Abstract
Deep-located tumor specific imaging has broad clinical applications in improving the accuracy of tumor diagnosis. Microwave-induced thermoacoustic imaging (MTAI), combining the high-contrast of microwave imaging with the high-resolution of ultrasound imaging, is a potential candidate for noninvasive tumor detection. Herein, a deep-located tumor specific MTAI method by tumor microenvironment (TME) activated nanoprobe is reported. In principle, manganous-manganic oxide-based nanoprobe can be triggered by TME with overexpressed glutathione and weak acidity, causing to release manganese ions and increase conductivity. With pulsed microwaves, manganese ions move repeatedly in gigahertz alternating electric field, resulting in a transient heating and thermoelastic expansion through the Joule effect, which yields a strong thermoacoustic (TA) wave in tumor site. In vitro and in vivo experiments demonstrate that manganous-manganic oxide-based nanoprobe could high-selectively amplify the TA signal in deep-located tumor. Our proposed tumor-specific MTAI method based on TME activation provides a potential approach for deep-located tumor detection.
Keywords: Manganous-manganic oxide, Glutathione, Deep-located tumor specific imaging, Tumor microenvironment, Microwave-induced thermoacoustic imaging
Abbreviations: MTAI, Microwave-induced thermoacoustic imaging; TME, Tumor microenvironment; TA, Thermoacoustic; GSH, Glutathione; ATP, Adenosine triphosphate; DMEM, Dulbecco’s modified Eagle’s medium; FBS, Fetal bovine serum; CCK-8, Cell counting kit-8; TEM, Transmission electron microscope; FTIR, Fourier transform infrared spectroscopy; CLSM, Confocal laser scanning microscopy; MNPs, Mn3O4-PEG-RGD nanoparticles; NMR, Nuclear magnetic resonance; Hcy, Homocysteine; CYS, Cysteine; HEK, Human emborynic kidney
1. Introduction
As one of the deadliest diseases, cancers is seriously threatening human health. According to WHO estimates, by 2035, the world could witness 24 million new cancer cases and 14.5 million cancer-related deaths a year [1]. The early, accurate diagnosis of tumors is of highly significance in the proper disease management and improving cancer survival rates, while tumor-specific imaging is a key to achieve this ambition.
Although investigators use many tumor-specific imaging methods—largely in the service of maintaining high target-to-background ratios—they fall into two general categories: targeted agents strategy and tumor microenvironment (TME) strategy [2], [3]. The targeted agents strategy is to achieve the accumulation of contrast agents at the tumor site by using targeted molecules that can interact with specific receptors, or using targeted antigens that can bind to the overexpressed antibodies [3], [4], [5], [6], [7]. Nevertheless, many efforts at tumor-specific imaging are fraught by nonspecific localization of the putative targeted agents, eliciting unacceptably high background noise. TME strategies based on the Warburg effect which results in a TME characterized by weak acidity, high glutathione (GSH), high adenosine triphosphate (ATP) and high hydrogen peroxide (H2O2) concentration, realizes the specific transformation of contrast agents with enhanced tumor imaging properties through certain physical or chemical changes [8], [9], [10]. At present, researchers have developed a series of TME contrast agents, such as manganese oxides that release Mn2+ ions under low pH and high GSH conditions to enhance T1 MRI [10], [11], [12], [13], [14], and metallic o-phenol polymers that can be hydrolyzed by ATP to achieve tumor specific fluorescence imaging [15]. Previous studies exhibited that TME strategy has the advantages of high selectivity and high target-background ratio, and is a promising tumor-specific imaging scheme. Nonetheless, due to the vulnerability of fluorescence imaging to background fluorescence interference and the limitation of penetration depth of light, the specific imaging of deep tumors was severely hampered. High resolution MRI requires longer scanning time and cannot be used for pregnant women and pacemakers. Therefore, there is an urgent need to develop new imaging techniques that can quickly and specifically detect deep-located tumors.
Microwave-induced thermoacoustic imaging (MTAI), a fast hybrid imaging technology, that integrates the high-sensitivity electromagnetic contrast of microwave and the high resolution of ultrasound in a single modality, has an extensive application prospect in deep-located tumor imaging [16], [17]. However, due to the limited difference in microwave absorption performance between tumor and normal tissues, MTAI faces significant challenges in clearly identification biological information such as the structure of solid tumors. Therefore, introducing contrast agents to achieve the wide application of MTAI in the fields of molecular and functional imaging is an inevitable trend. Among the exogenous contrast agents that have been reported [18], [19], [20], conductance loss nanoprobe (engineered saline nanodroplet [21]), dielectric loss nanoprobes (e.g. rGO [22], amino acids [23], WS2 [24], etc.), magnetic loss nanoprobes (e.g. Fe3O4 [25]) and dual-mechanism loss nanoprobes (e.g. defect-rich titanium nitride [26], Fe-filled carbon nanotube [27], etc.) have good microwave-acoustic conversion efficiency due to their unique electromagnetic properties. However, even when linked to targeted substances, such probes often accumulate in organs such as the liver and kidneys or other tissues, making it difficult to image tumors with high selectivity. Thus, specifically identifying deep-located tumors by MTAI remains a challenge.
Manganese tetroxide (Mn3O4), as a mixed valence manganese oxide, has been widely investigated in MRI [7], [8], [9], [28]. It could be reduced to manganese ions by GSH in weakly acidic TME [29], increasing the electrical conductivity of tumor sites and improving the microwave loss efficiency. In addition, the structure and physical properties of Mn3O4 determine its weak microwave absorption capacity. Moreover, PEG coated Mn3O4 has good biosafety, Mn (II) as an endogenous metal ion has good biocompatibility, and non-cardiovascular preparations containing manganese have been approved by the FDA [30], [31], [32]. Nanomaterials based on manganese tetroxide have the potential to realize endogenous response triggering deep-located tumor specific MTAI.
