Abstract
Pathogen control is a critical issue in the layer industry. Plant-based natural products are firmly replacing the undesirable use of antibiotics in animal production. The poultry industry embraced the opportunity to distance itself from the negative public perception of antibiotic use. In this study, we investigated the effects of a phytogenic product comprising of menthol, carvacrol and carvone on ileum gene expression profile in layers after 16 weeks of continual supplementation. Phytogen supplementation increased endocytosis and autophagy while showing significant predicted cardiovascular protective effects. Statistical comparison with over 100,000 manually curated and comparably reanalysed public datasets suggests that the phytogen effects are highly significantly comparable with transcriptomic effects of clinical antibiotics doxycycline and geldanamycin, and that phytogen can reverse transcriptomic effects of a range of viral diseases and malaria. Our data confirmed the hypothesis that similarly to the original essential oil type antimicrobial constituents of phytogenic products, there may be a range of benefits unrelated to their critical antimicrobial action, contributing to improved bird welfare.
Keywords: Ileum, RNAseq, Phytogen, Menthol, Carvacrol, Carvone
Ileum; RNAseq; Phytogen; Menthol; Carvacrol; Carvone.
1. Introduction
The use of Antibiotic Growth Promoters (AGPs) in the livestock industry was necessary to prevent disease outbreaks in production-intensive environments utilising the beneficial side-effects of antibiotics to promote the growth and performance of animals [1]. Pig and poultry production systems are well known for their high pathogen abundance and high stocking density, where pathogen control is critical for animal welfare and productivity [2].
In the last decade, the awareness of the rise in antimicrobial resistance (AMR) genes in hospital and animal production environments caused the banning of AGPs use as livestock supplements in many countries worldwide. World Health Organization (WHO) recognises emerging pathogens as a priority focus area with dire predictions that the death toll of AMR could be one person every three seconds by 2050 considering the fast rise in AMR worldwide [3].
The industry responded to the ban of antibiotic use with a range of new pathogen controlling products, and this time in the more natural use of plant antimicrobial products, also known as phytogenics. Although both layer and broiler production systems constitute a major part of the poultry industry, nutritional interventions affect broilers and layers differently. Broilers are juvenile birds exposed to pathogens over a much shorter time of up to 6 weeks, while layers with a typical production lifespan of ∼80 weeks have longer exposure and are more likely to develop long term effects, both beneficial and unwanted.
Investigated phytogenic product is comprised of menthol, carvacrol and carvone. Menthol is an organic compound found in mint-like plants but can also be synthesised on an industrial scale. Menthol is widely used, from mouthwashes to sweets and controversial menthol cigarettes. Although it is well known for its antimicrobial properties [4, 5], menthol also has a range of other effects including stimulating intestinal Ca absorption [6], it is beneficial for vascular and cardiovascular health [7], can help muscular recovery post exercise [8], and enhance exercise performance, especially under heat stress [9, 10] and influence thermal sensation via cooling effect [10], can improve thirst management [11], and it can be used in treatment of obesity [12]. Menthol reduces colitis-induced intestinal tumorigenesis and increases short-chain fatty acids [13], provides benefits in the endoscopic clarification of early gastric cancer [14], exerts analgesic effects via the enhancement of inhibitory synaptic transmission [15]. This suggests that, in addition to pathogen control, menthol could help birds manage heat stress, thirst, improve their cardiovascular and intestinal health and reduce the sensation of pain.
Carvacrol is a monoterpenoid phenol with a characteristic odour of oregano, where it represents the main beneficial component [16]. Carvacrol also has a range of benefits in addition to well documented antibacterial [17], anti–biofilm [18, 19], antifungal [20], anticoccidial [21], anti-parasitic [22], antimalarial [23] and insecticide [24, 25, 26] abilities. In addition, carvacrol is a strong antitumor agent [27], especially against tumours related to sex hormones such as breast [28], cervical [29] and prostate. It alleviates oxidative and LPS induced stress [30, 31], it is an anti-inflammatory and antioxidant in the respiratory system [32], strongly liver protective by slowing down liver fibrosis [33] and healing liver injury [34]. Carvacrol elevates vascular inflammation [35] and it is also cardioprotective [36]. Additionally, it has behavioural calming effects, most likely via inhibition of gut-brain axes [16], it is neuroprotective [37], alleviating neurodegeneration and depressive-like behaviours [30], and is considered for treatment of Alzheimer's disease [38]. Recently, carvacrol's known antiviral activity was in the spotlight again, with suggestions that it is a respectable candidate for the development of anti-COVID-19 drugs [39].
Carvone is a terpenoid found in many plant essential oils but is most abundant in caraway, spearmint, and dill. It is a known antimicrobial [40, 41], anti-inflammatory [42, 43], anti-arthritis [44], anticancer [45], liver-protective [46], cardioprotective [47], calming and neuroprotective [48, 49, 50] agent. It is also an anticonvulsant, suggested as a potential treatment for epilepsy [51]. The combination of these three powerful plant products in the form of commercial phytogenic supplement is expected to display a combination of the benefits of its basic ingredients. A wide range of these benefits was reported using this exact phytogenic product in a range of agriculture significant species such as fish [52, 53], cattle [54, 55], rabbit [56, 57], pig [58] and chicken [59].
Nutrigenomics is a science investigating the effects of food on gene expression, and it has experienced a significant revival with microarray technology being rapidly replaced with more accurate and informative RNAseq. Nutrigenomics methodologies are proving as the ultimate methodology for investigating general effects of food composition, supplements and additives on the host organ health and disease predisposition. Advances in bioinformatics and the rise in machine learning allow the use of transcriptomics to predict the long term effects nutritional intervention has on performance, immune system function, metabolism and organ toxicity, to name a few. In this manuscript, we present the nutrigenomic effects of prolonged use of this product on ileum transcriptomics in layers grown in the open-range production system.
2. Materials and methods
2.1. Animal trial and bird management
The study was approved by the Animal Ethics Committee of Central Queensland University under the approval number 0000020312.
The experiment was performed in a large commercial egg-producing farm, using the open range shed physically split into two sides with a wall (control and treatment), and each side housing 20,000 birds. The mesh wire separated the two treatments outdoor in the open range. The feed used was designed by the company nutritionist to meet the production and animal welfare requirements. The commercial phytogenic supplement is a mix of essential oil extracts and herbs with menthol, carvacrol and carvone as major ingredients. The supplement was added to the feed at the concentration of 150 g/t.
