates, through significant downregulation of the disintegrin and metalloproteinase Adamts1, which is critical for remodeling of extracellular matrix surrounding granulosa cells of preovulatory follicles [27]. In the same study, nevertheless, Ctgf cKO mice presented normal cumulus expansion and the decreased ovulation rates were attributed to defects in the expression of PGR-downstream genes related to the ovulation process. On the other hand, it has been demonstrated that supplementation of culture media with CTGF recombinant protein benefits ovine oocyte IVM and in vitro embryo production [40]. Briefly, CTGF and/or its combination with other factors significantly promoted cumulus cell expansion, inhibited oocyte/cumulus apoptosis, induced oocyte nuclear maturation, and improved early embryo developmental competence.
In a general context of Hippo effectors regulation, Hippo pathway activity presents a dynamic that differs from most cell signaling pathways. In the absence of Hippo signaling activity, unphosphorylated YAP/TAZ accumulate in the nucleus and form complexes with numerous transcription factors, particularly those of the TEAD family [25, 41]. When Hippo signaling is considered active, phosphorylation of YAP/TAZ results in their ubiquitination by β-TrCP and subsequent proteasomal degradation [42, 43] and/or phosphorylated YAP/TAZ can also bind proteins of the 14–3-3 family, resulting in their retention in the cytoplasm [44]. In the present study, the results strongly suggest that YAP/TAZ are post-translationally regulated in cumulus cells of bovine COCs following EGF signaling stimulation in vitro. Although the increase in mRNA levels for CTGF in CC post-EGF and FSH treatment can be considered a reliable indirect indicator of augmented YAP/TAZ-TEADs nuclear interaction, we did not assess if EGF and FSH effectively increased the YAP/TAZ-TEAD complex formation at the nuclear level in CC. Likewise, further studies will be required to determine if EGF and FSH modulate phosphorylation activity of both YAP and TAZ. Importantly, future studies would be required to determine if there are advantages of manipulating the augmentation of YAP/TAZ-TEADs interaction in cumulus cells during the early events of IVM protocol. So far, the possibility sounds encouraging. It has been reported that inhibition of YAP transcriptional activity during development of the bovine blastocyst in vitro, either by verteporfin or a YAP targeting GapmeR, reduced the percent of zygotes that became blastocysts and the proportion of blastocysts that hatched [45]. In addition, a recent study also in cattle confirmed that Hippo signaling pathway is involved in the initiation of bovine blastocyst formation [46].
Conclusions
The data presented herein provide considerable insight into the functional importance of a completely novel signaling pathway underlying cumulus expansion in monovulatory species. The Hippo pathway effectors YAP and TAZ interact with EGF-like signaling to regulate in vitro expansion-related events in bovine cumulus cells. The present study allowed us mainly to confirm that YAP/TAZ and/or CTGF are potential targets to improve the efficiency of in vitro maturation (IVM) protocols used by the industry of reproductive biotechnology in both animals and humans.
Acknowledgements
The authors are thankful to Mrs. Camila Azzolin de Souza and Mrs. Camila Cupper for their support in this study and to Frigorífico Silva for ovaries donation.
Abbreviations
- ADAM17
A disintegrin and metalloproteinase 17
- AREG
Amphiregulin
- CC
Cumulus cells
- COCs
Cumulus-oocyte complexes
- CTGF
Connective tissue growth factor
- DMSO
Dimethyl sulfoxide
- EGF
Epidermal growth factor
- EGFR
Epidermal growth factor receptor
- EREG
Epiregulin
- FSH
Follicle-stimulating hormone
- GC
Granulosa cells
- H2AFZ
Histone H2A.Z
- HAS2
Hyaluronan synthase 2
- IHC
Immunohistochemistry
- IVM
In vitro maturation
- LH
Luteinizing hormone
- PLAT
Plasminogen activator tissue-type A
- PTGS2
Prostaglandin endoperoxide synthase 2
- PTX3
Pentraxin-related protein 3
- RPS18
Ribosomal protein S18
- TAZ
Transcriptional co-activator with PDZ-binding motif
- TEAD
Transcriptional enhanced associate domain
- VP
Verteporfin
- YAP
Yes-associated protein 1
Author contribution
J.K., V.M.P., P.B.D.G., and G.Z. were involved in the study conception and design; J.K., V.M.P., E.C., D.M., L.G.A., Z.S., and A.Q.A. performed experiments and were involved in the acquisition and analyzes of data; G.Z., P.B.D.G., and B.G.G contributed with required reagents acquisition; J.K. wrote the main manuscript text and prepared the tables and figures; G.Z. and P.B.D.G. reviewed and edited the manuscript to be published.
