Highlights
-
•
Gap of public on-the-ground cancer data was addressed through questionnaires to professionals in the SEE region.
-
•
There is a lack of diagnostic imaging and radiotherapy modalities in the SEE region in comparison to Western Europe.
-
•
The Mortality to incidence ratio correlates inversely with the economic development and the availability of radiotherapy equipment.
-
•
The cancer incidence in SEE countries was correlate directly with the life expectancy and the availability of diagnostic equipment.
-
•
The need for reliable national and regional cancer registries in SEE countries for data collection and analysis has been emphasized.
Keywords: SEE region, Cancer patients, Diagnostic equipment, Radiation therapy equipment, SEEIIST, Cancer registries
Abstract
Background
The Southeast European (SEE) region of 10 countries and about 43 million people differs from Western Europe in that most SEE countries lack active cancer registries and have fewer diagnostic imaging devices and radiotherapy (RT) units. The main objective of this research is to initiate a common platform for gathering SEE regional cancer data from the ground up to help these countries develop common cancer management strategies.
Methods
To obtain detailed on-the-ground information, we developed separate questionnaires for two SEE groups: a) ONCO - oncologists regarding cancer treatment modalities and the availability of diagnostic imaging and radiotherapy equipment; and b) REG - national radiation protection and safety regulatory bodies regarding diagnostic imaging and radiotherapy equipment in SEE facilities.
Results
Based on responses from 13/17 ONCO participants (at least one from each country) and from 9/10 REG participants (all countries but Albania), cancer incidence rates are higher in those SEE countries that have greater access to diagnostic imaging equipment while cancer mortality-to-incidence (MIR) ratios are higher in countries that lack radiotherapy equipment.
Conclusion
By combining unique SEE region information with data available from major global databases, we demonstrated that the availability of diagnostic imaging and radiotherapy equipment in the SEE countries is related to their economic development. While immediate diagnostic imaging and radiation therapy capacity building is necessary, it is also essential to develop both national and SEE-regional cancer registries in order to understand the heterogeneity of each country’s needs and to establish regional collaborative strategies for combating cancer.
Nomenclature
Acronym list:
- DIRAC
DIrectory of RAdiotherapy Centres (DIRAC) IAEA
- GLOBOCAN
Global Cancer Observatory, IARC (estimates of incidence, mortality and prevalence for year 2020 in 185 countries or territories for 36 cancer types by sex and age group)
- GTFRCC
Global Task Force on Radiotherapy for Cancer Control
- HT
Hadron therapy
- IAEA
International Atomic Energy Agency (UN)
- IARC
International Agency for Research on Cancer (WHO)
- LMIC
Lower middle-income country (World Bank)
- MIC
Middle income countries (World Bank)
- MIR
Mortality-to-incidence ratio
- ONCO
Oncologists group that received questionnaires
- REG
Regulatory bodies that received questionnaires
- RT
Radiation therapy
- SEE
Southeast Europe
- SEEIIST
South East European International Institute for Sustainable Technologies
Introduction
Cancer is the second leading cause of death globally among noncommunicable diseases [1], [2]. According to the World Health Organization (WHO) the number of new cancer patients in the world is growing. In 2018, the number of new cancer cases was 18.1 million, 9.6 million patients died and 43.8 million people were living with cancer [3]. The number of new cancer cases worldwide each year is projected to rise to 27.5 million in 2040 [1], [3]. Approximately 65–70% of deaths from cancer occur in low- and middle-income countries where cancer is a significant cause of morbidity and mortality [4]. The demographic drivers of an increasing world population and increasing life expectancy in combination with progress in reducing deaths due to many other causes will continually increase the total number of deaths due to cancer.
Southeast European (SEE) countries are located in the Balkan peninsula and have a total population of about 43 million (M) inhabitants: Albania (3 M), Bosnia and Herzegovina (3.8 M), Bulgaria (7.1 M), Croatia (4.3 M), Greece (10.7 M), Kosovo1 (1.9 M), Montenegro (0.6 M), North Macedonia (2.1 M), Serbia (7.1 M) and Slovenia (2.1 M). Bulgaria, Croatia, Greece, and Slovenia are currently member states of the EU. The other six countries are either candidates or potential candidates for joining the EU. According to the World Bank [5] only three out of the 10 countries (Croatia, Slovenia and Greece) belong to the group of high-income countries, while the remaining seven are in the group of lower middle-income countries. The latter group of countries shows similarities and faces common challenges in fighting cancer and other health issues. The number of new cancer patients in the SEE region was estimated to be about 242,300 in 2020, while according to the most pessimistic projections, it was estimated to be 279.000 in 2030 [6]. It is commonly accepted that 50% of those patients would benefit from RT, namely 121.150 in 2020 and 139.500 in 2030. Using the linear regression model developed from empirical cancer incidence data taken from the SEE regional countries with national cancer statistics, as presented in the recent paper dedicated to planning for a hadron therapy centre for cancer patients in the SEE countries – the South-East European International Institute for Sustainable Technologies (SEEIIST) [6], it was estimated that 2900–3200 cancer patients per year in SEE countries would be eligible for hadron therapy (HT).
The fight against cancer is among the leading health priorities in the majority of EU member states and non-EU member states in the SEE region. It is essential to recognize that cancer care is a continuum and requires simultaneously investments in diagnostic imaging and treatment capacity [7]. Accurate assessment of the available healthcare resources and demand for treatment services, including RT, is essential for effective planning and responding to the increasing cancer burden [8]. Medical diagnostic imaging plays an essential role throughout the cancer care continuum from detection, diagnosis, treatment planning (especially in radiation oncology), assessment of treatment response and in long-term follow-up. At the suggestion of, and with help from the International Atomic Energy Agency (IAEA), the Lancet Oncology Commission on Medical Imaging and Nuclear Medicine was established in 2018 to examine the global access to diagnostic imaging and nuclear medicine for cancer care. The Commission issued its report in 2021 [7]. Data from this report are also presented in this paper.
