Abstract
Substitutions at position F171 of 6′-N-acetyltransferase type Ib cause variable loss of aminoglycoside resistance, indicating that this residue plays an important role in the structure and/or function of the enzyme.
A common mechanism of resistance to aminoglycosides is enzymatic modification by acetyltransferases (11). Although detailed studies on these enzymes have been limited, some mechanistic and mutational studies have been carried out (reviewed in references 4 and 11) and the three-dimensional structures of aminoglycoside 6′-N-acetyltransferase type Ii and aminoglycoside 3-N-acetyltransferase type Ia have been reported (15, 18). The aac(6′)-Ib gene, included in the transposon Tn1331, encodes the 6′-N-acetyltransferase type Ib [AAC(6′)-Ib] enzyme, which confers resistance to several aminoglycosides (13, 14). We recently isolated a mutant with the F171L modification, which resulted in a protein with reduced activity at 42°C toward aminoglycoside molecules that contain a substitution at the C-1 amino group of the deoxystreptamine moiety (10). This change in specificity at a higher temperature suggests that the phenylalanine at position 171 is important for the structure and/or function of the enzyme (10). It has been shown by mutagenesis analysis that certain positions in proteins are quite tolerant of amino acid substitutions (2). However, those positions with low tolerance for substitutions were shown to be important for the function of the proteins either by playing a direct role in the activity or by being required for the proper three-dimensional structure or stability (12). For example, residues with low tolerance for substitutions were in general the most important for lac repressor activity (2). Therefore, we studied several mutations at F171 in AAC(6′)-Ib to determine this residue’s tolerance for substitutions. Our results showed that amino acid F171 has a very low tolerance for amino acid substitutions, supporting the idea that F171 plays an important role in the activity or proper folding of AAC(6′)-Ib.
Methods.
Escherichia coli XL1-Blue (Stratagene) was used as plasmid host. The plasmid pJHCMW1 (16) was used for the mutagenesis experiments. The mutant F171L was generated by in vivo mutagenesis (10), and the other mutants were generated with the QuikChange site-directed mutagenesis kit (Stratagene) following the recommendations of the supplier. Nucleotide sequencing was performed at the California State University Northridge Sequencing Facility. MICs were determined by the E-test method (17) with commercial strips (AB Biodisk).
Results.
To determine the tolerance of AAC(6′)-Ib to substitutions at F171, we generated mutations to randomize the codon at position 171. Following mutagenesis, a random collection of cells harboring the mutant derivatives was plated onto L agar containing 30 μg of amikacin (AMK)/ml. DNA sequence analysis of several of the resultant colonies demonstrated that in all cases there was a phenylalanine residue at position 171, suggesting that the enzyme has a very low tolerance for substitutions at F171 if it is to remain capable of conferring resistance to AMK at this concentration on L agar plates. Following this, we selected colonies cultured in the absence of aminoglycosides and identified the amino acid encoded at position 171. E. coli strains carrying the plasmids harboring the mutated genes were analyzed by determining the MICs of several aminoglycoside antibiotics (Table 1). All nine mutant derivatives conferred substantially lower levels of resistance than did the wild type.
TABLE 1.
Susceptibility to aminoglycosides of plasmidless E. coli XL1-Blue and of the same strain harboring plasmids including the wild-type aac(6′)-Ib gene or mutant derivatives of the gene with substitutions for F171
| Plasmida | Amino acid 171 | MIC (μg/ml)b
|
||||
|---|---|---|---|---|---|---|
| KAN | AMK | NET | TOB | GEN | ||
| None | N.A.c | 0.5 | 0.25 | 0.19 | 0.25 | 0.064 |
| pJHCMW1 (wild type) | Phe | 128 | 24 | 64 | 32 | 0.75 |
| pDP1 | Leu | 64 | 8 | 12 | 12 | 0.19 |
| pCP213 | Met | 96 | 8 | 32 | 16 | 0.25 |
| pCP301 | Trp | 32 | 4 | 4 | 16 | 0.5 |
| pCP220 | Ile | 12 | 1 | 2 | 8 | 0.25 |
| pCP224 | Tyr | 8 | 1.5 | 1.5 | 6 | 0.19 |
| pCP210 | Gly | 3 | 0.75 | 0.75 | 2 | 0.19 |
| pCP262 | Ser | 0.75 | 0.38 | 0.5 | 0.5 | 0.064 |
| pSSF2 | Asn | 0.75 | 0.5 | 0.25 | 0.25 | 0.064 |
| pSSF8 | Lys | 0.75 | 0.5 | 0.09 | 0.5 | 0.064 |
Plasmid carried by E. coli XL1-Blue.
