Highlights
-
•
More intense proteolysis at 90 days caused an increase in antioxidant activity.
-
•
Peptide exhibited radical scavenging properties, reducing capacity and chelating effect.
-
•
Intense proteolysis caused lower angiotensin I-converting enzyme inhibitory activity.
-
•
Bioactive peptides were generated from αs1-casein and β-casein.
-
•
Ripening process of Gouda cheese results in a product with functional potential.
Keywords: Gouda cheese, Antioxidant, Antihypertensive, Bioactive peptides
Abstract
In Mexico, local ripened cheeses such as Chihuahua, Ranchero, and Cotija are produced, being consumed in great quantities together with imported cheeses. Proteolysis that takes place during ripening generates bioactive peptides; in this way the cheese acquires potential as a functional food. The ripening process of Gouda cheese was studied based on its bromatological and sensorial properties, bioactivity, and peptide profile. Ripened cheese met bromatological standard parameters and showed higher overall acceptability. After 90 days, bioactivity reached maximum values for radical scavenging (6.6%), ferric reducing power (11.2%), metal chelating effect (49%), and angiotensin I-converting enzyme inhibitory activity (66.2%). Eight peptides were identified, four from αS1-casein, f(1–9, 1–13, 1–14, and 25–36), and four from β-casein, f(11–28, 60–63, 193–209, and 197–205). Ripening of Gouda cheese results in a product with functional potential due to the presence of peptides with biological activity. Additionally, the methodology proposed in this work could be used by the dairy industry to monitor the manufacturing process and ripening of other types of cheeses.
Introduction
Ripening is one of the most important technological processes in the manufacture of cheeses, which consists of a series of biochemical and microbiological modifications generated by the metabolism of primary and adjunct cultures. The organoleptic and bromatological characteristics of the final product are positively modified (Khattab et al., 2019, Xiaochun et al., 2021). During ripening, several biochemical reactions take place, highlighting the metabolism of residual lactose, lactate and citrate, proteolysis, and lipolysis. As for the enzymes involved in maturation, these come from milk, coagulation, and starter cultures. In primary proteolysis, indigenous milk enzymes and those present in the coagulant play an important role. During secondary proteolysis, a great variety of peptides are released from the milk casein fraction (i.e., αS1-, αS2-, β-, and κ-casein) by proteolytic enzymes which mainly belong to microorganisms that participate in cheese manufacturing (primary and secondary starters, as well as adventitious microflora (Gan et al., 2016, Suzuki-Iwashima et al., 2020).
Bioactive peptides are defined as specific protein fragments that have a positive impact on body functions or conditions and may ultimately influence health. They usually range from 2 to 20 amino acids in length and have been derived from various plant and animal sources including milk, cheese, yogurt, fish, soybean, and kefir. Peptides derived from milk have the greatest potential to be used commercially. Bioactive peptides derived from milk have been shown to have various properties including antimicrobial, antihypertensive, opioid, antioxidant, antithrombotic, and mineral-binding effects (Bhat and Bhat, 2011, Sánchez and Vázquez, 2017).
Diet plays an important role in the development of healthy habits and is referred to as a modifiable risk factor for several noncommunicable chronic diseases. In this sense, various dairy products such as cream, butter, yogurt, kefir, and cheeses have been produced and consumed around the world for millennia. Functional dairy products are becoming more and more available, gaining increasing popularity. Consumers’ interest in personal health is one reason for establishing markets for functional dairy products. In the future, products aimed at special consumer groups will be developed and diversified (Sampson, 2015, Gasmalla et al., 2017, Konstantinidi and Koutelidakis, 2019).
Based on the health and biotechnological potential of peptides generated during ripening of cheese and the demand and need to diversify the supply of dairy products in Mexico, the aim of the present study was to investigate the bioactivity of ripening Gouda cheese as a radical scavenger, reducing agent, chelating agent, and antihypertensive, to examine its potential as a functional food.
Materials and methods
Materials
Cow milk was obtained from the Campus of Biological and Agricultural Sciences at the Autonomous University of Yucatan (Mexico). A mesophilic cheese culture CHN-22 (Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar diacetylactis, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, and Leuconostoc pseudomesenteroides) was procured from CHR-Hansen (Hoersholm, Denmark). All reagents used were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Manufacture of Gouda cheese
The milk quality was assessed by analysis of pH (pH meter Hanna model Hl99163), acidity (Automatic potentiometric titrator Kyoto electronics model AT-710B), density (Lactodensimeter Mantey model MANTE07012 USA), fat (Velp Scientific model SER 148 USA), and protein (Kjeldahl BÜCHI model K-350 Switzerland) according to methods established by the Official Mexican Standard NMX-F700-PROY-COFOCALEC-2012. Milk was pasteurized at 65 °C for 30 min. Cheese culture CHN-22 was added to milk at rate of 5 U/100 L of milk; temperature was kept constant at 30 ± 2 °C for 20 min. Calcium chloride (7 mL/100 L of milk) was added as a coadjutant of the coagulant process; coagulation required 45 min. Coagulated protein was heat-treated at 38 °C for 40 min. Whey was removed and cheese molding and pressing proceeded for 16 h. The ripening process was carried out for 90 days at 10 ± 2 °C and 90% relative humidity (Ripening chamber Mr. Winter model 31182-C USA). Cheese sanitary specifications were established according to the Official Mexican Standard NOM-243-SSA1-2010.
Effect of ripening on bromatological, physicochemical, and microbiological parameters
Bromatological parameters for ripe Gouda cheese were determined using official AOAC procedures (AOAC, 1997): nitrogen (method 954.01) (Kjeldahl BÜCHI model K-350 Switzerland); fat (920.39) (Velp Scientific model SER 148 USA); and moisture (925.09) (convection stove Novatech model E160-ED-ESP USA). Protein content was calculated as nitrogen × 6.25. pH (pH meter Hanna model Hl99163 USA) and water activity (Hygrometer Aqualab model CX-2 USA) were established according to the Official Mexican Standard NMX-F-317-NORMEX-2013. After each ripening time, cheese samples were stored in vacuum-sealed bags at − 10 °C and microbiological analysis pursuant to Official Mexican Standard NMX-F-317-NORMEX-2013 was performed. The parameters evaluated were fecal coliforms (50 CFU/g of sample), Staphylococcus aureus (100 CFU/g of sample), yeast and mold (500 CFU/g of sample), and Salmonella (absent/25 g of sample).
