Abstract
Objective
To summarize the currently available phase I and II clinical trials of the effects of nonoxynol-9 (N-9) on human sperm structure and functions.
Methods
A systematic review and meta-analysis aiming to evaluate the spermicidal activity of N-9 on motility, was conducted in PubMed, EMBASE, and Cochrane databases by 10 March 2021. The counted numbers of progressive motile (PR) sperm in cervical mucus and the vanguard sperm penetration distances were analyzed. Other effects on sperm structures and physiological activities were reviewed as well.
Results
In the pooled results, percentages or counted numbers of PR sperm decreased after the treatment of N-9. Vanguard sperm penetration distance was shortened in treated groups. N-9 has been confirmed to damage the structures of sperm, as well as other organelles like acrosome and mitochondria. The physiological activities such as generation of reactive oxygen species, superoxide dismutase activity, acrosin activity, and hemizona binding were all inhibited in the reviewed studies.
Conclusions
N-9 has several impacts on sperm owing to its potency in reducing sperm motility and cervical mucus penetration, as well as other functional competencies.
Lay summary
Nonoxynol-9 (N-9) has been used worldwide as a spermicide to kill sperm for more than 60 years but can cause side effects including vaginal irritation and can increase the rate of contraceptive failure. A detailed analysis of published literature aiming to evaluate the spermicidal activity of N-9 on sperm was carried out. In the pooled results, N-9 reduced the number of active sperm and the distance they traveled. It also caused damage to the structures of sperm and to the way the sperm acted and interacted with the egg. In conclusion, N-9 impacts on sperm in a number of ways that lead to sperm death and dysfunction.
Key Words: Nonoxynol-9, spermicide, progressive motility, vanguard sperm penetration distance, sperm function
Introduction
Spermicide is a chemical barrier contraceptive used before intercourse for birth control, which has been used for thousands of years (Duffy & Archer 2018). The basis of spermicidal action is to immobilize or disrupt the plasma membrane of the sperm, so as to avoid sperm–egg interaction (Ingram et al. 2006). Nonoxynol-9 (N-9) is widely used as an active ingredient in various spermicides. Spermicides containing N-9 do not require a prescription but may be available online or in the drugstore from low to high prices without age restrictions. It may be available from some health centers as well (Iyer & Poddar 2008).
N-9 is a membrane disruptive agent that accounts for its contraceptive property (Rosenberg et al. 2008). It lyses the sperm membrane through the interaction with the membrane lipid, leading to sperm immobilization (Burke et al. 2010). N-9 is a product that is relied upon by 0.1% of contracepting women globally either as a primary method or in conjunction with barriers such as the diaphragms and cervical caps that help position it more effectively (Farley 2002, United Nations, Department of Economic and Social Affairs, Population Division 2015). The dosage forms can be in the foam, gel, films, and suppositories (Iyer & Poddar 2008) (Table 1). N-9 spermicides in the form of gel or foam can act immediately, while the dosage form of film or suppository should be inserted at least 15 min for dissolving. However, when using these products, new application should be applied for another intercourse, and some products also require an extra 6 h before douching although douching is never recommended. A bioadhesive delivery system has also been applied in some products to release the N-9 contents. After attaching to the vaginal epithelial surface, the gel starts releasing N-9 slowly at a stable pace, and the protection has been claimed to last for as long as 24 h (Sangi-Haghpeykar et al. 1996).
Table 1.
Spermicide products containing N-9. Various deliver-9 products with different delivery systems and dosage options of N-9 are shown in the table, together with the directions for use.
| Product | Form | Content | Required insert time | Instruction | Duration | Availability |
|---|---|---|---|---|---|---|
| VCF® | Film | 28% | 15 min | In contact with the cervix | Last for 3 h | Available |
| VCF® | Foam | 12.5% | Less than 1 h; works immediately | Insert into vagina; wait at least 6 h for douching | – | Available |
| VCF® | Gel | 4% | Works immediately | Insert into vagina | Up to 1 h | Available |
| Gynol II® | Gel | 3% | Works immediately | Insert into vagina | Up to 1 h | Available |
| Conceptrol® | Gel | 4% | Works immediately | Insert into vagina | Up to 1 h | Available |
| ContraSeed® | Suppository | 100 mg | 15 min | Insert and lie against the cervix | Up to 1 h | Available |
| Today® | Sponge | 1000 mg | Works immediately | Wet sponge thoroughly and squeeze gently until sudsing; fold with dimple side inside; insert deeply into vagina with string loop on bottom end; wait 6 h before removing and avoid inserting for >30 h | 24 h | Available |
| Encare® | Suppository | 100 mg | At least 10 min | Insert into vagina; wait at least 6 h for douching | Up to 1 h | Not available |
| Advantage 24® | Gel | 3.5% | Less than 30 min | Insert into vagina; release steadily in the bioadhesive delivery system attaching to the vaginal epithelial surface | 24 h | Not available |
| Shur-Seal® | Gel | 2% | At least 10 min; works immediately | Insert into vagina; wait at least 6 h for douching | – | Not available |
| Delfen® | Foam | 12.5% | Up to 1 h | Insert into vagina; wait at least 6 h for douching | – | Not available |
N-9 containing spermicide were convincingly found to be lethal to chlamydia and gonococcal organisms in vitro (Singh et al. 1972, Kelly et al. 1985, Patton et al. 1992). However, in vivo, studies have demonstrated no such protection (Roddy et al. 1998, 2002). Several clinical studies have raised concerns that N-9 containing spermicides might increase human immunodeficiency virus transmission and acquisition (Kreiss et al. 1992, Van Damme et al. 2002, Musekiwa et al. 2020). At the same time, the failure rate of it is higher when used as a contraceptive alone compared with many other contraceptive methods, with a Pearl index of over 20 per hundred women-year (Burke et al. 2010). These have prompted World Health Organization (WHO) to limit candidates for use of N-9 spermicides (World Health Organization 2010b ).
While other products have been tested and one recently has been approved by the US Food and Drug Administration (FDA), N-9 is the most commonly used agent (Eisenberg 2020, Thomas et al. 2020). Its products constitute large proportions of the spermicides easily accessible in the market at affordable prices (Grimes et al. 2013). It can still be one of the options for women with a low risk of sexually transmitted diseases to prevent pregnancy. Phase I and II clinical trials studying the effects of N-9 focused on the sperm structure and functions. Sperm motility determines the ability of sperm to move toward the egg through the female reproductive tract. It is considered as the most important parameter to predict conception rate (Pasqualotto et al. 1999). Vanguard sperm penetration distance is also thought to have potential value in predicting IVF outcomes (De Geyter et al. 1988). Other sperm functions such as acrosome reaction and generation of reactive oxygen species (ROS), have also contributed to the pregnancy prediction (Ko et al. 2014, Tello-Mora et al. 2018). A holistic and up-to-date review is required to summarize the currently available phase I and II clinical trials to provide the knowledge foundation. We also want to find the inconstancies in the previous studies on N-9 and try to resolve the conflicts. Additionally, with the help of this review, we hope to provide new insights for further research on N-9. Thus, we conducted the systematic review and meta-analysis of published papers on functional mechanisms of N-9 on sperm structure and functions to update current understanding.
Materials and methods
This study was conducted according to the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Cochrane Handbook for Systematic Reviews of Interventions (Version 6) (Moher et al. 2009, Higgins et al. 2019). This meta-analysis was registered at the International Prospective Register of Systematic Reviews (no. CRD42021227646).
Search strategy
In order to conduct a comprehensive literature review, PubMed, EMBASE, and Cochrane Library databases were searched for eligible studies. All the relevant studies published before 10 March 2021 were included. The search strategies in the three databases were similar. We only used ‘Nonoxynol’ as the keyword or the medical subject headings (MeSH). The common synonyms of ‘Nonoxynol-9’ include ‘PEG-9 nonyl phenyl ether’ and ‘Tergitol NP-9’ (Wishart et al. 2018).
In PubMed, we used ‘Nonoxynol [MeSH Terms] OR PEG 9 nonyl phenyl ether OR PEG-9 nonyl phenyl ether OR Tergitol NP-9 OR Tergitol NP9’ as the search strategy. In EMBASE, we used ‘nonoxynol.mp. OR nonoxinol/ OR Tergitol NP9.mp. OR Tergitol NP-9.mp.’ as the search strategy. In Cochrane Library, ‘Nonoxynol’ was also used as the MeSH descriptors to explode all tress together with ‘PEG 9 nonyl phenyl ether’, ‘PEG-9 nonyl phenyl ether’, ‘Tergitol NP-9’, and ‘Tergitol NP9’. Additionally, the reference lists of the included studies were screened for more eligible studies.