RT is a critical and inseparable component of comprehensive cancer treatment and has a vital role in curing or palliating over 50% of patients with cancer [9], [10]. The importance of making RT available was emphasized in the 2015 Global Taskforce on Radiotherapy for Cancer Control (GTFRCC) report that highlighted the health, societal and economic benefits of increasing the global capacity for RT by 25% by 2025 [8]. Many low- and middle-income countries (LMICs) have inadequate or no RT centres. For example, it is projected that to meet the RT demand in LMICs over the next two to three decades, there will be a need for at least 5,000 additional RT machines just for 28 countries in Africa [11]. Applying the same methodology, it was estimated that there is a shortfall of approximately 30 RT machines in Albania, Bosnia and Herzegovina, Kosovo and North Macedonia.
Even though the number of facilities capable of treating cancer patients with protons and/or carbon ions, known as particle therapy (PT) or hadron therapy (HT), is growing worldwide, it is important to note that there is no particle therapy facility in the entire SEE region. The goal of the South East European International Institute for Sustainable Technologies – SEEIIST [12], [13], [14], currently in the phase of technical development, is to be able to provide particle therapy for cancer patients in the SEE region. In addition, SEEIIST will foster innovative technology to enhance clinical radiation therapy, radiation biology and radiation physics research in the SEE region. The goals of this research are to better define the scope of cancer challenges in the SEE region, to assess the availability of diagnostic imaging and RT services, and to raise awareness amongst policymakers and healthcare stakeholders to support, catalyse, and enable the infrastructure needed to markedly improve the treatment of cancer patients in the SEE region. This research paper will be of benefit to cancer care stakeholders in SEE countries for strategic planning and budgeting for diagnosis and treatment of their cancer patients and in planning for the SEEIIST HT facility.
Materials and methods
In the search for cancer data in the SEE region we encountered a challenge because the ten collaborating SEE countries, except for Slovenia, have only limited reliable cancer data. This was a problem because analyses and projections are crucial for estimating the future cancer burden [15]. The Global Cancer Observatory, GLOBOCAN, by IARC@WHO [16], [17], [18] was a valuable source of information for cancer incidence projections and for strategic planning related to each SEE country's cancer burden. However, as noted by GLOBOCAN, the quality and coverage of cancer data worldwide remains limited for LMICs [19]. Moreover, the GLOBOCAN projections for the SEE countries, which have no publicly available national cancer registries, are based on algorithms using the neighbouring countries’ data. The International Agency for Research on Cancer – IARC [17], was also a source of information for our study, highlighted the importance of evaluating, compiling and using the data from the Agency's cancer registry’s collaborators to make projections for the future. This project involving SEE countries was undertaken to add actual on-the-ground data which will serve both to enhance projected data from international sources and to help the SEE countries establish tumour registries and to make regional projections to guide cancer care.
In order to address the limited available cancer data within the SEE region in 2020, questionnaires were designed and sent to two key groups of respondents: 1) The ONCO group was comprised of the most prominent oncologists in the largest oncology centres in the 10 SEE countries with whom the first authors had already established a collaboration. Because of the lack of general response or only limited response from several initial key informants, the ONCO group was expanded to 17 key respondents. Eventually, data was collected from 13 ONCO respondents, covering all the ten SEE countries. The questionnaire consisted of six sets of questions regarding: a) the number of cancer patients in their country, b) the cancer treatment modalities used, c) the number of diagnostic imaging and RT units available, d) education and training opportunities, e) radiation safety and protection measures for the occupationally exposed RT staff and f) the number of cancer patients in the clinic of their affiliation, (Annex 1); 2) The key respondents of the REG group were representatives of the national regulatory bodies in the SEE countries. The questionnaire was created using Google-forms and was distributed by e-mails to the key informants. The reasoning behind data collection was presented at an ENLIGHT meeting with the SEE community [20] following which the questionnaire was distributed using e-mail communications and individual discussions. The REG key respondents from all ten SEE countries, except from Albania, provided data in response to the questionnaire. The questionnaire sent to the REG group requested information regarding the number of diagnostic imaging units (CT, mammography, PET/CT and GAMMA cameras), and the number and type of RT machines that are subject to regulatory licensing and inspection procedures, (Annex 2). To assess the reliability of the data obtained in our survey, we compared our results with the number of diagnostic imaging units reported by EUROSTAT and the number of RT machines reported by DIRAC (IAEA) for those countries.
In order to relate the cancer data to the general economic and health status of these countries, we retrieved the actual data on life expectancy at birth from the World Bank [21] and data on the Gross Domestic Product (GDP) per capita from data resources of the EUROSTAT GDP [22].
The selection of the Western European countries for comparison with the SEE cancer data was based on the (a) extreme values such as the cancer incidence recorded for Switzerland, France and Germany, and the RT units available in Switzerland and (b) nearest western neighbouring countries to the SEE region (such as Austria and Italy). Poland was selected due to the population size, similar to that of the SEE region (about 40 M). Belgium was selected as a smaller EU country with a population similar to that of Greece.
Here we present information on the cancer scenario in the SEE region based on the analysis of publicly available data (GLOBOCAN, DIRAC) and the data provided by the ONCO and REG groups. These data include information on the number of cancer patients, national cancer incidence and cancer mortality and the availability of diagnostic imaging and RT equipment.