2.2. Histology
The tissue samples were collected from the mid-ileum section and fixed in 10% buffered formalin solution. Fixation and paraffin embedding were outsourced to a commercial pathology laboratory (QML Pathology), and deparaffinisation, rehydration and staining with hematoxylin and eosin (H&E), were done by standard laboratory procedures. The slides were scanned in a commercial facility, and images were analysed using ZEISS ZEN software.
2.3. Transcriptomics
The RNA from tissue samples was isolated using TRIsure (Cat# BIO-38033, Bioline Meridian Bioscience, London, UK) and Isolate II RNA Mini Kit (Bioline, Cat# BIO-52072). Initially, around 100 mg of tissue was homogenised using OMNI tissue homogeniser TH (OMNI International, Kennesaw, GA, USA) with 1 ml of TRIsure and supernatant with RNA was separated from tissue residue with centrifugation. Phase separation with 200 μL of chloroform and centrifugation was done to separate RNA from DNA and protein in an aqueous phase. The RNA was then purified using Isolate II RNA Mini Kit (Bioline, Cat# BIO-52072) following manufacturer's protocol. The sequencing was performed in Azenta Life Sciences, China, on Novaseq 600 150bp PE., with the sequence library preparation using TruSeq RNA Library Prep Kit v2 #RS-122-2001 (Illumina). Raw sequence data from this study are available at NCBI Sequence Read Archive (SRA) database (https://www.ncbi.nlm.nih.gov/sra) under accession number PRJNA809869.
2.4. Data analysis, software and statistics
QIAGEN CLC Genomics Workbench (Qiagen, Hilden, Germany) and R were used to analyse RNA sequence data. Paired sequences were aligned to the chicken genome (Gallus_gallus-5.0), using CLC Genomics Workbench recommended settings to assign reads to genes and transcripts. The resulting gene count matrix was analysed with DeSeq2 in R to identify differentially expressed genes. The pathway analysis was down using Qiagen's Ingenuity Pathway Analysis (IPA) application using the IPA significance cut-offs as indicated in the results section below.
3. Results
3.1. Production parameters
Briefly, the birds on both control and phytogen supplemented food presented good performance and health with a steady egg production across the production cycle. Cumulative mortality and the cumulative number of dirty eggs were marginally lower in phytogen supplemented group, while other measured performance parameters such as rate of lay (ROL), egg production, feed consumption, feed conversion (kg of feed used to produce one dozen eggs), bird body weight, egg weight, eggshell thickness, eggshell Haugh units, yolk colour and eggshell strength were not affected. The table with all performance measures is provided in Supplementary Data File 1.
3.2. Sequencing quality control
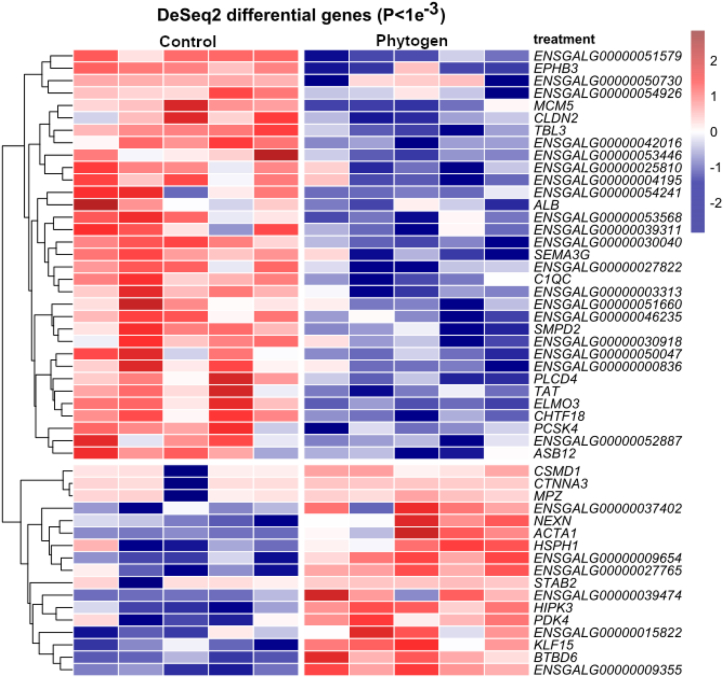
Five replicates from each control and phytogen were sequenced, aiming at 50 million quality-filtered sequences per sample. The total number of quality-filtered sequences per sample was 524.98 million sequences, with an average of 51.7 million for control (53.29, 50.28, 59.56, 46.43 and 48.79 million) and 53.27 million sequences per sample for phytogen (65.14, 56.2, 40.77, 52.71 and 51.44 million). All filtered sequences were 150 nt long, with zero ambiguous and minimal PHRED quality score of 20, and 97% sequences with PHRED >37. There were 14,410 genes represented with more than 10 sequences. Figure S1 of the Supplementary data shows DeSeq2 differential genes Volcano plot and PCA plot. There were 47 genes shown in Figure 1 heatmap, with DeSeq2 P < 0.001. The Qiagen's Ingenuity Pathway Analysis (IPA) software (Qiagen, Hilden, Germany) had annotations for the 11,582 genes from the dataset. Genes with absolute fold change >1.2 and DeSeq2 P < 0.05, totalling 549 genes, were considered in IPA statistical analysis. Histological analysis did not show significant differences in ileum epithelial morphology or villus height and crypt depth (Figure S2 of the Supplementary Data File 1).
Figure 1.
Heatmap showing the genes most highly differentially expressed (P < 0.001) between the control and phytogen treatment. The colour scale shows log2 transformed Deseq2 normalised gene abundance.
3.3. Pathway analysis
The Qiagen's Ingenuity Pathway Analysis (IPA) is based on over 10 million manually curated gene expression annotations in the Ingenuity Knowledge Base (IKB) [60] commercial database [7]. IPA was performed to identify significantly altered pathways (P < 0.01) and to differentiate if the pathways are activated (positive z-score) or inhibited (negative z-score). The IPA z-score statistics not only rely on whether the genes are up or down-regulated, but it also looks at the directionality of the interaction; for example, sometimes upregulation (of the pathway-inhibiting gene) can reduce the pathway. The complete list of the significantly altered pathways with P-values, z-scores and genes involved is provided in Supplementary Data File 1.
The most highly activated pathways (P < 0.05, z > 2) were FGF signalling, calcium signalling, G beta-gamma signalling, cell cycle: G2/M DNA damage checkpoint regulation, Huntington's disease signalling, white adipose tissue browning pathway, systemic lupus erythematosus in B cell signalling pathway, ILK signalling, AMPK signalling, integrin signalling, role of NFAT in cardiac hypertrophy, bladder cancer signalling, androgen signalling, glioma signalling, JAK/Stat signalling, CXCR4 signalling, telomerase signalling, HMGB1 signalling, Wnt/Î2-catenin signalling, ovarian cancer signalling, role of NFAT in the regulation of the immune response and colorectal cancer metastasis signalling, indication that the product was enhancing of a range of signalling pathways.