Eligibility criteria
We set inclusion criteria for considering studies in the systematic review and meta-analysis as listed: (i) Only mechanistic studies and no clinical efficacy trial data were included; (ii) In vitro studies were included where the sperm samples collected precoitally were all from healthy males aged over 18. Since the studies were conducted according to different versions of WHO guidelines, eligible studies only included participants with normal concentration and motility fulfilling the latest version. Eligible participants of in vivo studies were healthy, non-pregnant women (aged from 18 to 45 years) without known sensitivity to N-9 or the components contained in the products and had no risk of pregnancy. Before postcoital tests (PCTs), female participants were evaluated to be in the preovulatory period or had adequate cervical mucus. Sperm presence in the vaginal pool was considered as the false-negative control and presence in the cervical mucus was required; (iii) Spermicides containing N-9 were considered as the intervention, regardless of the forms and doses of N-9. The formulations could be in the forms of film, gel, foam, and suppository. Water, saline, phosphate buffer, DMSO, or Tyrode’s solution could be considered as the control group of in vitro studies. Personal baselines without any intervention were used as the control of the in vivo studies. Studies using N-9 together with other physical barriers were included to evaluate the synergistic effects as well; (iv) Sperm motility is one of the most important indicators to evaluate sperm quality, while only progressively motile (PR) sperm relates to the natural pregnancy. Sperm counts per high power field (HPF) or percentage of PR sperm in cervical mucus were considered as the primary outcome and vanguard sperm penetration distance was considered as the second outcome; (v) Other sperm function parameters such as acrosome reaction, hemizona binding, DNA integrity, and superoxide dismutase (SOD) activity were also included to evaluate the comprehensive functions; (vi) The longest time points were chosen if the studies were conducted at different time points.
We also set the exclusion criteria. Phase I and II clinical trials without control groups or using N-9 products as the control to confirm the efficacy of other chemicals were not included.
Study selection and data extraction
All studies in the databases were reviewed by two investigators (Xu and Zhao) independently in accordance with the titles, abstracts, and full texts to evaluate the eligibility. All the articles satisfying the inclusion criteria were selected to conduct the systematic review and meta-analysis. Disagreements emerging in the process of the screening were resolved by all authors.
Available data were extracted from the screened articles meeting the selection criteria to conduct the meta-analysis. The information extracted from the studies included authors, publication time, study mode, quality of semen, sample size, the concentration of N-9, type of control, treatment duration, and reported sperm parameters. The primary outcome was the sperm counts/HPF or percentage of PR sperms in cervical mucus. The secondary outcome was the vanguard sperm penetration distance.
Quality assessment
The biases of the articles were evaluated by the same two investigators (Xu and Zhao) independently using the ‘Risk Of Bias In Non-randomized Studies – of Interventions’ tool (ROBINS-I) (Sterne et al. 2016). The indicators included bias due to confounders, selection bias, bias in classification of intervention, bias due to deviations from intended interventions, bias due to missing data, bias in the measurement of outcomes, and bias in the selection of reported results. The disagreements in the process of the risk evaluation were also resolved by the third investigator (Chan).
Statistical analysis
In order to analyze the effects of N-9 on sperm function, the meta-analysis was performed using the Review Manager 5.4 (Cochrane Collaboration, Oxford, UK) software with available data extracted from the screened articles. Mean differences (MDs) and 95% CIs were used to analyze the continuous data of the primary and secondary outcomes. These were also applied in subgroup analysis.
The heterogeneity among the studies was tested with Cochran's Q test (Higgins et al. 2003). The confidence level was set at P = 0.1. I2 statistic was also performed to express and quantify the inconsistency of the results of studies. If the calculated I2 result met I2<50, showing that there was no statistically significant heterogeneity among the involved studies, the fixed model was used. Otherwise, the random-effects model was in use. A P -value of <0.05 was considered statistically significant. In some of the included studies, the s.d. was set as 0.000000001 since there were no variants among the groups (Mauck et al. 1997a, b, c, Schwartz et al. 2008, Mauck et al. 2017). The given s.e.m.was converted to s.d. by multiplying the square root of the sample size (Dunmire & Katz 1997a, b).
Additionally, Begg’s funnel plot was used to evaluate the publication bias of the studies. The symmetric shape indicated the precision of data and a small likelihood of publication bias.
Results
Meta-analysis of the effects of N-9 on sperm motility
Study selection
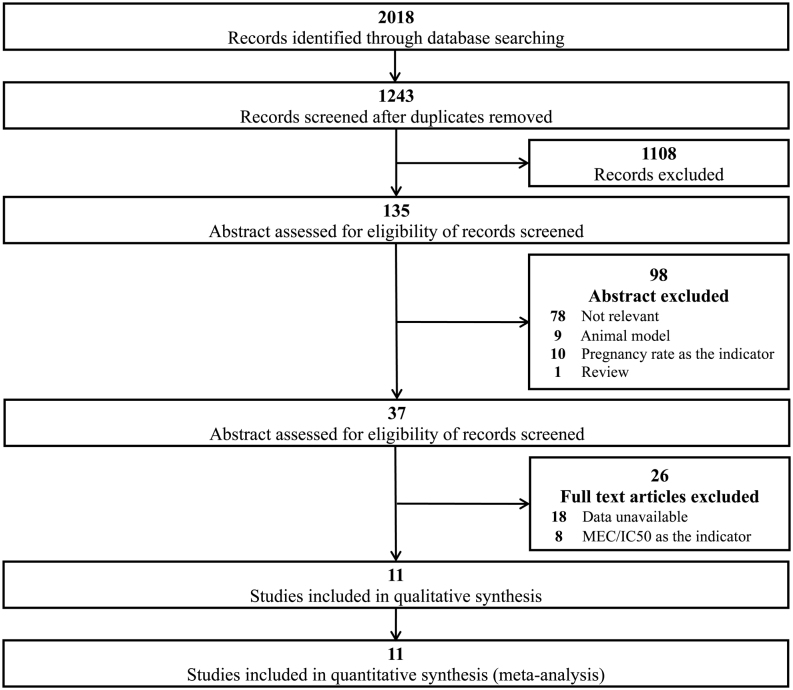
There were 2018 relevant studies obtained in the three databases, including 886 in PubMed, 1031 in EMBASE, and 101 in the Cochrane database. After removing the duplications, 1243 studies were screened based on titles and abstracts, and 1108 studies were excluded. The remaining 135 studies were assessed further for eligibility based on the abstracts first. Among the 135 articles, 98 of them were removed due to different reasons (78 irrelevant articles, 9 animal studies, 10 using pregnancy rate as the indicator, and 1 review article). The full-text assessments excluded 8 which used MEC or LD50 as the indicators and 18 of them without available data. At last, we included 11 eligible articles in the systematic review and meta-analysis. The flowchart of the study selection was shown in Fig. 1.
Figure 1.
Flowchart of study selection for the systematic review.
Descriptions of studies
The 11 eligible papers included 7 in vitro tests with 96 participants and 11 PCTs with 229 participants. Seven experiments in four papers measured the vanguard sperm penetration distances after treatment with N-9. The remaining studies measured the PR sperm count/HPF in cervical mucus. The characteristics and detailed information about the reported sperm parameters in included studies were extracted and shown in Table 2.
Table 2.