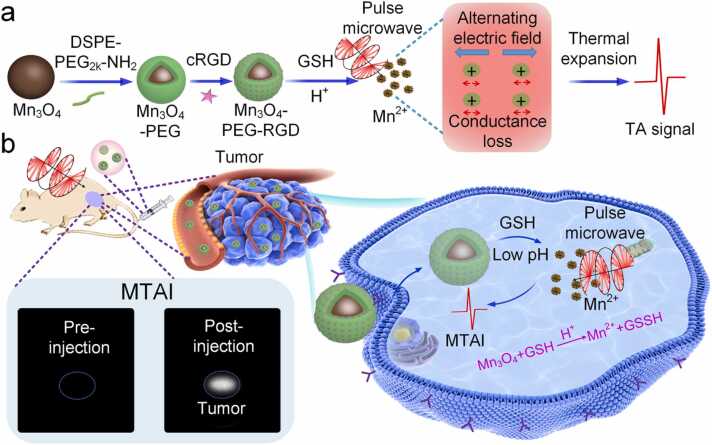
Herein, we propose a deep-located tumor specific MTAI protocol based on the endogenous triggering by TME. Through the interaction between TME and nanoparticles, the thermo-acoustic conversion efficiency and microwave loss capacity of tumor site are specifically changed, realizing thermoacoustic (TA) signal enhancement. In this paper, microwave absorption and TA properties of MNPs solution based on GSH triggering Mn2+ ions release were studied. Fig. 1 illustrates the synthesis of MNPs and the mechanism of deep-located tumor specific MTAI. To meliorate biocompatibility, Mn3O4-PEG was prepared by encapsulating Mn3O4 with hydrophilic and hydrophobic groups of PEG. Then, Mn3O4-PEG was labeled with cRGD to construct Mn3O4-PEG-RGD nanoparticle, triggering the efficient uptake of nanoparticles by tumor cells. After intravenous administration, MNPs targeted the tumor cell membrane through the specific binding of RGD and integrin. Subsequently, MNPs enters the cytoplasm via endocytosis and triggered by TME with weak acidity and high GSH to rapidly generate Mn2+ ions, which can increase the ions concentration in the tumor and improves the regional conductivity. Under pulsed microwave excitation, manganese ions move repeatedly in gigahertz alternating electric field, and the electric field is converted into internal energy through the Joule effect, which causes transient heating and thermoelastic expansion, yielding a strong TA wave and enhance the contrast of MTAI. Experimental results in vitro and in vivo with a breast tumor animal model indicate that TME-based endogenous triggering of Mn3O4 release of Mn2+ ions could improve the efficiency of thermo-acoustic conversion of tumor sites and achieve deep-located tumor specific MTAI.
Fig. 1.
(a) Schematic of Mn3O4-PEG-RGD synthesis and TA signal generation mechanism. (b) Schematic diagram tumor-specific MTAI.
2. Materials and methods
2.1. Materials
Mn3O4 was purchased from Hefei ZhongHang Nanometer Technology Development Co., Ltd. Anhydrous ethanol was purchased from Tianjin Baishi Chemical Industry Co., Ltd. Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin, and streptomycin were obtained from Gibco. MitoTracker Green was obtained from Beyotime Institute of Biotechnology (Shanghai, China). Cell counting kit-8 (CCK-8), Fluorescein isothiocyanate-annexin V (Annexin V-FITC), and calcein acetoxymethyl ester (calcein-AM), and propidium iodide (PI) chromophore were purchased from Dojindo Laboratories (Kumamoto, Japan). 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino (polyethylene glycol 2000)] (DSPE-PEG2k-NH2) was purchased from Shanghai Advanced Vehicle Technology Pharmaceutical Ltd (Shanghai, China). Water used in this study was deionized to a specific resistivity of 18.4 MΩ cm using a Milli-QSP reagent water system (Millipore). All other chemical reagents used in this study are analytically pure and can be used without further purification.
2.2. Preparation of Mn3O4 nanodots
The Mn3O4 crystal powder was crushed in ice water bath for 24 h by ultrasonic crusher (1200 W, 19–25 kHz). The obtained dispersion was centrifuged at 6000 RPM for 5 min, after that, the supernatant was centrifuged at 10,000 RPM for 20 min. The precipitation was cleaned with deionized water. After freeze drying, it was stored at room temperature for future use.
2.3. Preparation of Mn3O4-PEG
PEGylated Mn3O4 nanoparticles were synthesized through the oil-in-water microemulsion reaction method. Briefly, Mn3O4 nanodots (2 mg) were added into anhydrous ethanol (500 μL) and dispersed by ultrasonic for 30 min. DSPE-PEG2k-NH2 (4 mg) was dissolved in PBS (2 mL) and magnetically stirred at 2000 RPM for 30 min. The ethanol solution of Mn3O4 after ultrasound was injected into the PBS solution of DSPE-PEG2k-NH2 drop by drop, and Mn3O4-PEG was obtained by magnetic stirring at 2000 RPM for 12 h.
2.4. Preparation of Mn3O4-PEG-RGD
EDC (2 mg), NHS (2.5 mg) and cRGD (1 mg) were added into 1 mL PBS and magnetically stirred at 500 RPM for 2 h. Then, 1 mL Mn3O4-PEG (1 mg/mL) was added and magnetically stirred overnight at 500 RPM. After that, the free ions and molecules were removed by dialysis (3000 Da) for 24 h to obtain Mn3O4-PEG-RGD.
2.5. Characterization
JEM-2100 transmission electron microscope (TEM) was used to observe the morphology and structure of MNPs at 200 kV. The dynamic light scatterer (DLS, Malvern Zetasizer Nano-Zs 90, UK) was used to determine the size distribution of MNPs. The spectrophotometer (λ−35 UV-Visible spectrophotometer, PerkinElmer, MA,USA) is used to record the absorption spectrum of the materials. Fourier transform infrared spectroscopy (FTIR) (Bio-Rad FTS 6000, Bio-Rad Company, United States) was used to characterize the surface functional groups of Mn3O4-PEG-RGD nanoparticles. The nuclear magnetic resonance (NMR) contrast agent relaxation rate analyzer (MINPQ001, Shanghai Newmart Electronic Technology Co., LTD. Magnetic field intensity: 0.5 ± 0.08 T, magnetic field uniformity: ≤ 30 ppm, magnetic field stability: ≤ 200 Hz/hour, maximum sampling bandwidth: 2000 kHz, probe coil diameter: 15 mm) was used to measure the T1 relaxation time of the samples. The microplate reader (Thermo Fisher Instruments Inc.) was used to detect cell viability. Confocal laser scanning microscopy (CLSM) (ZEISS LSM 510 META, Germany) was used to observe MNPs uptake by cells.
2.6. Detection of dielectric properties
The vector network analyzer (VNA, AV36728-S) used in this experiment was purchased from the 41 Research Institute of China Electronics Technology Group Corporation (CETC). The microwave frequency adjustment range of VNA is 10 MHz to 26.5 GHz, and the frequency division value is 1 Hz. The dielectric data in this paper were measured using the instrument's default scan type linear scan. Complex permittivity spectra were obtained in the frequency range of 0.5–7 GHz using an N/3.5 mm (0.045–26.5 GHz bandwidth) dielectric probe suite. The test temperature was room temperature and each sample was measured three times. The conductivity meter (DDS-11A, Shanghai Yueping Scientific Instrument Co., LTD., China) is used to measure the conductivity of solution.