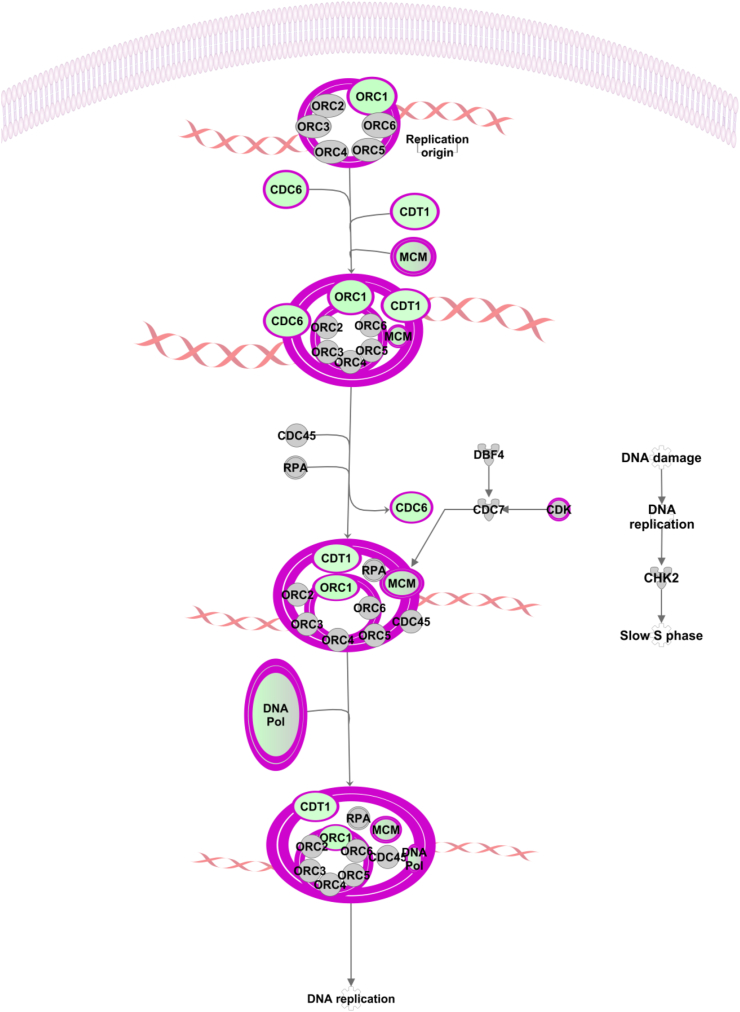
There were only two significantly (P < 0.05, z < -2) inhibited pathways: T cell exhaustion signalling pathway and cell cycle control of chromosomal replication presented in Figure 2.
Figure 2.
The cell cycle control of the chromosomal replication pathway was significantly (P = 3.46e−4) inhibited (z = -2.65). Genes and groups of genes coloured green are downregulated in the phytogen group.
3.4. Upstream regulators
IPA Upstream Analysis was used to predict overlapping effects on gene expression with all known receptors, micro-RNA, enzymes, transcription factors, chemicals, drugs, and other molecules annotated for their effects on gene expression in the IPK database. It is important to note that enzymes or transcription factors listed as activated or inhibited, in the same manner as chemicals or drugs, do not have to be expressed at all in the current dataset as long as they can regulate a significant subset of the genes in the same or the opposite way. They simply target the same genes. For example, if a transcription factor (that is not produced in chicken) from another host can regulate the same genes (based on the gene name) in the same direction, it would come up as activated in our treatment, indicating that they positively overlap in their effects on gene expression and could activate or enhance the effects of one another. For gene products like enzymes and transcription factors, Supplementary File 1 shows if they were present/expressed in our dataset and provide log2 fold change value.
In the order of z-scores, from the most activated, the upstream regulators significantly (P < 0.05, z > 2) activated include: l-asparaginase, SMARCA4, NUPR1, let-7, SMARCB1, tretinoin, TP53, RB1, calcitriol, GATA1, LEF1, 2-(4-amino-1-isopropyl-1H-pyrazolo[3,4-d]pyrimidin-3-yl)-1H-indol-5-ol, valproic acid, Rb, RET, medroxyprogesterone acetate, GDF2, TEAD1, progesterone, MRTFB, TCF3, SP1, AR, TEAD3, MEF2D, 2-amino-1-methyl-6-phenylimidazo-4-5-b-pyridine, CLEC4G, phorbol esters, MRTFA, doxorubicin, TGFB3, CDKN2A, IL10RA, ESR1, HSF1, PGR, SPI1, TEAD2, FASN, PDGFB, ciprofibrate, D-galactosamine, CXCL12, SOX10, RBL1, ZFP36, deferoxamine, fulvestrant, CDKN1B, Tcf7, BMP4, decitabine, troglitazone, ionomycin, DPP-23, TEAD4, Irgm1, TOB1, TRPS1.
Inhibited upstream regulators are the molecules that regulate gene expression in the opposite way to the treatment and could inhibit or deactivate one another. Significantly inhibited upstream regulators (P < 0.05, z < -2) include CSF2, TBX2, RABL6, ERBB2, EP400, MITF, SGPP2, PKD1, CCND1, camptothecin, 26s Proteasome, PTGER2, ZNF217, FFAR3, vitamin E, TFRC, REST, E2f, HGF, cycloheximide, FGF1, ACOX1, ACTB and histone deacetylase.
3.5. Diseases and functions
Based on the same principles as in pathways and upstream regulators, the diseases and functions analysis provides a list of activated and inhibited diseases and functions, while the toxicology function looks at possible activation or inhibition of toxic effects on major organs.
Highly phytogen-increased categories (P < 0.01, z > 2) include development of vasculature, transport of molecule, angiogenesis, vasculogenesis, engulfment of cells, migration of vascular endothelial cells, movement of vascular endothelial cells, aggregation of cells, endocytosis by eukaryotic cells, internalisation by tumour cell lines, cellular homeostasis, development of endothelial tissue, autophagy, engulfment of tumour cell lines, development of epithelial tissue, assembly of cells, the function of muscle, endocytosis, binding of lipid, while reduced diseases and functions include disorder of blood pressure, organismal death, morbidity or mortality, ploidy of cells, dysglycemia and amplification of centrosome.