Characteristics of the included studies. We included 11 eligible articles and summarized the characteristics of the mode, semen specimen information, sample size, intervention concentration, control, and duration. Reported sperm parameters included progressive motility in cervical mucus and vanguard sperm penetration distance. Values are mean ± s.d. or as specified.
| Reference | Mode | Sperm characteristics | Sample size | Duration (h) | Control | Intervention concentration | Reported sperm parameters (mm) | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Concentration | Motility | Treated | Control | Treated | Control | Treated | Control | ||||
| Vanguard sperm penetration distances | |||||||||||
| Sharman et al. (1986) | In vitro | >40 × 106/mL | >60% | 3 | 3 | 2 | Tyrode’s solution | % | % | ||
| 0.033 mg/mL | 28.2 ± 4.1 | 27.3 ± 4.1 | |||||||||
| 0.33 mg/mL | 13.4 ± 1.77 | 27.3 ± 4.1 | |||||||||
| 3.3 mg/mL | 7.86 ± 2.3 | 27.3 ± 4.1 | |||||||||
| Dunmire & Katz (1997b) | In vitro | >40 × 106/mL | >55% | 4 | 4 | >2 | 3 | Water | 100 mg/mL | 41.5 ± 1.0 | 44.2 ± 0.3 |
| Dunmire & Katz (1997a) | In vitro | >40 × 106/mL | >55% | 10 | 10 | >2 | 3 | 100 mg/mL | % | % | |
| Water | 40.4 ± 6 | 45.7 ± 4.1 | |||||||||
| Saline | 44.0 ± 2.8 | 49.3 ± 5.1 | |||||||||
| Mahony (2001) | In vitro | >100 × 106/mL | >50% | 5 | 5 | 4 | Saline | 5 mg/mL | 2 ± 0.4 | 39 ± 6.7 | |
| PR sperm count/HPF | |||||||||||
| Mauck et al. (1997a) | PCT | – | 10 | 10 | 2.5 ± 0.4 | 2.5 ± 0.6 | PB | VCF® (70 mg N-9) | 0±0 | 22.2 ± 20.2 | |
| Mauck et al. (1997c) | PCT | – | 10 | 10 | PB | % | % | ||||
| 2.8 ± 0.5 | 2.5 ± 0.5 | VCF® (70 mg N-9) | 0.5 ± 0.8 | 23.7 ± 26.7 | |||||||
| 2.1 ± 0.5 | 2.5 ± 0.5 | 100 mg N-9 | 0.6 ± 0.9 | 23.7 ± 26.7 | |||||||
| 2.5 ± 0.5 | 2.5 ± 0.5 | 130 mg N-9 | 0.9 ± 2.3 | 23.7 ± 26.7 | |||||||
| Mauck et al. (1997b) | PCT | – | 7 | 7 | PB | % | % | ||||
| 2.3 ± 0.4 | 2.5 ± 0.4 | Femcap® with N-9 | 0.2 ± 0.4 | 18.0 ± 20.5 | |||||||
| 2.6 ± 0.3 | 2.5 ± 0.4 | Diagram with N-9 | 0 ± 0 | 18.0 ± 20.5 | |||||||
| Ayotte & Colin (2002) | PCT | >20 × 106/mL | 5 | 9 | <3 h | PB | Protectaid® sponge (0.125%N-9) | 0.6 ± 1.1 | 17.8 ± 7.2 | ||
| Amaral et al. (2004) | PCT | – | 20 | 20 | <2 h | PB | 2% N-9 | 0.07 ± 0.23 | 17.94 ± 19.91 | ||
| Schwartz et al. (2008) | PCT | 2–3 h | PB | % | % | ||||||
| – | 13 | 14 | SILCS (metal)+N-9 | 0 ± 0 | 12.5 ± 8.8 | ||||||
| – | 8 | 14 | SILCS (polymer spring)+N-9 | 0 ± 0 | 12.5 ± 8.8 | ||||||
| Mauck et al. (2017) | PCT | – | 9 | 9 | 2–3 h | PB | Caya®+3% N-9 | 0.6 ± 1.1 | 22.5 ± 33.4 | ||
PB, personal baseline; PR, progressively motile; PCT, postcoital tests.
We measured seven types of potential biases in each paper. The judgment was explained by ‘low’, ‘moderate’, and ‘high’ risk. The risk of biases due to confounders were low in all studies. As to the bias in the selection of participants into the studies, all studies fulfilled strict criteria to select participants. Risks of biases due to the classification of intervention and selection of the reported results were both low in all studies. One study indicated unwanted outcomes in some participants, which might contribute to the bias due to deviation from intended interventions (Ayotte & Colin 2002). Biases due to missing data might be high in one study where some participants did not complete the tests (Schwartz et al. 2008). The risk of bias in the measurement of outcomes was low in all studies. Thus, the overall biases were low in all studies. The quality of the included studies was evaluated with the ROBINS-I tool in Table 3.
Table 3.
Risk of bias assessment using ROBINS-I. The quality of the included papers was evaluated by the ROBINS-I tool using the judgment of ‘low’, ‘moderate’, and ‘high’ risk. The overall biases of all the papers included were considered at low risks.
| Sharman et al. (1986) | Dunmire & Katz (1997b) | Dunmire & Katz (1997a) | Mahony (2001) | Mauck et al. (1997a) | Mauck et al. (1997c) | Mauck et al. (1997b) | Ayotte & Colin (2002) | Amaral et al. (2004) | Schwartz et al. (2008) | Mauck et al. (2017) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bias due to confounding | L | L | L | L | L | L | L | L | L | L | L |
| Bias in selection of participants into the study | L | L | L | L | L | L | L | L | L | L | L |
| Bias in classification of interventions | L | L | L | L | L | L | L | L | L | L | L |
| Bias due to deviations from intended interventions | L | L | L | L | L | L | L | H | L | L | L |
| Bias due to missing data | L | L | L | L | L | L | L | L | L | H | L |
| Bias in measurement of outcomes | L | L | L | L | L | L | L | L | L | L | L |
| Bias in selection of the reported result | L | L | L | L | L | L | L | L | L | L | L |
| Overall | L | L | L | L | L | L | L | L | L | L | L |
L, low risk; M, moderate risk; H, high risk.
Primary outcome – progressive motility in cervical mucus
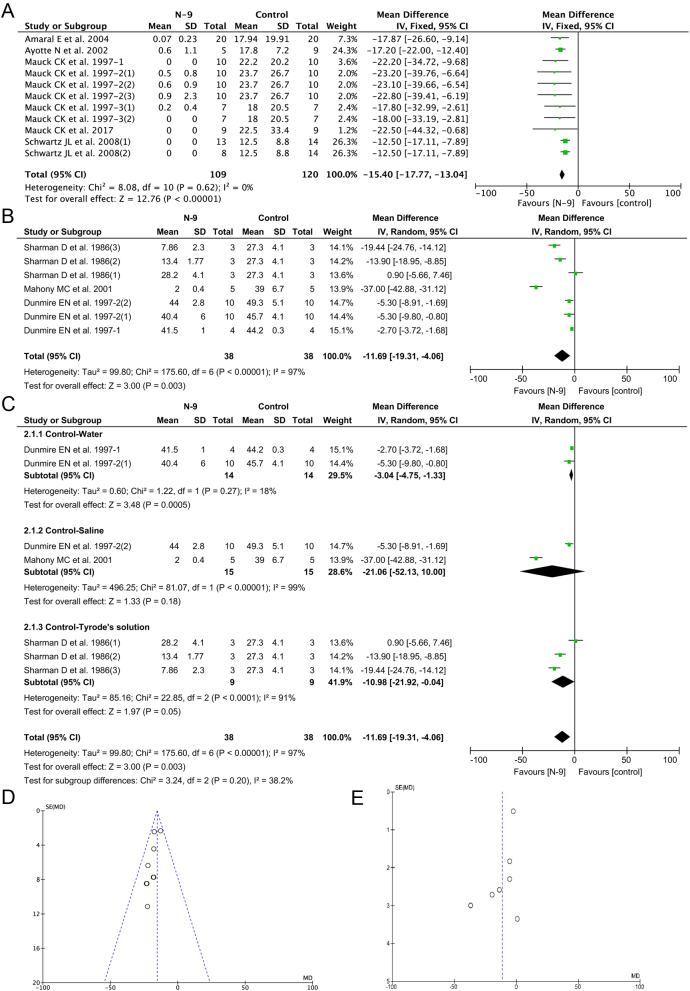
Sperm motility determines the ability of sperm to move toward the egg through the female reproductive tract. It is considered the most important parameter to predict conception rate (Pasqualotto et al. 1999). Sperm is classified as PR, non-progressively motile (NP), and immotile (World Health Organization 2010a). Therefore, progressive motility in the cervical mucus was chosen as the primary outcome. Eleven experiments in eight studies evaluated the PR sperm count/HPF or percentage in cervical mucus (Fig. 2A). The overall heterogeneity of these studies was large (I2= 0%), so the fixed effects model was applied. The pooled MD and 95% CI were −15.40 and −17.77 to −13.04 indicating a significant decrease in PR sperm after treatment with N-9. With N-9 intervention, the mean of sperm counts in the treated group was at least 15 lower than the mean of sperm counts in the control group. The results of the meta-analysis provided evidence that N-9 was effective in decreasing sperm motility in the cervical mucus.
