Abstract
Background and objective
Omentin-1 is an anti-inflammatory adipokine associated with metabolic disorders including insulin resistance, glucose intolerance and type 2 diabetes mellitus (T2DM). Diabetes is a crucial systemic risk factor for occurrence of periodontitis. Periodontitis is deliberated as a complication of diabetes. This study aimed to estimate salivary and serum levels of Omentin-1 in chronic periodontitis with or without diabetes mellitus before and after scaling and root planing (SRP) and also the level of glycemic control.
Materials and methods
Based on history and clinical parameters, 70 subjects were allocated into two groups i.e. chronic periodontitis (Group 1) and chronic periodontitis with well controlled T2DM (Group 2). Baseline saliva and serum samples were collected from both groups followed by full mouth SRP. Subjects were recalled 3 months post SRP for salivary and serum sample collection and measurement of clinical parameters. Serum as well as salivary samples collected were further analysed using enzyme-linked immunosorbent assay to determine the Omentin-1 concentration in each sample.
Results
The levels of Omentin-1 in saliva and serum were significantly lower in both the groups at baseline. Clinical parameters exhibited a significant reduction 3 months post SRP in both groups. Salivary and serum Omentin-1 levels significantly increased in both the groups, 3 months post SRP. HbA1c levels also reduced significantly post therapy.
Conclusion
A significant relation exists between salivary and serum Omentin-1 in chronic periodontitis and chronic periodontitis with T2DM. In consequence, Omentin-1 level can have possible application as inflammatory marker of T2DM, periodontal disease and treatment outcome.
Keywords: Adipokines, Chronic periodontitis, Diabetes mellitus, Glycated hemoglobin A, Insulin resistance, Omentin-1
1. Introduction
Periodontitis being a polymicrobial disease is mainly instigated by the plaque biofilm. The microbial dysbiosis within the biofilm results in a chronic, non-resolving and destructive inflammatory condition. The biofilm presents a microbial challenge leading to tissue damage (i.e. breakdown of periodontal ligament, pocketing and alveolar bone resorption) primarily caused by host inflammatory reaction.1 The periodontal destruction together with the host immune-inflammatory response in the periodontal tissues is furthermore altered by environmental factors, systemic diseases like diabetes mellitus and genetic susceptibility.2 Diabetes being a metabolic disorder is associated with numerous complications like retinopathy, nephropathy, cerebrovascular disease, stroke, macrovascular disease, increased risk of infections and periodontitis, which is now considered as a complications of diabetes mellitus (DM).3,4 Adipose tissue is an endocrine organ, capable of secreting hormones and cytokines called “adipocytokines”. The effects of adipokines are widespread with them playing a vital role in carbohydrate and lipid metabolism as well as in the pathogenesis of insulin resistance, diabetes and also in atherosclerosis, vascular endothelial dysfunction and inflammation.5 The influence of adipokines in the pathogenetic processes interlinking diabetes mellitus and periodontal disease (PD), are not well elucidated.
Omentin (Intelectin) identified by Yang et al., in 2003 from a visceral omental adipose tissue cDNA library is a novel fat depot-specific adipokine.6 Omentin-1 is the main form circulating in human blood and the circulating level is lower in patients with T2DM.7 Omentin exhibits anti-inflammatory, anti-diabetic and anti-atherogenic characteristics.8 This anti-inflammatory adipokine through its paracrine and endocrine influences is critical in regulation of insulin sensitivity by enhancing insulin sensitivity and glucose metabolism.7 Omentin-1 is linked to inflammatory conditions and is also linked to periodontitis and diabetes mellitus. Besides obesity, periodontal disease also down-regulates Omentin-1.9 GCF Omentin-1 levels are negatively influenced by inflammatory conditions like periodontitis.9,10 Scaling and root planing (SRP) remains the cornerstone of non-surgical periodontal therapy (NSPT) which reduces the bacterial burden. Some former studies and meta-analysis have perceived a considerable beneficial effect of NSPT on the glycemic control. No studies so far have assessed the salivary and serum levels of Omentin-1 in periodontitis and diabetes. Therefore, the current study was carried out to estimate and correlate salivary and serum Omentin-1 levels in patients with chronic periodontitis (CP) with and without type 2 diabetes mellitus before and after periodontal treatment and also to evaluate the potential effect of SRP on level of glycemic control.