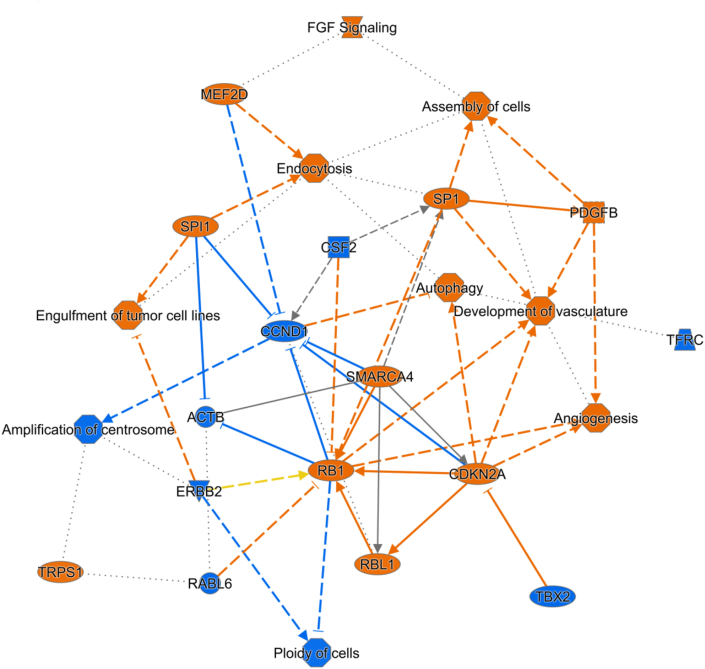
In toxicity analysis, we only noted two strongly phytogen-inhibited (P < 0.01, z < -2) categories: apoptosis of ventricular myocytes and congenital heart disease. Other significantly inhibited (P < 0.05, z < -1) toxicities include heart failure, pulmonary hypertension, dilation of left ventricle, cell death of tubular cells, hepatic steatosis, fibrosis of heart, atrial or ventricular septal defect, apoptosis of renal tubule, necrosis of cardiac muscle, myocardial infarction, hypoplasia of heart ventricle, apoptosis of kidney cells, cell death of liver cells. Figure 3 integrates the findings at the gene expression, upstream regulation and disease/functional profile into a network summarising the most significant effects of the phytogen. There were no predicted toxic functions significantly increased by phytogen.
Figure 3.
Network summarising transcriptomic effects of phytogen on genes, diseases and functions most altered by the phytogen supplementation. Orange colour indicates activation and blue inhibition.
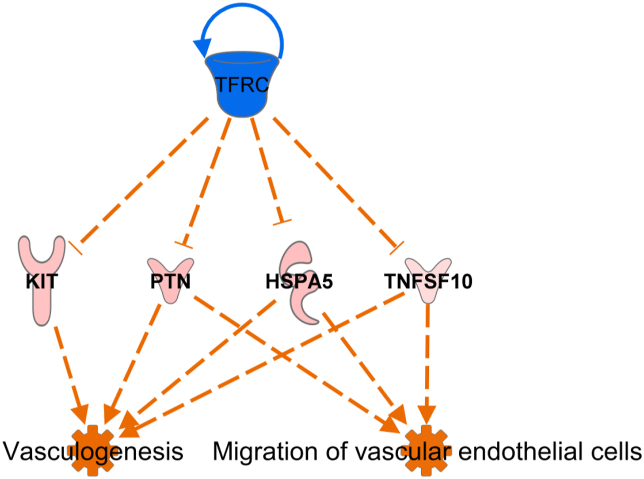
Both disease & function, and toxicology analysis indicate beneficial effects on the heart and cardiovascular system. Further, master regulator prediction analysis predicted RET, CXCL12, 26s Proteasome and SGPP2 and possible master regulators involved in increased autophagy transport and endocytosis (Supplementary File 1; Figure S3) while TFRC was predicted to be major vasculogenesis controller in phytogen supplemented birds (Figure 4).
Figure 4.
Transferrin receptor TFRC is one of the predicted master regulators of the vasculature related genes and functions. TFRC was downregulated by phytogen, and its inhibition is predicted to activate KIT, PTN, HSPA5 and TNFSF10 that were all upregulated by phytogen in the dataset. The upregulation of these genes leads to activation of vasculogenesis and migration of vascular endothelial cells.
3.6. Analysis match
Analysis Match is an IPA function that investigates the significance of overlap of the current dataset with over 100,000 manually curated publicly available gene expression datasets and any datasets from the authors' private datasets. This allows us to predict possible alternative uses of our treatment, for example, in our case, if the effects of phytogen have a positive overlap score with another dataset, like new drug clinical trial data, depending on the significance of overlap, it is likely that the phytogen would be able to reproduce a significant portion of the effects of the drug. If the public dataset on genes altered by certain diseases has significant negative overlap with our phytogen treatment, it is likely that the phytogen could be used to treat the disease or at least be offered as a supplementary treatment.
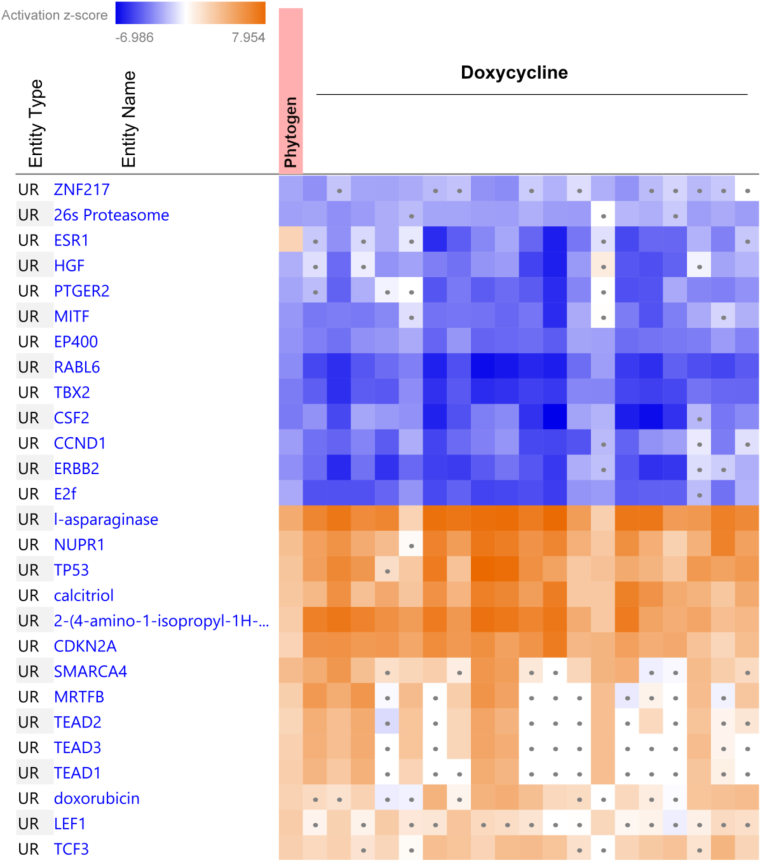
Among the treatments with the highest positive overlap were several known drugs; however, most interesting is a positive upstream regulator overlap with two clinical antibiotics, doxycycline and geldanamycin. There were 102 significantly (P < 0.01 and z > 10) overlapping doxycycline (Figure 5) and geldanamycin datasets (Supplementary File 1).
