Abstract
Background
The Hemophilia Joint Health Score (HJHS) was developed and validated to detect arthropathy in children. Additional evidence is required to show validity in adults. We studied the convergent and discriminant construct validity of the HJHS version 2.1(HJHSv2.1) in adults with hemophilia. A secondary aim was to define age‐related normative adult HJHSv2.1 reference values.
Methods
We studied 192 adults with hemophilia, and 120 healthy adults in four age‐matched groups—18 to 29, 30 to 40, 41 to 50, and >50 years—at nine centers. Trained physiotherapists scored the HJHS and World Federation of Hemophilia (WFH) joint score. Health history, the Functional Independence Scale of Hemophilia (FISH), Hemophilia Activities List (HAL), and Short‐Form McGill Pain Questionnaire (SF‐MPQ) were also collected.
Results
The median age was 35.0 years. Of participants with hemophilia, 68% had severe, 14% moderate, and 18% mild disease. The HJHS correlated strongly with WFH score (Spearman’s rho [rs ] = .95, P < .001). Moderate correlations were seen between the FISH (rs = .50, P < .001) and SF‐MPQ Present Pain Intensity (rs = .50, P < .001), while a modest correlation was found with the HAL (rs = −.37, P < .001). The HJHS significantly differentiated between age groups (Kruskal‐Wallis T = 35.02, P < .001) and disease severity in participants with hemophilia. The HJHS had high internal reliability (Cronbach’s α = .88). We identified duration of swelling as a redundant item in the HJHS.
Conclusions
The HJHS shows evidence of strong convergent and discriminant construct validity to detect arthropathy in adults with hemophilia and is well suited for use in this population.
Keywords: adult, arthropathy, hemarthrosis, hemophilia, validity of results
Essentials.
The HJHS version 2.1 (HJHSv2.1) instrument was valid for arthropathy in adults with hemophilia.
HJHS scores progressively worsen with age.
Age‐related HJHSv2.1 reference values are presented for healthy adults.
The HJHS is suitable for use in adults with hemophilia.
1. INTRODUCTION
In persons with hemophilia, spontaneous and/or trauma‐related hemarthrosis and bleeding into muscles commonly leads to arthropathy and joint dysfunction. 1 Hemophilic arthropathy is characterized by chronic proliferative synovitis with associated chronic osteochondral changes in the large joints, especially affecting the elbows, knees, and ankles (the “index“ joints). 1 , 2 While the early and regular prophylactic administration of factor concentrates very significantly decreases the yearly rate of hemarthroses associated with hemophilia, preventing the pernicious development of joint damage that may accompany subclinical bleeding or inadequate prophylaxis is more challenging. Structural joint damage can be assessed by imaging, but radiological methods have limitations such as lack of sensitivity to early joint damage in the case of plain x‐rays, and high cost or the need for specialized equipment for magnetic resonance imaging (MRI) or ultrasound. 3 , 4 The objective measurement of joint health by physical examination therefore remains of paramount importance for care teams in evaluating the impact of clinical interventions, and even more so as innovative but costly approaches to correcting hemostatic deficiencies are introduced. 5 , 6
Over the past 20 years, considerable efforts have been directed to improving the original World Federation of Hemophilia (WFH) Orthopedic Joint Score (also known as the Gilbert Score). The Hemophilia Joint Health Score (HJHS) was specifically developed by the Physical Therapy Expert Working Group of the International Prophylaxis Study Group to detect early joint changes in boys aged 4 to 18 years with hemophilia. 7 , 8 It built on the early work by clinical teams in Denver and Stockholm, who were trying to improve the detection of subtle changes in joint health in persons with hemophilia. The HJHS is derived from a physical examination of the index joints by a health care professional who should have training and expertise in the conduct of a detailed musculoskeletal (MSK) examination. It is a measure of joint health and sits within the World Health Organization International Classification of Function and Disability domain of body structure and function (ie, impairment). The current HJHS version 2.1 comprises an assessment of specific features, or items, of the six index joints and an assessment of global gait. For each of the six joints, the following items are scored: swelling (scored 0‐3), duration of swelling (0‐1), muscle atrophy (0‐2), crepitus on motion (0‐2), flexion loss (0‐3), extension loss (0‐3), joint pain (0‐2), and strength (0‐4). The maximum score for an individual index joint is 20. Gait is scored 0 to 4. The maximum HJHS total score is 124, with a higher score indicating worse joint health. The measurement properties of various physical examination instruments used in hemophilia, including the HJHS, have recently been extensively reviewed and assessed. The authors concluded that the body of evidence supporting the use of the HJHS in clinical practice was particularly strong in pediatric populations and in intensively treated young adults. However, they found that the validity of the HJHS in adults needed to be further investigated. 9
We hypothesized that the Hemophilia Joint Health Score (HJHSv2.1) is valid for use in adults with hemophilia. The primary study objective was, therefore, to investigate the convergent construct validity and discriminant construct validity of the HJHSv2.1 in adults with hemophilia by examining correlations with relevant constructs such as functional independence (as determined by the Functional Independence Scale of Hemophilia [FISH], and Hemophilia Activities List [HAL]) and pain (as determined by the Short‐Form McGill Pain Questionnaire [SF‐MPQ]), as well as observed differences in scores between known groups in whom we expect differences. A secondary objective was to explore if any modifications, item reduction, or addenda to the tool are required for the adult population. In addition, little is known regarding the properties of the HJHS in healthy adults. Another important secondary objective of this study was to define age‐related HJHS scores in healthy men without hemophilia to establish normative adult reference values.