In our calculations of the mortality-to-incidence ratio (MIR), some of the missing ONCO data on mortality due to cancer were supplemented by GLOBOCAN data.
Results
Comparison of survey data between ONCO and REG
Validation of the reliability of the data obtained from the ONCO and REG groups was essential prior to the data analysis. Fig. 1 (a) presents a comparative chart of the ONCO versus REG data regarding availability of the diagnostic machines subject to regulatory bodies’ licencing procedures (in particular the CT units), the numbers match for some SEE countries but substantially differ for others. Hence, the reliable set of data regarding diagnostic units (CT, mammograph units and Gamma cameras) are considered to be the numbers provided by REG key informants. MRI machines do not use ionizing radiation sources and thus are not subject to licencing procedures. For this reason, in our analysis the only valid on-the-ground data source was considered to be the set provided by the ONCO key respondents. Fig. 1(b) is a chart comparing the ONCO versus REG data related to availability of RT treatment equipment (in particular, linear accelerators – LINACS). As evident, the ONCO versus REG data perfectly match for most of the countries, but there were some mismatches, it was later clarified that these differences originate from the long commissioning phase of the machines in the clinical departments that follows the regulatory procedures. For instance, North Macedonia faced difficulties adapting the space in the existing hospitals for the newly purchased equipment while Montenegro is in the process of adding one more LINAC in the single oncology centre in the country. For this reason, the REG data set is considered relevant for determining the actual number of LINACS in the country. The unique source of data from the ONCO group regarding the incidence of all cancers, except for non-melanoma skin cancers (NMSC), was subject of analysis and comparison with the GLOBOCAN data (Fig. 2).
Fig. 1.
(a) Comparative chart of the ONCO versus REG data related to the availability of (a) diagnostic imaging machines, (in particular, CT units); (b) RT equipment (in particular, LINACs).
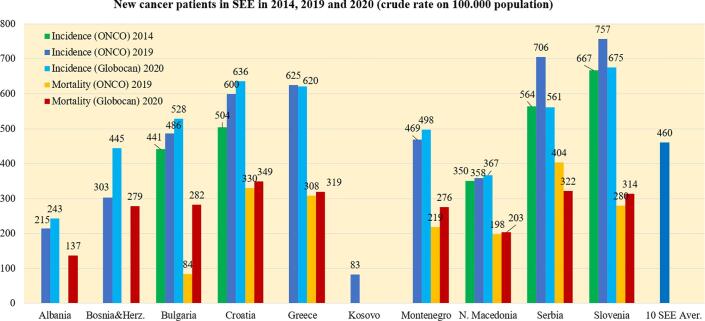
Fig. 2.
Crude incidence rates for all cancers except NMSC, all ages, both sexes, average values in 100,000 population (in 2014, 2019 and 2020) and mortality rates (in 2019 and 2020) in the SEE countries and the SEE region as a whole.
It should be noted that in Figure l below, REG key respondent from Albania did not provide an answer.
Cancer patients in the SEE region
Previous research showed that the number of new cancer patients in the SEE region was about 242,300 in 2020 and is projected to be approximately 279,000 in 2030 [6]. A limited picture of this incidence trend is shown in Fig. 2 where the data provided by the ONCO group for 5 SEE countries in 2014 and 2019 (2018 for Slovenia) shows an increase in the incidence of all cancers during that time interval. Although the cancer incidence reported by the ONCO group for many countries differs considerably from the incidence reported by them to GLOBOCAN in 2020, there was slightly better agreement in the cancer mortality reported to GLOBOCAN in 2020 with the data from the ONCO group in the seven SEE countries that provided data for 2019.
Although the average crude cancer incidence rates in the SEE region for 2019 (460/100,000) is about 30% lower than that of Germany (740/100,000) and Switzerland (661/100,000), the crude incidence rates in Slovenia and Serbia are comparable to the rates in the latter countries. The lowest cancer incidence rates in 2019 were reported for Albania, Bosnia and Herzegovina, and Kosovo. Possible reasons for variations in these rates among the SEE countries will be discussed below.
As can be seen from the calculated data in Fig. 2 (ONCO data for 2019) the mortality-to-incidence ratio (MIR) ranges from 0.47 to 0.57 for the SEE countries except for the ratio of 0.37 for Slovenia. According to the GLOBOCAN data from 2020, the MIR for the SEE countries ranges from 0.51 to 0.57 except for Bosnia & Herzegovina (0.63) and Slovenia (0.47). Considerations regarding the SEE mortality rates that are almost all higher than those in Western European countries are discussed below.
Availability of cancer treatment modalities in the SEE region
Data acquired from the ONCO group indicated that all the SEE countries offer the following cancer treatment modalities: chemotherapy, radiotherapy, immunotherapy, hormonal therapy and targeted drug therapy. In 2019 gene therapy for treating some cancers was available only in Croatia, Slovenia and in Bosnia and Herzegovina. Cryoablation procedures for cancer treatment were available in Montenegro, Bulgaria, Serbia, Albania, Kosovo and Greece. Of the 6 SEE countries providing data on the use of chemotherapy (Croatia, Kosovo, Montenegro, North Macedonia, Serbia and Slovenia), from 10% to 39% of patients received chemotherapy at some time and averaged 24%. The percentage of cancer patients receiving RT as a part of their treatment course in the six countries that provided data (Bulgaria, Croatia, Montenegro, North Macedonia, Serbia and Slovenia) varied from 24% (Serbia) to 42% (Montenegro) and averaged 32.7%. These percentages in the use of RT are far lower than that of the high-income European countries, known to be treating about 50% of their cancer patients with RT [17].