Figure 5.
The heatmap of positive overlap of current phytogen dataset with the top 20 most significantly correlated doxycycline datasets. The top overlapping upstream regulators (UR) with z-score lower than two are considered insignificant and indicated with a dot.
It is important to note that this is an overlap of transcriptomic effects of antibiotics on tissues in different mammals with the effects of phytogen on chicken ileum gene expression, and it does not imply an overlap of effects on bacteria, ie., it does not mean that phytogen acts as doxycycline on gut bacteria.
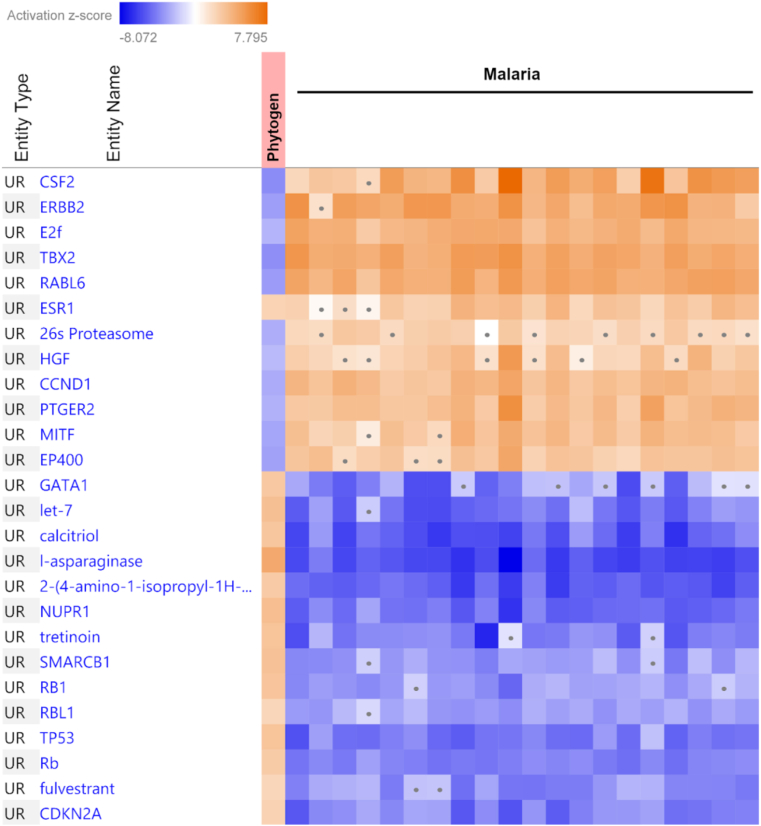
The datasets that significantly negatively overlapped with phytogen transcriptomic mode of action included 60 public datasets on malaria (P-value from 0.01 to 3.7e−39). The top 20 hits are presented in Figure 6. Significantly negative overlap with viral infections including influenza, dengue and Zika virus is provided in Supplementary File 1.
Figure 6.
The heatmap of negative overlap of current phytogen dataset with the top 20 most significantly correlated malaria datasets. The top overlapping upstream regulators (UR). The z-score > -2 was considered insignificant and indicated on the heatmap with a dot.
4. Discussion
Nutrigenomic research that focuses on a mixed ingredient product can show effects different to outcomes of single original ingredients due to more complex product to host interactions. The phytogenic product we investigated contains three base compounds that have multiple strong health beneficial effects to a level that outshines their central purpose of antimicrobial action. Here we detected a combination of benefits reported in products’ individual ingredients strongly presented in the chicken ileum transcriptomics.
One of the most affected pathways was Cell Cycle Control of Chromosomal Replication (Figure 2). There were seven downregulated genes in this pathway CDC6, CDK2, CDT1, MCM5, ORC1, POLE and TOP2A (Supplementary data and Figure 2), and they are all robust regulators of different stages of cell cycle and their upregulation is associated with increased cancer susceptibility. For example, CDC6 or cell division cycle 6 protein, is essential for the initiation of DNA replication and functions as a regulator at the early steps of DNA replication (Entrez Gene Summary) [61], and according to IPK database, it is associated with over 30 cancer categories including liver cancer, invasive breast cancer, epithelial and colon cancer [60]. Cyclin dependent kinase 2 (CDK2) regulates progression through the cell cycle, and there are 13 registered CDK inhibitors used as anticancer drugs, including alvocidib, dinaciclib and roscovitine. MCM5 is also involved in the initiation of DNA replication. POLE or DNA polymerase epsilon, is involved in DNA repair and chromosomal replication. DNA topoisomerase II alpha (TOP2A) is associated with more than 200 cancer categories, and more than 100 registered drugs target this protein as inhibitors or chain breakers. Cancer is characterised with out of control cell growth cycle, and inhibition of these 7 cell cycle associated genes that already have a range of anticancer inhibitors developed, is in support of anticancer effects reported for all 3 major ingredients of the phytogenic product [13, 27, 45].
Among significantly activated (P < 0.01 and z-score > 2) upstream regulators that have a comparative effect on gene regulation to the phytogen and could enhance each other's mode of action, we are reporting a number of anticancer drugs, including tretinoin, doxorubicin, hormonal antineoplastic fulvestrant, in line with the known anticancer effects of the main components. Activated effects of calcium regulator calcitriol is in agreement with menthol stimulation of calcium absorption [6]. Additionally, activation of antiepileptic and mood stabilising drug valproic acid is in agreement with antiepileptic effects of carvone [62] and mood stabilising with antidepressant effects of menthol [63] and carvacrol [30]. Activation of effects of antiarthritic drug infliximab is in alignment with anti-arthritis effects of carvone [44].