2. Methodology
Patients between 35 and 60 years and having a minimum of 20 teeth, visiting the Department of Periodontology and Oral Implantology at Vydehi Institute of Dental Science and Research Center, Bangalore were screened to recruit the 70 subjects necessary for the study. 35 systemically healthy subjects with chronic periodontitis clinically diagnosed based on American Academy Periodontology (1999) classification, having clinical attachment loss ≥ 3 m in 30% of the sites and probing pocket depth ≥5 mm with atleast 6 teeth were included in Group 1. 35 CP patients (based on American Academy Periodontology 1999 classification) with well controlled T2DM having HbA1C <7% and patients with T2DM diagnosed at-least one year prior to the study using diagnostic criteria of the American Diabetes Association were included in Group 2. Patients with any other systemic diseases, pregnancy or lactation, current or former smokers, history of periodontal therapy within past 6 months, history of antibiotic and anti-inflammatory drug intake within past 3 months and with any active intraoral lesions were excluded from the study. Ethical clearance from the Institutional Review Board of Vydehi Institute of Dental Sciences and Research Centre, Bangalore was obtained. Written informed consent was obtained from all the participants.
3. Clinical protocol
Collection of Saliva- 5 ml of unstimulated whole saliva was collected from each subject for the study by passive drool method between 9:00 a.m. to 10:00 a.m. to minimize diurnal disparities in the collected sample. Collected samples were centrifuged at 5000 rpm for 10 min. The supernatant was immediately transferred to a sterile plastic vial and stored at −80°C.
Collection of Serum- 5 ml of blood was collected from the antecubital fossa by venipuncture using a 20-gauge needle with a 5 ml syringe and incubated at room temperature in upright position for 30–45 min (no longer than 60 min) to allow clotting. Serum and blood were separated by centrifuging at 5000 rpm for 10 min. The extracted serum was immediately transferred to a sterile plastic vial and stored at −80°C until the time of assay.
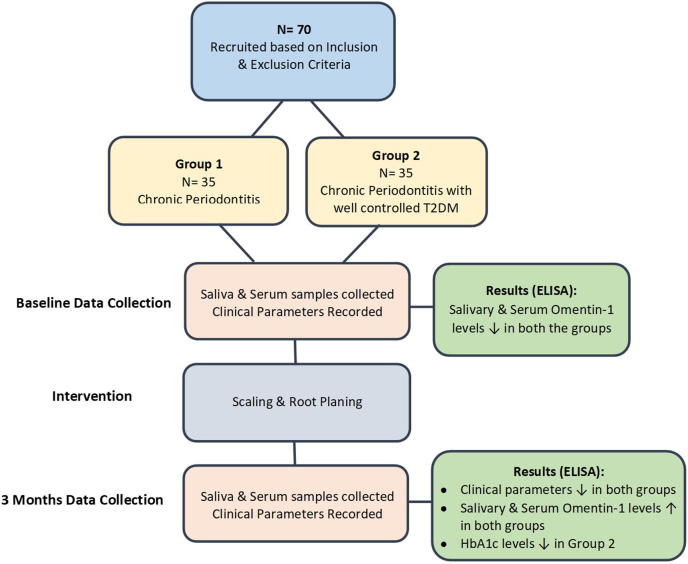
Post sample collection all the clinical parameters i.e. Plaque index (PI), Gingival index (GI), Percentage of bleeding sites (BOP), Probing Pocket Depth (PPD) and Clinical Attachment Level (CAL) were recorded at baseline and at 3 months. Patients in both groups, received periodontal therapy in the form of SRP followed by oral hygiene instructions (OHI) immediately after the baseline sample collection. Salivary and the serum samples were collected from the patients at baseline and 3 months post SRP (Fig. 1). Levels of Omentin-1 in saliva and serum was measured by enzyme-linked immunosorbent assay (ELISA) method.
Fig. 1.
Graphical abstract.
4. ELISA protocol
Omentin-1 levels were measured by ELISA method by using commercially available kit (Human Omentin-1 kit- Genlisa™ ELISA, Krishgen Biosystems, Mumbai, India). This kit uses a double-antibody sandwich ELISA to assay the levels of Omentin-1 in the samples. Monoclonal antibodies were pre-coated onto microwells. 40μl of diluted saliva and/or serum samples and standards were pipetted into microwells and Human Omentin-1 present in the sample were bound by the antibodies. 10μl Biotin labeled antibody was then added, followed by 50μl Streptavidin-HRP which was pipetted and incubated at 37°C for 60 min to form a complex. After washing the microwells in order to remove any non-specific binding, the substrate solution i.e. 3,3′,5,5′-Tetramethylbenzidine (TMB) was added to the microwells. The colour develops proportionately to amount of Human Omentin-1 in sample. Colour development was then stopped by addition of 50μl of stop solution. The colour of solution changed from blue to yellow. Blank well was calculated as zero, the absorbance was read at 450 nm.