2. METHODS
2.1. Study design and participants
This was a cross‐sectional study approved by the Research Ethics Boards at The Hospital for Sick Children, Toronto (data coordination center), CHU Sainte‐Justine, Montreal, Canada (central coordination site), and the eight additional participating sites (Cliniques Universitaires St‐Luc, Haemostasis & Thrombosis Unit, Brussels, Belgium; Christian Medical College, Vellore, India;, Hemophilia and Thrombosis Center, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; Indiana Hemophilia and Thrombosis Center, Indianapolis, IN, USA; The Center for Inherited Blood Disorders [CIBD], Orange, CA, USA; the Centre for Haemostasis and Thrombosis, Guy’s and St Thomas’ NHS Foundation Trust, London, UK; Haemophilia, Haemostasis and Thrombosis Centre, Basingstoke, UK; and Royal Free Hospital, Katherine Dormandy Haemophilia and Thrombosis Centre, London, UK). Informed consent was obtained from all participants.
Adults aged ≥18 years diagnosed with hemophilia A and B (factor VIII and IX deficiency) of any severity were eligible for this study. The ISTH definitions of severity of hemophilia were used in our study and analysis. 10 Participants with a past or current history of inhibitors, infections such as hepatitis C and/or HIV, or prior arthroplasty or arthrodesis could be recruited into the study. Candidates were excluded on the basis of comorbid illnesses such as rheumatoid arthritis, muscular dystrophy, neurologic disorders, or other non–hemophilia‐related significant MSK conditions that may have independently affected the HJHS score, as were candidates who were unable to comply with the study protocol or deemed inappropriate by the investigators. As per guidelines in the HJHSv2.1 instruction manuals, candidates within 2 weeks of an acute joint bleed were excluded or the study procedure was postponed until the eligibility criteria were met.
Age‐matched healthy men without hemophilia were also recruited. Again, participants with conditions such as rheumatoid arthritis, muscular dystrophy, neurologic disorders, or other significant MSK conditions that may have independently affected the HJHS score were excluded. However, subjects with mild osteoarthritis, mild sequelae from injury or minor joint surgeries such as tendon repair, or other mild nonspecific degenerative conditions were allowed to participate.
Participants from both groups were stratified into four age groups: 18 to 29, 30 to 40, 41 to 50, and >50 years. Study sites were allocated similar recruitment targets based on age and hemophilia severity. Participants with hemophilia were identified using clinic databases and were approached by their treatment team shortly before or at the time of their regularly scheduled clinic visit via letter, telephone, or in person with no selection criteria other than age and a confirmed diagnosis of hemophilia. Adults without hemophilia were recruited through flyers, posters, or institutional recruitment websites.
2.2. Study procedures and measures
All study procedures were generally performed during one visit to the study site. General demographic, occupation, sport activities, and health information about factor levels, history of an inhibitor, number and types of bleeds in the preceding 12 months, factor prophylaxis regimen, regular use of MSK aids, coinfections, medications, history of arthritis, joint replacement, and any joint or limb surgeries were obtained through an interview and review of the patient health chart.
All participants underwent one MSK examination performed by physiotherapists specialized in hemophilia care and with long‐standing experience in use of the HJHS. In the case of participants with hemophilia, the HJHS, WFH (Gilbert) and FISH were performed by the same physiotherapist, who was blinded to the scores of the other measures, except for the Vellore, India, study site, where the FISH score was obtained by an occupational therapist. In the healthy participants without hemophilia, only the HJHS was completed. The HAL and SF‐MPQ are self‐reported measures that participants with hemophilia completed independently.
Data collection was completed in case report forms by the research team members at each study site and sent to the central data coordination center for entry into a secure electronic database. Data quality was ensured by thorough double verification of the received data in addition to query responses provided by each study site.
2.2.1. Hemophilia Joint Health Score version 2.1
The HJHSv2.1 was performed according to the instruction manual and video available through registration on the web site http://ipsg.ca/hjhs‐portal. Items from the original HJHS version 1.0, specifically axial alignment and instability, were also collected for review in this specific population. These two items were scored independently from the HJHSv2.1 total score to determine if they warranted inclusion in an additional module that is targeted for use in adults with hemophilia. Participants who had undergone previous joint replacement surgery had those joints scored using the same item definitions and grading as nonoperated joints.
2.2.2. WFH Orthopaedic Joint score
The WFH score was calculated by study physiotherapists from the same raw data obtained while performing the HJHSv2.1 and using the scoring sheet available from the WFH website. 11 , 12
2.2.3. Functional Independence Score in Hemophilia
An objective assessment of participants’ ability to perform activities was scored using a validated performance‐based measure, the FISH, which includes the assessment of eight activities: eating, grooming, dressing, chair transfer, squatting, walking, step climbing, and running. 13 , 14 , 15 The score was calculated on the basis of the materials provided online at http://www.wfh.org/en/page.aspx?pid=884.
2.2.4. Hemophilia Activities List
The HAL is a tool that measures the impact of hemophilia on self‐perceived functional abilities in adults. It is a self‐administered questionnaire originally developed in Dutch and has validated versions in the languages used in this study. It consists of 42 items in seven domains: lying down/sitting/kneeling/standing, functions of the legs, functions of the arms, use of transportation, self‐care, household tasks, and leisure activities and sport. 16 , 17 It was used in all participants with hemophilia with the exception of the Vellore, India, study site where investigators had previously found it to be unreliable in their population. 15
2.2.5. Short‐Form McGill Pain Questionnaire
The SF‐MPQ is a self‐administered pain questionnaire and consists of 15 descriptors that are rated on an intensity scale: 0 (none) to 3 (severe). The Pain Rating Index includes three pain scores, derived from the sum of intensity rank values of the words chosen in each of the dimensions of pain: sensory (11 items), affective (4 items), and total descriptors. The short form includes the selection of a representative group of words in each category. The SF‐MPQ also includes a visual analog scale known as the Present Pain Intensity scale. It has been validated and is available in more than 80 languages. 18
2.3. Analysis and sample size
For this analysis, correlation values >0.8 are considered to indicate strong correlation, whereas values of 0.4 to 0.8 are considered moderate and 0.2 to 0.39 are considered modest.