2.7. Microwave-induced TA signal and imaging
The 1.2 GHz microwave generator (BW-1200hPT; Microelectromechanical technology (Xi 'an, China) Co., LTD.) transmits 0.5 μs microwave pulse at 10 Hz repetition frequency under computer control. The peak power of pulsed microwave is 250 kW, the size of the waveguide (model: WR650) used in the imaging process is 8.3 × 16.5 cm2, and the microwave energy density is E = (250 × 0.5)/(8.3 × 16.5) mJ/cm2 = 0.912 mJ/cm2. During imaging, the sample to be imaged was placed in a plastic container immersed in a coupling oil and coupled with TA wave. Under the excitation of pulsed microwave, the sample generated TA signal, and the TA signal was recorded by circular ultrasonic detector (256 elements; radius 7.5 cm, center frequency 2.5 MHz, bandwidth 60%). The data acquisition system (DAS) transmits, amplifies, filters, transforms and caches signals from each element. DAS consists of two 32-channel acquisition cards (NI5752; National Instruments) with a sampling rate of 50 MHz. Finally, the maximum intensity projection algorithm is used to process the TA data to reconstruct the 2D image.
2.8. Cell culture
EMT6 cells, MCF-7 cells and 239T cells were cultured in DMEM containing 1% penicillin/streptomycin (PS) and 10% fetal bovine serum (FBS) in a humidified cell incubator at with 5% CO2 and 37 ℃.
2.9. Cell viability
Evaluation in vitro cytotoxicity was assessed using colorimetric tetrazole assay for CCK-8. EMT6 cells were cultured in 96-well microplates (5 × 103 per well, 100 μL) in a humidified atmosphere of 5% CO2 at 37 ℃ overnight. The solutions of MNPs at 0 µg/mL (PBS), 12.5 µg/mL, 25 µg/mL, 50 µg/mL, 100 µg/mL and 200 µg/mL were incubated for 24 h. After that, the cell viability was tested according to the CCK-8 assay.
2.10. In vitro hemolysis test
To assess the cytotoxicity of MNPs in vitro, fresh mouse blood samples were centrifuged and diluted with PBS to obtain a 2% red blood cells suspension. Then, 0.5 mL red blood cells were mixed with MNPs at a concentration of 0–200 µg/mL (the solvent was PBS). A mixture of 0.5 mL of deionized water and 0.5 mL of cells was used to obtain a positive control group, and 0.5 mL of PBS and 0.5 mL of cells were mixed to obtain a negative control group. Each experimental group contained three parallel experiments in duplicate. The mixture of each group was placed at 37 ℃ for 3 h and then centrifuged. The absorbance at 570 nm of the supernatant was detected by a UV–vis spectrometer. The calculation formula of hemolysis rate:
where , and are the absorbance of the sample to be tested, the negative sample, and the positive sample at 570 nm [33].
2.11. In vitro cell uptake and flow cytometry analysis
In vitro cell targeting and uptake test, MCF-7 cells and EMT6 cells were cultured in confocal dishes (1 ×105 cells/well) for 24 h and incubated in serum-free medium containing different nanoparticles (Mn3O4-PEG-RGD-Cy5.5 and Mn3O4-PEG-Cy5.5) for 3 h. After that, the cells were then washed three times with PBS to remove uningested nanoparticles. CLSM (ZEIESS LSM SH120, Germany) was used to observe the morphology and fluorescence information of each cell sample. Cells were sampled (10,000 cells) and tested on FACS Canto II flow cytometry (Becton Dickinson, Mountain View, CA, USA).
2.12. Animal models
The PBS suspension of EMT6 cells (1 × 106, 100 μL) was injected subcutaneously into the back of Balb/c mice. After one week, the tumor volume had grown to about 80 mm3, thus a typical tumor back graft model was successfully constructed.
2.13. In vivo toxicity
All animal experiments were in accordance with the protocol rules approved by the Animal Protection and Utilization Committee of SCNU. The mice in this study were healthy male Balb/ C mice aged 4–5 weeks. First, mice were randomly divided into 4 groups with 3 mice in each group. The mice in the four groups were injected with different concentrations of MNPs (0, 10, 20, 40 mg/kg) in the tail vein. After that, they were fed under the same conditions for 21 days, weighed every two days, and dissected after 21 days. H&E staining sections were made, and the appearance of the tissue was observed under light microscope.
2.14. Pharmacokinetics of MNPs in vivo
EMT6 tumor-bearing mice were injected with MNPS-CY5.5 (100 g/mL) through tail vein, and fluorescence imaging was performed at different time points (0, 1, 3, 6, 12, 24, 48 h after injection) using a two-color infrared fluorescence imaging system (Odyssey Li-COR, USA). After that, tumor-bearing mice injected with MNPs for different time periods were dissected. Their viscera and tumor tissues were fluorescently imaged.
2.15. In vivo TA imaging
EMT6 tumor-bearing mice were injected with MNPs solution 100 μL (200 μg/mL) when the tumor grew to about 80 mm3. Then the mice were anesthetized with isoflurane, and the TA signals of live mouse tumors at different times were collected by TA imaging system. Finally, the maximum intensity projection algorithm was used to process the TA signal and reconstruct the two-dimensional tumor image.