Figure 2.
Meta-analysis forest plots and funnel plots.
Secondary outcome – vanguard sperm penetration distance
The in vitro sperm–mucus penetration test evaluates the sperm quality by measuring the distance that sperm swim up in the cervical mucus (De Geyter et al. 1988). The vanguard sperm penetration distance is shown to have positive associations with fertility (Murase et al. 1990). The vanguard sperm penetration distance used in our analysis is the distance between the foremost sperm and the mucus interface at the departure point of the treatment in the capillary tube. Seven experiments from four papers evaluated the vanguard sperm penetration distances, including 76 participants (Fig. 2B). In the pooled analysis of the vanguard sperm penetration distances, the mean difference was −11.69 with 95% CI −19.31 to −4.06. Since the heterogeneity was high with I2= 97%, a random-effects model was used. Sperm treated with N-9 had significantly shorter penetration distances compared with those in the control group (P = 0.003).
The stratified meta-analysis of vanguard sperm penetration distance was based on the different controls (Fig. 2C). Among the experiments, two used water as control, and another two used saline as control. The remaining three experiments used Tyrode’s solution as control. The pooled MDs (95% CIs) of the three different controls were −3.04 (−4.75 to −1.33), −21.06 (−52.13 to 10.00), and −10.98 (−21.92 to −0.04) respectively. There were significant differences in the experiments using water and Tyrode’s solution as the controls, but no difference in the experiments using saline as the control. Among the experiments with water and Tyrode’s solution as control, the penetration distances in the N-9-treated group were shorter than those in the control groups.
In the stratified meta-analyses, N-9 significantly reduced vanguard sperm penetration distance only when water or Tyrode’s solution, but not saline as the medium. Among the three media used in controls, Tyrode’s solution should be the most suitable as it provides isosmotic loading and the nutrition needed by sperm.
Publication bias
The publication biases were shown in funnel plots (Fig. 2D and E). Deviation from the funnel plots implicated the significant presence of the publication biases. The symmetric funnel-shaped distribution of the progressive motility in cervical mucus demonstrated that the data sets behaved well with less likelihood of publication bias, while the non-symmetric funnel-shaped distribution of vanguard sperm penetration distances indicated more likelihood of publication bias.
Effect of N-9 on other mechanisms of sperm function
A summary of the studies on the mechanisms of N-9 on human sperm function which cannot be analyzed quantifiably is shown in Table 4.
Table 4.
Summary of the functional mechanisms of N-9 on sperm. The table showed the studies focusing on the effects of N-9 on the viability, motility characteristics, acrosome intactness, acrosin activity, sperm membrane, other organelles, and other aspects of human sperm.
| Reference | Sperm | Sample size | N-9 concentration | Descriptions of the effect of N-9 | |
|---|---|---|---|---|---|
| Concentration | Motility | ||||
| Viability | |||||
| Jain et al. (2010) | >65 × 106/mL | >70% | – | 500 μg/mL | Necrosis in nearly 90% of sperm; viable sperm cell populations fell to ~2%. |
| Shah et al. (2005) | – | – | 10 | – | Near 0% viability at 0.08 mg/mL. |
| Ping et al. (2005) | >15 × 106/mL | – | 15 | 0.5, 0.25, 0.125, and 0.0625 mg/mL | 0% viability within 30 min at ≥0.25 mg/mL; 0% viability within 20 s at 0.5mg/mL |
| Jain et al. (2009) | >60 × 106/mL | >75% | – | – | Necrosis induction in a significant number of sperm nonspecifically. |
| Motility characteristics | |||||
| Centola (1998) | – | 6 | 2000, 1000, 100, 20, 10 μg/mL | 0% motility at 2000–20 μg/mL, 6.5 ± 5.74% motility at 10 μg/mL; 0 progressive velocity at all doses except at 10 μg/mL (8.2 ± 5.5), with a significant decrease compared to fresh (46.7 ± 4.03); significant decrease of hyperactivation by N-9 (0–0.3 ± 0.3%). | |
| Dunmire & Katz (1994) | >60 × 106/mL | >50% | 6 | 0, 15, 30, 45, and 60 μg/mL | Significant decreases in motility, LIN, and VSL (P < 0.005); significant increase in MAD (P < 0.001); slight decrease in VCL (P < 0.1). |
| Dunmire & Katz (1997a) | >40 × 106/mL | >55% | 9 | – | Significant decrease in MOT (P < 10−5); significant decrease in ALH (P < 0.01) for N9-saline treatment; significant decreases in LIN, VCL, VSL (P < 0.01), and significant increase in MAD (P < 0.05) for N9-saline andwater treatment. |
| Mahony (2001) | >100 × 106/mL | >50% | 5 | 5 mg/mL | Very significant effect on all sperm motility characteristics, VCL, ALH, and LIN (P< 0.01). |
| Zaïri et al. (2013) | – | 20 | 5–200 μg/mL | 0% motility at ≥100 μg/mL. | |
| Zairi et al. (2008) | – | 50 | 0–500 μg/mL | 0% motility at ≥100 μg/mL. | |
| White et al. (1995) | >20 × 106/mL | >40% | >50 | 5, 50, and 500 μg/mL | No significant effect on human sperm motility at 5 μg/mL; significant (P < 0.01) reduction of motility at 50 μg/mL; complete stop in all sperm movement within 1 min at 500 μg/mL. |
| Srivastava & Coutinho (2010) | – | 8 | – | Significant effect on sperm motility at a dose of 50 µM. | |
| Louis & Pearson (1985) | – | 6 | – | Decreased motility with the increasing concentration. | |
| Lee et al. (1996) | >20 × 106/mL | >50% | – | 0.050, 075, 0.1, 0.125, 0.15, and 0.175 mg/mL | Decreased motility with the increasing concentration. |
| Harrison & Chantler (1998) | >15 × 106/mL | >40% | 5 | 0.2, 0.4, and 0.6 mg/mL | 0% motility at ≥0.4 mg/mL. |
| Shah et al. (2005) | – | 10 | – | Decreased motility with the increasing concentration. | |
| Ping et al. (2005) | >15 × 106/mL | 15 | 0.5, 0.25, 0.125, and 0.0625 mg/mL | 0% motility at ≥0.25 mg/mL within 20 s; 0% motility at ≥0.125 mg/mL in 30 min. | |
| Acrosome intactness and acrosin activity | |||||
| Centola (1998) | – | 6 | 2000, 1000, 100, 20, and 10 μg/mL | Complete break down and release of the acrosomal contents at all doses (16 ± 2.6–26 ± 6.0% RITC+; 73.3 ± 4.8–83.7 ± 2.6% RITC−). | |
| Harrison & Chantler (1998) | >15 × 106/mL | >40% | 44 | 0.2, 0.4, and 0.6 mg/mL | Loss of the acrosomal structure. |
| Xia et al. (2020) | – | 40 mg/mL | No intact acrosome from a weak fluorescence strap that appeared in the equatorial zone. | ||
| Wilborn et al. (1983) | – | 30 | 0.05, 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, and 10.0% | Damage in acrosomal membrane complex, which ranged from vesiculations to complete obliteration; not always affected post-acrosomal membrane. | |
| Schill & Wolff (1981) | – | – | 0.05% | Missed acrosomal membrane complex including equatorial segment. | |
| Müller-Esterl & Schill (1982) | – | – | 0.05–1% | Gelatin film method (acrosin activity): complete prevention gelatin lysis at 0.05–1% N-9; moderate halo formation at 0.001–0.01%; completely missed halo formation at 0.05–1%. | |
| Sperm membrane and other organelles | |||||
| Harrison & Chantler (1998) | >15 × 106/mL | >40% | 44 | 0.2, 0.4, and 0.6 mg/mL | Severely damaged membrane organization; partial dissolution of plasma membrane; partly exposed nucleus. |
| Jain et al. (2010) | >65 × 106/mL | >70% | – | 500 μg/mL | Significant (P< 0.001) depolarization of plasma membrane potential. |
| Xia et al. (2020) | – | – | 40 mg/mL | Rare appearance of spermatozoa with a swollen tail; deconstruction of membrane permeability; leakage of cytoplasm. | |
| Wilborn et al. (1983) | – | 30 | 0.05, 0.1, 0.25, 0.5, 1.0, 2.5, 5.0, and 10.0% | Destruction of the cell membrane of the neck; absence of midpiece membrane; extirpated mitochondria of the midpiece and exposed fibers in approximately 25% of sperm; vesiculations as the first evidence of all membrane damage; loose and detached membranes then; membrane of the tail not always affected. | |
| Thompson et al. (1996) | – | – | 0.01, 0.02, 0.03, and 0.04% | Increase of sperm cell permeabilization. | |
| Shah et al. (2005) | – | 10 | – | Membrane perturbation and disruption. | |
| Schill & Wolff (1981) | – | – | 0.05% | Completely removed plasma membrane from head to the end piece; appearance of swollen, break, discontinuous nuclear membrane; enlarged space between the nucleus and the nuclear membrane; swollen chromatin structures in some sperm and nuclear decondensation in others; missed cytoplasm in the neck region and the middle piece; appearance of monolayer membrane of the mitochondria instead of bilaminar membrane; empty interior of the mitochondria or containing a fine granular material and disappearance of normal cristae. | |
| Lakshmi et al. (2008) | >60 × 106/mL | >75% | – | 0.05% | Physiological damage of sperm membrane. |
| Others | |||||
| Jain et al. (2010) | >65 × 106/mL | >70% | – | 500 μg/mL | Intracellular pH of human sperm: Significant (P< 0.01) intracellular acidification of human sperm at 20 μg/mL;ROS generation: Significant snduction of ROS (P< 0.001) in human sperm;SOD activity: significant inhibition of SOD activity of human sperm (P< 0.01);Sperm dyenin ATPase activity: no significant changes in dyenin ATPase activity which provides motor energy to sperm;Tyrosine phosphorylation: visibly significant inhibition of tyrosine phosphorylation in human sperm;Hemizona assay: a potent inhibition of sperm-zona pellucida binding: the index was 10. |
Linearity-LIN, Straight line velocity-VSL, Mean angular displacement-MAD, Curvilinear velocity-VCL, Motility- MOT, Amplitude of lateral head displacement-ALH, Rhodamine isothio- cyanate conjugated-RITC, Reactive Oxygen Species-ROS, Superoxide dismutase-SOD.