Based on the inhibited upstream regulators, the phytogenic product is likely to mutually reverse or inhibit the effects of anticancer drug camptothecin, immunomodulator triptolide and fungicide cycloheximide. It was previously reported that oregano supplementation of broilers resulted in massive perturbations of genes and categories involved in cancer, with predicted reduction of genitourinary cancers like breast and prostate while some other cancers transcriptional profiles were activated [16]. Similar effects on the specific types of cancers and sex hormone related regulators and drugs are noted, with an overlap of carvacrol being one of the main chemicals in both oregano and phytogenic product investigated here. Immunomodulator triptolide is effective against polycystic kidney disease and pancreatic cancer, but its severe toxicity limits its potential use. Inhibition of this potent toxin could indicate antitoxic effects against other plant-derived toxins.
Neurotransmitter dopamine transcriptional regulation effects were inhibited by the phytogen. This is interesting as all three components of the phytogenic product have a range of anti-depressive and calming effects, as presented in the introduction section. Dopamine has major role in mental health and either too much or too little of dopamine will cause significant health issues. For example, it is believed that schizophrenia originates from a hyperactive dopamine system. The roles of dopamine include drug addiction, Parkinson disease, obesity, gut health immune system and much more, reviewed in Franco et al. [64].
Insulin resistance drug 10E,12Z-octadecadienoic acid and several other drugs listed in Supplementary data could be inhibited or activated using phytogen. Although this has little relevance to poultry use of phytogens, it reinstates the need to be cautious with the use of presumably healthy natural products and essential oils in serious medical conditions or in combination with prescription drugs in humans.
The most exciting results came from disease and function transcriptional enrichment analysis. Among strongly activated functions, we can note that the phytogen did not increase the predisposition to any of the diseases; on the contrary, it enhanced a range of beneficial functions such as angiogenesis and vasculogenesis, homeostasis, engulfment of tumour cells, the function of muscle and autophagy to name a few (Supplementary data) while inhibiting non-desirable functions like dysglycemia (abnormalities in blood glucose levels), morbidity and mortality, disorders of blood pressure and more. Vasculogenesis and other cardiovascular beneficial functions enhanced by phytogen are in agreement with previously reported cardioprotective effects of phytogens main ingredients menthol and carvacrol [7, 36]. Reduction in mortality related functions is in agreement with our trial data showing consistently reduced mortality in phytogen shed from the second week of phytogen administration.
Increase in autophagy (P = 1.37e−4, z-score = 2.3) function is encouraging. Autophagy is believed to occur in the absence of external food sources when the body responds by eating itself, destroying and recycling its own damaged and old cell to rebuild a new, more energy-efficient, younger self. Autophagy is a burning topic in human health, with 3171 manuscripts published in the first eight months of 2021 with the word autophagy in the title (PubMed). Since the award of the 2016 Nobel Prize in Physiology or Medicine to Japanese researcher Yoshinori Ohsumi for his work on defining autophagy and a major health regenerative process [65], extreme fasting to induce autophagy for health benefits is now often suggested in nearly every known disease, including cancer [66] and COVID-19 [67]. Severe fasting to induce autophagy became the latest obsession of healthy lifestyle activists [68], and fasting to induce intestinal autophagy was recently done in broilers [69]. Increasing autophagy using phytogen without fasting can be regarded as one of the major health benefits of phytogen supplementation with a high potential for implications in animal welfare.
Finally, the results of the analysis match did not come as a surprise. The prediction that the phytogen has transcriptional effects highly similar to those of well-known antibiotics is something that is universally accepted by farmers who see phytogens as a viable antibiotic alternative. The predicted anti-malarial effects across 60 datasets are in alignment with the study suggesting carvacrol or its potential derivate could be used as a potent anti-malarial agent against Plasmodium falciparum [23], and antiviral effects of the main components of the phytogen were previously discussed.
5. Conclusions
Advances in nutrigenomic studies are revealing a new understanding of how complex the nutrigenomic effects of potent natural chemicals can be. This research brings caution to the use of phytogens and essential oils as human supplements due to potential interference with preexisting conditions or prescription drugs. On the other hand, extra health benefits provided by the present pathogen for poultry are yet to be thoroughly evaluated. Autophagy without fasting, anticancer effects, gastrointestinal health-promoting, cellular and vascular regeneration and immunoprotection are all highly relevant to poultry welfare and more so in highly health challenging, pathogen exposed open and free-range productions setups, perceived by consumers as more bird beneficial than they are in reality.
Declarations
Author contribution statement
Yadav S. Bajagai: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Friedrich Petranyi; Darwin Horyanto; Romeo Batacan Jr; Edina Lobo; Xipeng Ren; Maria M. Whitton; Sung J. Yu; Advait Kayal: Analyzed and interpreted the data.
Dragana Stanley: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Central Queensland University Merit Grant scheme.