Convergent construct validity was considered to have been established if all the correlations (Spearman’s rho [rs ]), as follows, were achieved. We hypothesized that the correlation between the total HJHS score and the WFH score would be strong, as they address the same construct. We hypothesized a moderate correlation when the HJHS is compared to the FISH and modest to moderate negative correlation with HAL, given the fact that these measures address activity limitation and (in the case of the HAL) participation rather that specifically structure and function of the joints. Finally, we expected a modest to moderate correlation with the subscales of the SF‐MPQ, as features of pain are related to the types of damage picked up by the HJHS. No correction was made for multiple tests of correlation, as all of the convergent construct validity hypotheses had to be as predicted to conclude convergent construct validity and because we were concerned with the magnitude of the correlation and not the P values in this analysis.
Discriminant (known groups) construct validity was considered to have been established if the HJHS scores were significantly higher in older adults than in younger adults and in participants with severe hemophilia compared to those with mild or moderate disease (as assessed by the Kruskal‐Wallis nonparametric analysis of variance).
To explore if we could reduce the items of the HJHS in adults by removing those that contributed poorly to the assessment of the construct of joint impairment, we examined their internal reliability using Cronbach’s α and the item‐total correlations. Cronbach’s α should be between 0.7 and 0.9; with a higher value indicating very good internal reliability. Item‐total correlations calculated with rs <.3 indicated that an item did not contribute to measurement of the target construct (joint health in the structure and function domain). Redundant items were also examined by correlations using rs . Interitem correlations calculated with rs <.2 indicated items with a weak correlation and >.9 indicated potential item redundancy. Exploratory analysis using item‐total correlations calculated with rs were also completed with HJHS items previously removed from earlier versions (instability and axial alignment). Finally, items with nonzero scores in <15% of participants were considered rarely endorsed.
Our target sample size of 200 persons with hemophilia and 120 healthy adults was calculated to show, with over 95% power at α = .05, that an observed correlation of 0.6 (moderate) is statistically different from a correlation of <0.4 (modest); this would mean that our sample size was adequate to explore all our convergent construct validity hypotheses.
3. RESULTS
This study was conducted between 2016 and 2018. We enrolled 192 adults with hemophilia (median age, 35.0 years; range, 18‐82) and 120 age‐matched healthy adults without hemophilia (median age, 35.0 years; range, 18.0‐69.0) in this study. The demographic and clinical characteristics of the hemophilia cohort are provided in Table 1. Among the participants with hemophilia, 130 (68%) had severe, 26 (14%) had moderate, and 34 (18%) had mild disease. With regard to treatment regimens, 85 (44%) were on episodic treatment at the time of study, 38 (20%) were considered to have been on primary prophylaxis since early childhood, and 69 (36%) were on other types of prophylaxis regimens. The prevalence of treatment regimen (episodic vs prophylaxis) varied as expected on the basis of age and disease severity. Of the participants with hemophilia, 31 (16%) had a history of joint replacement, and 59 (31%) had had some form of surgical, chemical, or radioisotope synovectomy. Being involved in a sport or other form of physical activity in the preceding 3 months was reported by 102 (53%) of participants with hemophilia. Table 1 provides more detailed information by age category.
TABLE 1.
Demographic and clinical characteristics of the hemophilia study cohort
| Age cohorts, year | 18‐29 | 30‐40 | 41‐50 | >50 | Total |
|---|---|---|---|---|---|
| (N = 65) | (N = 51) | (N = 26) | (N = 50) | (N = 192) | |
| Type | |||||
| Hemophilia A | 60 (92.3) | 47 (92.2) | 22 (84.6) | 35 (70) | 164 (85.4) |
| Hemophilia B | 5 (7.7) | 4 (7.8) | 4 (15.4) | 15 (30) | 28 (14.6) |
| Factor level | |||||
| <1% | 45 (69.2) | 37 (72.5) | 18 (69.2) | 30 (60) | 130 (67.7) |
| 1%‐5% | 10 (15.4) | 5 (9.8) | 4 (15.4) | 7 (14) | 26 (13.5) |
| >5% | 9 (13.8) | 9 (17.6) | 4 (15.4) | 12 (24) | 34 (17.7) |
| Prophylaxis regimen | |||||
| Episodic (“on‐demand”) | 24 (36.9) | 27 (52.9) | 12 (46.1) | 22 (44) | 85 (44.3) |
| Primary prophylaxis | 25 (38.5) | 12 (23.5) | 1 (3.8) | 0 (0) | 38 (19.8) |
| Other prophylaxis | 16 (24.6) | 12 (23.5) | 13 (50) | 28 (56) | 69 (35.9) |
| Inhibitors | |||||
| Current inhibitor | 4 (6.1) | 2 (3.9) | 2 (7.7) | 1 (2) | 9 (4.7) |
| Past inhibitor | 8 (12.3) | 5 (9.8) | 2 (7.7) | 2 (4) | 17 (8.9) |
| Comorbid conditions | |||||
| HIV | 0 (0) | 2 (3.9) | 7 (29.9) | 11 (22) | 20 (10.4) |
| Prior or active hepatitis C virus | 0 (0) | 6 (11.8) | 7 (26.9) | 6 (12) | 19 (9.9) |
| Other comorbid conditions | 2 (3.1) | 12 (23.5) | 12 (46.1) | 30 (60) | 56 (29.2) |
| Medications (regularly) | |||||
| Nonsteroidal anti‐inflammatory drugs | 1 (1.5) | 2 (3.9) | 6 (23.1) | 3 (6) | 12 (6.3) |
| Opioid analgesics | 2(3.1) | 0 (0) | 2 (7.7) | 7 (14) | 11 (5.7) |
| Other analgesics (eg, acetaminophen) | 1 (1.5) | 1 (2) | 4 (15.4) | 18 (18.4) | 24 (12.5) |
| Reporting use of aids (eg, brace, splint, cane) | 10 (15.4) | 17 (33.3) | 6 (23.1) | 15 (30) | 48 (25) |
| Prior joint replacement | |||||
| Elbow | 0 (0) | 0 (0) | 0 (0) | 1 (2) | 1 (0.5) |
| Knee | 1 (1.5) | 5 (9.8) | 7 (26.9) | 22 (44) | 35 (18.2) |
| Ankle | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Hip | 0 (0) | 1 (2) | 1 (3.8) | 4 (8) | 6 (3.1) |
| Prior synovectomy | 14 (21.5) | 18 (35.3) | 12 (46.2) | 15 (30) | 59 (30.7) |
| Bleeds | |||||
| Reporting any bleeds in the 4 wk before study participation | 11 (16.9) | 10 (19.6) | 9 (34.6) | 10 (20) | 40 (20.8) |
| Reporting joint bleeds in the 12 months before study participation | 12 (18.5) | 12 (23.5) | 8 (30.8) | 9 (18) | 41 (21.4) |
| Employment status | |||||
| Student | 24 (36.9) | 1 (2) | 0 (0) | 0 (0) | 25 (13.0) |
| Employed | 36 (55.4) | 44 (86.3) | 22 (84.6) | 26 (52.0) | 128 (66.7) |
| Unemployed | 5 (7.7) | 5 (9.8) | 2 (7.7) | 7 (14.0) | 19 (9.9) |
| Retired | 0 (0) | 0 (0) | 2 (7.7) | 16 (32.0) | 18 (9.4) |
| Unknown | 0 (0) | 1 (2) | 0 (0) | 1 (2) | 2 (1.0) |
All values represent numbers and their associated percentage.