Effects on viability and motility characteristics
Studies showed that N-9 treatment resulted in significantly reduced sperm viability to nearly 0% even at a low concentration (Ping et al. 2005, Shah et al. 2005, Jain et al. 2009, 2010), mainly by inducing necrosis in the majority of sperm (Jain et al. 2010).
As to the effects on motility characteristics, many studies showed the ability of N-9 to interfere with sperm motility even at a low concentration (Louis & Pearson 1985, Dunmire & Katz 1994, 1997a, White et al. 1995, Lee et al. 1996, Centola 1998, Harrison & Chantler 1998, Mahony 2001, Ping et al. 2005, Shah et al. 2005, Zairi et al. 2008, Srivastava & Coutinho 2010, Zaïri et al. 2013). Three of them also evaluated motility parameters by computer-assisted sperm analysis and showed that N-9 treatment significantly reduced straight- line velocity (VSL), curvilinear velocity (VCL), linearity (LIN), and amplitude of lateral head displacement (ALH), and increased mean angular displacement (MAD) (Dunmire & Katz 1994, 1997a, Mahony 2001) (Supplementary data 1, see section on supplementary materials given at the end of this article). VSL, VCL, LIN, and ALH have been reported to positively associate with fertility, while MAD shows a negative correlation (King et al. 2000, Youn et al. 2011). For example, The increase of ALH after semen preparation is beneficial to the pregnancy outcome (Fréour et al. 2010). Additionally, the level of hyperactivation was inhibited significantly as well after treatment with N-9 (Centola 1998).
Effects on sperm acrosome
Five studies provided information on acrosome intactness, together with one study focusing on acrosin activity. Studies evaluating the acrosome structure all gave the conclusions that N-9 damaged the acrosomal membrane complex, resulting in the release of the acrosome enzymes, and the sperm structure was even found to break down completely since 10μg/ml (Schill & Wolff 1981, Wilborn et al. 1983, Centola 1998, Harrison & Chantler 1998, Xia et al. 2020). Although the release of the acrosomal enzymes contributes to penetrating the zona pellucida, it has also been found that the activity of the enzymes was inhibited at the same time. The gelatin film method was used to evaluate the acrosin activity after the N-9 treatment. The complete prevention of gelation lysis after N-9 treatment indicated the influence on acrosin activity, even at the lowest concentration of 0.05% (Müller-Esterl & Schill 1982). Among the studies, the missed acrosomal structure was also found in the equatorial zone, and the damage was presented in the form of vesiculations to complete obliteration (Schill & Wolff 1981, Xia et al. 2020). After an acrosome reaction, the forepart of the sperm is only covered by the inner acrosome membrane. For the further binding with the oolemma, the post-acrosomal membrane plays a critical role (Singh 2014). However, the post-acrosomal membrane was not always affected by the N-9 treatment (Wilborn et al. 1983).
Effects on sperm membrane integrity
Effects of N-9 on sperm membrane were reported. Severe necrosis was observed, and it began with membrane disruption (Shah et al. 2005, Jain et al. 2010). Severe damage of cell membrane organization was observed (Schill & Wolff 1981, Wilborn et al. 1983, Harrison & Chantler 1998, Shah et al. 2005, Lakshmi et al. 2008). Vesiculations would appear first during the damage caused by N-9, and then the membranes would start loosening and detaching (Wilborn et al. 1983). However, the tail membrane was not always influenced by N-9, and spermatozoa with a swollen tail were rarely seen (Xia et al. 2020). At the same time, the membrane permeability was deconstructed, and it would be increased by N-9 treatment, together with the significant depolarization of plasma membrane potential (Thompson et al. 1996, Jain et al. 2010, Xia et al. 2020). After membrane disruption, leakage of cytoplasm and further disruption of the nuclear membrane with enlargement of the nuclear space occurred (Schill & Wolff 1981, Xia et al. 2020). Then, the nucleus was partly exposed and swollen chromatin structures or nuclear decondensation could be seen (Schill & Wolff 1981, Harrison & Chantler 1998). Additionally, two studies reported the effects on mitochondria. N-9 treatment turned the bilaminar membrane of mitochondria into monolayer, and the interior became empty with the production of granular materials and the disappearance of normal cristae (Schill & Wolff 1981). Mitochondria might also be extirpated by N-9 directly (Wilborn et al. 1983).
Other aspects
The effects of N-9 on other aspects such as generation of ROS, the activity of SOD, and sperm dynein ATPase were reviewed in the study by Jain et al. as well. When treated with N-9, a significant inhibition could be seen in the tyrosine phosphorylation, which is associated with sperm capacitation. The significant suppression could also be seen in the activity of SOD, which plays an important role in cell metabolism. As to the ROS generation, the result showed that it was induced significantly, which might lead to oxidate stress which damages cell structure. When treated by N-9 at 500 μg/mL, sperm–zona pellucida binding was inhibited potently. Additionally, significant intracellular acidification of human sperm could be seen at a low concentration of N-9 and acidic pH is associated with decreased motility and capacitation (Zhou et al. 2015). However, the activity of sperm dynein ATPase, which provides energy support to sperm did not exhibit significant changes (Jain et al. 2010).
Discussion
Nonoxynol-9 has been used as a spermicide worldwide for more than 60 years. In order to better understand the mechanisms of N-9 on various aspects of sperm functions, a holistic and up-to-date review was conducted.
In this systematic review and meta-analysis, we included 11 eligible studies to assess the impacts of N-9 on sperm with the changes in numbers of PR sperm in cervical mucus demonstrated by PCT in in vivo studies as the primary outcome and vanguard sperm penetration distances in in vitro studies as the secondary outcome. The primary result showed that, in cervical mucus, the number of PR sperm per HPF decreased significantly after N-9 treatment when compared with the personal baselines. As one meta-analysis indicates that vanguard sperm penetration distance has low accuracy in evaluating sperm motility compared with sperm count/HPF, this was taken as the secondary outcome (Ola et al. 2003). The vanguard sperm penetration distance in in vitro tests was shorter after the N-9 treatment when compared with the control.