3. Results and discussion
3.1. Synthesis and characterization of MNPs
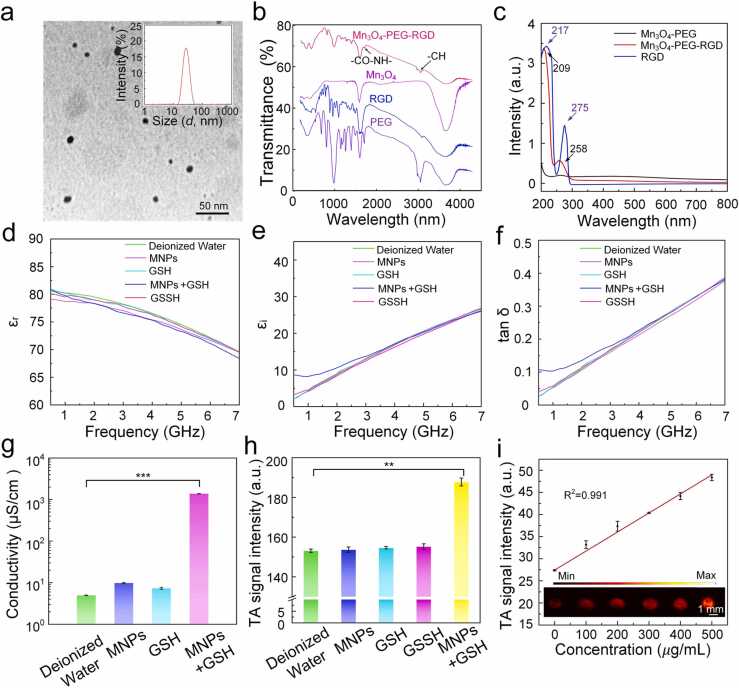
Ultrasonic crushing method was used to obtain the Mn3O4 nanodots with a diameter about 10 nm (Fig. 2a). The Mn3O4 nanodots were coated with DSPE-PEG2k-NH2 for improving biosafety and biocompatibility. For safety applications in the biomedical field, the nanodots were coated with. Moreover, RGD was linked by amidation reaction to achieve the purpose of specific targeting to the tumor cells. TEM revealed that MNPs had well-dispersed spherical morphology with an average hydration diameter of 100 nm (Fig. S1a). Under these conditions, the hydrodynamic size and polymer dispersity index (PDI) of the MNPs was almost unchanged in 48 h, indicating good stability (Fig. S1b, c). The photos of MNPs solution more directly proves the dispersion stability of MNPs (Fig. S1d). The FTIR of Mn3O4, PEG, RGD, and Mn3O4-PEG-RGD were verified the formation of Mn3O4-PEG-RGD (Fig. 2b). The absorption peak at 2880 cm−1 is attributed to the characteristic peak of the vibration of the -CH bond in PEG, indicating that PEG has successfully modified Mn3O4 surface. The absorption peak at 1660 cm−1 of Mn3O4-PEG-RGD spectrum is the characteristic peak of stretching vibration of -CO-NH-, exhibiting the successful synthesis of Mn3O4-PEG-RGD. The successful preparation of MNPs was further demonstrated by UV–vis spectra. As shown in Fig. 2c, the 217 nm and 275 nm characteristic peaks of RGD were blue shifted to 209 nm and 258 nm, indicating that RGD is successfully linked to Mn3O4-PEG.
Fig. 2.
Characterization of MNPs. (a) TEM images of Mn3O4 nanodots. (b) FTIR of PEG, RGD, Mn3O4, and Mn3O4-PEG-RGD. (c) UV–vis absorption spectra of Mn3O4-PEG (black), RGD (blue), and Mn3O4-PEG-RGD (red). (d) The real, (e) imaginary parts of complex relative permittivity, (f) loss tangent (tan δ) and (g) conductivity of deionized water, MNPs, GSH, MNPs+GSH, and GSSH (oxidized glutathione) solutions at the same concentration. (h) TA signal intensity of deionized water, MNPs, GSSH, GSH and MNPs+GSH solutions at the same concentration. (i) In vitro TA maximal amplitude projection images at diverse concentration. The error bars represent standard deviation obtained from ten measurements. Data are shown as mean ± SD (n = 3), **p < 0.01, ***p < 0.001.
3.2. TA effect of MNPs
The complex permittivity () was used to evaluate the microwave loss properties of the solution after the reaction of MNPs with GSH. The imaginary part () reflected the dissipation of electrical energy within medium, the real part () reflected the storage of electrical energy. The dielectric loss factor () provides a standard parameter to measure the ratio of energy loss and conservation in materials [34], [35], [36], [37]. The higher the value of the dielectric dissipation factor, the better the microwave absorption properties of the material. The and of the complex permittivity of MNPs+GSH, MNPs, GSH, GSSH, and deionized water in 0.5–7 GHz region were measured. The experimental results showed that MNPs+GSH solution had a high dielectric constant with a strong dielectric loss, suggesting excellent microwave absorption (Fig. 2d, e, and f). To testify that the improvement of dielectric virtual part and microwave absorption performance comes from the increase of direct current (DC) conductivity () caused by the specific response of MNPs and GSH, the DC conductivity of MNPs+GSH, MNPs, GSH, and deionized water was tested. The conductivity of MNPs + GSH solution was much higher than that of MNPs, GSH and deionized water (Fig. 2g). The above results manifested that MNPs have good microwave absorption performance after responding to GSH.
To further prove that the reduction of MNPs by GSH enables to enhance the microwave-acoustic conversion efficiency, the TA signal of MNPs + GSH solution was compared to the MNPs, GSH and deionized water. The results revealed that the TA signal generated by the MNPs + GSH solution was much stronger than that generated by MNPs and GSH at the same concentration (Figs. 2h, 1 mg/mL). The quantitative results showed that the TA signal generated by MNPs + GSH was 25%, 26%, 24% higher than that deionized water, the same concentration of MNPs and GSH respectively, which is agreement with the corresponding dielectric parameters. Moreover, GSSH solution at the same concentration did not enhance TA signal, which reflected the generation of TA signal from Mn2+ rather than GSSH. In addition, the concentration-dependent linear correlation of TA intensity and MTAI contrast revealed that the microwave absorption efficiency of the reaction of MNPs with GSH was stable and great (Fig. 2i). In contrast, at the same mass concentration (1 mg/mL), the TA signals of PEG+GSH and RGD+GSH were not increased compared with that of deionized water (Fig. S2), which proved that Mn3O4 + GSH did not lose TA signal and showed good microwave stability.
3.3. Ions release and TA effects in an in vitro TME
To demonstrate the tumor-specific MTAI capacity of MNPs by responding specifically to the TME, we assessed the nuclear magnetic resonance (NMR) longitudinal relaxation time (T1), conductivity and dielectric loss angle of MNPs over time in an in vitro TME model, where T1 reflected the release of Mn2+ ions since Mn2+ with five unpaired 3d electrons is a great T1-shortening agent in MR imaging [38], [39], [40], conductivity () and characterized electrical parameters related to microwave loss. Fig. 3a–c showed that with the increase of GSH concentration from 0 mM to 10 mM, the NMR longitudinal relaxation time (T1), conductivity, and of MNPs solution are grew continuously. Moreover, when the GSH concentration is 0 mM, the NMR and electrical parameters of MNP didn’t change significantly with time. These results indicated that MNPs could specifically respond to GSH to release Mn2+ and improve microwave loss capacity. Fig. 3d–f showed that when the concentration of GSH was 10 mM, as the solution pH decreased from blood physiological environment (pH = 7.4) to tumor intracellular condition (pH = 6.0–6.5) and lysosomal (pH = 5.0) in tumor cells, the NMR longitudinal relaxation time (T1), electrical conductivity and of MNPs solution also changed faster and faster. The results manifested that the acidic environment in tumor sites could accelerate the specific response of MNPs to GSH so as to boost the enhancement microwave loss capacity of tumor site. These above results preliminarily proves that MNPs have the potential to realize tumor-specific MTAI.
Fig. 3.
Response of MNPs to TME. (a) The longitudinal relaxation time (T1), (b) dielectric loss Angle, and (c) conductivity of MNPs solution over time in different GSH concentrations (pH = 7.4). (d) The longitudinal relaxation time (T1), (e) dielectric loss angle, and (f) conductivity of MNPs solution over time in different pH environments (GSH = 10 mM).