Sensory evaluation of ripe Gouda cheese
A sensory evaluation assay was performed with 40 untrained panelists (both genders) who were instructed before evaluation with basic rules for the process (no smoking, not eating at least 2 h before evaluation). Each panelist was served 20 g of cheese on a small white plate coded with a random three-digit number. The panelists were asked to use low-salt crackers and water to clean their palates between the samples. The general acceptance, color, odor, texture, and flavor were evaluated on a 9-point unstructured hedonic scale ranging from 1 (dislike very much) to 9 (like very much). Commercial cheese was used as a control.
Characterization of proteolysis during ripening
Total soluble protein, amino nitrogen, and peptide content in water-soluble extracts
One gram of grated Gouda cheese was mixed with 5 mL of distilled water and homogenized. The mixtures were centrifuged at 12,000 g for 20 min in a refrigerated centrifuge at 5 °C (Refrigerated centrifuge Hettich model 320R Germany). The fat layers were removed, and the water extracts were filtered through Whatman No. 1 filter paper. To further remove any impurities, the water-soluble extracts were filtered through a 0.22 μm pore size filter. Water-soluble extracts (WSEs) of Gouda cheese were evaluated for their soluble protein nitrogen (Bradford, 1976), amino nitrogen (Atanasova et al., 2021) and peptide nitrogen content, calculated using:
Gel permeation chromatography analysis of water-soluble extracts of cheese samples
A filtration column (0.7 × 70 cm) was packed with Sephadex G-25 (Sigma-Aldrich) and equilibrated by monitoring pH and absorbance at 280 nm. Then 1 mL of WSEs was injected; the flow rate was 0.75 mL/min (Vioque et al., 2004).
SDS-PAGE of water-soluble extracts of cheese samples
The extent of proteolysis of cheese samples was assessed using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) performed according to the method of Schagger and Jagow (1987) on a 15% slab gel (separating gel) with slight modification. Samples were prepared by mixing WSEs of Gouda cheese at different ripening stages with an equal volume of sample buffer (pH 6.8). A vertical electrophoresis unit (Bio-Rad Midi USA) was used, and the samples were run at 70 V for 2 h. The gels were stained using plate nitrate and the polypeptides detected were identified using a low molecular weight kit (Bio-Rad, Cat: 161-0303).
Effect of ripening on biological activity
Radical scavenging assay
The free radical scavenging activity of WSEs was determined using ABTS radical cation according to a previously described method with minor modifications (Soleymanzadeh et al., 2016). To generate the ABTS radical, ABTS was incubated with potassium persulfate for 16 h in the dark at room temperature. ABTS radical was adjusted to an absorbance of 0.70 ± 0.02 at 734 nm by dilution with ethanol; 30 μL of WSEs was mixed with 2970 μL of the ABTS radical solution. The decrease of absorbance was monitored at 734 nm (Thermo Scientific UV–VIS Visible Spectrophotometer Lab Equipment 360–1000 nm 4 nm 721 N USA) after 7 min. All assays were carried out in triplicate, and the values represent the means.
where Abs control represents the initial ABTS absorbance and Abs sample represents the ABTS absorbance in the presence of WSEs.
Ferric reducing power assay
The ferric reducing power assay was performed according to the method reported by Khan et al. (2019) with some modifications. A 250 μL of sample was added to 250 mL of phosphate buffer (0.2 M, pH 6.6) and 250 mL of potassium ferricyanide (1%); the mixture was incubated at 50 °C for 20 min. Trichloroacetic acid (10%; 250 mL) was added and the mixture was centrifuged at 3000 rpm for 10 min. A 500 μL portion of the supernatant were collected, and 400 μL of distilled water and 100 μL of ferric (0.1%) chloride were added; the mixture was incubated at 50 °C for 10 min. After 10 min at room temperature, absorbance was determined at 700 nm (Thermo Scientific UV–VIS Visible Spectrophotometer Lab Equipment 360–1000 nm 4 nm 721 N USA). Reducing power was calculated as follows:.
where AbsM is the absorbance in the presence of sample or control and AbsB is the absorbance of the blank.
Chelating capacity
The methodology reported by Meister-Meira et al. (2012) was used to determine chelating activity. Blank: 1.2 mL of acetate sodium buffer 50 mM (pH 6) over 25 μL of pyrocatechol violet 4.0 mM. Control: 1.1 mL of acetate buffer 50 mM (pH 6), 100 μL pattern Cu (II) (40 mg/mL), and 25 μL of 4 mM pyrocatechol violet sodium solution. Samples: 1.0 mL of acetate buffer 50 mM (pH 6), 100 μL pattern Cu (II) (40 mg/mL), and 25 μL of pyrocatechol 4.0 mM solution sodium was allowed to react for 5 min at room temperature and then 100 μL of sample (containing 200 mg of sample) was added. A decrease in absorbance at 632 nm (Thermo Scientific UV–VIS Visible Spectrophotometer Lab Equipment 360–1000 nm 4 nm 721 N USA) shows that there is chelating ability.
where AbsVPCuM is the absorbance of pyrocatechol violet complex Cu (II) in the presence of sample and AbsVPCu is the absorbance of pyrocatechol violet complex Cu (II).
Angiotensin-converting enzyme inhibition assay
Angiotensin-converting enzyme (ACE) inhibitory activity was measured using the method of Nilsen et al. (2016). Hippuryl-l-histidyl-l-leucine (HHL) is hydrolyzed by ACE to yield hippuric acid and histidyl-leucine. This method relies on the colorimetric reaction of hippuric acid with 2,4,6-trichloro-s-triazine (TT) in a 0.5 mL incubation mixture containing 40 mmol potassium phosphate buffer (pH 8.3), 30 mmol sodium chloride, 0.3% HHL in potassium phosphate buffer (pH 8.3), and 100 mU/mL ACE. The mixture was incubated at 37 °C for 30 min and the reaction was terminated by adding 360 µL of TT in dioxane and 720 µL of 0.2 M potassium phosphate buffer (pH 8.3). After centrifuging the reaction mixture at 10,000 rpm for 10 min, enzymatic activity was determined in the supernatant by measuring absorbance at 382 nm (Thermo Scientific UV–VIS Visible Spectrophotometer Lab Equipment 360–1000 nm 4 nm 721 N USA). The activity of each sample was tested in triplicate. The positive control of the reaction was carried out by adding only substrate, ACE, and water (blank). The blank was prepared with only substrate and water (ACE volume was replaced by an equal amount of water).