Availability of diagnostic imaging and RT equipment for cancer patients in SEE countries
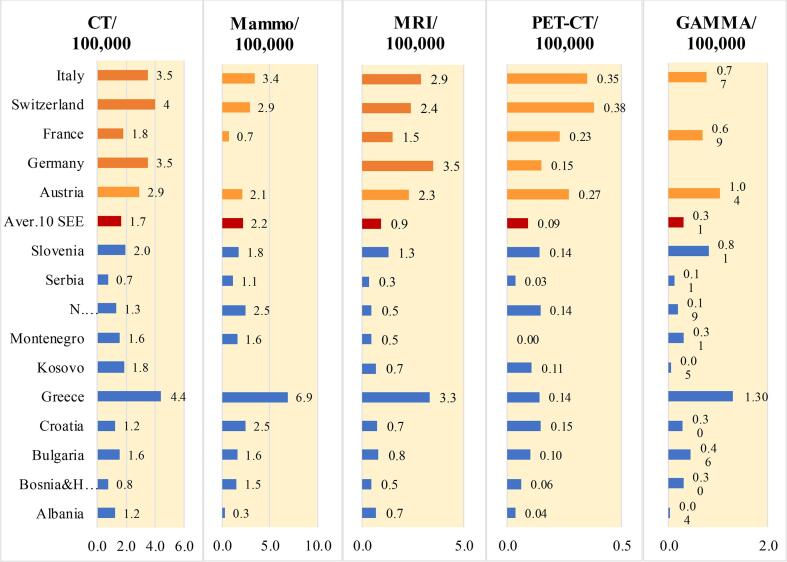
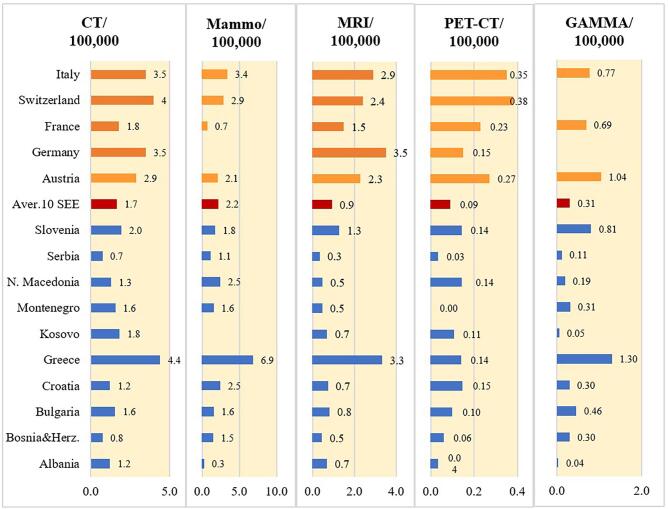
The availability of diagnostic imaging equipment in SEE countries is presented as the density of computed tomography (CT), mammography, magnetic resonance imaging (MRI), positron emission tomography (PET-CT) and gamma camera units in terms of the number of units per 100,000 inhabitants. All of the information regarding diagnostic imaging equipment presented in Fig. 3 was provided by regulatory (REG) respondents to our surveys except for Albania (ONCO). Likewise, all the MRI data originated from the ONCO respondents. For comparison, diagnostic imaging and RT equipment data for five Western European countries (Austria, France, Germany, Italy and Switzerland) available at EUROSTAT [23] were added to Figs. 3. As evident from Fig. 3a, the density of each type of diagnostic modality is much lower in most SEE countries than in the Western European countries chosen for comparison. A notable exception is Greece which enjoys a high availability of diagnostic imaging equipment per 100,000 population, comparable or even higher than that of many of the Western European countries shown in Fig. 3 Generally, the density of diagnostic imaging equipment in the SEE countries that are members of the EU (Bulgaria, Croatia, Greece and Slovenia) is higher than the other SEE countries. Based on our survey, there is a notable lack of diagnostic imaging equipment in Albania and Kosovo.
Fig. 3a.
Density of the available diagnostic imaging equipment (CT, mammography, MRI, PET-CT and gamma cameras) shown as the ratio of the number of diagnostic imaging units per 100,000 inhabitants in the SEE countries and several Western European countries.
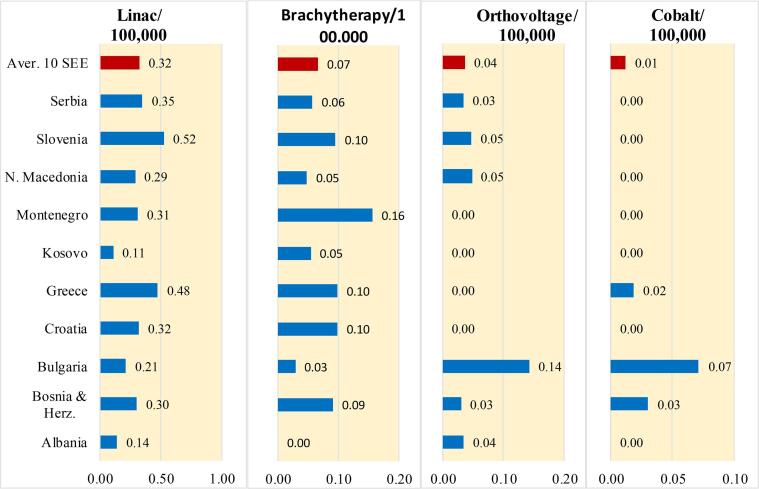
As noted above, between 24% and 42% of cancer patients in the SEE countries are treated with RT while approximately 50% of cancer patients can benefit from RT. This low rate of treatment of cancer patients with RT in these countries may indicate a shortage of RT equipment and/or a lower utilization rate of the available RT equipment. Fig. 3b, shows data for linear accelerators (LINACS) for all countries however for brachytherapy data from 8 countries is presented, since data were not available for all of the SEE countries. Fortunately, cobalt-60 machines are not a common treatment option in the majority of the SEE countries because LINACs offer technical superiority in terms of accuracy of the dose delivered to patients. In addition, the high-activity radioactive sources in cobalt-60 machines raise security concerns, especially in politically unstable countries [24]. The information presented in Fig. 3b, shows that the density of LINACs in Albania and Kosovo is the lowest in the SEE region, similar to the low density of diagnostic imaging machines (Fig. 3a).