3.4. In vitro tumor cells-specific MTAI and in stiu tumor-specific MTAI
To verify the specific imaging ability of MNPs for tumor cells and tumor tissues, primarily, the GSH specificity was evaluated by incubating MNPs with different types of oxidizing and reducing substances, including GSH, H2O2, hydroxyl radical (-OH), homocysteine (Hcy), hypochlorite (-OCl) and cysteine (CYS). Fig. 4a shows that the MNPs can specifically response to GSH, with no obvious response to other substances. Then, to prove that specific MTAI can be achieved based on the interaction between TME and Mn3O4 at the cell level in vitro, it is necessary to exclude the difference in the number of nanoparticles entering EMT6 and 239 T cells (a variant of human embryonic kidney (HEK) cells) caused by RGD. EMT6 cells and 239 T cells with the same cell density were cultured in two separate petri dishes in cell culture medium containing Mn3O4-PEG (200 μg/mL), and MTAI was performed on them respectively. The results revealed that the MTAI signal intensity of EMT6 +Mn3O4-PEG group was significantly improved, while the MTAI of 239 T cells showed no obvious change after the addition of Mn3O4-PEG (Fig. 4b, c). At the same time, we carried out MTAI for deionized water, GSH solution, MNPs solution and MNPs+GSH solution. The TA signal quantitative analysis of the samples illustrated that, compared with deionized water, GSH and MNPs the TA signal generated by the MNPs+GSH solution increased by about 20%, which was caused by the reaction of Mn3O4 with GSH to release ions (Fig. S3). These results proved that MNPs can achieve tumor cells-specific MTAI in vitro. And beyond that, we further performed MTAI on the thigh and tumor of mice injected with MNPs in situ. After 2 h injection, the imaging effect and signal intensity of the tumor were significantly enhanced, while the thigh treated with the same treatment was not significantly changed, further demonstrating the superior tumor-specific MTAI ability of MNPs (Fig. 4d, e).
Fig. 4.
In vitro tumor cells-specific MTAI and in stiu tumor-specific MTAI. (a) TA signal intensity of MNPs after incubation with various types of oxidizing and reducing substances for 2 h. (b) MTAI of EMT6 cells and 239 T cells with different treatments. Scale bars 1 mm. (c) Quantitative analysis of b. (d) MTAI of in vivo breast tumor and thigh obtained before and 2 h after in stiu injection of MNPs solution (200 μg/mL, 100 μL). (e) Statistical results of d.
3.5. Biocompatibility of MNPs in vitro and in vivo
For evaluating the in vitro and in vivo biosafety of MNPs, initially, the in vitro cytotoxicity of MNPs was first detected using standard CCK-8. Experimental results demonstrate that MNPs didn’t show any obvious toxicity that affected the survival of the EMT6 cells (Fig. S4). After that, the biocompatibility of MNPs under physiological conditions such as blood circulation was evaluated by hemolysis test. Fig. S6 shows that the hemolysis rates of MNPs under all test contents were < 5%. The quantitative results were consistent with the visual images. Except for the positive control group, no obvious hemolysis occurred in all experimental groups, indicating that MNPs has obvious biocompatibility in blood circulation [41], [42], [43]. Finally, to further ensure the potential of further clinical application of MNP, we systematically investigated its biocompatibility and biosafety in vivo. The behavior and body weight data of mice injected with different amounts of MNPs were continuously monitored and recorded for 21 days (Fig. S7a), and the results showed that the mice in each group continued to grow normally without any obvious abnormalities. In addition, no abnormal changes or behaviors were observed. H&E staining of heart, liver, spleen, lung, and kidney in each group showed no significant difference, further confirming that MNPs had no toxic and side effects on mouse organs (Fig. S7b). These results proved that MNPs had excellent safety profiles for biomedical utilization.
3.6. The cytomembrane targeting capability, and biodistribution of MNPs
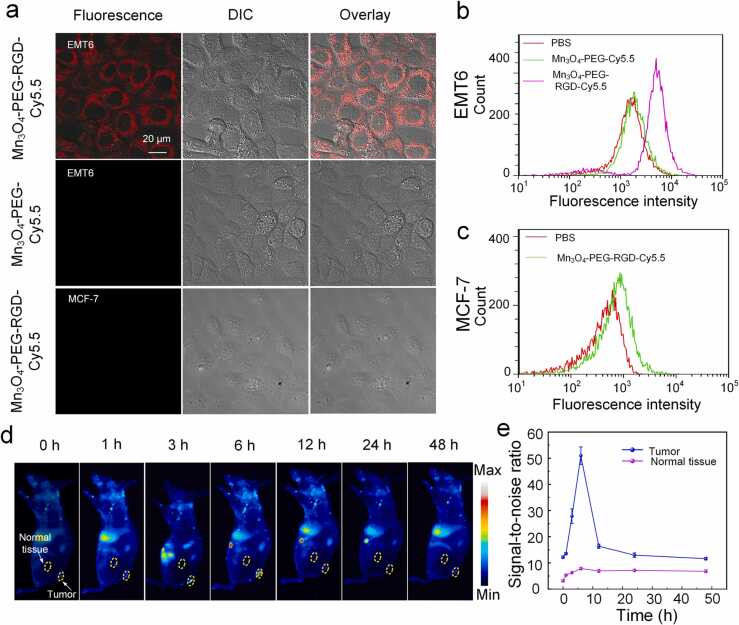
RGD is modified on nanoparticles to promote more aggregation of nanoparticles at the tumor site and achieve better tumor-specific imaging performance. Then, to evaluate the specific targeting ability of MNPs to EMT6 cells, firstly, the cellular uptake of Mn3O4-PEG-RGD-Cy5.5 was investigated with flow cytometry. The fluorescence value of EMT6 cells reached the maximum value after 4 h co-incubation (Fig. S5), indicating that 4 h was the best time to detect the targeting ability of Mn3O4-PEG-RGD. Then, the subcellular localization of Mn3O4-PEG-RGD-Cy5.5 was investigated in cultured tumor cells. The nanoparticles were incubated with EMT6 cells (overexpressing integrin ) and MCF-7 cells (underexpressing integrin ). As an additional control, EMT6 cells were incubated with Mn3O4-PEG-Cy5.5 (without the RGD targeting molecule), then, all cells were cultured and treated under the same conditions. After that, confocal microscopy was used to detect the strong fluorescence signal on the membrane of EMT6 cells incubated with Mn3O4-PEG-RGD-Cy5.5 (Fig. 5a), while there was very minimal signal in the MCF-7 cells incubated with Mn3O4-PEG-RGD-Cy5.5 and the EMT6 cells incubated with Mn3O4-PEG-Cy5.5. Then flow cytometry was used to further study the targeting of nanoparticles. The uptake of Mn3O4-PEG-RGD-Cy5.5 in EMT6 cells was significantly higher than that in Mn3O4-PEG-Cy5.5 group (Fig. 5b). Cell uptake was minimal in MCF-7 cells treated with Mn3O4-PEG-RGD-Cy5.5 (Fig. 5c).