MICs were determined by the E-test. GEN, gentamicin.
N.A., not applicable.
Comparison of two mutants with F171I (pCP220) and F171L (pDP1) substitutions indicated that, despite the very similar structures of leucine and isoleucine, they behaved differently not only from the wild type but also from each other. E. coli harboring the plasmid pCP220 (F171I mutant derivative) showed considerably lower MICs of AMK, kanamycin (KAN), netilmicin (NET), and tobramicin (TOB) than E. coli(pDP1) (Table 1). The ratios of the MICs of AMK, KAN, NET, and TOB for the mutant F171L to those for the mutant F171I were 8, 5.3, 6, and 1.5, respectively. Substitutions with the nonpolar amino acids tryptophan (pCP301) and methionine (pCP213) resulted in mutant derivatives that were able to confer detectable levels of resistance to AMK, KAN, NET, and TOB, although the levels were considerably lower than those conferred by the wild type (Table 1). E. coli harboring pCP224, the plasmid carrying the gene with a tyrosine substitution, showed a very low, albeit detectable, level of resistance to AMK, KAN, NET, and TOB (Table 1). The mutant derivatives F171G (plasmid pCP210), F171K (plasmid pSSF8), F171N (plasmid pSSF2), and F171S (plasmid pCP262) were unable to confer resistance against the tested aminoglycosides (Table 1).
Discussion.
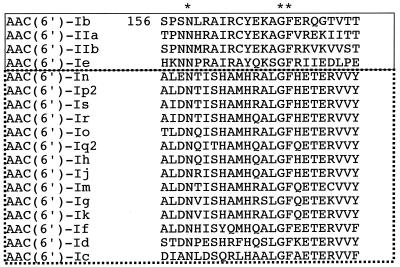
A thorough understanding of the structure and function of the AAC(6′)-Ib protein may play an important role in future rational drug design. To address this possibility we recently initiated an analysis of the aac(6′)-Ib gene by mutagenesis (10). This approach has been used to perform structure-function analysis of numerous proteins (1, 3, 5, 6, 9). In this paper, we show that amino acid substitutions at F171 result in enzyme derivatives that conferred substantially reduced or no resistance to various aminoglycosides. This low tolerance to substitutions suggests that F171 may be important for the enzymatic function either by playing a direct role in the activity or by being required for the proper three-dimensional structure or stability. F171 is one of the most conserved residues within a region designated as motif B in two subgroups of AAC(6′) enzymes (Fig. 1) (8, 11). Although the role played by this motif is still not clear (18), Lin et al. (7) have recently postulated that F174 [equivalent to AAC(6′)-Ib F171] in motif B of tGCN5 is part of the hydrophobic core and may be involved in binding acetyl coenzyme A. Elucidation of the three-dimensional structure of AAC(6′)-Ib will allow the determination of the role of F171 in the function and/or specificity of this enzyme.
FIG. 1.
Alignment of amino acid sequences in motif B, one of the conserved regions among AAC(6′) enzymes (11) and other acetyltransferases (8). The top four sequences are of the four members of AAC(6′) subgroup 1, and the sequences of the members of subgroup 2 are outlined by the dotted line. The asterisks indicate the amino acids that are conserved in all of the amino acid sequences of AAC(6′)-I subgroups 1 and 2.
Nucleotide sequence accession number.
The nucleotide sequences of the mutants have been deposited in the GenBank sequence library (accession no. AF139864-71).
Acknowledgments
This work was supported by Public Health Service Grant AI39738 from the National Institutes of Health. D.P. and R.C. were recipients of Undergraduate Research Creativity Awards.
We thank George Miller and Karen Shaw (Schering-Plough Research Institute) for generously providing netilmicin. We thank Julian Davies for suggestions and reading of the manuscript.