The effects of N-9 on other functional mechanisms of the human sperm were also reviewed. Briefly, N-9 disrupted the plasma membrane and resulted in the increase of cell permeabilization and leakage of the cell contents, followed by damage of the nuclear membrane (Schill & Wolff 1981, Thompson et al. 1996, Harrison & Chantler 1998, Xia et al. 2020). The nucleus exposed to N-9 would experience swollen chromatin structures or nuclear decondensation (Schill & Wolff 1981). During this process, N-9 also disrupts the organelles of the sperm-like acrosome in the sperm head and mitochondria in the midpiece (Schill & Wolff 1981, Wilborn et al. 1983, Centola 1998, Harrison & Chantler 1998, Xia et al. 2020). Inhibition of acrosin also impacts penetration through the zona pellucida (Müller-Esterl & Schill 1982). Additionally, some other physiological activities like ROS generation, SOD activity, tyrosine phosphorylation, and hemizona binding were all altered (Jain et al. 2010). With the disruption to the structures and physiological activities, the viability and motility were affected in different facets, contributing additional functional mechanisms on top of the effect on sperm motility.
This meta-analysis has several limitations: (i) The number of the studies and the sample sizes were limited, and some studies only contained several participants; (ii) In our meta-analysis, only the sperm motility and vanguard sperm penetration distances were included. Other parameters such as viability, kinematic characteristics, and physiological activities were only described qualitatively without numerical data; (iii) Different studies made use of N-9 in different forms and in different concentrations. It has been shown that the efficacy of N-9 has associations with the dose but on the other hand, a high dose may contribute to a higher risk of vaginal irritation (Raymond et al. 2004). We did not do stratified analysis based on the concentration of the treatment due to the high degree of variations; (iv) In all in vitro tests, seven experiments in four papers used three different controls, which might increase the heterogeneity among the experiments. Also, the anisosmotic environment when using water as the control might cause variations. 5) The studies included varied from 1986 to 2017 and they used different WHO guidelines. We included studies whose given parameters fulfilled the latest version, while the given parameters were not integrated. It is worth noting that although only one study used the latest edition the rest followed the earlier edition of WHO semen analysis manual so might result in coherent parameters.
Up to date, scientists have put forward many alternatives to replace N-9 in spermicides. For example, tideglusib, a protein from Ricinus communis, desgalactotigonin, and bivittoside D from Bohadschia vitiensis have been considered as potential spermicides in animal models (Nithya et al. 2012, Chakraborty et al. 2014, Chen et al. 2019). Combinations of N-9 and propranolol or polyvinylpyrrolidone both showed a complementary effect to achieve a complete cessation of sperm motility (White et al. 1995, Fowler et al. 2003). In 2020, FDA approved a new contraceptive vaginal pH modifier without N-9, which kills the sperm by changing the vagina acidity. It is also confirmed to have higher efficacy compared with N-9 products, and even lower the risk of gonorrhea and chlamydia infections (Chappell et al. 2021). The latest review on N-9 has been published for more than ten years and none of the reviews emphasized on functional mechanisms of N-9 on human sperm. A comprehensive understanding of the N-9 on sperm structures and functions can benefit the further contraceptive targeting.
Since it has been confirmed to be effective in killing sperm, more research can be done to explore complementary agents like anti-microbiotics or other agents which can change the vagina environment to achieve the goal of preventing pregnancy more effectively and to confer non-contraceptive benefits such as microbicidal effects. In conclusion, N-9 has several effects on sperm owing to its potency in reducing sperm motility and cervical mucus penetration, as well as other functional competencies.
Supplementary Material
Declaration of interest
Raymond Hang Wun Li is an Associate Editor of Reproduction and Fertility. Raymond Hang Wun Li was not involved in the review or editorial process for this paper, on which he is listed as an author. The other authors declare no conflict of interest.
Funding
This work was supported by Right Pearl Limited, research contract TR1914524.
Author contribution statement
M X and M Z reviewed the studies and analyzed the data. M X drafted the manuscript, and R H W L and D Y L C edited it. Z L, J P W C, T C L, and D Y L C supervised the project.
Acknowledgement
The authors thank Mr Daniel Kwan for his generous support in contraception research.
References
- Amaral E, Perdigão A, Souza MH, Mauck C, Waller D, Zaneveld L, Faúndes A.2004Postcoital testing after the use of a bio-adhesive acid buffering gel (ACIDFORM) and a 2% nonoxynol-9 product. Contraception 70492–497. ( 10.1016/j.contraception.2004.06.007) [DOI] [PubMed] [Google Scholar]
- Ayotte N, Colin P.2002Spermicidal activity of a new contraceptive sponge. Advances in Therapy 19219–228. ( 10.1007/BF02850362) [DOI] [PubMed] [Google Scholar]
- Burke AE, Barnhart K, Jensen JT, Creinin MD, Walsh TL, Wan LS, Westhoff C, Thomas M, Archer D, Wu Het al. 2010A randomized trial of the contraceptive efficacy, acceptability, and safety of C31G and nonoxynol-9 spermicidal gels. Obstetrics and Gynecology 116 1265–1273. ( 10.1097/AOG.0b013e3181fc3b1a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centola GM.1998Dose-response effects of gramicidin-D, EDTA, and nonoxynol-9 on sperm motion parameters and acrosome status. Contraception 5835–38. ( 10.1016/s0010-7824(9800057-2) [DOI] [PubMed] [Google Scholar]
- Chakraborty D, Maity A, Jha T, Mondal NB.2014Spermicidal and contraceptive potential of desgalactotigonin: a prospective alternative of nonoxynol-9. PLoS ONE 9 e107164. ( 10.1371/journal.pone.0107164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell BT, Mena LA, Maximos B, Mollan S, Culwell K, Howard B.2021EVO100 prevents chlamydia and gonorrhea in women at high risk of infection. American Journal of Obstetrics and Gynecology 225162.e1–162.e14. ( 10.1016/j.ajog.2021.03.005) [DOI] [PubMed] [Google Scholar]
- Chen Z, Shu N, Wang Y, Yang Y, Shao Z, Tian F, Xia M, Wang Z, Wang X, Feng X.2019Tideglusib, a prospective alternative to nonoxynol-9 contraceptive. Contraception 1 100007. ( 10.1016/j.conx.2019.100007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Geyter C, Bals-Pratsch M, Doeren M, Yeung CH, Grunert JH, Bordt J, Schneider HP, Nieschlag E.1988Human and bovine cervical mucus penetration as a test of sperm function for in-vitro fertilization. Human Reproduction 3948–954. ( 10.1093/oxfordjournals.humrep.a136824) [DOI] [PubMed] [Google Scholar]
- Duffy DM, Archer DF.2018Female contraception. In Encyclopedia of Reproduction, 2nd ed. Ed Skinner MK.Oxford: Academic Press. [Google Scholar]
- Dunmire EN, Katz DF.1994Kinematic response of human spermatozoa to nonoxynol-9. Biology of Reproduction 50903–911. ( 10.1095/biolreprod50.4.903) [DOI] [PubMed] [Google Scholar]
- Dunmire EN, Katz DF.1997aAlteration of human sperm kinematics in cervical mucus due to nonoxynol-9. Contraception 55209–217. ( 10.1016/s0010-7824(9700009-7) [DOI] [PubMed] [Google Scholar]
- Dunmire EN, Katz DF.1997bMeasurement and modulation of nonoxynol-9 diffusion and bioactivity against spermatozoa in human cervical mucus. Contraception 55115–122. ( 10.1016/s0010-7824(9600281-8) [DOI] [PubMed] [Google Scholar]