5. Statistical analysis
Estimation of sample size was by G Power software v. 3.1.9.2. Considering the effect size to be measured (d) at 80% for Two-tailed hypotheses, power of the study at 80%, Confidence Interval at 95% and the margin of the error at 5%, the total sample size needed was 52, which was rounded off to 60. However, each study group comprised of 35 samples [35 samples x 2 groups = 70 samples].
Statistical Package for Social Sciences [SPSS] for Windows Version 22.0 (Released 2013. Armonk, NY: IBM Corp) was used to perform statistical analysis. Descriptive analysis was done using frequency and proportions for categorical variables, whereas mean and SD was used for continuous variables. Independent Student t-test and Student paired t-test was used to compare the mean values of different clinical parameters and salivary and serum Omentin-1 levels amongst the groups at baseline and 3 months period. Pearson's correlation test was done to evaluate relationship between salivary and serum Omentin-1 levels and clinical parameters at baseline and 3 months period. Stepwise multiple linear regression analysis was used for analysing salivary and serum levels of Omentin-1 in both groups at baseline and 3 months period. The level of significance was set at P < 0.05.
6. Results
Intragroup comparison of clinical parameters (i.e. GI, PI, BOP, PPD and CAL) between baseline and 3 months post intervention in Group 1 and 2 using student paired t-test (Table 1) exhibited a statistically significant reduction in all the clinical parameters in both the Groups between baseline and 3 months post treatment at p < 0.001. Mean HbA1c levels (%) between baseline and 3 months in Group 2 (Table 1) also indicated a significant change in HbA1c percentage 3 months after SRP as compared to baseline at p < 0.001. Comparison of salivary and serum Omentin-1 levels between the baseline at 3 months post intervention in Group 1 and Group 2 (Table 1) showed that there was a statistically significant difference between baseline and 3 months post SRP at p < 0.001.
Table 1.
Comparison of mean values of different parameters between baseline and 3 months period in Group 1 and Group 2 using Student Paired t-Test.
| Group | Parameters | Time | N | Mean | SD | Mean Diff | P-Value |
|---|---|---|---|---|---|---|---|
| Group 1 | GI | Baseline | 35 | 2.43 | 0.20 | 1.25 | <0.001a |
| 3 Months | 35 | 1.18 | 0.09 | ||||
| PI | Baseline | 35 | 2.49 | 0.24 | 1.19 | <0.001a | |
| 3 Months | 35 | 1.30 | 0.14 | ||||
| BOP | Baseline | 35 | 94.82 | 3.38 | 43.51 | <0.001a | |
| 3 Months | 35 | 51.31 | 2.35 | ||||
| PPD | Baseline | 35 | 6.18 | 0.75 | 1.83 | <0.001a | |
| 3 Months | 35 | 4.35 | 0.61 | ||||
| CAL | Baseline | 35 | 5.52 | 0.66 | 1.26 | <0.001a | |
| 3 Months | 35 | 4.26 | 0.44 | ||||
| HbA1c | – | – | – | – | – | – | |
| Salivary Omentin-1 (ng/ml) | Baseline | 35 | 514.893 | 123.613 | −333.732 | <0.001a | |
| 3 Months | 35 | 846.625 | 173.340 | ||||
| Serum Omentin-1 (ng/ml) | Baseline | 35 | 192.165 | 59.173 | −642.935 | <0.001a | |
| 3 Months | 35 | 835.100 | 125.557 | ||||
| Group 2 | GI | Baseline | 35 | 2.41 | 0.36 | 1.03 | <0.001a |
| 3 Months | 35 | 1.38 | 0.29 | ||||
| PI | Baseline | 35 | 2.43 | 0.29 | 1.04 | <0.001a | |
| 3 Months | 35 | 1.39 | 0.16 | ||||
| BOP | Baseline | 35 | 95.45 | 2.26 | 42.09 | <0.001a | |
| 3 Months | 35 | 53.37 | 1.22 | ||||
| PPD | Baseline | 35 | 6.02 | 0.49 | 1.25 | <0.001a | |
| 3 Months | 35 | 4.77 | 0.46 | ||||
| CAL | Baseline | 35 | 5.35 | 0.32 | 0.73 | <0.001a | |
| 3 Months | 35 | 4.62 | 0.26 | ||||
| HbA1c | Baseline | 35 | 6.45 | 0.28 | 0.32 | <0.001a | |
| 3 Months | 35 | 6.13 | 0.29 | ||||
| Salivary Omentin-1 (ng/ml) | Baseline | 35 | 472.293 | 111.929 | −296.066 | <0.001a | |
| 3 Months | 35 | 768.359 | 73.744 | ||||
| Serum Omentin-1 (ng/ml) | Baseline | 35 | 181.243 | 28.379 | −555.577 | <0.001a | |
| 3 Months | 35 | 736.820 | 145.440 |
- Statistically Significant.