Note Episodic “on‐demand”: Treatment at the time of a bleed. Patient may be infusing factor from time to time before a risky activity to prevent bleeding. Primary prophylaxis: Factor infusions given regularly at least once a week to prevent bleeding and its consequences in a patient with no established joint disease—usually starting in the first or second year of life, before the third year, or after a spontaneous joint bleed. This option was selected for patients that have been on uninterrupted primary prophylaxis since childhood, as defined above. Any other prophylaxis: Factor infusions given regularly at least once a week in order to prevent bleeding. Bleeds: include both spontaneous and traumatic bleeds.
Table 2 provides the demographic and clinical characteristics of the healthy participants without hemophilia. Of those, 17 (14%) reported having had various surgery on their limbs, but none had undergone a joint replacement. Among the participants without hemophilia of all age categories, 95 (79%) reported engaging in sports and other physical activities over the preceding 3 months.
TABLE 2.
Demographic and clinical characteristics of the healthy nonhemophilia study cohort
| Age cohorts, y | 18‐29 | 30‐40 | 41‐50 | >50 |
|---|---|---|---|---|
| (N = 40) | (N = 35) | (N = 22) | (N = 23) | |
| Prior significant limb injury | 11 (27.5) | 13 (37.1) | 5 (22.7) | 9 (39.1) |
| Prior limb surgery | ||||
| Wrist | 0 (0.0) | 0 (0.0) | 1 (4.5) | 1 (4.3) |
| Shoulder | 0 (0.0) | 1 (2.9) | 0 (0.0) | 0 (0.0) |
| Knee | 4 (10.0) | 3 (8.6) | 1 (4.5) | 0 (0.0) |
| Ankle | 0 (0.0) | 2 (5.7) | 0 (0.0) | 0 (0.0) |
| Hip | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.3) |
| Other | 0 (0.0) | 1 (2.9) | 0 (0.0) | 2 (8.6) |
| Prior joint replacement | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Physical activities | ||||
| Past 3 months | 37 (92.5) | 29 (82.6) | 14 (63.6) | 15 (65.2) |
| Leisure sports/activities | 26 (65.0) | 24 (68.6) | 10 (45.5) | 13 (56.5) |
| Organized sports (team or league) | 8 (20) | 4 (11.4) | 4 (18.2) | 0 (0.0) |
| Unknown | 3 (7.5) | 1 (2.9) | 0 (0.0) | 2 (8.7) |
All values represent numbers and their associated percentage.
Occupations for both participants with and participants without hemophilia were categorized into the 10 categories of the Canadian National Occupational Classification version 1. 19 This information is available in Appendix S1.
The HJHS total scores had a wide span in all age categories and increased with age (Figure 1). The median (interquartile range [IQR]) of HJHS total score in the adults with hemophilia was 9.0 (2.0‐21.0) in participants aged 18 to 29 years, 27.0 (8.0‐38.0) in participants aged 30 to 40 years, 36.0 (20.50‐48.0) in participants aged 41 to 50 years, and 37.0 (17.0‐50.8) in those aged >50 years.
FIGURE 1.
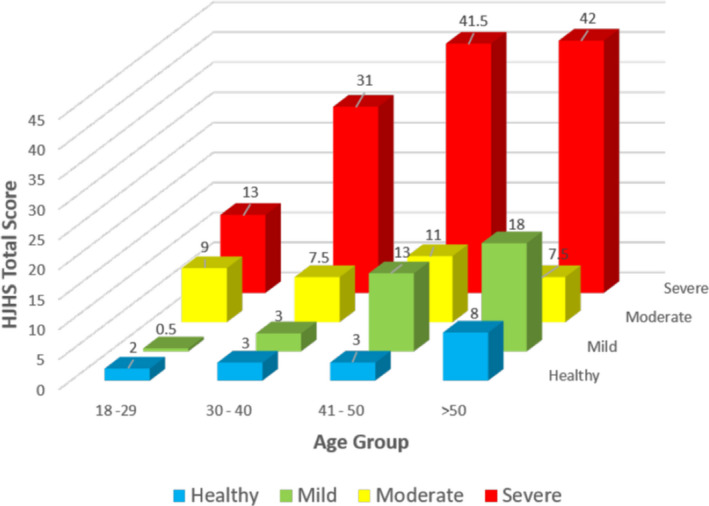
The Hemophilia Joint Health Score total scores in the hemophilia and healthy cohorts by age group and severity
In comparison, the HJHS total scores in the healthy adults without hemophilia were significantly lower. Median (IQR) HJHS total scores were 2.0 (0.75‐5.0) in those aged 18 to 29 years; 3.0 (1.5‐6.0) in those 30 to 40 years; 3.0 (2.0‐6.0) in those 41 to 50 years, and 8.0 (3.0‐12.5) in those >50 years. HJHS scores increased as a function of age in healthy adults without hemophilia, with rs = .34 (P < .001; Table 3).