- Eisenberg DL.2020Expanding Contraceptive Choices for Women: The Vaginal pH Modulator. In Supplement to OBG management. [Google Scholar]
- Fowler PT, Doncel GF, Bummer PM, Digenis GA.2003Coprecipitation of nonoxynol-9 with polyvinylpyrrolidone to decrease vaginal irritation potential while maintaining spermicidal potency. AAPS PharmSciTech 4E30. ( 10.1208/pt040330) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréour T, Jean M, Mirallié S, Dubourdieu S, Barrière P.2010Computer-assisted sperm analysis (CASA) parameters and their evolution during preparation as predictors of pregnancy in intrauterine insemination with frozen-thawed donor semen cycles. European Journal of Obstetrics, Gynecology, and Reproductive Biology 149186–189. ( 10.1016/j.ejogrb.2009.12.029) [DOI] [PubMed] [Google Scholar]
- Grimes DA, Lopez LM, Raymond EG, Halpern V, Nanda K, Schulz KF.2013Spermicide used alone for contraception. Cochrane Database of Systematic Reviews CD005218. ( 10.1002/14651858.CD005218.pub4) [DOI] [PubMed] [Google Scholar]
- Harrison C, Chantler E.1998The effect of nonoxynol-9 and chlorhexidine on HIV and sperm in vitro. International Journal of STD and AIDS 992–97. ( 10.1258/0956462981921747) [DOI] [PubMed] [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG.2003Measuring inconsistency in meta-analyses. BMJ 327557–560. ( 10.1136/bmj.327.7414.557) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA.2019Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram MJ, Zeller E, Moss GP, Hall CE.2006A potential anti-implantation and spermicidal strategy: putative derivatives of nonoxynol-9 and anti-inflammatory agents and their spermicidal activity. European Journal of Contraception and Reproductive Health Care 11258–261. ( 10.1080/13625180600907487) [DOI] [PubMed] [Google Scholar]
- Iyer V, Poddar SS.2008Update on nonoxynol-9 as vaginal spermicide. European Journal of Contraception and Reproductive Health Care 13339–350. ( 10.1080/13625180802263515) [DOI] [PubMed] [Google Scholar]
- Jain RK, Jain A, Maikhuri JP, Sharma VL, Dwivedi AK, Kiran Kumar ST, Mitra K, Bajpai VK, Gupta G.2009In vitro testing of rationally designed spermicides for selectively targeting human sperm in vagina to ensure safe contraception. Human Reproduction 24590–601. ( 10.1093/humrep/den415) [DOI] [PubMed] [Google Scholar]
- Jain RK, Jain A, Kumar R, Verma V, Maikhuri JP, Sharma VL, Mitra K, Batra S, Gupta G.2010Functional attenuation of human sperm by novel, non-surfactant spermicides: precise targeting of membrane physiology without affecting structure. Human Reproduction 251165–1176. ( 10.1093/humrep/deq036) [DOI] [PubMed] [Google Scholar]
- Kelly JP, Reynolds RB, Stagno S, Louv WC, Alexander WJ.1985In vitro activity of the spermicide nonoxynol-9 against Chlamydia trachomatis. Antimicrobial Agents and Chemotherapy 27760–762. ( 10.1128/AAC.27.5.760) [DOI] [PMC free article] [PubMed] [Google Scholar]
- King LM, Holsberger DR, Donoghue AM.2000Correlation of CASA velocity and linearity parameters with sperm mobility phenotype in turkeys. Journal of Andrology 2165–71. ( 10.1002/j.1939-4640.2000.tb03277.x) [DOI] [PubMed] [Google Scholar]
- Ko EY, Sabanegh ES, Agarwal A.2014Male infertility testing: reactive oxygen species and antioxidant capacity. Fertility and Sterility 1021518–1527. ( 10.1016/j.fertnstert.2014.10.020) [DOI] [PubMed] [Google Scholar]
- Kreiss J, Ngugi E, Holmes K, Ndinya-Achola J, Waiyaki P, Roberts PL, Ruminjo I, Sajabi R, Kimata J, Fleming TR.1992Efficacy of Nonoxynol 9 contraceptive sponge use in preventing heterosexual acquisition of HIV in Nairobi prostitutes. JAMA 268477–482. ( 10.1001/jama.1992.03490040053025) [DOI] [PubMed] [Google Scholar]
- Lakshmi V, Saxena A, Mishra SK, Raghubir R, Srivastava MN, Jain RK, Maikhuri JP, Gupta G.2008Spermicidal activity of bivittoside D from Bohadschia vitiensis. Archives of Medical Research 39631–638. ( 10.1016/j.arcmed.2008.06.007) [DOI] [PubMed] [Google Scholar]
- Lee CH, Bagdon R, Chien YW.1996Comparative in vitro spermicidal activity of chelating agents and synergistic effect with nonoxynol-9 on human sperm functionality. Journal of Pharmaceutical Sciences 8591–95. ( 10.1021/js9501876) [DOI] [PubMed] [Google Scholar]
- Louis SM, Pearson RM.1985A comparison of the effects of nonoxynol-9 and chlorhexidine on sperm motility. Contraception 32199–205. ( 10.1016/0010-7824(8590108-8) [DOI] [PubMed] [Google Scholar]
- Mahony MC.2001Evaluation of the effect of a cervical cap device on sperm functional characteristics in vitro. Andrologia 33207–213. ( 10.1046/j.1439-0272.2001.00426.x) [DOI] [PubMed] [Google Scholar]
- Mauck CK, Baker JM, Barr SP, Abercrombie TJ, Archer DF.1997aA phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9: postcoital testing and colposcopy. Contraception 5689–96. ( 10.1016/s0010-7824(9700097-8) [DOI] [PubMed] [Google Scholar]
- Mauck CK, Baker JM, Barr SP, Johanson W, Archer DF.1997bA phase I study of femcap® used with and without spermicide: postcoital testing. Contraception 56111–115. ( 10.1016/s0010-7824(9700098-x) [DOI] [PubMed] [Google Scholar]
- Mauck CK, Baker JM, Barr SP, Johanson WM, Archer DF.1997cA phase I comparative study of three contraceptive vaginal films containing nonoxynol-9: postcoital testing and colposcopy. Contraception 5697–102. ( 10.1016/s0010-7824(9700099-1) [DOI] [PubMed] [Google Scholar]
- Mauck CK, Brache V, Kimble T, Thurman A, Cochon L, Littlefield S, Linton K, Doncel GF, Schwartz JL.2017A phase I randomized postcoital testing and safety study of the Caya diaphragm used with 3% Nonoxynol-9 gel, ContraGel or no gel. Contraception 96124–130. ( 10.1016/j.contraception.2017.05.016) [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. & PRISMA Group 2009Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6 e1000097. ( 10.1371/journal.pmed.1000097) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller-Esterl W, Schill WB.1982Sperm acrosin: liberation from the acrosome and activity of the free proteinase in the presence of Nonoxinol-9. Andrologia 14309–316. ( 10.1111/j.1439-0272.1982.tb02267.x) [DOI] [PubMed] [Google Scholar]
- Murase T, Okuda K, Sato K.1990Assessment of bull fertility using a mucus penetration test and a human chorionic gonadotrophin stimulation test. Theriogenology 34801–812. ( 10.1016/0093-691x(9090552-5) [DOI] [PubMed] [Google Scholar]
- Musekiwa A, Fernando NB, Abariga SA.2020Effectiveness of vaginal microbicides in preventing HIV transmission. Tropical Medicine and International Health 25790–802. ( 10.1111/tmi.13401) [DOI] [PubMed] [Google Scholar]
- Nithya RS, Anuja MM, Rajamanickam C, Indira M.2012Rat sperm immobilisation effects of a protein from Ricinus communis (Linn.): an in vitro comparative study with nonoxynol-9. Andrologia 44381–387. ( 10.1111/j.1439-0272.2012.01291.x) [DOI] [PubMed] [Google Scholar]
- Ola B, Afnan M, Papaioannou S, Sharif K, Bjoèrndahl L, Coomarasamy A.2003Accuracy of sperm–cervical mucus penetration tests in evaluating sperm motility in semen: a systematic quantitative review. Human Reproduction 181037–1046. ( 10.1093/humrep/deg209) [DOI] [PubMed] [Google Scholar]