Pearson's correlation test done to evaluate relationship between salivary and serum Omentin-1 levels and clinical parameters in Group 1 and Group 2 at baseline and at 3 months post SRP (Table 2). Both groups indicated a significant moderate negative correlation between salivary Omentin-1 levels and the clinical parameters both at baseline and at 3 months. A significant moderate negative correlation was illustrated between the serum Omentin-1 levels and the clinical parameters in both the Groups at baseline and in Group 1 at 3 months and a weak negative correlation was illustrated between the serum Omentin-1 levels and the clinical parameters in Group 2 at 3 months. Therefore, as values of the clinical parameters increased serum and salivary Omentin-1 levels decreased thus indicating an inverse correlation between them. Also, as the serum levels increased the salivary levels also increased which indicates a positive correlation between the two.
Table 2.
Pearson's correlation test to assess relationship between Salivary and Serum Omentin-1 levels and clinical parameters at baseline and 3 months period in Group 1 and Group 2.
| Variable | Time | Groups | Values | GI | PI | BOP | PPD | CAL | HbA1c | SE |
|---|---|---|---|---|---|---|---|---|---|---|
| Salivary Omentin-1 | Baseline | Group 1 | r | −0.48 | −0.44 | −0.46 | −0.49 | −0.47 | .. | 0.67 |
| P-Value | 0.004a | 0.006a | 0.006a | 0.003a | 0.003a | .. | <0.001a | |||
| Group 2 | r | −0.51 | −0.48 | −0.49 | −0.54 | −0.52 | −0.05 | 0.69 | ||
| P-Value | 0.002a | 0.003a | 0.003a | 0.001a | 0.001a | 0.77 | <0.001a | |||
| Serum Omentin-1 | Baseline | Group 1 | r | −0.41 | −0.4 | −0.41 | −0.43 | −0.42 | .. | 0.67 |
| P-Value | 0.01a | 0.01a | 0.01a | 0.01a | 0.01a | .. | <0.001a | |||
| Group 2 | r | −0.49 | −0.45 | −0.46 | −0.44 | −0.45 | −0.11 | 0.69 | ||
| P-Value | 0.003a | 0.006a | 0.006a | 0.008a | 0.006a | 0.69 | <0.001a | |||
| Salivary Omentin-1 | 3 months | Group 1 | r | −0.45 | −0.41 | −0.47 | −0.51 | −0.5 | .. | 0.68 |
| P-Value | 0.005a | 0.02a | 0.004a | 0.002a | 0.002a | .. | <0.001a | |||
| Group 2 | r | −0.46 | −0.41 | −0.45 | −0.47 | −0.44 | −0.19 | 0.67 | ||
| P-Value | 0.006a | 0.02a | 0.005a | 0.005a | 0.006a | 0.27 | <0.001a | |||
| Serum Omentin-1 | 3 months | Group 1 | r | −0.40 | −0.41 | −0.41 | −0.46 | −0.47 | .. | 0.68 |
| P-Value | 0.02a | 0.02a | 0.01a | 0.005a | 0.005a | .. | <0.001a | |||
| Group 2 | r | −0.35 | −0.41 | −0.36 | −0.44 | −0.34 | −0.29 | 0.67 | ||
| P-Value | 0.04a | 0.03a | 0.04a | 0.005a | 0.04a | 0.09 | <0.001a |
‘r’- correlation coefficients.
Minus sign denotes negative correlation.
Correlation coefficient range.
0.0 - No correlation.
0.01–0.20 - Very Weak Correlation.
0.21–0.40 - Weak Correlation.
0.41–0.60 - Moderate Correlation.
0.61–0.80 - Strong Correlation.