Data availability statement
Data associated with this study has been deposited at NCBI SRA database under the accession number PRJNA809869.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The data was analysed using the Marie Curie High-Performance Computing System at Central Queensland University. We wish to acknowledge and appreciate the help Jason Bell provided in all aspects of High-Performance Computing.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Marshall B.M., Levy S.B. Food animals and antimicrobials: impacts on human health. Clin. Microbiol. Rev. 2011;24(4):718–733. doi: 10.1128/CMR.00002-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chattopadhyay M.K. Use of antibiotics as feed additives: a burning question. Front. Microbiol. 2014;5:334. doi: 10.3389/fmicb.2014.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O'Neill . 2016. J. The Review On Antimicrobial Resistance. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations.http://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdfhttp://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf Available at: Available from: [Google Scholar]
- 4.Qiu J., et al. Menthol diminishes Staphylococcus aureus virulence-associated extracellular proteins expression. Appl. Microbiol. Biotechnol. 2011;90(2):705–712. doi: 10.1007/s00253-011-3122-9. [DOI] [PubMed] [Google Scholar]
- 5.Shahverdi A.R., et al. Inhibition of nitrofurantoin reduction by menthol leads to enhanced antimicrobial activity. J. Chemother. 2003;15(5):449–453. doi: 10.1179/joc.2003.15.5.449. [DOI] [PubMed] [Google Scholar]
- 6.Geiger S., et al. Menthol stimulates calcium absorption in the rumen but not in the jejunum of sheep. J. Dairy Sci. 2021;104(3):3067–3081. doi: 10.3168/jds.2020-19372. [DOI] [PubMed] [Google Scholar]
- 7.Silva H. Current Knowledge on the vascular effects of menthol. Front. Physiol. 2020;11:298. doi: 10.3389/fphys.2020.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillis D.J., et al. Influence of menthol on recovery from exercise-induced muscle damage. J. Strength Condit Res. 2020;34(2):451–462. doi: 10.1519/JSC.0000000000002833. [DOI] [PubMed] [Google Scholar]
- 9.Hu Y., Li M. [Menthol promotes cytokine secretion in human bronchial epithelial BEAS-2B cells by activating transient receptor potential melastatin 8 (TRPM8)] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2021;37(7):577–584. [PubMed] [Google Scholar]
- 10.Jeffries O., Waldron M. The effects of menthol on exercise performance and thermal sensation: a meta-analysis. J. Sci. Med. Sport. 2019;22(6):707–715. doi: 10.1016/j.jsams.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Garcia A.K.A., et al. Menthol chewing gum on preoperative thirst management: randomised clinical trial. Rev. Lat. Am. Enfermag. 2019;27:e3180. doi: 10.1590/1518-8345.3070.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sanders O.D., Rajagopal J.A., Rajagopal L. Menthol to induce non-shivering thermogenesis via TRPM8/PKA signaling for treatment of obesity. J. Obes. Metab. Syndr. 2021;30(1):4–11. doi: 10.7570/jomes20038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luo L., et al. The effect of menthol supplement diet on colitis-induced colon tumorigenesis and intestinal microbiota. Am. J. Transl. Res. 2021;13(1):38–56. [PMC free article] [PubMed] [Google Scholar]
- 14.Kikuchi H., et al. Effectiveness of L-menthol spray application on lesions for the endoscopic clarification of early gastric cancer: evaluation by the color difference. Digestion. 2021;102(2):274–282. doi: 10.1159/000504667. [DOI] [PubMed] [Google Scholar]
- 15.Choi I.S., et al. Menthol facilitates excitatory and inhibitory synaptic transmission in rat medullary dorsal horn neurons. Brain Res. 2021;1750:147149. doi: 10.1016/j.brainres.2020.147149. [DOI] [PubMed] [Google Scholar]
- 16.Bajagai Y.S., et al. Prolonged continual consumption of oregano herb interferes with the action of steroid hormones and several drugs, and effects signaling across the brain-gut axis. Food Funct. 2021;12(2):726–738. doi: 10.1039/d0fo02988b. [DOI] [PubMed] [Google Scholar]
- 17.Kachur K., Suntres Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020;60(18):3042–3053. doi: 10.1080/10408398.2019.1675585. [DOI] [PubMed] [Google Scholar]
- 18.Nostro A., et al. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007;56(Pt 4):519–523. doi: 10.1099/jmm.0.46804-0. [DOI] [PubMed] [Google Scholar]
- 19.Walczak M., et al. Potential of carvacrol and thymol in reducing biofilm formation on technical surfaces. Molecules. 2021;26(9) doi: 10.3390/molecules26092723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang K., et al. Synthesis and antifungal activity of carvacrol and thymol esters with heteroaromatic carboxylic acids. Nat. Prod. Res. 2019;33(13):1924–1930. doi: 10.1080/14786419.2018.1480618. [DOI] [PubMed] [Google Scholar]
- 21.Felici M., et al. In vitro anticoccidial activity of thymol, carvacrol, and saponins. Poultry Sci. 2020;99(11):5350–5355. doi: 10.1016/j.psj.2020.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen G.Q., et al. Synthesis of sulfonate derivatives of carvacrol and thymol as anti-oomycetes agents. J. Asian Nat. Prod. Res. 2021;23(7):692–702. doi: 10.1080/10286020.2020.1758675. [DOI] [PubMed] [Google Scholar]
- 23.Uddin A., et al. Identification and structure-activity relationship (SAR) studies of carvacrol derivatives as potential anti-malarial against Plasmodium falciparum falcipain-2 protease. Bioorg. Chem. 2020;103:104142. doi: 10.1016/j.bioorg.2020.104142. [DOI] [PubMed] [Google Scholar]
- 24.Goncalves R.R.P., et al. Nat Prod Res; 2020. Acetylation of Carvacrol Raises its Efficacy against Engorged Cattle Ticks Rhipicephalus (Boophilus) Microplus (Acari: Ixodidae) pp. 1–5. [DOI] [PubMed] [Google Scholar]
- 25.Youssefi M.R., et al. Efficacy of two monoterpenoids, carvacrol and thymol, and their combinations against eggs and larvae of the west nile vector Culex pipiens. Molecules. 2019;24(10) doi: 10.3390/molecules24101867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Y., et al. Efficacy of Origanum vulgare essential oil and carvacrol against the housefly, Musca domestica L. (Diptera: muscidae) Environ. Sci. Pollut. Res. Int. 2019;26(23):23824–23831. doi: 10.1007/s11356-019-05671-4. [DOI] [PubMed] [Google Scholar]
- 27.Sampaio L.A., et al. Antitumor effects of carvacrol and thymol: a systematic review. Front. Pharmacol. 2021;12:702487. doi: 10.3389/fphar.2021.702487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li L., et al. Carvacrol affects breast cancer cells through TRPM7 mediated cell cycle regulation. Life Sci. 2021;266:118894. doi: 10.1016/j.lfs.2020.118894. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad A., Ansari I.A. Carvacrol exhibits chemopreventive potential against cervical cancer cells via caspase-dependent apoptosis and abrogation of cell cycle progression. Anti Cancer Agents Med. Chem. 2020 doi: 10.2174/1871520621999201230201258. [DOI] [PubMed] [Google Scholar]