Mass spectrometry analysis
The WSEs of protein obtained during the ripening process were characterized using matrix-assisted laser desorption/ionization mass spectrometry (MALDI-TOF MS System Sciex model ZenoTOF 7600 system USA). In the present work, the lyophilized samples of WSEs (1 mg) were dissolved in 1 mL of distilled water; 1.5 μL was mixed with 1.5 μL of a saturated solution of dihydroxybenzoic acid 2,5-(DHB) and placed in a plate for further analysis. This experiment was performed at The Center for Nanoscience and Micro and Nanotechnologies, National Polytechnic Institute, Professional Unit Adolfo Lopez Mateos, Zacatenco, Mexico.
Statistical analysis
The means of the results were evaluated using analysis of variance (ANOVA) and the Tukey test was used to compare differences (p < 0.05) among the bromatological and physicochemical parameters, sensory evaluations, characterization of proteolysis, and biological activity. All analyses were processed with Statgraphics Plus version 5.1 software (USA).
Results and discussion
Effect of ripening on bromatological, physicochemical, and microbiological parameters
Moisture content, together with the percentage of mineral salts and pH parameters, is a determining factor for the quality of cheese. The results indicate that the moisture content remained stable during ripening (Table 1), which was conducted under controlled conditions of temperature (10 ± 2 °C) and relative humidity (90%). During the ripening process, the fat percentage decreased from 33.9% at baseline to 26.6% at the end of the process. During ripening, lipolytic enzymes present in lactic acid bacteria (LAB) can hydrolyze a substrate to generate free fatty acid esters, triacylglycerides, diacylglycerides, and monoacylglycerides (Khan et al., 2019, Xiaochun et al., 2021). The former would explain the decreases in fat content observed from day 30, reaching a total reduction of 21.54% in total fat content at day 90.
Table 1.
Moisture, fat, protein, pH, Aw, and nitrogen content during proteolysis of Gouda cheese at different ripening times.
| Ripening (Days) | Moisture (%) | Fat (%) | Protein (%) | pH | Aw | Nitrogen content during proteolysis (μg/mL) |
||
|---|---|---|---|---|---|---|---|---|
| Soluble protein nitrogen | Amino acid nitrogen | Peptide nitrogen | ||||||
| 0 | 44.52 ± 0.23b | 33.89 ± 0.07c | 21.96 ± 0.61a | 5.17 ± 0.01c | 0.97 ± 0.01a | 249.16 ± 0.32b | 0.612 ± 0.05a | 248.54 ± 0.33a |
| 30 | 44.59 ± 0.19b | 29.79 ± 0.16b | 23.19 ± 0.94a | 4.87 ± 0.02a | 0.98 ± 0.01a | 247.86 ± 0.32a | 0.624 ± 0.03b | 247.23 ± 0.32a |
| 60 | 43.46 ± 0.17a | 26.04 ± 0.80a | 22.41 ± 0.86a | 5.04 ± 0.04b | 0.97 ± 0.04a | 259.55 ± 0.46c | 0.627 ± 0.03b | 258.92 ± 0.46b |
| 90 | 46.47 ± 0.32c | 26.59 ± 0.02a | 21.81 ± 0.68a | 5.24 ± 0.02d | 0.97 ± 0.02a | 260.52 ± 0.32c | 0.645 ± 0.02c | 259.87 ± 0.32b |
Data are mean ± SD of three determinations.
Different letters indicate significant difference (p < 0.05).
In respect to total protein (%) during 90 days of ripening, no significant differences between the samples (Table 1) were found. Although during ripening proteolysis modifies the molecular weight of proteins by generating polypeptides, peptides, and free amino acids from caseins, the total amount of nitrogen quantified in the proximal analysis does not change.
During the ripening process, pH decreased after 30 days, and then it increased again and remained constant until the end of ripening (Table 1). Changes in pH can be attributed to the production of lactic acid due to glycolysis. Thermophilic cultures have moderate acidifying activity that is dependent on the strain. This would explain what was observed in this work, as during the first 30 days of ripening, a decrease in pH was observed and then a rise was seen towards the end of the process. Aw remained without significant changes (Table 1). This is related to the conditions of the maturation process; the temperature and relative humidity were set to 10 ± 2 °C and 90%, respectively. Such conditions prevent excessive loss of water, keeping the Aw stable.
The cheese manufacturing process has a high risk of contamination by environmental pathogens, this risk is particularly high during ripening due to open-air exposure of the product. Currently, no post-manufacturing or post-ripening disposal steps are in place to control food safety risks associated with contamination. The assays of microbiological quality carried out during ripening process always indicate that, the parameters evaluated did not exceed the maximum limits established according to the Official Mexican Standard NMX-F-317-NORMEX-2013: fecal coliforms <50 CFU/g of sample, Staphylococcus aureu s<100 CFU/g of sample, yeast and mold <500 CFU/g of sample, and Salmonella absent/25 g of sample. The number of bacteria present in reflects the hygiene conditions under which the milk was processed and determines the period of preservation of the cheese. The results show that hygienic practices were appropriate during the ripening process, so it can be considered that the pasteurization of milk was efficient. This indicates that the ripe Gouda cheese could be safe and suitable product for consumption.
Sensory evaluation of ripe cheese
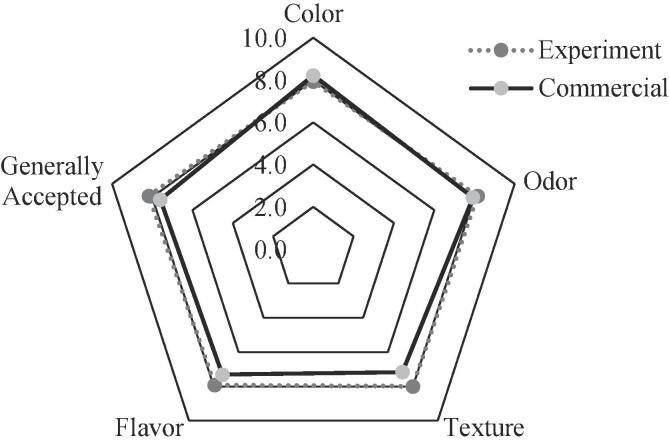
Sensory profiles are necessary to establish standardized parameters of sensory quality, in the case of dairy products such as cheese, these parameters are essential to understand the acceptability by the consumer (de Ramírez-Rivera et al., 2017). The results of the sensory evaluation of the ripe Gouda cheese and the commercial cheese used as conventional reference product are shown in Fig. 1. In general terms, the products were perceived positively by the panelists, receiving both very similar ratings. However, the rating chart indicates that the ripe Gouda cheese scored higher for flavor, texture, and acceptance, after 90 days of storage. This is relevant, considering that Gouda cheese was subjected to a ripe process to enhance its biological properties, as peptides were released during the process. In this sense, the results indicate that ripening not only improves the sensory properties but could also increase the functional potential of the cheese.