Funding
This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant RGPIN-2018–06470 (to Dr. Zamberlam) and by fellowships from the National Council for Scientific and Technological Development (CNPq; Brazil), Coordination for the Improvement of Higher Education Personnel (CAPES; Brazil) and by the grants from Rio Grande do Sul State Research Support Foundation (FAPERGS – Edital 06/2019; 19/2551–0002275-1) & CNPq, Edital 16/2551–0000494-3; Brazil (to Dr. Gonçalves).
Data availability
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Code availability
Not applicable.
Declarations
Ethics approval/consent to participate
All ovaries were obtained from a local slaughterhouse and in vitro procedures were approved by the Ethics and Animal Welfare Committee of the Federal University of Santa Maria, Brazil/not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Fortune JE. Ovarian follicular growth and development in mammals. Biol Reprod. 1994;50(2):225–232. doi: 10.1095/biolreprod50.2.225. [DOI] [PubMed] [Google Scholar]
- 2.Bromer JG, Cetinkaya MB, Arici A: Pretreatments before the induction of ovulation in assisted reproduction technologies: evidence-based medicine in 2007. Ann N Y Acad Sci 2008, 1127 31–40 10.1196/annals.1434.004 [DOI] [PubMed]
- 3.Davis BJ, Lennard DE, Lee CA, Tiano HF, Morham SG, Wetsel WC, Langenbach R. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1beta. Endocrinology. 1999;140(6):2685–2695. doi: 10.1210/endo.140.6.6715. [DOI] [PubMed] [Google Scholar]
- 4.Espey LL. Ovulation as an inflammatory reaction–a hypothesis. Biol Reprod. 1980;22(1):73–106. doi: 10.1095/biolreprod22.1.73. [DOI] [PubMed] [Google Scholar]
- 5.Shimada M, Hernandez-Gonzalez I, Gonzalez-Robayna I, Richards JS. Paracrine and autocrine regulation of epidermal growth factor-like factors in cumulus oocyte complexes and granulosa cells: key roles for prostaglandin synthase 2 and progesterone receptor. Mol Endocrinol. 2006;20(6):1352–1365. doi: 10.1210/me.2005-0504. [DOI] [PubMed] [Google Scholar]
- 6.Russell DL, Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update. 2007;13(3):289–312. doi: 10.1093/humupd/dml062. [DOI] [PubMed] [Google Scholar]
- 7.Portela VM, Zamberlam G, Goncalves PB, de Oliveira JF, Price CA. Role of angiotensin II in the periovulatory epidermal growth factor-like cascade in bovine granulosa cells in vitro. Biol Reprod. 2011;85(6):1167–1174. doi: 10.1095/biolreprod.111.094193. [DOI] [PubMed] [Google Scholar]
- 8.Park J-Y, Su Y-Q, Ariga M, Law E, Jin SLC, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303(5658):682–684. doi: 10.1126/science.1092463. [DOI] [PubMed] [Google Scholar]
- 9.Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24(2):402–411. doi: 10.1210/me.2009-0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heath E, Tahri D, Andermarcher E, Schofield P, Fleming S, Boulter CA. Abnormal skeletal and cardiac development, cardiomyopathy, muscle atrophy and cataracts in mice with a targeted disruption of the Nov (Ccn3) gene. BMC Deve Biol. 2008;8:18. doi: 10.1186/1471-213x-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–2738. doi: 10.1158/0008-5472.Can-10-2711. [DOI] [PubMed] [Google Scholar]
- 12.Mauviel A, Nallet-Staub F, Varelas X. Integrating developmental signals: a Hippo in the (path)way. Oncogene. 2012;31(14):1743–1756. doi: 10.1038/onc.2011.363. [DOI] [PubMed] [Google Scholar]
- 13.Malik AR, Liszewska E, Jaworski J. Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system. Front Cell Neurosci. 2015;9:237–237. doi: 10.3389/fncel.2015.00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dos Santos EC, Lalonde-Larue A, Antoniazzi AQ, Barreta MH, Price CA, Dias Gonçalves PB, Portela VM, Zamberlam G. YAP signaling in preovulatory granulosa cells is critical for the functioning of the EGF network during ovulation. Mol Cell Endocrinol. 2021;541:111524. 10.1016/j.mce.2021.111524 [DOI] [PubMed]