TABLE 3.
Percentiles of the HJHS total score in the healthy adults without hemophilia
| Age cohorts, y | HJHS total score | |||
|---|---|---|---|---|
| 18‐29 | 30‐40 | 41‐50 | >50 | |
| N | 40 | 35 | 22 | 23 |
| Median | 2.0 | 3.0 | 3.0 | 8.0 |
| Minimum | 0.0 | 0.0 | 0.0 | 0.0 |
| Maximum | 13.0 | 15.0 | 12.0 | 34.0 |
| 2.5th percentile | 0.0 | 0.0 | 0.0 | 1.1 |
| 5th percentile | 0.0 | 0.0 | 0.0 | 2.0 |
| 25th percentile | 0.8 | 1.5 | 2.0 | 3.0 |
| 50th percentile (median) | 2.0 | 3.0 | 3.0 | 8.0 |
| 75th percentile | 5.0 | 6.0 | 6.0 | 12.5 |
| 95th percentile | 9.2 | 12.2 | 10.8 | 19.4 |
| 97.5th percentile | 12.0 | 15.0 | 11.5 | 26.3 |
Abbreviation: HJHS, Hemophilia Joint Health Score.
The Mann‐Whitney U test indicated that the HJHS total score was significantly greater in adults with hemophilia compared to healthy men without hemophilia: in participants with hemophilia aged 18 to 29 years (U = 1973.0, P < .001); 30 to 40 years (U = 1486.0, P < .001); 41 to 50 years (U = 549.5, P < .001); and >50 years (U = 1034.5, P < .001). The descriptive results for all other outcome measures completed by the hemophilia cohort are provided in Table S1.
3.1. Validation
As hypothesized, there was a very high correlation between the HJHS total scores and the WFH Gilbert scores. Moderate to modest correlations were seen between the HJHS and the pain (SF‐MPQ), function (FISH), and activity (HAL) measures (Table 4), as predicted.
TABLE 4.
Spearman’s correlation between the HJHS total score and WFH (Gilbert) score, SF‐MPQ, FISH, and HAL
| WFH total score | SF‐MPQ | Fish total score | HAL total score | ||||
|---|---|---|---|---|---|---|---|
| Sensory PRI | Affective PRI | SF‐MPQ VAS scale | PPI score | ||||
| HJHSv2.1 total score (P ≤.001) | 0.95 | 0.52 | 0.38 | 0.47 | 0.50 | 0.50 | −0.37 |
Abbreviations: FISH, Functional Independence Score of Hemophilia; HAL, Hemophilia Activities List; HJHS, Hemophilia Joint Health Score; PPI, Present Pain Intensity; PRI, Pain Rating Index; SF‐MPQ, Short‐Form – McGill Pain Questionnaire; WFH, World Federation of Hemophilia Physical Examination (Gilbert) Score.
The HJHS total score significantly differentiated between age groups (Kruskal‐Wallis T = 35.02, P < .001) and disease severity in persons with hemophilia. In participants aged 18 to 29 years, the median HJHS total scores were, respectively, 0.5, 9.0, and 13.0 in participants with mild, moderate, and severe hemophilia (Kruskal‐Wallis T = 6.939, P = .03); comparable values were 3.0, 27.5, and 31 in those aged 30 to 40 years (Kruskal‐Wallis T = 11.439, P = 0.003); 13.0, 29.5, and 41.5 in those aged 41 to 50 years (Kruskal‐Wallis T = 6.211, P = 0.05); and 18.0, 29.0, and 42.0 in those aged >50 years (Kruskal‐Wallis T = 9259, P = .010; Figure 1).
3.2. Internal reliability and possible item reduction
Overall, the HJHSv2.1 items had a high degree of internal reliability (Cronbach’s α = .88). Item score–total score correlations showed that almost all HJHS items (muscle atrophy, crepitus, flexion and extension loss, joint pain, and strength) were highly correlated, except for the items swelling and duration of swelling, which were only moderately correlated. 20
As an exploratory analysis, axial alignment and instability, items included in the initial HJHS version (version 1.0), were examined to determine if they were useful items to include in the adult hemophilia population. Both axial alignment (rs = .215; P = .003) and instability (rs = .227; P = .002) were poorly correlated with the HJHSv2.1 total score, and also resulted in an interitem correlation of rs = .229, which was less than all other HJHSv2.1 items (Table 5).
TABLE 5.
Item score–total score correlation
| HJHSv2.1 item | Item score–total score correlation (Spearman’s rho) |
|---|---|
| HJHS swelling (total) | .58 |
| HJHS duration of swelling | .60 |
| HJHS muscle atrophy (total) | .84 |
| HJHS crepitus (total) | .76 |
| HJHS flexion loss (total) | .85 |
| HJHS extension loss (total) | .89 |
| HJHS joint pain (total) | .76 |
| HJHS strength (total) | .79 |
| Global gait score | .80 |
All items had a P value < .001.
Abbreviation: HJHS, Hemophilia Joint Health Score.
The items in the HJHSv2.1 had a moderate correlation with each other. Two items, swelling and duration of swelling, were very highly correlated (rs = .95; P ≤ .001) indicating a high degree of redundancy.