Fig. 5.
Experiments to test the targeting ability and biodistribution of MNPs. (a) Cell uptake of Mn3O4-PEG-RGD-Cy5.5 in EMT6 cells and MCF-7 cells and Mn3O4-PEG-Cy5.5 in EMT6 cells. The fluorescence images were taken by confocal microscopy. (b) Flow cytometry analysis of EMT6 cells incubated with PBS, Mn3O4-PEG-Cy5.5, and Mn3O4-PEG-RGD-Cy5.5. (c) Flow cytometry analysis of MCF-7 cells incubated with PBS and Mn3O4-PEG-RGD-Cy5.5. (d) In vivo fluorescence imaging of mice with EMT6 tumor upon treatment with MNPs-Cy5.5 (200 μg/mL, 100 μL) at different time intervals. (e) The fluorescence SNR in tumors after treatment with MNPs-Cy5.5 at different time intervals.
To determine the pharmacokinetics and biological distribution of MNPs in vivo, on a systemic level, the preferential accumulation of MNPs in tumorous regions was investigated. The biodistribution of MNPs was inspected using an in vivo fluorescence imaging system at various time points post-injection (Fig. 5d). Compared with other time points, the strongest fluorescence signal was observed at 6 h after injection, demonstrating the ability of nanoparticles to effectively target tumor (Fig. 5e). Fluorescence quantitative analysis of tumor and important organs (heart, liver, spleen, lung and kidney) resected at different time points (0, 3, 6, 12 h) (Fig. S8) were also consistent with the above result, and the maximum time point of MNPs aggregation in tumor site was 6 h after injection.
3.7. In vivo deep-located tumor specific MTAI of MNPs
To further investigate the tumor-specific MTAI performance of MNPs in vivo, we employed EMT6 tumor-bearing mice as an animal model to assess evaluate the MTAI performance of MNPs in vivo. TA signal increased gradually after intravenous infusion of MNPs, indicating a time-dependent accumulation of MNPs in the tumor under RGD targeting (Fig. 6a). The TA signal in tumor peaked 6 h after MNPs injection (2-fold enhancement) and then decreased with time (1.1-fold enhancement at 48 h) (Fig. 6c). This phenomenon is consistent with the sum of the fluorescence quantitative analysis. These results indicated that MNPs is an excellent tumor-specific MTAI probe in vivo.
Fig. 6.
Specific MTAI of breast tumor in vivo. (a) MTAI of EMT6 tumor-bearing mice after intravenous administration of MNPs over prolonged time intervals. (b) Specific MTAI of deep-located breast tumor before and 6 h after MNPs solution injection (500 μg/mL). (c) Schematic illustration of deep-located tumor MTAI. (d) TA signal intensity of (a). (e) Statistical results of (b). Data are shown as mean ± SD (n = 3), **p < 0.01.
To demonstrate the ability of this protocol to achieve specific imaging of deep-located tumors, the MTAI of tumor covering 5 cm of adipose tissue was performed in vivo (Fig. 6b). After MNPs injection, TA signal was increased (from 38.3 to 65.8) (Fig. 6d). Those results further prove that this scheme has deep-located tumor specific imaging capacity.
However, at present, the scheme might not be applied in clinic. First of all, MTAI technology needs to be improved in terms of improving resolution, implementing imaging and simplifying the complexity of equipment operation before clinical application. Secondly, although MNPs can achieve specific imaging of deep-located tumors in animal models, its biosafety needs further study and TA signal enhancement efficiency needs to be further improved. In addition, currently only 5 cm deep-located tumor imaging has been evaluated in the experiment, and the feasibility of deeper tumor imaging remains to be further explored.
4. Conclusions
In summary, we have developed a deep-located tumor specific imaging protocol that utilizes endogenous excitation to illuminate deep-located tumor tissues with high selectivity. Our study manifests that RGD labeled MNPs can be internalized into tumor cells through integrin-mediated endocytosis to implement MNPs aggregation in tumor cells. More importantly, MNPs can specifically respond to TME with weak acidity and high GSH concentration to cause rapid release of Mn2+ ions, thus specifically enhancing microwave loss at the tumor site. Combined with the deep penetration of microwave in tissue, specific MTAI for deep-located tumors was achieved. Meanwhile, MNPs hardly change the background TA signals generated by other normal tissues, which are mainly derived from the polarization loss (from water and other polarized molecules) and conductivity loss (from Na+, Cl-, etc.). In addition, the Mn2+ ion produced is an endogenous metal element, which plays an important role in numerous life processes such as anti-aging and bone promotion, and has long-term biocompatibility. The corresponding in vitro and in vivo experiments undoubtedly elucidate the unique advantages of TME-activated MTAI in realizing the specific imaging of deep-located tumors, providing a new research direction for the specific detection of deep-located tumors.
Funding
This research was supported by the National Natural Science Foundation of China, China (Grant Nos. 62075066); the Science and Technology Planning Project of Guangdong Province, China (Grant Nos. 2019A1515012054); and the Science and Technology Program of Guangzhou, China (No. 2019050001).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Biographies

Shanxiang Zhang is currently a Master student at the College of Biophotonics, South China Normal University, China. His research interests are microwave thermoacoustic imaging of tumors and contrast agents.

Wenjing Li is currently a Master student at the College of Biophotonics, South China Normal University, China. Her research interest is microwave thermoacoustic therapy of tumors.

Xiaoyu Chen is currently a Master student at the College of Biophotonics, South China Normal University, China. Her research interest is high efficiency thermoacoustic agent for assisting pulsed microwave imaging guided therapy system.

Mingyang Ren is currently a Master student at the College of Biophotonics, South China Normal University, China. His research interests are microwave thermoacoustic imaging system and device.

Huimin Zhang is currently a Master student at the College of Biophotonics, South China Normal University, China. Her research interests are microwave thermoacoustic imaging system and device.

Huan Qin is an associate professor at the College of Biophotonics, South China Normal University, China. He received his Ph.D. degree in Optics from South China Normal University in 2015. His research interests include microwave thermoacoustic imaging, photoacoustic molecular imaging and their biomedical applications.