Fig. 3b.
Density of the available of operating RT machines (LINACs, brachytherapy, cobalt-60) in the SEE countries (REG data except for Albania, ONCO). For comparison, according to Eurostat, Switzerland is a European leader with 1.91 RT machines per 100,000 population. Austria and Italy have 0.55 and 0.75 RT machines per 100,000 population, respectively.
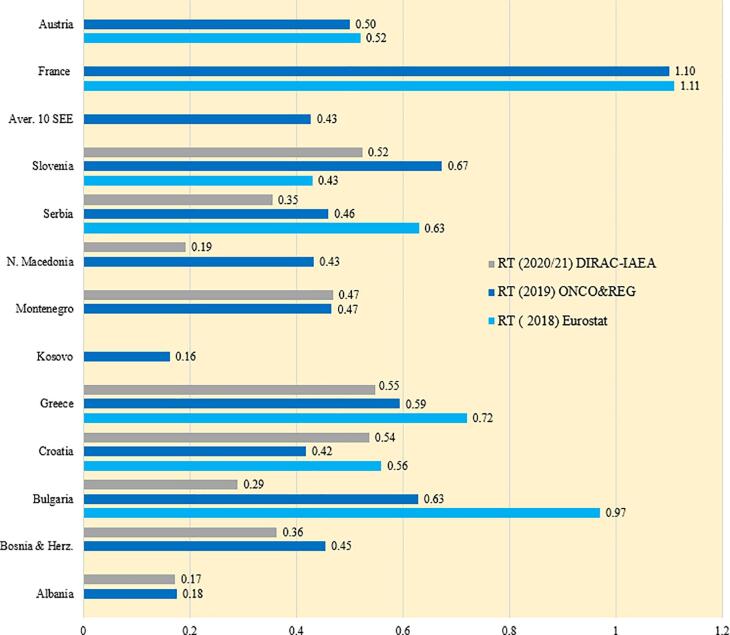
The information shown in Fig. 4 regarding the density of all available RT units (sum of LINACs, brachytherapy equipment, and cobalt-60 machines) in the SEE countries originate from three sources: REG data (already given in Fig. 3b) and from DIRAC (IAEA) and Eurostat that are publicly available [25], [26]. The differing numbers in Fig. 4 highlight the challenges encountered when trying to collect accurate and reliable data in the SEE countries.
Fig. 4.
The density of RT machines (total number of available RT machines per 100,000 population), including LINACs, brachytherapy units, and cobalt-60 machines in the SEE countries. Herein REG data were used except for Albania (ONCO).
Based upon data provided in responses to our surveys (REG/ONCO), Croatia, Slovenia, Bulgaria, Montenegro and Greece with 0.81, 0.62, 0.63, 0.63 and 0.61 RT units per 100,000 population, respectively, are the SEE regional leaders. Albania and Kosovo have the least RT equipment (densities of 0.14 and 0.22, respectively). The density of RT equipment in the remainder of the SEE countries ranges from 0.34 to 0.55. For comparison, according to Eurostat, Switzerland is a European leader with 1.91 RT machines per 100,000 population. Austria and Italy have 0.55 and 0.75 RT machines per 100,000 population, respectively.
According to the recognized recommendations, the optimum number of RT machines per 100,000 population is 0.6 [27], [28]. Our research shows that most of the SEE countries are below this optimum and that Albania and Kosovo are considerably below (RT equipment density of about 0.2). Keeping in mind that the availability of RT machines positively impacts the outcome of treatment, especially the survival rate, of the cancer population, the SEE countries' cancer-fighting strategies must incorporate plans for a rapid increase in the availability of RT equipment.
Correlation between cancer incidence, life expectancy and the availability of diagnostic imaging equipment
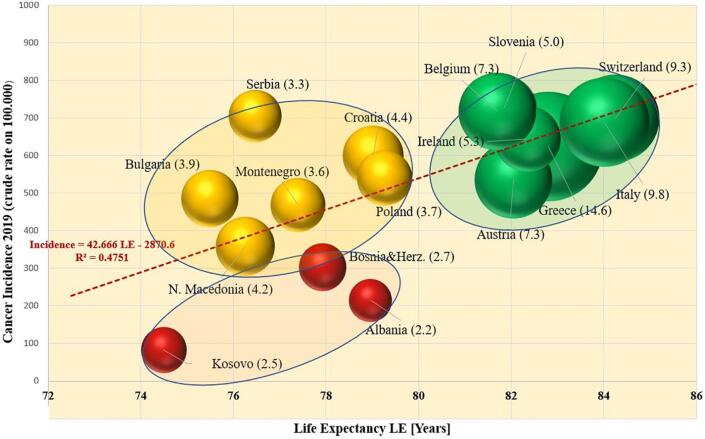
As presented in Fig. 2, the reported crude incidence of cancer per 100,000 population, except for NMSC, is higher in Western European countries than is the average of the SEE countries. Here we present the correlation between the reported cancer incidence rates and life expectancy with the availability of diagnostic imaging equipment. Fig. 5 is a multiparameter chart on which each country is represented by a sphere whose location in the figure is a function of cancer incidence and life expectancy while the radius of the sphere is proportional to the density of the country’s diagnostic imaging equipment (sum of CT, MRI and mammography units per 100,000 population). It is evident that there are three clusters of countries in Fig. 6. The cluster of green spheres is formed by countries with the availability of at least 5 diagnostic equipment machines per 100,000 population. These countries (Austria, Belgium, Greece, Italy, Ireland, Slovenia and Switzerland) report high cancer incidence rates and life expectancy between 82 and 85 years. The yellow cluster of countries is formed by SEE countries for which the life expectancy is 75–79 years and for which there are 3.0–4.9 diagnostic imaging machines per 100,000 population. Finally, the cluster of red-coloured SEE countries (Albania, Bosnia and Herzegovina, and Kosovo) have less than 3.0 diagnostic machines per 100,000 population. The cancer incidence in these countries appears to be substantially lower (below 300 diagnosed per 100,000 population) than in the other SEE countries (yellow and green from 350 to over 700 cancers diagnosed per 100,000 population). The red cluster in Fig. 5 is formed by countries with life expectancy within the same interval as the countries in the yellow cluster, but their diagnostic imaging capacity is below 3.0 machines per 100,000 population.