In all four age categories for persons with hemophilia, every HJHS item had an abnormal (nonzero) score for at least 40% of the participants. The HJHS items with the largest proportion of a zero score (normal) were: strength in 40 (61.5%) participants aged 18 to 29 years; swelling and duration of swelling in 25 (49.0%) participants aged 30 to 40 years; extension loss in 6 (23.1%) participants aged 41 to 50 years; and duration of swelling in 18 (36%) participants aged >50 years. Of note, maximum scores in the item global gait were obtained in >60% of the participants in the age categories 41 to 50 years and >50 years.
In contrast, in healthy adults of all age categories, the HJHS items had an abnormal (nonzero) score in <30% of participants, with the exception of crepitus, which was present in >60% of the of participants. In healthy adults aged >50 years, more items had abnormal scores: swelling (52.2%), duration of swelling (60.9%), muscle atrophy (56.5%), and global gait (52%). In addition, only two healthy participants obtained a maximum score in global gait, and both were aged >50 years. The HJHS items with the largest proportion of zero (normal) scores in healthy adults aged 18 to 29, 30 to 40, and 41 to 50 years were strength (n = 94; range, 95.0%‐100%) and joint pain (n = 91; range, 90.1%‐95.0%).
4. DISCUSSION
We have found the HJHS to be a valid joint assessment tool with high internal reliability in the adult hemophilia population. Moreover, the HJHS significantly differentiated between age groups and disease severity, confirming its discriminant construct validity. As expected, the HJHS and the WFH Gilbert physical examination joint score showed excellent correlation. Together with the moderate correlations between the HJHS total score and both pain (SF‐MPQ) and functional independence (FISH) in the participants with hemophilia, this establishes the convergent construct validity of the HJHS as we initially postulated.
A limitation of our study, however, relates to fact that the same physiotherapist computed both the HJHS and WFH joint score using the same raw physical measurements based on the standardized set of instructions provided with the HJHS tool. In regular practice, no such instructions are provided to arrive at the WFH joint score. We are of the opinion that this improved the performance of the WFH joint score, which was judged to be relatively insensitive in a recent review 9 and which explains in part the high correlation between the two scores.
Interrater and test‐retest reliability has previously been demonstrated for the HJHS 9 and was not in the scope of the current study. In teenagers and young adults, other investigators have shown a low correlation between HJHS and MRI scores and a high correlation with Petterson scores. 21 , 22 Studying the correlation of HJHS and imaging scores was also outside the scope of this investigation. Investigators in the United Kingdom and the Netherlands have previously studied the relationship between the HJHS total score and functional abilities as assessed by the HAL in adults with hemophilia 23 and von Willebrand disease. 24 In both studies, strong correlations were demonstrated between the HJHS and HAL. A larger study in the United States investigated the correlations between four patient‐reported outcome instruments, including the HAL, in adults with hemophilia. 25 An optional HJHS examination was performed on a subset of the subjects, and only a moderate correlation was found between HJHS scores and HAL, similar to our findings. Although these three studies were not designed specifically to establish the convergent and discriminant construct validity of the HJHSv2.1, their results all generally support our conclusions.
It is interesting to note that the HJHS total scores of participants with mild and moderate hemophilia aged >40 years were similar. However, moderate hemophilia is much less prevalent than the severe and mild forms. It can be associated with a range of bleeding phenotypes and represented the smallest group in our cohort. The median HJHS total score for a given age group with moderate hemophilia should be interpreted with caution.
The HJHS has been criticized by many as too time consuming for routine clinical practice, and efforts are under way to determine if a shorter version could be developed. 26 To that end, we confirmed that all HJHS items displayed internal reliability but did not all contribute in a relevant way to the assessment of the joints. We found that the duration of swelling and swelling items were very highly correlated suggesting that only one item (swelling) may need to be scored for adults. This finding is consistent with the conclusions of a recent review of previously published data by a panel of experts. 26 In addition, our analysis shows that axial alignment and joint instability were rarely endorsed and contributed little to the overall score; therefore, the exclusion of these items in the HJHS 2.1 seems reasonable in adults as well.
We purposefully elected to include participants with hemophilia who had undergone joint arthroplasty or fusion in our study, and those joints were scored with the same scale for all HJHS items as nonoperated joints. It can be expected that prior surgery had a significant impact either positively or negatively on the scores of all HJHS items (such as flexion loss, extension loss, joint pain, etc) for those operated joints. The same could be argued for prior synovectomy or other types of therapeutic interventions. Participants with prior joint replacements represented 31% and 54% of the participants in the cohorts aged 41 to 50 and >50, respectively, a proportion comparable to data reported by others. 27 , 28 Including patients with arthroplasty or joint fusion does not alter our conclusion with regard to the construct validity of the HJHS as a measure of joint health at one point in time. Further analysis of our data on operated joints will be performed in the future to assess how scoring criteria might be adapted on the basis of the type of surgery.
Reference ranges for the nonhemophilia population are required to discriminate between HJHS scores due to hemophilia‐related complications and progressive MSK issues resulting from age progression. We have shown, based on our examination of 120 healthy adult participants, that the normative adult reference values of the HJHS total score in healthy men extends up to 12 (95th percentile) in healthy men aged ≤50, and up to 19 (95th percentile) in those aged >50. A history of minor joint injury or surgery is very prevalent in men who would otherwise be considered healthy and is difficult to recall or document accurately over a lifetime. 12 We therefore chose not to exclude from our healthy cohort participants with such a history and consider our healthy control group to be appropriate.
These data are important for the accurate interpretation of joint outcomes using the HJHS in cohorts of adults with hemophilia. We plan to compare the goniometric measures of joint mobility we obtained in our healthy participants by age cohorts with the normative tables provided with the HJHS instruction manual and those available in the literature.