Da Xing is a professor at the College of Biophotonics, South China Normal University, China. He received his Ph.D. degree in laser engineering from Harbin Institute of Technology in 1988. His research interests include photoacoustic spectroscopy functional imaging and its application in clinical medicine, optical detection of single molecule behavior in vivo and its application in cell biology, and laser technology.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.pacs.2022.100347.
Contributor Information
Da Xing, Email: xingda@scnu.edu.cn.
Huan Qin, Email: qinghuan@scnu.edu.cn.
Appendix A. Supplementary material
Supplementary material
.
References
- 1.Liu H.-W., Chen L., Xu C., Li Z., Zhang H., Zhang X.-B., Tan W. Recent progresses in small-molecule enzymatic fluorescent probes for cancer imaging. Chem. Soc. Rev. 2018;47:7140–7180. doi: 10.1039/c7cs00862g. [DOI] [PubMed] [Google Scholar]
- 2.Bhang H.C., Gabrielson K.L., Laterra J., Fisher P.B., Pomper M.G. Tumor-specific imaging through progression elevated Gene-3 promoter-driven gene expression. Nat. Med. 2010;17:123–129. doi: 10.1038/nm.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biscaglia F., Ripani G., Rajendran S., Benna C., Mocellin S., Bocchinfuso G., Meneghetti M., Palleschi A., Gobbo M. Gold nanoparticle aggregates functionalized with cyclic RGD peptides for targeting and imaging of colorectal cancer cells. ACS Appl. Nano Mater. 2019;2:6436–6444. [Google Scholar]
- 4.Liang Z., Wang Q., Liao H., Zhao M., Lee J., Yang C., Li F., Ling D. Artificially engineered antiferromagnetic nanoprobes for ultra-sensitive histopathological level magnetic resonance imaging. Nat. Commun. 2021;12:3840. doi: 10.1038/s41467-021-24055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao F., Zhou Y., Liu X., Yu C.H. Podosome formation promotes plasma membrane invagination and integrin-β3 endocytosis on a viscous RGD-membrane. Commun. Biol. 2020;3:117. doi: 10.1038/s42003-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marco P., Lappano R., De Francesco E.M., Cirillo F., Pupo M., Avino S., Vivacqua A., Abonante S., Picard D., Maggiolini M. GPER signalling in both cancer-associated fibroblasts and breast cancer cells mediates a feedforward IL1β/IL1R1 response. Sci. Rep. 2016;6:24354. doi: 10.1038/srep24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prossnitz E.R., Barton M. Estrogen biology: new insights into GPER function and clinical opportunities. Mol. Cell. Endocrinol. 2014;389:71–83. doi: 10.1016/j.mce.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swietach P., Vaughan-Jones R.D., Harris A.L., Hulikova A. The chemistry, physiology and pathology of pH in cancer. Philos. Trans. R. Soc. B. 2014;369 doi: 10.1098/rstb.2013.0099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulikova A., Harris A.L., Vaughan-Jones R.D., Swietach P. Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J. Cell. Physiol. 2013;228:743–752. doi: 10.1002/jcp.24221. [DOI] [PubMed] [Google Scholar]
- 10.Yang L., Chueng S.D., Li Y., Patel M., Rathnam C., Dey G., Wang L., Cai L., Lee K.B. A biodegradable hybrid inorganic nanoscaffold for advanced stem cell therapy. Nat. Commun. 2018;9:3147. doi: 10.1038/s41467-018-05599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He D., Hai L., He X., Yang X., Li H.-W. Glutathione-activatable and O2/Mn2+-evolving nanocomposite for highly efficient and selective photodynamic and gene-silencing dual therapy. Adv. Funct. Mater. 2017;27 [Google Scholar]
- 12.Ding B., Shao S., Jiang F., Dang P., Sun C., Huang S., Ma P. a, Jin D., Kheraif A.A.A., Lin J. MnO2-disguised upconversion hybrid nanocomposite: an ideal architecture for tumor microenvironment-triggered UCL/MR bioimaging and enhanced chemodynamic therapy. Chem. Mater. 2019;31:2651–2660. [Google Scholar]
- 13.Wan S.-S., Cheng Q., Zeng X., Zhang X.-Z. A Mn(III)-sealed metal-organic framework nanosystem for redox-unlocked tumor theranostics. ACS Nano. 2019;13:6561–6571. doi: 10.1021/acsnano.9b00300. [DOI] [PubMed] [Google Scholar]
- 14.Liu C., Wang D., Zhang S., Cheng Y., Yang F., Xing Y., Xu T., Dong H., Zhang X. Biodegradable biomimic copper/manganese silicate nanospheres for chemodynamic/photodynamic synergistic therapy with simultaneous glutathione depletion and hypoxia relief. ACS Nano. 2019;13:4267–4277. doi: 10.1021/acsnano.8b09387. [DOI] [PubMed] [Google Scholar]
- 15.Ximendes E., Marin R., Shen Y., Ruiz D., Gómez-Cerezo D., Rodríguez-Sevilla P., Lifante J., Viveros-Méndez P.X., Gámez F., García-Soriano D., et al. Infrared-emitting multimodal nanostructures for controlled in vivo magnetic hyperthermia. Adv. Mater. 2021 doi: 10.1002/adma.202100077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding W., Ji Z., Ye F., Lou C., Xing D. Near-field microwave distribution measurement with a point detector base on thermoacoustic effect. IEEE Trans. Microw. Theory Tech. 2015;63:3272–3276. [Google Scholar]
- 17.Luo W., Ji Z., Yang S., Xing D. Microwave-pumped electric-dipole resonance absorption for noninvasive functional imaging. Phys. Rev. Appl. 2018;10:02004. [Google Scholar]
- 18.Fang W., Shi Y., Xing D. Vacancy-defect-dipole amplifies the thermoacoustic conversion efficiency of carbonnanoprobes. Nano Res. 2020;13:2413–2419. [Google Scholar]
- 19.Wu D., Huang L., Jiang M.S., Jiang H. Contrast agents for photoacoustic and thermoacoustic imaging: a review. Int. J. Mol. Sci. 2014;15:23616–23639. doi: 10.3390/ijms151223616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogunlade O., Beard P. Exogenous contrast agents for thermoacoustic imaging: an investigation into the underlying sources of contrast. Med. Phys. 2015;42:170–180. doi: 10.1118/1.4903277. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y.-S., Zhao Y., Beinat C., Zlitni A., Hsu E.-C., Chen D.-H., Achterberg F., Wang H., Stoyanova T., Dionne J., Gambhir S.S. Ultra-high-frequency radio-frequency acoustic molecular imaging with saline nanodroplets in living subjects. Nat. Nanotechnol. 2021;16:717–724. doi: 10.1038/s41565-021-00869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C., Qin B., Qin H., Xing D. Increasing dielectric loss of a graphene oxide nanoparticle to enhance the microwave thermoacoustic imaging contrast of breast tumor. Nanoscale. 2019;11:22222–22229. doi: 10.1039/c9nr06549k. [DOI] [PubMed] [Google Scholar]