- Pasqualotto EB, Daitch JA, Hendin BN, Falcone T, Thomas AJ, Nelson DR, Agarwal A.1999Relationship of total motile sperm count and percentage motile sperm to successful pregnancy rates following intrauterine insemination. Journal of Assisted Reproduction and Genetics 16476–482. ( 10.1023/a:1020598916080) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton DL, Wang SK, Kuo CC.1992In vitro activity of Nonoxynol 9 on HeLa 229 cells and primary monkey cervical epithelial cells infected with Chlamydia trachomatis. Antimicrobial Agents and Chemotherapy 361478–1482. ( 10.1128/AAC.36.7.1478) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ping X, Zhuo C, Lijun X, Fuer L.2005Spermicidal effect of Jieze No. 1 in combination with nonoxynol-9 in vitro. Journal of Huazhong University of Science and Technology 25225–228. ( 10.1007/BF02873584) [DOI] [PubMed] [Google Scholar]
- Raymond EG, Chen PL, Luoto J. & Spermicide Trial Group 2004Contraceptive effectiveness and safety of five nonoxynol-9 spermicides: a randomized trial. Obstetrics and Gynecology 103430–439. ( 10.1097/01.AOG.0000113620.18395.0b) [DOI] [PubMed] [Google Scholar]
- Roddy RE, Zekeng L, Ryan KA, Tamoufe U, Weir SS, Wong EL.1998A controlled trial of Nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. New England Journal of Medicine 339504–510. ( 10.1056/NEJM199808203390803) [DOI] [PubMed] [Google Scholar]
- Roddy RE, Zekeng L, Ryan KA, Tamoufé U, Tweedy KG.2002Effect of nonoxynol-9 gel on urogenital gonorrhea and chlamydial infection: a randomized controlled trial. JAMA 2871117–1122. ( 10.1001/jama.287.9.1117) [DOI] [PubMed] [Google Scholar]
- Rosenberg ZF, Mitchnick M, Coplan P.2008Vaginal microbicides against HIV. In Global HIV/AIDS Medicine. Elsevier. [Google Scholar]
- Sangi-Haghpeykar H, Poindexter AN, Levine H, Group AS.1996Sperm transport and survival post-application of a new spermicide contraceptive. Advantage 24 Study Group. Contraception 53353–356. ( 10.1016/0010-7824(9600084-4) [DOI] [PubMed] [Google Scholar]
- Schill WB, Wolff HH.1981Ultrastructure of human spermatozoa in the presence of the spermicide nonoxinol-9 and a vaginal contraceptive containing nonoxinol-9. Andrologia 1342–49. ( 10.1111/j.1439-0272.1981.tb00006.x) [DOI] [PubMed] [Google Scholar]
- Schwartz JL, Ballagh SA, Creinin MD, Rountree RW, Kilbourne-Brook M, Mauck CK, Callahan MM.2008SILCS diaphragm: postcoital testing of a new single-size contraceptive device. Contraception 78237–244. ( 10.1016/j.contraception.2008.04.118) [DOI] [PubMed] [Google Scholar]
- Shah V, Doncel GF, Seyoum T, Eaton KM, Zalenskaya I, Hagver R, Azim A, Gross R.2005Sophorolipids, microbial glycolipids with anti-human immunodeficiency virus and sperm-immobilizing activities. Antimicrobial Agents and Chemotherapy 494093–4100. ( 10.1128/AAC.49.10.4093-4100.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman D, Chantler E, Dukes M, Hutchinson FG, Elstein M.1986Comparison of the action of nonoxynol-9 and chlorhexidine on sperm. Fertility and Sterility 45259–264. ( 10.1016/s0015-0282(1649165-x) [DOI] [PubMed] [Google Scholar]
- Singh I.2014Human Embryology. JP Medical Ltd. [Google Scholar]
- Singh B, Cutler JC, Utidjian HM.1972Studies on development of a vaginal preparation providing both prophylaxis against venereal disease, other genital infections and contraception: III. In vitro effect of vaginal contraceptive and selected vaginal preparations of Candida albicans and Trichomonas vaginalis. Contraception 5401–411. ( 10.1016/0010-7824(7290032-7) [DOI] [PubMed] [Google Scholar]
- Srivastava S, Coutinho E.2010Adrenergic antagonist propranolol as a novel, effective spermicide: an NMR study. International Journal of Pharmacy and Pharmaceutical Sciences 4196–200. [Google Scholar]
- Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron Iet al. 2016Robins-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355i4919. ( 10.1136/bmj.i4919) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tello-Mora P, Hernández-Cadena L, Pedraza J, López-Bayghen E, Quintanilla-Vega B.2018Acrosome reaction and chromatin integrity as additional parameters of semen analysis to predict fertilization and blastocyst rates. Reproductive Biology and Endocrinology 16102. ( 10.1186/s12958-018-0408-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas MA, Chappell BT, Maximos B, Culwell KR, Dart C, Howard B.2020A novel vaginal pH regulator: results from the phase 3 AMPOWER contraception clinical trial. Contraception 2 100031. ( 10.1016/j.conx.2020.100031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson KA, Malamud D, Storey BT.1996Assessment of the anti-microbial agent C31G as a spermicide: comparison with nonoxynol-9. Contraception 53313–318. ( 10.1016/S0010-7824(9600066-2) [DOI] [PubMed] [Google Scholar]
- United Nations , Department of Economic and Social Affairs , Population Division 2015Trends in Contraceptive Use Worldwide 2015. ST/ESA/SER. A/349. https://www.un.org/development/desa/pd/sites/www.un.org.development.desa.pd/files/undesa_pd_report_2015_trends_contraceptive_use.pdf [Google Scholar]
- Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Tshibaka LM, Ettiègne-Traoré V, Uaheowitchai Cet al. 2002Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet 360971–977. ( 10.1016/s0140-6736(0211079-8) [DOI] [PubMed] [Google Scholar]
- White DR, Clarkson JS, Ratnasooriya WD, Aitken RJ.1995Complementary effects of propranolol and nonoxynol-9 upon human sperm motility. Contraception 52241–247. ( 10.1016/0010-7824(9500190-l) [DOI] [PubMed] [Google Scholar]
- Wilborn WH, Hahn DW, Mcguire JJ.1983Scanning electron microscopy of human spermatozoa after incubation with the spermicide nonoxynol-9. Fertility and Sterility 39717–719. ( 10.1016/s0015-0282(1647074-3) [DOI] [PubMed] [Google Scholar]
- Wishart DS, Feunang YD, Guo AC, Lo EJ, Marcu A, Grant JR, Sajed T, Johnson D, Li C, Sayeeda Zet al. 2018DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Research 46D1074–D1082. ( 10.1093/nar/gkx1037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization 2002WHO/CONRAD technical consultation on nonoxynol-9, World Health Organization, Geneva, 9–10 October 2001: summary report. Reproductive Health Matters 10175–181. ( 10.1016/s0968-8080(0200085-x) [DOI] [PubMed] [Google Scholar]
- World Health O rganization 2010aWHO Laboratory Manual for the Examination and Processing of Human Semen. World Health Organization. [Google Scholar]
- World Health Organization 2010bMedical Eligibility Criteria for Contraceptive Use. World Health Organization. [Google Scholar]
- Xia M, Yang M, Wang Y, Tian F, Hu J, Yang W, Tao S, Lu L, Ding X, Jiang Set al. 2020dl-Mandelic acid exhibits high sperm-immobilizing activity and low vaginal irritation: a potential non-surfactant spermicide for contraception. Biomedicine and Pharmacotherapy 126110104. ( 10.1016/j.biopha.2020.110104) [DOI] [PubMed] [Google Scholar]
- Youn JS, Cha SH, Park CW, Yang KM, Kim JY, Koong MK, Kang IS, Song IO, Han SC.2011Predictive value of sperm motility characteristics assessed by computer-assisted sperm analysis in intrauterine insemination with superovulation in couples with unexplained infertility. Clinical and Experimental Reproductive Medicine 38 47–52. ( 10.5653/cerm.2011.38.1.47) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zairi A, Serres C, Tangy F, Jouannet P, Hani K.2008In vitro spermicidal activity of peptides from amphibian skin: dermaseptin S4 and derivatives. Bioorganic and Medicinal Chemistry 16266–275. ( 10.1016/j.bmc.2007.09.045) [DOI] [PubMed] [Google Scholar]
- Zaïri A, Tangy F, Hani K.2013Dermaseptin S4 derivative K4K20S4: a potential candidate for development of a new microbicide contraceptive agent – an in vitro study. European Journal of Contraception and Reproductive Health Care 1879–87. ( 10.3109/13625187.2013.769950) [DOI] [PubMed] [Google Scholar]
- Zhou J, Chen L, Li J, Li H, Hong Z, Xie M, Chen S, Yao B.2015The semen pH affects sperm motility and capacitation. PLoS ONE 10 e0132974. ( 10.1371/journal.pone.0132974) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a