Fig. 1.
Sensory profile of ripe (90 days) Gouda cheese compared with a commercial product.
Starter cultures have a great influence on the sensory properties of cheese. Under certain conditions, bacteria such as Lactococcus lactis, which is part of the cheese starter culture evaluated in this study, rapidly ferment available carbon sources in milk such as lactic acid and some saccharides into other organic compounds, such as formate, acetate, and ethanol, which can negatively influence the sensory properties of unripened cheeses (Květoslava et al., 2019). In contrast, the profile of secondary proteolysis products found in ripening cheeses made with some specific LAB strains have a satisfactory impact on sensory characteristics, particularly flavor (Květoslava et al., 2019). The results of the sensory evaluation indicate that the bacterial culture and the ripening process positively influenced the sensory characteristics of Gouda cheese.
Characterization of proteolysis during ripening
Total soluble protein, amino nitrogen, and peptide content in water-soluble extracts
Cheese ripening involves complex biochemical and chemical changes. Proteolysis can be caused by coagulant residues, milk-specific enzymes, starter culture enzymes, and secondary microbiota enzymes. In this study, proteolysis was monitored by determining the total soluble protein content, the amino nitrogen content, and the peptide nitrogen content (Table 1). The content of soluble protein representing primary proteolysis in cheese during ripening was found to increase significantly (p < 0.05) with an increase from day 60 to day 90. Compounds derived from proteolysis are represented by peptides of various sizes and free amino acids. In this study the content of peptide nitrogen increased significantly from (p < 0.05) with an increase from day 60 to day 90. The results obtained indicate that more than 95% of the nitrogen present in the soluble extracts obtained during proteolysis are represented by peptide nitrogen with a limited content of free amino acids.
The hydrolytic processes of food proteins are accompanied by an increase in biological functionality as the peptide fragments that were encrypted in proteins can exert their activity once they are released. The proteolysis process releases peptides with specific bioactivities, including antimicrobial, antihypertensive, immunomodulatory, analgesic, and antioxidant activities that can positively affect major human body systems (Meister-Meira et al., 2012, Hernández-Ledesma et al., 2014, Sánchez and Vázquez, 2017). While quantifying the total soluble protein, amino nitrogen, and peptide content provides an overview of proteolytic behavior during the ripening process, it does not explain the changes in molecular weight of the peptides generated. It was therefore necessary to supplement the results obtained with chromatographic and electrophoretic techniques.
Gel permeation chromatography
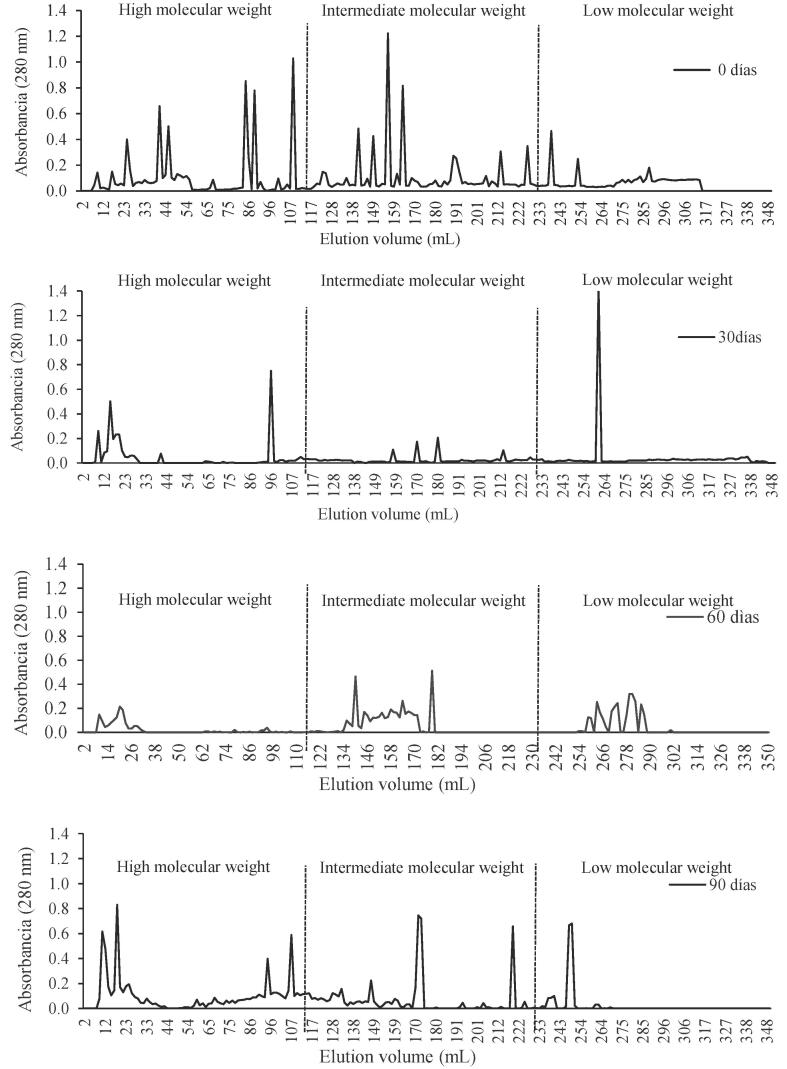
Gel filtration chromatography depends on the differential migration of dissolved solutes through gels that have pores of defined sizes. For gel filtration samples have been taken at different stages of ripening. The results of gel filtration are shown in Fig. 2. For comparison, in each case, chromatograms corresponding to 0, 30, 60, and 90 days of ripening are shown in series. Taking into consideration the elution volume, chromatograms were divided into three zones according to their molecular weight: 1) high molecular weight (0–116 mL), 2) intermediate molecular weight (116–232 mL), and low molecular weight (232–348 mL).
Fig. 2.
Elution profiles of water-soluble protein extracts of Gouda cheese at different ripening times.