- 15.Sun T, Diaz FJ. Ovulatory signals alter granulosa cell behavior through YAP1 signaling. Reprod Biol Endocrinol. 2019;17(1):113–113. doi: 10.1186/s12958-019-0552-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell BK, Souza C, Gong J, Webb R, Kendall N, Marsters P, Robinson G, Mitchell A, Telfer EE, Baird DT. Domestic ruminants as models for the elucidation of the mechanisms controlling ovarian follicle development in humans. Reproduction (Cambridge, England) Suppl. 2003;61:429–443. [PubMed] [Google Scholar]
- 17.Leibfried L, First NL. Characterization of bovine follicular oocytes and their ability to mature in vitro. J Anim Sci. 1979;48(1):76–86. doi: 10.2527/jas1979.48176x. [DOI] [PubMed] [Google Scholar]
- 18.Stefanello JR, Barreta MH, Porciuncula PM, Arruda JN, Oliveira JF, Oliveira MA, Gonçalves PB. Effect of angiotensin II with follicle cells and insulin-like growth factor-I or insulin on bovine oocyte maturation and embryo development. Theriogenology. 2006;66(9):2068–2076. doi: 10.1016/j.theriogenology.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Barreta MH, Oliveira JF, Ferreira R, Antoniazzi AQ, Gasperin BG, Sandri LR, Gonçalves PB. Evidence that the effect of angiotensin II on bovine oocyte nuclear maturation is mediated by prostaglandins E2 and F2alpha. Reproduction. 2008;136(6):733–740. doi: 10.1530/rep-08-0268. [DOI] [PubMed] [Google Scholar]
- 20.De Cesaro MP, Macedo MP, Santos JT, Rosa PR, Ludke CA, Rissi VB, Gasperin BG, Gonçalves PB. Natriuretic peptides stimulate oocyte meiotic resumption in bovine. Anim Reprod Sci. 2015;159:52–59. doi: 10.1016/j.anireprosci.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 21.Liu-Chittenden Y, Huang B, Shim JS, Chen Q, Lee SJ, Anders RA, Liu JO, Pan D. Genetic and pharmacological disruption of the TEAD-YAP complex suppresses the oncogenic activity of YAP. Genes Dev. 2012;26(12):1300–1305. doi: 10.1101/gad.192856.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acids Res. 2001;29(9):e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price JC, Sheldon IM. Granulosa cells from emerged antral follicles of the bovine ovary initiate inflammation in response to bacterial pathogen-associated molecular patterns via Toll-like receptor pathways. Biol Reprod. 2013;89(5):119. doi: 10.1095/biolreprod.113.110965. [DOI] [PubMed] [Google Scholar]
- 24.Portela VM, Zamberlam G, Price CA. Cell plating density alters the ratio of estrogenic to progestagenic enzyme gene expression in cultured granulosa cells. Fertil Steril. 2010;93(6):2050–2055. doi: 10.1016/j.fertnstert.2009.01.151. [DOI] [PubMed] [Google Scholar]
- 25.Meng Z, Moroishi T, Guan KL. Mechanisms of Hippo pathway regulation. Genes Dev. 2016;30(1):1–17. doi: 10.1101/gad.274027.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halder G, Johnson RL. Hippo signaling: growth control and beyond. Development. 2011;138(1):9–22. doi: 10.1242/dev.045500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagashima T, Kim J, Li Q, Lydon JP, DeMayo FJ, Lyons KM, Matzuk MM. Connective tissue growth factor is required for normal follicle development and ovulation. Mol Endocrinol. 2011;25(10):1740–1759. doi: 10.1210/me.2011-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plewes MR, Hou X, Zhang P, Liang A, Hua G, Wood JR, Cupp AS, Lv X, Wang C, Davis JS. Yes-associated protein 1 is required for proliferation and function of bovine granulosa cells in vitro. Biol Reprod. 2019;101(5):1001–1017. doi: 10.1093/biolre/ioz139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu LL, Su T, Luo RC, Zheng YH, Huang J, Zhong ZS, Nie J, Zheng LP. Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J Cell Physiol. 2019;234(2):1578–1587. doi: 10.1002/jcp.27024. [DOI] [PubMed] [Google Scholar]
- 30.Lv X, He C, Huang C, Wang H, Hua G, Wang Z, Zhou J, Chen X, Ma B, Timm BK, et al. Timely expression and activation of YAP1 in granulosa cells is essential for ovarian follicle development. FASEB J. 2019;33(9):10049–10064. doi: 10.1096/fj.201900179RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsoi M, Morin M, Rico C, Johnson RL, Paquet M, Gévry N, Boerboom D. Lats1 and Lats2 are required for ovarian granulosa cell fate maintenance. FASEB J. 2019;33(10):10819–10832. doi: 10.1096/fj.201900609R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-alpha-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144(10):4376–4384. doi: 10.1210/en.2003-0487. [DOI] [PubMed] [Google Scholar]