Our study sites were hemophilia treatment centers with well‐developed and experienced MSK teams, including physiotherapists already very familiar with the use of HJHS who followed the instructions provided to standardize its use. As in any study, the input of the HJHS scores in a large database led to queries and corrections in the process of data cleansing to an extent that might not be reproduced in real‐world practice, even in large hemophilia clinics or registries. Eight of our nine study sites were situated in North America or Europe, and one was in India. Our cohort may not be representative of areas where health care for persons with hemophilia is more limited. Studying the performance of the HJHS in a larger sample of adults with limited access to hemostatic agents will be important to assist with the development of individualized treatment plans and data collection for patient advocacy initiatives.
Although we included countries known to have race diversity and multicultural populations, our study did not collect race or ethnicity information, which limits our ability to identify specific racial and ethnic minority groups. This would be important and useful information for future research.
Future studies of the HJHS may consider the application of the weighted HJHS scoring system proposed by Ribeiro and colleagues 29 to provide impactful HJHS total scores emphasizing the most important clinical indicators on joint health. Further studies specifically designed to quantify the responsiveness of the HJHS to clinical interventions are also greatly needed.
In conclusion, we have demonstrated excellent convergent and discriminant construct validity of the HJHS in the adult population. The adult normative values obtained from this study will provide context on HJHS scores resulting specifically from bleed‐related damage and not age‐related joint impairment as is seen in healthy adults without hemophilia. Future modifications of the HJHS for adults may be improved by removing or modifying certain items, leading to a validated single MSK assessment tool across the life span of persons with hemophilia suitable for clinical and research use.
RELATIONSHIP DISCLOSURE
The International Prophylaxis Study Group (IPSG) is funded by educational grants to the Hospital for Sick Children (“SickKids”) Foundation from Bayer, Novo Nordisk Health Care AG, Pfizer, Sanofi, Takeda and Spark Therapeutics. None of the industry partners of the IPSG were involved with the design or conduct of the work reported in this article, and the opinions reported in the manuscript are those of the authors alone. In addition, no professional or paid medical writer was used in the development of this article.
AUTHOR CONTRIBUTIONS
JS‐L contributed to study concept, led study operations, clinical data collection, analysis and interpretation of data, writing and revisions to the manuscript, and review and approval of the final version of the manuscript. AA contributed to study operations, analysis and interpretation of data, writing and revisions to the manuscript, and review and approval of the final version of the manuscript. SF contributed to study concept, musculoskeletal and clinical data collection, manuscript revisions, and review and approval of the final version of the manuscript. MT contributed to musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. SC contributed to musculoskeletal and clinical data collection, manuscript revisions, and review and approval of the final version of the manuscript. NZ contributed to study concept, musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. PM contributed to musculoskeletal data collection; manuscript revisions, and review and approval of the final version of the manuscript. SL contributed to musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. GH contributed to musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. SA contributed to musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. AJW contributed to musculoskeletal data collection, manuscript revisions, and review and approval of the final version of the manuscript. MM‐J contributed to study concept; clinical data collection, and review and approval of the final version of the manuscript. JJ contributed to clinical data collection, manuscript revisions, and review and approval of the final version of the manuscript. SA contributed to clinical data collection and review and approval of the final version of the manuscript. PC contributed to clinical data collection, manuscript revisions, and review and approval of the final version of the manuscript. CH contributed to clinical data collection and review and approval of the final version of the manuscript. DN contributed to clinical data collection and review and approval of the final version of the manuscript. NB contributed to clinical data collection, manuscript revisions, and review and approval of the final version of the manuscript. SM contributed to clinical data collection and review and approval of the final version of the manuscript. PH contributed to study concept, musculoskeletal data review, manuscript revisions, and review and approval of the final version of the manuscript. VSB contributed to study concept and design, analysis and interpretation of data, revisions to the manuscript, and review of the final version of the manuscript. BMF contributed to study concept, design, and operations; analysis and interpretation of data; revisions to the manuscript; and review and approval of the final version of the manuscript.
Supporting information
Supplementary Material
Supplementary Material
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the members of the Executive of the International Prophylaxis Study Group (Drs Lou Aledort, Rolf Ljung, and Victor Blanchette) and additional members of the Musculoskeletal Health Expert Working Group of the International Prophylaxis Study Group (Janjaap van der Net, Pia Petrini, Melanie Bladen, Sylvia Thomas, and Magnus Aspandahl) for their useful comments regarding this article. The authors also thank Laura Tiseo and Natasha Nairadoo for assistance with data entry and verification at The Hospital for Sick Children; Tiffany Kaltenmark, DPT, and Fred Loeffler, PT, DPT, LAT, ATC, from the Indiana Hemophilia and Thrombosis Center Indianapolis, Indiana, for their assistance with data collection; and Prof. Catherine Lambert from the Haemostasis and Thrombosis of Cliniques universitaires Saint‐Luc, Brussels, Belgium, for her assistance with data collection.