Fig. 5.
Cancer incidence as a function of the life expectancy of the population in the respective country. All the data refer to 2019. Green spheres denote the density of diagnostic imaging equipment being greater than 5 units per 100,000 population; yellow indicates density between 3 and 5 units per 100,000, and red below 3 units per 100,000. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
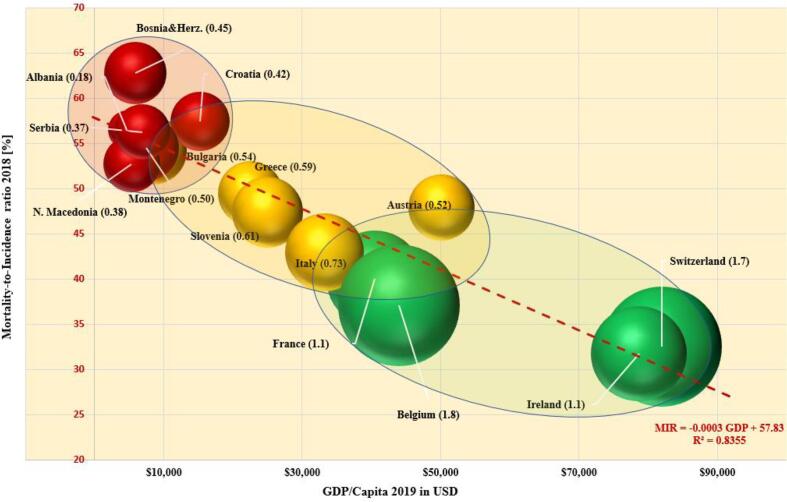
Fig. 6.
Dependence of the MIR on the GDP per capita and the density of conventional RT equipment. The radius of the spheres is proportional to the density of RT equipment per 100,000 population in the respective countries. The number in the parentheses represents the LINAC density in the country. MIR data are from 2018. The GDP per capita are from 2019.
Since the life expectancies in the countries in the red cluster (Albania, Bosnia and Herzegovina, and Kosovo) lie in the same age range as the countries in the yellow cluster (Bulgaria, Montenegro, N. Macedonia, Serbia and Croatia), one would expect their crude cancer incidence rates to be in the same range, which is not the case. The apparent mismatch in the range of cancer incidence rates between the red and yellow clusters of SEE countries suggests that the “incidence” reported for the red countries may not reflect the actual incidence of cancer within their populations. Hence, one could consider that the reported values represent only the “detected new cancers” rather than the real “incidence of new cancers” because of a lack of cancer screening capacity.
In Fig. 5 the dependence of the cancer incidence has been modelled with a linear regression model from the life expectancy (R2 = 0.48). The strength of the correlation (described with R2) grew considerably to R2 = 0.79 if the data of the countries of the red cluster (Albania, Bosnia and Herzegovina, and Kosovo) are disregarded when creating the model, having in mind that their real cancer incidence rates may be different from the detected new cancer cases.
Correlation between mortality-to-incidence ratio, GDP/capita and the availability of RT equipment
The mortality-to-incidence ratio (MIR) for cancer was calculated for each SEE country. MIR, an indicator linked the with patient survival rate, represents the percentage of cancers with a fatal outcome and reflects the effectiveness of cancer management in each country. For comparison, the MIR of several Western European countries is shown in Fig. 6, a multiparameter chart that presents MIR as a function of GDP per capita in 2019 (according to the World Bank, 2019) for each country. Each country’s pair of MIR-GDP values positions a sphere whose radius is proportional to the density of the available RT equipment (sum of LINACS, cobalt-60 units and brachytherapy machines per 100,000 population) in that country (RT density is the number in parenthesis). In Fig. 6, the spheres representing the countries form three clusters (green, yellow and red). The applied linear regression model shows a linear dependence of the MIR with the GDP per capita (R2 = 0.84). The cluster of countries represented by red spheres (Albania, Bosnia and Herzegovina, Bulgaria, Croatia, Montenegro, N. Macedonia and Serbia) have MIR values between 50% and 67% and GDP per capita less than $20,000 (middle income countries). The density of RT equipment in this cluster varies in a high range, starting from 0.14 RT machines per 100,000 population for Albania and ending with 0.81 for Croatia. In this group it appears that there is now proportionality between the available equipment and the level of the economic development. The second cluster, formed by yellow spheres (Greece, Italy and Slovenia), represents countries with a MIR between 37% and 50%, a GDP per capita between $20,000 and $35,000 and a density of RT machines between 0.6 and 0.8 RT machines per 100,000 population. For comparison, a cluster of high income Western European countries with a GDP per capita higher than $40,000 (Austria, Belgium, France, Ireland and Switzerland) is represented by green spheres. All these countries, are characterised by a relatively low MIR (between 30% and 40%), and a density of RT equipment greater than 1.0. The only exception in this cluster is Austria with MIR about 47% and RT density of 0.55 machines per 100,000. It is noteworthy that the MIR for Bosnia and Herzegovina (63%) is about twice as high as that of Ireland and Switzerland (∼32%).