0.81–1.00 - Very Strong Correlation.
Statistically Significant.
Table 3 shows stepwise multiple linear regression for salivary and serum Omentin-1 levels in Group 1 and Group 2 at baseline and at 3 months. At baseline for every 1 mm PPD increase, the salivary Omentin-1 levels and serum Omentin-1 levels significantly decreased by 33.190 ng/ml (p < 0.001) and 16.031 ng/ml (p = 0.03) respectively in Group 1 and 20.473 ng/ml (p < 0.001) and 12.651 ng/ml (p = 0.01) respectively in Group 2. The variability in salivary and serum levels of Omentin-1 for PPD at baseline was 36% and 28% respectively in Group 1 and 33% and 24% respectively in Group 2. At 3 months for every 1 mm CAL decrease in Group 1, the salivary Omentin-1 levels significantly increased by 43.459 ng/ml (p < 0.001) and the variability was 34%. At 3 months for every 1 mm PPD decrease, the salivary Omentin-1 levels in Group 2 significantly increased by 40.569 ng/ml (p = 0.002) and the serum Omentin-1 levels significantly increased by 37.404 ng/ml (p < 0.001) and 34.808 ng/ml (p = 0.006) in Group 1 and Group 2 respectively. The variability in salivary Omentin-1 levels for PPD in Group 2 was 26% and the variability in serum Omentin-1 levels for PPD was 35% and 22% in Group 1 and Group 2 respectively.
Table 3.
Stepwise multiple linear regression analysis for Salivary and Serum Omentin-1 levels in Group 1 and Group 2 at baseline and 3 months.
| Variable | Time | Groups | IV | b | SE | t | P-value | R2 |
|---|---|---|---|---|---|---|---|---|
| Salivary Omentin-1 | Baseline | Group 1 | Constant | 612.808 | 92.160 | 8.161 | <0.001a | 0.36 |
| PD | −33.190 | 18.318 | −4.263 | <0.001a | ||||
| Group 2 | Constant | 866.438 | 109.850 | 8.644 | <0.001a | 0.33 | ||
| PD | −20.473 | 10.260 | −4.041 | <0.001a | ||||
| Serum Omentin-1 | Baseline | Group 1 | Constant | 336.938 | 115.293 | 5.691 | <0.001a | 0.28 |
| PD | −16.031 | 8.705 | −2.353 | 0.03a | ||||
| Group 2 | Constant | 270.237 | 106.285 | 8.295 | <0.001a | 0.24 | ||
| PD | −12.651 | 7.679 | −2.724 | 0.01a | ||||
| Salivary Omentin-1 | 3 months | Group 1 | Constant | 299.473 | 102.896 | 8.141 | <0.001a | 0.34 |
| CAL | −43.459 | 17.613 | −4.107 | <0.001a | ||||
| Group 2 | Constant | 249.675 | 90.557 | 5.235 | <0.001a | 0.26 | ||
| PD | −40.569 | 17.994 | −3.432 | 0.002a | ||||
| Serum Omentin-1 | 3 months | Group 1 | Constant | 185.584 | 89.915 | 9.896 | <0.001a | 0.35 |
| PD | −37.404 | 18.653 | −4.223 | <0.001a | ||||
| Group 2 | Constant | 2.090 | 0.126 | 8.978 | <0.001a | 0.22 | ||
| PD | −34.808 | 16.618 | −2.970 | 0.006a |
Statistically Significant.
7. Discussion
Diabetes was initially proved as a risk factor for development of periodontitis followed by proposal of an inverse impact i.e. periodontitis could also function as a risk factor for DM which is supported by several studies.1,11,12 Hence this would propose a multifaceted two-way association between them creating a vicious circle which mutually exacerbates both diseases when they occur in the same individual.13 Adipose tissue secreted adipokines being bioactive molecules control insulin sensitivity, energy expenditure, inflammation and also healing. They are believed to act through their impact on insulin sensitivity and thereby fluctuations of these hormones are linked with T2DM. Ogawa et al. described impact of adipokines on the pathogenesis of periodontitis and T2DM.14 Some adipokines like leptin and omentin have an anti-inflammatory effect on the tissues while others like resistin are pro-inflammatory in nature.