At the beginning of ripening, the chromatogram exhibited 17 peaks. More intense peaks were observed in the zones of high and intermediate molecular weight; less intense peaks were observed in the zone of low molecular weight. After 90 days of ripening, the peaks of high and intermediate molecular weight decreased, and those of lower molecular weight increased. This coincides with the results obtained from the quantification of peptide nitrogen, indicating that the proteolytic process mainly generates peptides of various molecular sizes. In addition to gel filtration, electrophoresis was used to characterize proteolysis during ripening.
SDS-PAGE of water-soluble protein extracts
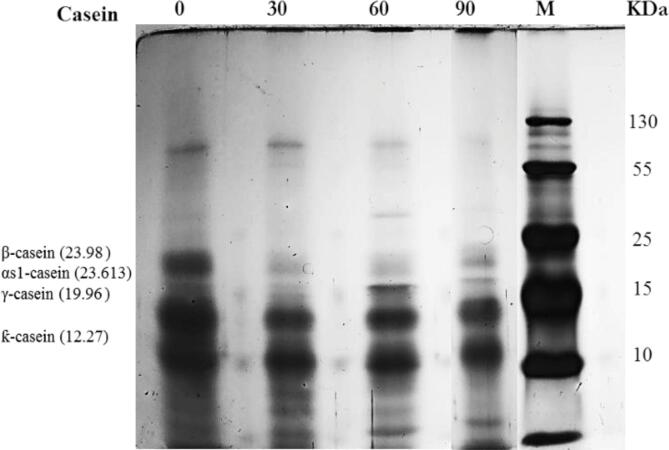
SDS-PAGE of WSEs was performed to determine the extent of proteolysis during ripening from 0 to 90 days; electrophoretic patterns are presented in Fig. 3. At first, the objective was to determine if there were differences as ripening time advanced and to study the possible changes that may occur due to the proteolytic activity. The electrophoretic patterns were characterized by.
Fig. 3.
SDS-PAGE electrophoresis of water-soluble protein extracts of Gouda cheese at different ripening times. 0, 30, 60, and 90 (days of ripening). M (Molecular Weight Marker).
an increasing number of bands as ripening time elapsed, which is consistent with the increase in total soluble protein/peptide nitrogen values. In general, no qualitative differences were observed during the first month of cheese ripening; this is attributed to the agents responsible for proteolysis at this level (primary proteolysis) being rennet and plasmin, which act mainly on the α-casein on the outside of the casein micelle. However, from the second month, the migration of high-weight bands was observed.
As shown in Fig. 3, the soluble protein extracted from unripened cheese shows a band of high molecular weight, which disappears after 60 days of ripening. In all samples, three bands with molecular weights lower than 40 kDa remain present during ripening. Caseins that are located within this range can be β-caseins, αS1-casein, γ-casein, and β-casein fragments (1–105) and κ-caseins. According to Khan et al. (2019), peptides derived from β-casein and αS1-casein are potential radical scavengers. Therefore, the peptides released during maturation could exhibit diverse biological activities, which will be evaluated by means of in vitro assays.
Effect of ripening on biological activity
As evident from the previous section, peptides were formed during ripening of the cheese prepared. Some of these peptides may exert bioactivity including antioxidant activity and antihypertensive. Furthermore, secondary proteolysis during cheese ripening also leads to the formation of other peptides with antioxidant activity (Khan et al., 2019).
Radical scavenging assay
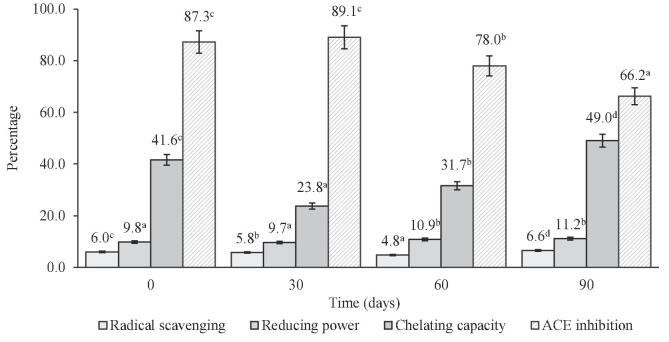
Free radical scavenging activity was determined by the DPPH free radical scavenging assay. DPPH is a stable free radical having maximum absorption at 517 nm that accepts an electron or hydrogen atom to become a stable diamagnetic molecule. In the presence of a substance capable of donating a hydrogen atom, its free radical nature is lost and hence the reduction in DPPH radical is determined by a decrease in its absorbance at 517 nm. In this study, the radical scavenging, reducing power, and chelating activity of water-soluble peptides were measured and are presented in Fig. 4.
Fig. 4.
Antioxidant and ACE inhibition activities of water-soluble extracts of Gouda cheese at different ripening times. Data are mean ± SD of three determinations. Different letters in same columns indicate significant difference (p < 0.05).
During ripening, radical scavenging decreased slightly significantly (P < 0.05) until 60 days of ripening. Then, the average initial radical scavenging activity of water protein soluble extracts increased, by 9.09% (90 days of ripening). The observed behavior is consistent with the change in antioxidant properties of the soluble protein of Cheddar cheese reported by Barać et al. (2016) for water-soluble and protein fractions of white cheese at different stages of ripening. Although free radical scavenging activity is present at all ripening times, it was relatively low, reaching maximum values of 6.6%. The poor radical scavenging capacity may be because the present peptides do not exhibit this activity. In this sense, for the scavenging of free radicals, the donation of a proton or an electron is required, if the structure of the amino acids present in the peptide is not adequate, the scavenging of radicals will be reduced.
Ferric reducing power assay
Reducing power measures, the potential antioxidant activity of bioactive compounds in different products, including peptides. In this assay, the presence of antioxidants caused reduction of the Fe3+/ferricyanide complex to the ferrous form, and the yellow color of the test solution changed to various shades of green and blue depending on the reducing power of each compound. Changes in Fe2+ were then monitored by measuring the formation of Perl’s Prussian blue at 700 nm. The ability of an antioxidant to keep a transition metal in its reduced state is its reducing potential. The reducing potential was affected by the level of ripeness (Fig. 4); the average initial value of the reducing potential of water-soluble extracts increased, by only 12.5% (90 day). Bioactive compounds having reducing power indicates that they are electron donors and can reduce the oxidized intermediates of lipid peroxidation processes, so that they can act as primary and secondary antioxidants. Although free radical scavenging activity is present at all ripening times, it was relatively low, reaching maximum values of 11.2%. During maturation, proteolysis reduces the molecular weight of proteins, however, this does not affect the reducing power since it depends more on the specific amino acids in the peptides and not on the molecular weight. In this way the amino acids Met, Cys, Tyr, Met, Trp, His and Lys can improve ferric reducing potential, due to the low reducing activity of the soluble protein extracts during maturation, it can be considered that the generated peptides are not abundant in the aforementioned amino acids.