- 23.Zhai S., Hu X., Ji Z., Qin H., Wang Z., Hu Y., Xing D. Pulsed microwave-pumped drug-free thermoacoustic therapy by highly biocompatible and safe metabolic polyarginine probes. Nano Lett. 2019;19:1728–1735. doi: 10.1021/acs.nanolett.8b04723. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L., Qin H., Zeng F., Wu Z., Wu L., Zhao S., Qin H., Xing D. A stimulated liquid-gas phase transition nanoprobe dedicated to enhance the microwave thermoacoustic imaging contrast of breast tumors. Nanoscale. 2020;12:16034–16040. doi: 10.1039/d0nr04441e. [DOI] [PubMed] [Google Scholar]
- 25.Wen L., Yang S., Zhong J., Zhou Q., Xing D. Thermoacoustic imaging and therapy guidance based on ultra-short pulsed microwave pumped thermoelastic effect induced with superparamagnetic iron oxide nanoparticles. Theranostics. 2017;7:1976–1989. doi: 10.7150/thno.17846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Z., Zeng F., Zhang L., Zhao S., Wu L., Qin H., Xing D. Defect-rich titanium nitride nanoparticle with high microwave-acoustic conversion efficiency for thermoacoustic imaging-guided deep tumor therapy. Nano Res. 2021;14:2717–2727. [Google Scholar]
- 27.Ding W., Lou C., Qiu J., Zhao Z., Zhou Q., Liang M., Ji Z., Yang S., Xing D. Targeted Fe-filled carbon nanotube as a multifunctional contrast agent for thermoacoustic and magnetic resonance imaging of tumor in living mice. nanomed: nanotechnol. Biol. Med. 2016;12:235–244. doi: 10.1016/j.nano.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 28.Lee J., Kumari N., Kim S.M., Kim S., Jeon K.-W., Im G.H., Jang M.-S., Lee W.J., Lee J.H., Lee I.S. Anchoring ligand-effect on bright contrast-enhancing property of hollow Mn3O4 nanoparticle in T1-weighted magnetic resonance imaging. Chem. Mater. 2018;30:4056–4064. [Google Scholar]
- 29.Ding B., Zheng P., Ma P., Lin J. Manganese oxide nanomaterials: synthesis, properties, and theranostic applications. Adv. Mater. 2020;32 doi: 10.1002/adma.201905823. e1905823. [DOI] [PubMed] [Google Scholar]
- 30.Briley-Saebo K.C., Nguyen T.H., Saeboe A.M., Cho Y.S., Ryu S.K., Volkova E.R., Dickson S., Leibundgut G., Wiesner P., Green S., et al. In vivo detection of oxidation-specific epitopes in atherosclerotic lesions using biocompatible manganese molecular magnetic imaging probes. J. Am. Coll. Cardiol. 2012;59:1043. doi: 10.1016/j.jacc.2011.10.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adhikari A., Mondal S., Das M., Biswas P., Pal U., Darbar S., Bhattacharya S.S., Pal D., Saha-Dasgupta T., Das A.K., et al. Incorporation of a biocompatible nanozyme in cellular antioxidant enzyme cascade reverses huntington’s like disorder in preclinical model. Adv. Healthc. Mater. 2020;10 doi: 10.1002/adhm.202001736. [DOI] [PubMed] [Google Scholar]
- 32.Cheng Y., Cheng C., Yao J., Yu Y., Liu Y., Zhang H., Miao L., Wei H. Mn3O4 Nanozyme for inflammatory bowel disease therapy. Adv. Ther. 2021;4(9) [Google Scholar]
- 33.Shi H., Liu T., Fu C., Li L., Tan L., Wang J., Ren X., Wang J.J., Meng X. Insights into a microwave susceptible agent for minimally invasive microwave tumor thermal therapy. Biomaterials. 2015;44:91–102. doi: 10.1016/j.biomaterials.2014.12.035. [DOI] [PubMed] [Google Scholar]
- 34.Abbas S.M., Chandra M., Verma A., Chatterjee R., Goel T.C. Complex permittivity and microwave absorption properties of a composite dielectric absorber. compos. Part A: Appl. Sci. Manuf. 2006;37:2148–2154. [Google Scholar]
- 35.Michielssen E., Sajer J.-M., Ranjithan S., Mittra R. Design of lightweight, broad-band microwave absorbers using genetic algorithms. IEEE Trans. Microw. Theory Tech. 1993;41:1024–1031. [Google Scholar]
- 36.Yu X., Yi B., Liu F., Wang X. Prediction of the dielectric dissipation factor tan δ of polymers with an ANN model based on the DFT calculation. React. Funct. Polym. 2008;68:1557–1562. [Google Scholar]
- 37.Nie L., Ou Z., Yang S., Xing D. Thermoacoustic molecular tomography with magnetic nanoparticle contrast agents for targeted tumor detection. Med. Phys. 2010;37:4193–4200. doi: 10.1118/1.3466696. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.M., Im G.H., Lee D.G., Lee J.H., Lee W.J., Lee I.S. Mn2+-doped silica nanoparticles for hepatocyte-targeted detection of liver cancer in T1-weighted MRI. Biomaterials. 2013;34:8941–8948. doi: 10.1016/j.biomaterials.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 39.Bock N.A., Kocharyan A., Silva A.C. Manganese-enhanced MRI visualizes V1 in the non-human primate visual cortex. NMR Biomed. 2009;22:730–736. doi: 10.1002/nbm.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang G., Xu L., Chao Y., Xu J., Sun X., Wu Y., Peng R., Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li M., Tang Z., Lv S., Song W., Hong H., Jing X., Zhang Y., Chen X. Cisplatin crosslinked pH-sensitive nanoparticles for efficient delivery of doxorubicin. Biomaterials. 2014;35:3851–3864. doi: 10.1016/j.biomaterials.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 42.Yang X., Niu Y., Zhao N., Mao C., Xu F., Biocleavable A. Pullulan-based vector via ATRP for liver cell-targeting gene delivery. Biomaterials. 2014;35:3873–3884. doi: 10.1016/j.biomaterials.2014.01.036. [DOI] [PubMed] [Google Scholar]
- 43.Mou F., Chen C., Ma H., Yin Y., Wu Q., Guan J. Self-propelled micromotors driven by the magnesium-water reaction and their hemolytic properties. Angew. Chem. Int. Ed. 2013;52:7208–7212. doi: 10.1002/anie.201300913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material