Omentin-1 of visceral stromal cells, up-regulates insulin sensitivity of adipocytes. Its circulating levels are lesser in patients demonstrating degraded glucose tolerance and T2DM.10 A systematic review and meta-analysis concluded that reduced concentration of Omentin-1 can be a vital indicator for T2DM.15 To the best of our knowledge no studies so far have assessed the salivary and serum levels of Omentin-1 in periodontitis and diabetes. Therefore, the current study was undertaken to investigate Omentin-1 as a biomarker in periodontal disease and diabetes mellitus.
In the present study, the mean values of different clinical parameters (GI, PI, BOP, PPD, CAL) were significantly higher at baseline compared to 3 months period in both the groups. A significant reduction in all the clinical parameters was noted in Group 1 at 3 months. This showed that periodontal inflammation decreased after NSPT. A significant reduction in GI, PI, BOP was observed after non-surgical periodontal therapy in Group 2 which showed the clinical effectiveness of NSPT in chronic periodontitis with T2DM. A significant reduction in PPD and CAL was also noted in chronic periodontitis with T2DM after NSPT. The improvement in clinical parameters was due to the reduced bacterial load in the subgingival environment which in turn results in reduced periodontal inflammation. The consequence of subgingival bacterial load reduction is decreased levels of circulating bacteria and its products. Statistically significant decrease in mean percentage of HbA1c was reflected in Group 2 between baseline and 3 months post NSPT. The reduction in HbA1c levels of 0.3% in the current study after NSPT in Group 2 shows improvement in glycemic control which was also observed in other studies done by Simpson TC et al.16 and Madianos PN et al.17 Improvement in glycemic control with NSPT may relate to a substantial improvement in general health of the individuals and in doing so reduces the risk of complications.
Salivary and serum Omentin-1 levels at baseline were significantly lower as compared to 3 months period in both groups. Additionally, the Omentin-1 levels were lower in CP with T2DM group when compared to CP group at baseline, but these differences failed to reach statistical significance. In vitro observations have demonstrated that, insulin signal transduction is enhanced by Omentin via activation of Atk/Protein Kinase B and thus augments insulin– mediated glucose transport in adipocytes.6 It has also been substantiated that human omental adipose tissue exhibited dose dependent reduction in expression of Omentin-1 on administration of glucose and insulin.6,15,18 Thus it can be understood that Omentin is significant for glucose metabolism and plasma glucose as well as insulin can directly or indirectly moderate Omentin synthesis. It is conceivable that the reduced serum Omentin-1 levels witnessed in patients having impaired glucose regulation might possibly reduce insulin stimulated glucose uptake in adipocytes or similar insulin sensitive tissues and hence contributing to insulin resistance and development of diabetes.19 In chronic periodontitis local inflammatory conditions present in periodontium cause localized release of inflammatory mediators. Presence of DM immunologically modifies these mediators significantly increasing the quantities of cytokines present in periodontal tissues, and in turn aggravating the disease.20 This could explain why the Omentin levels in CP with DM group were lower than that of CP group in the current study. These findings may suggest that Omentin-1 levels might indicate chronic inflammation and hence periodontitis may affect Omentin-1 levels in patients with DM.21 Omentin probably plays an important part in the pathogenesis of these conditions.
In the present study it was noted that the Omentin-1 levels in saliva and serum were significantly higher after non-surgical periodontal therapy in both the groups which was similar to studies by Balli U et al.9 and Dogan SB et al.10 It is likely that the decreased serum Omentin-1 levels observed in CP and patients with impaired glucose metabolism may perhaps impact insulin resistance in T2DM. Omentin-1 levels are possibly linked to inflammation and negatively influenced by it, as their levels are lower in individuals with chronic inflammatory disease and T2DM.9,10 In addition, non-surgical periodontal therapy increased levels of Omentin-1 suggesting its anti-inflammatory role in periodontitis.9
The Pearson's correlation between salivary and serum Omentin-1 levels and clinical parameters showed that as the values of the clinical parameters increased, serum and salivary Omentin-1 levels declined thus indicating an inverse correlation. Also, as the serum levels increased, levels in saliva also increased which indicates a positive correlation amongst the two. No other studies have so far been done which correlated salivary and serum Omentin-1 levels.