Chelating capacity
The antioxidant activity of peptides is also attributed to their ability to chelate transition metal ions, such as those of iron and copper, which have been proposed as the catalysts for the initial formation of reactive oxygen species. The chelating capacity of water-soluble extracts decreased slightly significantly (P < 0.05) until 60 days of ripening (Fig. 4). Then, the average initial radical scavenging activity increased, by 15.10% (90 day-ripened cheese). Many proteins whose specific biological function is not to store or transport metals are still capable of chelating metals. This is because amino acid residues such as histidine, glutamic acid, aspartic acid, and phosphorylated serine and threonine are known to bind metals. If these amino acids are exposed on the surface of the proteins, they can chelate metals. Proteolysis during the ripening process increases antioxidant activity by disrupting the tertiary structure of proteins; this increases the solvent accessibility of amino acid residues that can scavenge free radicals and chelate prooxidative metals. The results obtained in present study indicate that the WSEs of ripe Gouda cheese contain peptides that exhibit antioxidant capacity by several antioxidant mechanisms: radical scavenging, reducing ability, and chelation of divalent metallic cations. It is relevant that these extracts exhibited their antioxidant capacity at low concentration (0.1 μg/mL of protein).
Angiotensin-converting enzyme (ACE) inhibition assay
The inhibitory activity of ACE is generally analyzed by in vitro assays, which means that they are performed at a constant concentration of the enzyme in the presence of a range of concentrations of the sample and a synthetic substrate consisting of di- and tri-amino-substituted peptides, such as HHL, detection being performed spectrophotometrically. The ACE-inhibitory activity of WSEs of Gouda cheese samples collected at 1-month intervals up to 3 months was compared by determining the percentage of enzyme inhibition at a concentration of 0.05 μg/mL of protein (Fig. 4).
During ripening, ACE inhibition decreased significantly (P < 0.05) after 60 days of ripening. Finally, the average initial radical scavenging activity decreased, by 24.16% (90 day-ripened cheese). The observed trend was consistent with the change in ACE-inhibitory capacity of WSEs of Cheddar cheeses made with adjunct cultures at different stages of ripening reported by Gupta et al. (2013). Biological activity like antioxidant and ACE-inhibitory activity increases as proteolysis proceeds but only to a certain extent. The presence of active peptides that are naturally formed in cheese depends on a delicate equilibrium between their formation and their degradation by the proteolytic systems involved in cheese ripening (Vargas-Bello-Pérez et al., 2019, Guha et al., 2021). The above behavior coincides with that observed in the present study.
Mass spectrometry analysis
Four peptides were generated from β-casein and αS1-casein (Table 2). During the first 30 days of ripening, seven peptides were identified in cheese WSEs: three fragments from αS1-casein, f(1–9, 1–13, and 1–14), and four from β-casein, f(11–28, 60–63, 193–209, and 197–205). After 60 and 90 days of ripening, only six peptides were detected: four fragments from αS1-casein, f(1–9, 1–13, 1–14, and 25–36), and two from β-casein, f(60–63 and 197–205).
Table 2.
Antioxidant and ACE-inhibitors peptides identified by Mass spectrometry analysis during ripening of Gouda cheese.
| Time of ripening (days) |
Fragment | Sequence | Biological activity | Reference | ||
|---|---|---|---|---|---|---|
| 30 |
60 |
90 |
||||
| MW | ||||||
| 793.045 | 793.165 | 793.223 | β-casein f(60–63) | YPFP | ANPR | In this work |
| 907.279 | β-casein f(197–205) | VLGPVRGPF | ANPR | In this work | ||
| 1142.887 | 1143.061 | 1143.140 | αs1-casein f(1–9) | RPKHPIKHQ | Antioxidant ACE-Inhibitor |
Ardö et al. (2009)Chen et al. (2010) |
| 1309.912 | 1309.127 | αs1-casein f(25–36) | VAPFPEVFGKEK | ANPR | In this work | |
| 1538.802 | 1539.660 | 1538.678 | αs1-casein f(1–13) | RPKHPIKHQGLPQ | Antioxidant ACE-Inhibitor |
Ardö et al. (2009)Chen y col. (2010) |
| 1668.126 | 1668.985 | 1669.250 | αs1-casein f(1–14) | RPKHPIKHQGLPQE | Antioxidant | Ardö et al. (2007) |
| 1880.614 | 1881.291 | 1884.132 | β-casein f(193–209) | YQEPVLGPVRGPFPIIV | Antioxidant ACE-Inhibitor |
Hayes et al. (2007) Birkemo et al. (2008) |
| 2352.372 | β-casein f(11–28)4P | EIVESLSSSEESITRINK | ANPR | In this work | ||
| MW = Molecular weight. ANPR = Activity not previously reported. | ||||||
To hydrolyze casein, LAB use their cell-envelope proteinase (CEP), which is linked to peptidoglycan at the cell wall by the action of sortase (Chang et al., 2012, Vargas-Bello-Pérez et al., 2019). To date, six types of CEP in LAB have been reported: PrtP for Lactococcus lactis, PrtR for Lactobacillus rhamnosus, PrtS for Streptococcus thermophilus, PrtL for Lactobacillus lactis, PrtH for Lactobacillus helveticus, and PrtB for Lactobacillus bulgaricus; these facilitate bacterial growth themselves and produce peptides (Espeche-Turbay et al., 2009). In this sense, the hydrophilic region at the N-terminal region of β-casein was resistant to hydrolysis by the culture used in this study (Lactococcus lactis subsp. cremoris, Lactococcus lactis subsp. lactis biovar diacetylactis, Lactococcus lactis subsp. lactis, Leuconostoc mesenteroides, and Leuconostoc pseudomesenteroides), because only one peptide was generated from this region, f(11–28). The results of the present study show that the hydrophobic region was more accessible to the CEP of the bacterial culture, as three peptides were generated, f(60–63, 193–209, and 197–205).