- 33.Su YQ, Denegre JM, Wigglesworth K, Pendola FL, O’Brien MJ, Eppig JJ. Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol. 2003;263(1):126–138. doi: 10.1016/s0012-1606(03)00437-8. [DOI] [PubMed] [Google Scholar]
- 34.Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, Doni A, Bastone A, Mantovani G, Beck Peccoz P, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131(7):1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- 35.Yang R, Wu Y, Zou J, Zhou J, Wang M, Hao X, Cui H. The Hippo transducer TAZ promotes cell proliferation and tumor formation of glioblastoma cells through EGFR pathway. Oncotarget. 2016;7(24):36255–36265. doi: 10.18632/oncotarget.9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrade D, Mehta M, Griffith J, Panneerselvam J, Srivastava A, Kim T-D, Janknecht R, Herman T, Ramesh R, Munshi A. YAP1 inhibition radiosensitizes triple negative breast cancer cells by targeting the DNA damage response and cell survival pathways. Oncotarget. 2017;8(58):98495–98508. doi: 10.18632/oncotarget.21913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao L, Guan H, Song C, Wang Y, Liu C, Cai C, Zhu H, Liu H, Zhao L, Xiao J. YAP1 is essential for osteoclastogenesis through a TEADs-dependent mechanism. Bone. 2018;110:177–186. doi: 10.1016/j.bone.2018.01.035. [DOI] [PubMed] [Google Scholar]
- 38.Song S, Honjo S, Jin J, Chang SS, Scott AW, Chen Q, Kalhor N, Correa AM, Hofstetter WL, Albarracin CT, et al. The Hippo coactivator YAP1 mediates EGFR overexpression and confers chemoresistance in esophageal cancer. Clin Cancer Res. 2015;21(11):2580–2590. doi: 10.1158/1078-0432.Ccr-14-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tome-Garcia J, Erfani P, Nudelman G, Tsankov AM, Katsyv I, Tejero R, Bin Z, Walsh M, Friedel RH, Zaslavsky E, et al. Analysis of chromatin accessibility uncovers TEAD1 as a regulator of migration in human glioblastoma. Nat Commun. 2018;9(1):4020. doi: 10.1038/s41467-018-06258-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang DH, Ren J, Zhou CJ, Han Z, Wang L, Liang CG. Supplementation with CTGF, SDF1, NGF, and HGF promotes ovine in vitro oocyte maturation and early embryo development. Domest Anim Endocrinol. 2018;65:38–48. doi: 10.1016/j.domaniend.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Zhao B, Tumaneng K, Guan KL. The Hippo pathway in organ size control, tissue regeneration and stem cell self-renewal. Nat Cell Biol. 2011;13(8):877–883. doi: 10.1038/ncb2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu CY, Zha ZY, Zhou X, Zhang H, Huang W, Zhao D, Li T, Chan SW, Lim CJ, Hong W, et al. The hippo tumor pathway promotes TAZ degradation by phosphorylating a phosphodegron and recruiting the SCF{beta}-TrCP E3 ligase. J Biol Chem. 2010;285(48):37159–37169. doi: 10.1074/jbc.M110.152942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao B, Li L, Tumaneng K, Wang CY, Guan KL. A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF(beta-TRCP) Genes Dev. 2010;24(1):72–85. doi: 10.1101/gad.1843810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 2007;21(21):2747–2761. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Negrón-Pérez VM, Hansen PJ. Role of yes-associated protein 1, angiomotin, and mitogen-activated kinase kinase 1/2 in development of the bovine blastocyst. Biol Reprod. 2018;98(2):170–183. doi: 10.1093/biolre/iox172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu B, van Tol HTA, Oei CHY, Stout TAE, Roelen BAJ 2021 Lysophosphatidic acid accelerates bovine in vitro-produced blastocyst formation through the Hippo/YAP pathway. Int J Mol Sci. 22(11) 10.3390/ijms22115915 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request. All data generated or analyzed during this study are included in this published article.
Not applicable.