There is evidence to that Omentin-1 is a better indicator for prognosis of DM and hypothetical mechanisms have been suggested to show that Omentin-1 secretion may contribute to pathophysiology of diabetes.15 Direct hypothetical mechanism with regard to Omentin-1 suggests that it modifies insulin sensitivity and secretion and hence has an effect on organs like adipose, brain, muscle, liver and other tissues.15 Similarly, few experimental studies have shown that Omentin-1 activates Adenosine 5-monophosphate (AMP)- activated protein kinase (AMPK) via insulin receptor substrate (IRS) by restricting the rapamycin (mTOR-p70S6K).22 Also, adiponectin gene expression and Zinc-alpha 2-glycoprotein (ZAG) mRNA expression is upregulated by Omentin-1 which can directly stimulate lipolysis through interaction with AMPK.23 Indirect hypothetical mechanisms are linked to influence of Omentin-1 on adipose tissue accumulation, inflammation and adverse fat distribution which in turn contribute or affect glucose metabolism.15 Currently literature has shown paradoxical relations between serum Omentin and diabetes as some studies do not support such an association.24,25
The present study showed that Omentin-1 was identified in saliva and serum of patients with CP with or without diabetes. The results indicated that salivary and serum Omentin-1 levels increased after NSPT and there was significant correlation with clinical parameters in chronic periodontitis patients with or without T2DM. Omentin-1 can be measured in a saliva sample and hence be used as a non-invasive test and might have probable role as diagnostic, prognostic marker of T2DM, periodontal disease and response to therapy. However, additional studies are essential for improved understanding the pathological mechanisms that link this adipokine to periodontal disease and T2DM.
8. Conclusion
A significant relation exists between salivary and serum Omentin-1 in CP and CP with T2DM. Thus, Omentin-1 level could have a probable application as an inflammatory marker of diabetes mellitus, periodontal disease and treatment outcome.
Funding
No funds, grants, or other support was received.
Declaration of competing interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.The authors have no relevant financial or non-financial interests to disclose.
Contributor Information
Avexilla Dooxa Nongrum, Email: anvexilla308@gmail.com.
Sanjeela R. Guru, Email: sanjeelaguru@yahoo.co.in.
Nisha K J, Email: nisharejath@gmail.com.
Suchetha Aghanashini, Email: suchetha70@gmail.com.
References
- 1.Preshaw P.M., Bissett B.M. Periodontitis and diabetes. Br Dent J. 2019;227(7):577–584. doi: 10.1038/s41415-019-0794-5. [DOI] [PubMed] [Google Scholar]
- 2.Kornman K. Host modulation as a therapeutic strategy in the treatment of periodontal disease. Clin Infect Dis. 1999;28(3):520–526. doi: 10.1086/515165. [DOI] [PubMed] [Google Scholar]
- 3.Stratton I.M., Adler A.I., Neil H.A., et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321(7258):405–412. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loe H. Periodontal disease. The sixth complication of diabetes mellitus. Diabetes Care. 1993;16(1):329–334. [PubMed] [Google Scholar]
- 5.Jung U.J., Choi M.S. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang R.Z., Lee M.J., Hu H., et al. Identification of omentin as a novel depot-specific adipokine in human adipose tissue: possible role in modulating insulin action. Am J Physiol Endocrinol Metab. 2006;290(6):E1253–E1261. doi: 10.1152/ajpendo.00572.2004. [DOI] [PubMed] [Google Scholar]
- 7.Pan H.Y., Guo L., Li Q. Changes of serum omentin-1 levels in normal subjects and in patients with impaired glucose regulation and with newly diagnosed and untreated type 2 diabetes. Diabetes Res Clin Pract. 2010;88(1):29–33. doi: 10.1016/j.diabres.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Arman Y., Kirna K., Ugurlukisi B., et al. The effects of blood glucose regulation in omentin-1 levels among diabetic patients. Exp Clin Endocrinol Diabetes. 2017;125(4):262–266. doi: 10.1055/s-0042-118862. [DOI] [PubMed] [Google Scholar]