In the case of αS1-casein, the hydrophobic region was more accessible to the CEP of the bacterial culture, as four peptides were generated, f(1–9, 1–13, 1–14, and 25–36). No peptides were identified from αS2-casein and κ-casein in this study. Investigation of the accessibility of regions containing protein structure seems to be an appropriate method to explain susceptibility to hydrolysis (Miclo et al., 2012, Vargas-Bello-Pérez et al., 2019). In this sense, αS2-casein could be more resistant to hydrolysis due to the formation of a tetrameric structure, providing protection in some regions. Glycan chains from the glycomacropeptide region at the C-terminus of κ-casein may protect against hydrolysis in this region because they confer hydrophilic properties and negative charge, increasing electrostatic repulsions (Chang et al., 2012, Vargas-Bello-Pérez et al., 2019).
Studies have been focusing on the biological properties of milk proteins, which possess physiological effects due to the numerous bioactive peptides that are encrypted within intact proteins (Bhat and Bhat, 2011, Guha et al., 2021). During cheese ripening, proteinases degrade caseins, releasing peptides. These peptides have different bioactivity in the digestive, cardiovascular, immune, and nervous systems (Sánchez & Vázquez, 2017). As shown in Table 2, one peptide, YQEPVLGPVRGPFPIIV, among those generated from β-casein identified through the analysis in this work, has been reported to exhibit antioxidant and ACE-inhibitory properties (Nilsen et al., 2016). Regarding the other three peptides, YPFP, VLGPVRGPF, and EIVESLSSSEESITRINK, this is the first time that antioxidant and ACE-inhibitory activity has been reported for these sequences. Two peptides generated from αS1-casein identified through the analysis in this work (Table 2), RPKHPIKHQ and RPKHPIKHQGLPQ, have been reported to exhibit antioxidant and ACE-inhibitory properties (Ardö et al., 2009, Guha et al., 2021). One peptide, RPKHPIKHQGLPQE, has been reported to exhibit antioxidant properties (Ardö et al., 2009, Guha et al., 2021). Regarding the other peptide, VAPFPEVFGKEK, this is the first time that antioxidant and ACE-inhibitory activity has been reported for this sequence.
Conclusions
In the present study, the ripening process of Gouda cheese was studied based on its bromatological and sensorial properties, bioactivity, and peptide profile. Bromatological, physicochemical, and microbiological parameters do not change throughout the ripening process. Sensory analysis showed that ripe Gouda cheese was more acceptable than a commercial cheese when comparing general acceptance, color, odor, texture, and flavor. Antioxidant and ACE-inhibitory activity of casein-derived peptides obtained during ripening of Gouda cheese was evaluated by in vitro assays of radical scavenging, reducing power, chelation of prooxidant cations, and ACE-inhibitory capacity. Eight peptide sequences were identified in soluble protein extracts through MS analysis, four fragments from αS1-casein, f(1–9, 1–13, 1–14, and 25–36), and four from β-casein, f(11–28, 60–63, 193–209, and 197–205). The results indicated that the ripening process of Gouda cheese results in a product suitable as a functional food once biological activity studies were carried out during processes such as packaging, transportation, and storage. As well as preclinical and clinical studies of safety and effect that verify the influence of ripened cheese on physiological mechanisms of the organism. Additionally, the methodology proposed in this work could be used by the dairy industry to monitor the manufacturing process and ripening of other types of cheese.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Association of Official Analytical Chemists (AOAC) (1997). Official methods of analysis. Arlington, VA: AOAC. Secs. 920.39, 925.09, 954.01.
- Ardö Y., Pripp A.H., Lillevang S.K. Impact of heat-treated Lactobacillus helveticus on bioactive peptides in low-fat, semi-hard cheese. Australian Journal of Dairy Technology. 2009;64:58–62. [Google Scholar]
- Atanasova J., Dalgalarrondo M., Iliev I., Moncheva P., Todorov S.D., Ivanova I.V. Formation of free amino acids and bioactive peptides during the ripening of bulgarian white brined cheeses. Probiotics and Antimicrobial Proteins. 2021;13:261–272. doi: 10.1007/s12602-020-09669-0. [DOI] [PubMed] [Google Scholar]
- Barać M., Smiljanić M., Žilić S., Pešić M., Stanojević S., Vasić M., Vučić T. Protein profiles and total antioxidant capacity of water soluble and insoluble protein fractions of white cow cheese at different stage of ripening. Mljekarstvo. 2016;66:187–197. doi: 10.1111/ijfs.13091. [DOI] [Google Scholar]
- Bhat Z.F., Bhat H. Milk and dairy products as functional foods: A Review. International Journal of Dairy Science. 2011;6:1–12. doi: 10.3923/ijds.2011.1.12. [DOI] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chang O.K., Perrin W., Galia F., Saulnier L., Miclo E., Roux A.…Humbert A.D. Release of the cell envelope protease PrtS in the growth medium of Streptococcus thermophilus 4F44. International Dairy Journal. 2012;23:91–98. doi: 10.1016/j.idairyj.2011.10.014. [DOI] [Google Scholar]
- Espeche-Turbay M.B., Savoy D.E., Giori G., Hebert E.M. Release of the cell-envelope-associated proteinase of Lactobacillus delbrueckii subspecies lactis CRL 581 is dependent upon pH and temperature. Journal of Agriculture and Food Chemistry. 2009;57:8607–8611. doi: 10.1021/jf901531q. [DOI] [PubMed] [Google Scholar]
- Gan H.H., Yan B., Linforth R.S.T., Fisk I.D. Development and validation of an APCI-MS/GC-MS approach for the classification and prediction of Cheddar cheese maturity. Food Chemistry. 2016;190:442–447. doi: 10.1016/j.foodchem.2015.05.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasmalla M.A.A., Tessema H.A., Salaheldin A., Alahmad K., Hassanin H.A.M., Aboshora W. Health benefits of milk and functional dairy products. MOJ Food Process Technology. 2017;4(4) doi: 10.15406/mojfpt.2017.04.00099. [DOI] [Google Scholar]