Discussion
The number of new cancer patients in the SEE region was estimated to be 242,300 in 2020 and it was projected to be 279,000 in 2030 [6]. As noted above, the accuracy of the reported number of cancers diagnosed in each SEE country may vary considerably in the absence of national cancer registries in a majority of the countries. To begin to fill this gap we queried two groups: oncologists (ONCO) and regulatory bodies (REG) with questionnaires (in Annexes 1 and 2). With the understanding that the cancer data from the global databases often involve calculated estimates, the finer detail from the ONCO and REG questionnaires supports the need to develop individual country and regional registries, see Fig. 1.
The cancer incidence is strongly correlated with the diagnostic imaging capacity of the respective countries as shown by the higher incidence of cancer in the countries with greater than five diagnostic imaging devices per 100,000 population (Fig. 5). The higher cancer incidence in Western European countries could also be ascribed to their ageing populations that are prone to develop cancer. The higher incidence of cancer in the more developed SEE countries could also be due to their higher density of diagnostic imaging equipment and to their cancer screening programs. At the other extreme, the lower incidence of cancer in the countries in the red cluster (Albania, Bosnia and Herzogovina and Kosovo) could be ascribed to their low density of diagnostic imaging machines as well as other possible reasons such as the different demographic structure of the SEE region from Western Europe, the lack of screening programs, the lack of qualified medical personnel as well as social and other parameters.
The mortality-to-incidence ratio (MIR) appears to serve as an indicator of the capacity of a country to manage cancer effectively. As shown in Fig. 6, MIR appears to be related to the economic power of the country expressed by gross domestic product (GDP) per capita and, hence, the country's capability to provide an optimum number of RT machines and the associated trained professional human resources to utilize the equipment. The multiparameter analysis of this research clearly shows the clustering behaviour of MIR linked to GDP per capita.
Conclusion
A central purpose of this study was to illustrate both the challenge and the importance of obtaining reliable and reproducible data in the SEE countries regarding the incidence of and mortality from cancer as well as access of cancer patients to diagnostic imaging services and to RT equipment. Importantly, while using available information from global data sets is necessary to fill obvious current gaps in data on technology and personnel, the more comprehensive data we obtained in this study – which also could serve as an incentive for the development of cancer registries - are needed for SEE countries to make strategic planning decisions to improve cancer care such as through providing greater availability of diagnostic imaging equipment and RT services for their 40 million inhabitants. This more comprehensive information on SEE countries is also needed to strengthen planning for the SEEIIST particle therapy facility, a unique project designed to help the SEE region develop a collaborative approach to cancer care.
The capacity for detection and treatment of cancers in countries within the SEE region is diverse. It varies greatly depending on the level of economic development of the country and, as shown above, on the availability of (a) diagnostic imaging equipment that is essential for detecting cancers, especially at an early stage, and (b) RT equipment that, when properly utilized, will improve cancer treatment outcomes, especially a higher percentage of cured patients. Bearing in mind that only 24% − 42% of patients with cancer in SEE countries undergo RT as part or all of their treatment, it will take both an investment in diagnostic imaging and RT equipment as well as training of personnel for the SEE countries to achieve the “benchmark” for cancer care demonstrated by high-income Western European countries where at least 50% of patients with cancer undergo RT as part or all of their cancer treatment.
While the obvious shortfall in technology in many SEE countries needs to be addressed, the importance of accurate data will be essential for strategic planning to improve cancer treatment outcomes in the SEE countries and also for the multinational strategy behind the SEEIIST HT project. A major goal of this study is to stimulate discussions in SEE countries of steps that can be taken to improve the outcome of patients treated for cancer. A remarkably useful consequence of this effort and that of the SEEIIST Institute is identifying the relevant partners and building collaborations within the SEE region among those committed to improving cancer care. Developing cancer strategies and regional networks while optimizing the usage of conventional RT equipment should go in parallel with building the SEEIIST Institute. Economic measures are needed to mitigate the differences in cancer management capacities in the lower-income SEE countries. It is vital to organize and enhance the ability within the SEE region to obtain reliable cancer data by establishing SEE national cancer registries and to use that data to develop national cancer management strategies. From an international perspective, these national registries could join in a shared network to create a SEE regional cancer registry. As mentioned above, enhancement of the early detection of cancer and all other aspects of cancer management in the SEE countries is essential. It is of paramount importance for SEE countries to take a comprehensive approach in this fight against cancer. An increase in the number of well-equipped RT centres should be accompanied by screening programs, public awareness-raising programs and the training and education of staff to increase the number of highly qualified professionals who are essential throughout all phases of managing the increasing burden of cancer.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors would like to express thanks to David Pistenmaa and Norman Coleman from International Cancer Expert Corps for their advice and excellent editorial assistance. The questionnaire design of this work has been completed during the stay of the first and corresponding author, Mimoza Ristova, as an associate scientist in CERN, Geneva in 2020, fundied from the EU DG-RTD via a special instrument ‘Service Facility in Support of the Strategic Development of International Cooperation in Research and Innovation N°30-CE-0838742/00-87’.