- 9.Balli U., Bozkurt Dogan S., Ongoz Dede F., Sertoglu E., Keles G.C. The levels of visceral adipose tissue-derived serpin, omentin-1 and tumor necrosis factor-α in the gingival crevicular fluid of obese patients following periodontal therapy. J Oral Sci. 2016;58(4):465–473. doi: 10.2334/josnusd.16-0212. [DOI] [PubMed] [Google Scholar]
- 10.Dogan S.B., Dede F.O., Balli U., Sertoglu E. Levels of vaspin and omentin-1 in gingival crevicular fluid as potential markers of inflammation in patients with chronic periodontitis and type 2 diabetes mellitus. J Oral Sci. 2016;58(3):379–389. doi: 10.2334/josnusd.15-0731. [DOI] [PubMed] [Google Scholar]
- 11.Lalla E., Papapanou P.N. Diabetes mellitus and periodontitis: a tale of two common interrelated diseases. Nat Rev Endocrinol. 2011;7(12):738–748. doi: 10.1038/nrendo.2011.106. [DOI] [PubMed] [Google Scholar]
- 12.Chapple I.L. Genco R; working group 2 of the joint EFP/AAP workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol. 2013;84(4 Suppl):S106–S112. doi: 10.1902/jop.2013.1340011. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.Z., Yuan Y.H., Liu H.H., et al. Epidemiologic relationship between periodontitis and type 2 diabetes mellitus. BMC Oral Health. 2020;20(1):204. doi: 10.1186/s12903-020-01180-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogawa H., Damrongrungruang T., Hori S., et al. Effect of periodontal treatment on adipokines in type 2 diabetes. World J Diabetes. 2014;5(6):924–931. doi: 10.4239/wjd.v5.i6.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan X., Kaminga A.C., Wen S.W., Acheampong K., Liu A. Omentin-1 in diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2019;14(12) doi: 10.1371/journal.pone.0226292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simpson T.C., Needleman I., Wild S.H., Moles D.R., Mills E.J. Treatment of periodontal disease for glycaemic control in people with diabetes. Cochrane Database Syst Rev. 2010;5:CD004714. doi: 10.1002/14651858.CD004714.pub2. [DOI] [PubMed] [Google Scholar]
- 17.Madianos P.N., Koromantzos P.A. An update of the evidence on the potential impact of periodontal therapy on diabetes outcomes. J Clin Periodontol. 2018;45(2):188–195. doi: 10.1111/jcpe.12836. [DOI] [PubMed] [Google Scholar]
- 18.Tan B.K., Adya R., Farhatullah S., et al. Omentin-1, a novel adipokine, is decreased in overweight insulin-resistant women with polycystic ovary syndrome: ex vivo and in vivo regulation of omentin-1 by insulin and glucose. Diabetes. 2008;57(4):801–808. doi: 10.2337/db07-0990. [DOI] [PubMed] [Google Scholar]
- 19.Nanda B., Mahapatra S., Devi N., Swain S., Padhy R.K., Rattan R. Study of serum omentin-1 in relation to insulin resistance in type II diabetes mellitus. IOSR J Dent Med Sci. 2015;14(12):12–21. [Google Scholar]
- 20.Taiyeb-Ali T.B., Raman R.P., Vaithilingam R.D. Relationship between periodontal disease and diabetes mellitus: an Asian prespective. Periodontol. 2000 2011;56(1):258–268. doi: 10.1111/j.1600-0757.2010.00370.x. [DOI] [PubMed] [Google Scholar]
- 21.Sarhat E.R., Rmaid Z.J., Jabir T.H. Changes of salivary interleukine- 17, Apelin, Omentin and Vaspin levels in normal subjects and diabetic patients with chronic periodontitis. Ann Trop Med Publ Health. 2020;23:S404. [Google Scholar]
- 22.Hernandez-Diaz A., Arana-Martinez J.C., Carbo R., Espinosa-Cervantes R., Omentin Sanchez-Munoz F. Role in insulin resistance, inflammation and cardiovascular protection. Arch Cardiol Mex. 2016;86(3):233–243. doi: 10.1016/j.acmx.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 23.Ceperuelo-Mallafre V., Naf S., Escote X., et al. Circulating and adipose tissue gene expression of zinc-alpha2-glycoprotein in obesity: its relationship with adipokine and lipolytic gene markers in subcutaneous and visceral fat. J Clin Endocrinol Metab. 2009;94(12):5062–5069. doi: 10.1210/jc.2009-0764. [DOI] [PubMed] [Google Scholar]
- 24.Moreno-Navarrete J.M., Ortega F., Castro A., Sabater M., Ricart W., Fernandez-Real J.M. Circulating omentin as a novel biomarker of endothelial dysfunction. Obesity. 2011;19(8):1552–1559. doi: 10.1038/oby.2010.351. [DOI] [PubMed] [Google Scholar]
- 25.Gateva A., Assyov Y., Tsakova A., Kamenov Z. Classical (adiponectin, leptin, resistin) and new (chemerin, vaspin, omentin) adipocytokines in patients with prediabetes. Horm Mol Biol Clin Invest. 2018;34(1):10. doi: 10.1515/hmbci-2017-0031. [DOI] [PubMed] [Google Scholar]