- Guha S., Sharma H., Deshwal G.K., Rao P.S. A comprehensive review on bioactive peptides derived from milk and milk products of minor dairy species. Food Production, Processing and Nutrition. 2021;3:2. doi: 10.1186/s43014-020-00045-7. [DOI] [Google Scholar]
- Gupta A., Mann B., Kumar R., Sangwan B.R. ACE-Inhibitory activity of Cheddar cheeses made with adjunct cultures at different stages of ripening. Advances in Dairy Research. 2013;1:1. doi: 10.4172/2329-888X.1000102. [DOI] [Google Scholar]
- Hernández-Ledesma B., García-Nebot M.J., Fernández-Tomé S., Amigo L., Recio I. Dairy protein hydrolysates: Peptides for health benefits. International Dairy Journal. 2014;38(2):82–100. doi: 10.1016/j.idairyj.2013.11.004. [DOI] [Google Scholar]
- Khan I.T., Nadeem M., Imran M., Ullah R., Ajmal M., Jaspa M.H. Antioxidant properties of Milk and dairy products: A comprehensive review of the current knowledge. Lipids in Health and Disease. 2019;18:41. doi: 10.1186/s12944-019-0969-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattab A.R., Guirguis H.A., Tawfik S.M., Farag M.A. Cheese ripening: A review on modern technologies towards flavor enhancement, process acceleration and improved quality assessment. Trends in Food Science & Technology. 2019;88:343–360. doi: 10.1016/j.tifs.2019.03.009. [DOI] [Google Scholar]
- Konstantinidi M., Koutelidakis A.E. Functional foods and bioactive compounds: A review of its possible role on weight management and obesity's metabolic consequences. Medicines. 2019;6(3):94. doi: 10.3390/medicines6030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Květoslava S., Stanislav K., Miroslav F., Pavla B. Influence of starter culture to sensory quality of edam cheese during ripening. Journal of Microbiology, Biotechnology and Food Sciences. 2019;9:442–446. doi: 10.15414/jmbfs.2019.9.special.422-426. [DOI] [Google Scholar]
- Meister-Meira S.M., Daroit D.J., Etges-Helfer V., Folmer-Corrêa A.P., Segalin J., Carro S., Brandellia A. Bioactive peptides in water-soluble extracts of ovine cheeses from Southern Brazil and Uruguay. Food Research International. 2012;48(1):322–329. doi: 10.1016/j.foodres.2012.05.009. [DOI] [Google Scholar]
- Miclo L.E., Roux M., Genay E., Brusseaux C., Poirson N., Jameh C., Perrin A.D. Variability of hydrolysis of β-, αs1-, and αs2-caseins by 10 strains of Streptococcus thermophilus and resulting bioactive peptides. Journal of Agriculture and Food Chemistry. 2012;60:554–565. doi: 10.1021/jf202176d. [DOI] [PubMed] [Google Scholar]
- Nilsen R., Pripp A.H., Høstmark A.T., Haug A., Skeie S. Effect of a cheese rich in angiotensin-converting enzyme-inhibiting peptides (Gamalost®) and a Gouda-type cheese on blood pressure: Results of a randomised trial. Food and Nutrition Research. 2016;60 doi: 10.3402/fnr.v60.32017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NMX-F-317-NORMEX-2013. Norma Oficial Mexicana. Alimentos. Determinación de pH en Alimentos y Bebidas no Alcohólicas. Método Potenciométrico. Método de Ensayo (Prueba).
- NOM-243-SSA1-2010. Normal Oficial Mexicana. Productos y Servicios. Leche, Fórmula Láctea, Producto Lácteo Combinado y Derivados Lácteos. Disposiciones y Especificaciones Sanitarias. Métodos de Prueba.
- PROY-NMX-F700-COFOCALEC-2012. Proyecto de Norma Mexicana. Sistema producto leche. Alimento. Lácteo. Leche cruda de vaca. Especificaciones fisicoquímicas, sanitarias. Métodos de prueba.
- Ramírez-Rivera, E. de J., Ramón-Canul, L.G., Díaz-Rivera, P., Juárez-Barrientos, J.M., Herman-Lara, E., Prinyawiwatkul, W., Herrera-Corredor, J.A. (2017). Sensory profiles of artisan goat cheeses as influenced by the cultural context and the type of panel. International Journal of Food Science and Technology, 52(8), 1789–1800. https://doi.org/10.1111/ijfs.13452.
- Sampson S. Functional foods: The connection between nutrition, health, and food science. Journal of Nutrition Education and Behavior. 2015;47(1):117. doi: 10.1016/j.jneb.2014.07.005. [DOI] [Google Scholar]
- Sánchez A., Vázquez A. Bioactive peptides: A review. Food Quality and Safety. 2017;1(1):29–46. doi: 10.1093/fqsafe/fyx006. [DOI] [Google Scholar]
- Schagger H., Jagow V. Tricine sodium dodecyl sulfate polyacrylamide gel electrophoresis for separation of protein in range from 1 to 100 kDa. Analytical Biochemistry. 1987;166:368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Soleymanzadeh N., Mirdamadi S., Kianirad M. Antioxidant activity of camel and bovine milk fermented by lactic acid bacteria isolated from traditional fermented camel milk (Chal) Dairy Science and Technology. 2016;96:443–457. doi: 10.1007/s13594-016-0278-1. [DOI] [Google Scholar]
- Suzuki-Iwashima, A., Matsuura, H., Iwasawa, A., & Shiota, M. (2020). Metabolomics analyses of the combined effects of lactic acid bacteria and Penicillium camemberti on the generation of volatile compounds in model mold-surface-ripened cheeses. Journal of Bioscience and Bioengineering, 129, 333–347. https://doi.org/doi: 10.1016/j.jbiosc.2019.09.005. [DOI] [PubMed]
- Vioque J., Megías C., Yust M., Pedroche J., Lquari H., Girón-Calle J.…Millán F. Purification of an ACE inhibitory peptide alters hydrolysis of sunflower (Helianthus annuus L.) protein Isolates. Journal of Agriculture and Food Chemistry. 2004;52:1928–1932. doi: 10.1021/jf034707r. [DOI] [PubMed] [Google Scholar]
- Vargas-Bello-Pérez E., Márquez-Hernández R., Hernández-Castellano L. Bioactive peptides from milk: Animal determinants and their implications in human health. Journal of Dairy Research. 2019;86(2):136–144. doi: 10.1017/S0022029919000384. [DOI] [PubMed] [Google Scholar]
- Xiaochun Z., Xuewei S., Bin W. Review on the general cheese processing technology, flavor biochemical pathways and the influence of yeasts in cheese. Frontiers in Microbiology. 2021;12 doi: 10.3389/fmicb.2021.703284. [DOI] [PMC free article] [PubMed] [Google Scholar]