Footnotes
In this document the designation to Kosovo is without prejudice to positions on status and is in line with UNSC 1244/1999 and the ICJ opinion on the Kosovo Declaration.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ctro.2022.03.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018:GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Hunter D.J., Reddy K.S. Non-communicable disease. N Engl J Med. 2013;397:1336–1343. doi: 10.1056/NEJMra1109345. [DOI] [PubMed] [Google Scholar]
- 3.Worldwide cancer incidence statistics | Cancer Research UK, www.cancerresearchuk.org. [Accessed Online: October 2021].
- 4.WHO. https://www.who.int/news-room/fact-sheets/detail/cancer. [Accessed Online: May 2021].
- 5.World Bank. https://blogs.worldbank.org/opendata/new-world-bank-country-classifications-income-level-2020-2021. [Accessed Online: October 2021].
- 6.Ristova M., Gershan V., Amaldi U., Schopper H., Dosanjh M. Cancer patients in the countries of SEE (the Balkans) region and prospective of the Particle Therapy. Adv Radiat Oncol. 2021 doi: 10.1016/j.adro.2021.100772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hricak H., Abdel-Wahab M., Atun R., Paez M.L.D., M, Brink JA, et al. Medical imaging and nuclear medicine.Lancet Oncology. Commission. 2021;22:e136–e172. doi: 10.1016/S1470-2045(20)30751-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Atun R., Jaffray D.A., Barton M.B., Bray F., Baumann M., Vikram B., et al. Expanding global access to radiotherapy. Lancet Oncol. 2015;16(10):1153–1186. doi: 10.1016/S1470-2045(15)00222-3. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Wahab M., Bourque J.-M., Pynda Y., et al. Status of radiotherapy resources in Africa: An International Energy Agency analysis. Lancet Oncol. 2013;4:168–175. doi: 10.1016/S1470-2045(12)70532-6. [DOI] [PubMed] [Google Scholar]
- 10.Barton M.B., Jacob S., Shafiq J., Wong K., Thompson S.R., Hanna T.P., et al. Estimating the demand for radiotherapy from the evidence: a review of changes from 2003 to 2012. Radiother Oncol. 2014;112(1):140–144. doi: 10.1016/j.radonc.2014.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Ige T.A., Jenkins A., Burt G., Angal-Kalinin D., McIntosh P., Coleman C.N., et al. Surveying the challenges to improve linear accelerator-based radiation therapy in Africa: a unique collaborative platform of All 28 African countries offering such treatment. Radiational Oncology. 2021;33(12):e521–e529. doi: 10.1016/j.clon.2021.05.008. [DOI] [PubMed] [Google Scholar]
- 12.SEEIIST. https://seeiist.eu/. [Accessed Online: October 2021].
- 13.Amaldi U, Balosso J, Dosanjh M, Overgaard J, Scholz M, Singers-Sorensen B., A Facility for Tumour Therapy and Biomedical Research.: CERN Yellow Reports, 2019: Monographs: CERN-2019-002. https://doi.org/10.23731/CYRM-2019-002.
- 14.Amaldi U, Benedetto E, Damjanovic S, Dosanjh M et al, South East European International Institute for Sustainable Technologies (SEEIIST): Review. Frontiers in Physics, Applied Nuclear Physics at Accelerators, 2021, Vol. 29 January. https://doi.org/10.3389/fphy.2020.567466.
- 15.Plummer M, de Martle C, Vignat J, et al., Global burden of cancer attributable to infection in 2012: a synthetic analysis. Lancet Glob Health, 2016, Vol. 4, pp. e609 – e616, 2016. doi: 10.1016/S2214-109X(16)30143-7. [DOI] [PubMed]
- 16.IARC. https://gco.iarc.fr/. [Accessed Online: October 2021].
- 17.IARC. https://screening.iarc.fr/[Accessed Online: October 2021].
- 18.Borras J.M., Lievens Y., Barton M., Corral J., Ferlay J., Bray F., et al. How many new cancer patients in Europe will require radiotherapy by 2025? An ESTRO-HERO analysis. Radiother Oncol. 2016;119(1):5–11. doi: 10.1016/j.radonc.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Sankaranarayanan R. Screening for cancer in low-and middle-income countries. Ann Glob Health. 2014;80:412–417. doi: 10.1016/j.aogh.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 20.ENLIGHT for SEEIIST in South-East Europe: https://indico.cern.ch/event/933746/.
- 21.World Bank Data-Life Expectancy; https://data.worldbank.org/indicator/SP.DYN.LE00.IN.
- 22.EUROSTAT-GDP. [Accessed Online: October 2021] https://ec.europa.eu/eurostat/databrowser/view/NAMA_10_GDP/.
- 23.EUROSTAT-Medical technology. https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=hlth_rs_equip&lang=en.
- 24.Pomper M, Dalnoki-Veress F, Moore G., Treatment, Not Terror: Strategies to enhance external beam therapy in developing countries while permanently reducing the risk of radiological terrorism, 2016. http://www.stanleyfoundation.org/publication.
- 25.IAEA-DIRAC https://dirac.iaea.org/ [Accessed Online: October, 2021].
- 26.EUROSTAT-Equipment. https://ec.europa.eu/eurostat/databrowser/view/HLTH_RS_EQUIP/default/table?lang=en.
- 27.IAEA. Planning National Radiotherapy Services: A Practical Tool, No.14, 2010. https://www.iaea.org/publications/8419/planning-national-radiotherapy-services-a-practical-tool.
- 28.IAEA. Radiotherapy In Cancer Care: Facing The Global Challenge, 2017. https://www.iaea.org/publications/10627/radiotherapy-in-cancer-care-facing-the-global-challenge.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.