St‐Louis J, Abad A, Funk S, et al. The Hemophilia Joint Health Score version 2.1 Validation in Adult Patients Study: A multicenter international study. Res Pract Thromb Haemost. 2022;6:e12690. doi: 10.1002/rth2.12690
Handling Editor: Pantep Angchaisuksiri
REFERENCES
- 1. Rodriguez‐Merchan EC. Musculoskeletal complications of hemophilia. HSS J. 2010;6:37‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Valentino LA. Blood‐induced joint disease: the pathophysiology of hemophilic arthropathy. J Thromb Haemost. 2010;8:1895‐1902. [DOI] [PubMed] [Google Scholar]
- 3. Oldenburg J. Optimal treatment strategies for hemophilia: achievements and limitations of current prophylactic regimens. Blood. 2015;125:2038‐2044. [DOI] [PubMed] [Google Scholar]
- 4. Fischer K, Poonnoose P, Dunn AL, et al. Choosing outcome assessment tools in haemophilia care and research: a multidisciplinary perspective. Haemophilia. 2017;23(1):11‐24. [DOI] [PubMed] [Google Scholar]
- 5. Kuijlaars IAR, Timmer MA, de Kleijn P, Pisters MF, Fischer K. Monitoring joint health in haemophilia: factors associated with deterioration. Haemophilia. 2017;23:934‐940. [DOI] [PubMed] [Google Scholar]
- 6. Srivastava A, Santagostino E, Dougall A, et al. WFH guidelines for the management of hemophilia, 3rd edition. Haemophilia. 2020;26(suppl 6):1‐158. [DOI] [PubMed] [Google Scholar]
- 7. Hilliard P, Funk S, Zourikian N, et al. Hemophilia joint health score reliability study. Haemophilia. 2006;12:518‐525. [DOI] [PubMed] [Google Scholar]
- 8. Feldman BM, Funk SM, Bergstrom BM, et al. Validation of a new pediatric joint scoring system from the International hemophilia prophylaxis study group: validity of the hemophilia joint health score. Arthritis Care Res (Hoboken). 2011;63:223‐230. [DOI] [PubMed] [Google Scholar]
- 9. Gouw SC, Timmer MA, Srivastava A, et al. Measurement of joint health in persons with haemophilia: a systematic review of the measurement properties of haemophilia‐specific instruments. Haemophilia. 2019;25:e1‐e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Blanchette VS, Key NS, Ljung LR, et al. Definitions in hemophilia: communication from the SSC of the ISTH. J Thromb Haemost. 2014;12:1935‐1939. [DOI] [PubMed] [Google Scholar]
- 11. Gilbert MS. Prophylaxis: musculoskeletal evaluation. Semin Hematol. 1993;30:3‐6. [PubMed] [Google Scholar]
- 12. Gilbert MS, Orthopedic Advisory Committee of WFH . World Federation of Hemophilia Physical Examination Score (also called the Gilbert Score). 2011.
- 13. Poonnoose PM, Manigandan C, Thomas R, et al. Functional independence score in haemophilia: a new performance‐based instrument to measure disability. Haemophilia. 2005;11:598‐602. [DOI] [PubMed] [Google Scholar]
- 14. Poonnoose PM, Srivastava A. Functional assessment of arthropathy–an international perspective. Semin Hematol. 2006;43:S27‐32. [DOI] [PubMed] [Google Scholar]
- 15. Poonnoose PM, Thomas R, Keshava SN, et al. Psychometric analysis of the Functional Independence Score in Haemophilia (FISH). Haemophilia. 2007;13:620‐626. [DOI] [PubMed] [Google Scholar]
- 16. van Genderen FR, van Meeteren NL, van der Bom JG, et al. Functional consequences of haemophilia in adults: the development of the haemophilia activities list. Haemophilia. 2004;10:565‐571. [DOI] [PubMed] [Google Scholar]
- 17. van Genderen FR, Westers P, Heijnen L, et al. Measuring patients’ perceptions on their functional abilities: validation of the haemophilia activities list. Haemophilia. 2006;12:36‐46. [DOI] [PubMed] [Google Scholar]
- 18. Melzack R. The short‐form McGill pain questionnaire. Pain. 1987;30:191‐197. [DOI] [PubMed] [Google Scholar]
- 19. Statistics Canada . National Occupational Classification (NOC). 2016. https://www.statcan.gc.ca/en/subjects/standard/noc/2016/indexV1.3
- 20. Malec LM, Cheng D, Witmer CM, et al. The impact of extended half‐life factor concentrates on prophylaxis for severe hemophilia in the United States. Am J Hematol. 2020;95:960‐965. [DOI] [PubMed] [Google Scholar]
- 21. Fischer K, de Kleijn P. Using the Haemophilia Joint Health Score for assessment of teenagers and young adults: exploring reliability and validity. Haemophilia. 2013;19:944‐950. [DOI] [PubMed] [Google Scholar]
- 22. Den Uijl IE, De Schepper AM, Camerlinck M, Grobbee DE, Fischer K. Magnetic resonance imaging in teenagers and young adults with limited haemophilic arthropathy: baseline results from a prospective study. Haemophilia. 2011;17:926‐930. [DOI] [PubMed] [Google Scholar]
- 23. McLaughlin P, Morris R, Chowdary P. Investigating the relationship between the HJHS and HAL in routine clinical practice: a retrospective review. Haemophilia. 2018;24:988‐994. [DOI] [PubMed] [Google Scholar]
- 24. van Galen K, Timmer M, de Kleijn P, et al. Joint assessment in von Willebrand disease. Thromb Haemost. 2017;117(08):1465‐1470. [DOI] [PubMed] [Google Scholar]
- 25. Batt K, Recht M, Cooper DL, Iyer NN, Kempton CL. Construct validity of patient‐reported outcome instruments in US adults with hemophilia: results from the Pain, Functional Impairment, and Quality of life (P‐FiQ) study. Patient Prefer Adher. 2017;11:1369‐1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kuijlaars IAR, van der Net J, Feldman BM, et al. Evaluating international Haemophilia Joint Health Score (HJHS) results combined with expert opinion: options for a shorter HJHS. Haemophilia. 2020;26:1072‐1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tagariello G, Iorio A, Santagostino E, et al. Comparison of the rates of joint arthroplasty in patients with severe factor VIII and IX deficiency: an index of different clinical severity of the 2 coagulation disorders. Blood. 2009;114:779‐784. [DOI] [PubMed] [Google Scholar]
- 28. Osooli M, Steen Carlsson K, Astermark J, Berntorp E. Surgery and survival in birth cohorts with severe haemophilia and differences in access to replacement therapy: the Malmo experience. Haemophilia. 2017;23:e403‐e408. [DOI] [PubMed] [Google Scholar]
- 29. Ribeiro T, Abad A, Feldman BM. Developing a new scoring scheme for the hemophilia joint health score 2.1. Res Pract Thromb Haemost. 2019;3(3):405‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material
Supplementary Material
Supplementary Material