REFERENCES
- 1.Bonomo R, Knox J, Rudin S, Shlaes D. Construction and characterization of an OHIO-1 β-lactamase bearing Met69Ile and Gly238Ser mutations. Antimicrob Agents Chemother. 1997;41:1940–1943. doi: 10.1128/aac.41.9.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bowie J, Reidhaar-Olson J, Lim W, Sauer R. Deciphering the message in protein sequences: tolerance to amino acid substitutions. Science. 1990;247:1306–1310. doi: 10.1126/science.2315699. [DOI] [PubMed] [Google Scholar]
- 3.Carnoy C, Moseley S. Mutational analysis of receptor binding mediated by the Dr family of Escherichia coli adhesins. Mol Microbiol. 1997;23:365–379. doi: 10.1046/j.1365-2958.1997.2231590.x. [DOI] [PubMed] [Google Scholar]
- 4.Davies J, Wright G. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 5.Flory N, Gorman M, Coutinho P, Ford C, Relly P. Thermosensitive mutants of Aspergillus awamori glucoamylase by random mutagenesis: inactivation kinetics and structural interpretation. Protein Eng. 1994;7:1005–1012. doi: 10.1093/protein/7.8.1005. [DOI] [PubMed] [Google Scholar]
- 6.Holm L, Koivula A, Lehtovaara P, Hemminki A, Knowles J. Random mutagenesis used to probe the structure and function of Bacillus stearothermophilus alpha-amylase. Protein Eng. 1990;3:181–191. doi: 10.1093/protein/3.3.181. [DOI] [PubMed] [Google Scholar]
- 7.Lin Y, Fletcher M, Zhou J, Allis C, Wagner G. Solution structure of the catalytic domain of GCN5 histone acetyltransferase bound to coenzyme A. Nature. 1999;400:86–89. doi: 10.1038/21922. [DOI] [PubMed] [Google Scholar]
- 8.Neuwald A F, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 9.Palzkill T, Botstein D. Identification of amino acid substitutions that alter the substrate specificity of TEM-1 β-lactamase. J Bacteriol. 1992;174:5237–5243. doi: 10.1128/jb.174.16.5237-5243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Panaite D, Tolmasky M. Characterization of mutants of the 6′-N-acetyltransferase encoded by the multiresistance transposon Tn1331: effect of Phe171-to-Leu171 and Tyr80-to-Cys80 substitutions. Plasmid. 1998;39:123–133. doi: 10.1006/plas.1997.1330. [DOI] [PubMed] [Google Scholar]
- 11.Shaw K, Rather P, Hare R, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shortle D. Probing the determinants of protein folding and stability with amino acid substitutions. J Biol Chem. 1989;264:5315–5318. [PubMed] [Google Scholar]
- 13.Tolmasky M E, Crosa J H. Tn1331, a novel multiresistance transposon encoding resistance to amikacin and ampicillin in Klebsiella pneumoniae. Antimicrob Agents Chemother. 1987;31:1955–1960. doi: 10.1128/aac.31.12.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolmasky M E, Roberts M, Woloj M, Crosa J H. Molecular cloning of amikacin resistance determinants from a Klebsiella pneumoniae plasmid. Antimicrob Agents Chemother. 1986;30:315–320. doi: 10.1128/aac.30.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf E, Vassilev A, Makino Y, Sali A, Nakatani Y, Burley S. Crystal structure of a GCN5-related N-acetyltransferase: Serratia marcescens aminoglycoside 3-N-acetyltransferase. Cell. 1998;94:439–449. doi: 10.1016/s0092-8674(00)81585-8. [DOI] [PubMed] [Google Scholar]
- 16.Woloj M, Tolmasky M E, Roberts M, Crosa J H. Plasmid-encoded amikacin resistance in multiresistant strains of Klebsiella pneumoniae isolated from neonates with meningitis. Antimicrob Agents Chemother. 1986;29:315–319. doi: 10.1128/aac.29.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woods G, Washington J. Antibacterial susceptibility tests: dilution and disk diffusion methods. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C.: American Society for Microbiology; 1995. pp. 1327–1341. [Google Scholar]
- 18.Wybenga-Groot L, Draker K, Wright G, Berghius A. Crystal structure of an aminoglycoside 6′-N-acetyltransferase: defining the GCN5-related N-acetyltransferase superfamily fold. Structure. 1999;7:497–507. doi: 10.1016/s0969-2126(99)80066-5. [DOI] [PubMed] [Google Scholar]