- 30.Naeem K., et al. Natural dietary supplement, carvacrol, alleviates LPS-induced oxidative stress, neurodegeneration, and depressive-like behaviors via the Nrf2/HO-1 pathway. J. Inflamm. Res. 2021;14:1313–1329. doi: 10.2147/JIR.S294413. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Mortazavi A., et al. The effects of carvacrol on oxidative stress, inflammation, and liver function indicators in a systemic inflammation model induced by lipopolysaccharide in rats. Int. J. Vitam. Nutr. Res. 2021:1–11. doi: 10.1024/0300-9831/a000711. [DOI] [PubMed] [Google Scholar]
- 32.de Carvalho F.O., et al. Anti-inflammatory and antioxidant activity of carvacrol in the respiratory system: a systematic review and meta-analysis. Phytother Res. 2020;34(9):2214–2229. doi: 10.1002/ptr.6688. [DOI] [PubMed] [Google Scholar]
- 33.El-Gendy Z.A., et al. Carvacrol hinders the progression of hepatic fibrosis via targeting autotaxin and thioredoxin in thioacetamide-induced liver fibrosis in rat. Hum. Exp. Toxicol. 2021 doi: 10.1177/09603271211026729. 9603271211026729. [DOI] [PubMed] [Google Scholar]
- 34.Zhao W., et al. Protective effect of carvacrol on liver injury in type 2 diabetic db/db mice. Mol. Med. Rep. 2021;24(5) doi: 10.3892/mmr.2021.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao W., et al. Carvacrol may alleviate vascular inflammation in diabetic db/db mice. Int. J. Mol. Med. 2020;46(3):977–988. doi: 10.3892/ijmm.2020.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hou N., et al. Carvacrol attenuates diabetic cardiomyopathy by modulating the PI3K/AKT/GLUT4 pathway in diabetic mice. Front. Pharmacol. 2019;10:998. doi: 10.3389/fphar.2019.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zamanian M., et al. CNS Neurol Disord Drug Targets; 2021. Carvacrol as a Potential Neuroprotective Agent for Neurological Diseases: A Systematic Review Article. [DOI] [PubMed] [Google Scholar]
- 38.de Souza M.M., et al. Structure-activity relationships of sulfonamides derived from carvacrol and their potential for the treatment of Alzheimer's disease. RSC Med. Chem. 2020;11(2):307–316. doi: 10.1039/d0md00009d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javed H., et al. Carvacrol, a plant metabolite targeting viral protease (M(pro)) and ACE2 in host cells can Be a possible candidate for COVID-19. Front. Plant Sci. 2020;11:601335. doi: 10.3389/fpls.2020.601335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan Y.W., et al. Plasma polymerised carvone as an antibacterial and biocompatible coating. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;68:861–871. doi: 10.1016/j.msec.2016.07.040. [DOI] [PubMed] [Google Scholar]
- 41.Mun S.H., et al. In vitro anti-MRSA activity of carvone with gentamicin. Exp. Ther. Med. 2014;7(4):891–896. doi: 10.3892/etm.2014.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sousa C., et al. Elucidation of the mechanism underlying the anti-inflammatory properties of (S)-(+)-Carvone identifies a novel class of sirtuin-1 activators in a murine macrophage cell line. Biomedicines. 2021;9(7) doi: 10.3390/biomedicines9070777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu X., et al. Protective effect of D-carvone against dextran sulfate sodium induced ulcerative colitis in balb/c mice and LPS induced RAW cells via the inhibition of COX-2 and TNF-alpha. J. Environ. Pathol. Toxicol. Oncol. 2020;39(3):235–245. doi: 10.1615/JEnvironPatholToxicolOncol.2020031860. [DOI] [PubMed] [Google Scholar]
- 44.Chen G., et al. Antiarthritic activity of D-carvone against complete Freund's adjuvant-induced arthritis in rats through modulation of inflammatory cytokines. KOREAN J. PHYSIOL. PHARMACOL. 2020;24(6):453–462. doi: 10.4196/kjpp.2020.24.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ding X., Chen H. Anticancer effects of Carvone in myeloma cells is mediated through the inhibition of p38 MAPK signalling pathway, apoptosis induction and inhibition of cell invasion. J. BUON. 2018;23(3):747–751. [PubMed] [Google Scholar]
- 46.Asle-Rousta M., Amini R., Aghazadeh S. Carvone suppresses oxidative stress and inflammation in the liver of immobilised rats. Arch. Physiol. Biochem. 2020:1–6. doi: 10.1080/13813455.2020.1851726. [DOI] [PubMed] [Google Scholar]
- 47.Abbas M.M., Kandil Y.I., Abbas M.A. R-(-)-carvone attenuated doxorubicin induced cardiotoxicity in vivo and potentiated its anticancer toxicity in vitro. Balkan Med. J. 2020;37(2):98–103. doi: 10.4274/balkanmedj.galenos.2019.2019.7.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aydin E., Turkez H., Keles M.S. Potential anticancer activity of carvone in N2a neuroblastoma cell line. Toxicol. Ind. Health. 2015;31(8):764–772. doi: 10.1177/0748233713484660. [DOI] [PubMed] [Google Scholar]
- 49.Sanchez-Borzone M., Delgado-Marin L., Garcia D.A. Inhibitory effects of carvone isomers on the GABAA receptor in primary cultures of rat cortical neurons. Chirality. 2014;26(8):368–372. doi: 10.1002/chir.22328. [DOI] [PubMed] [Google Scholar]
- 50.Goncalves J.C., et al. Distinct effects of carvone analogues on the isolated nerve of rats. Eur. J. Pharmacol. 2010;645(1-3):108–112. doi: 10.1016/j.ejphar.2010.07.027. [DOI] [PubMed] [Google Scholar]
- 51.Marques T.H., et al. Anticonvulsant effects of acute treatment with cyane-carvone at repeated oral doses in epilepsy models. Pharmacol. Biochem. Behav. 2014;124:421–424. doi: 10.1016/j.pbb.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Kord M.I., et al. The immunostimulatory effects of commercial feed additives on growth performance, non-specific immune response, antioxidants assay, and intestinal morphometry of nile tilapia, Oreochromis niloticus. Front. Physiol. 2021;12:627499. doi: 10.3389/fphys.2021.627499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peterson B.C., et al. Phytogenic feed-additive effects on channel catfish rhamnose-binding lectin levels, and susceptibility to Edwardsiella ictaluri. Dis. Aquat. Org. 2018;129(2):99–106. doi: 10.3354/dao03235. [DOI] [PubMed] [Google Scholar]
- 54.Brand T., et al. Impact of a phytogenic feed additive on growth performance, feed intake, and carcass traits of finishing steers. Transl. Anim. Sci. 2019;3(4):1162–1172. doi: 10.1093/tas/txz109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Akbarian-Tefaghi M., Ghasemi E., Khorvash M. Performance, rumen fermentation and blood metabolites of dairy calves fed starter mixtures supplemented with herbal plants, essential oils or monensin. J. Anim. Physiol. Anim. Nutr. 2018;102(3):630–638. doi: 10.1111/jpn.12842. [DOI] [PubMed] [Google Scholar]
- 56.Mattioli S., et al. The effect of dietary Digestarom(R) herbal supplementation on rabbit meat fatty acid profile, lipid oxidation and antioxidant content. Meat Sci. 2016;121:238–242. doi: 10.1016/j.meatsci.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 57.Celia C., et al. Effect of pre- and post-weaning dietary supplementation with Digestarom(R) herbal formulation on rabbit carcass traits and meat quality. Meat Sci. 2016;118:89–95. doi: 10.1016/j.meatsci.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 58.Nowland T.L., et al. Maternal supplementation with phytogenic additives influenced the faecal microbiota and reproductive potential in sows. Amb. Express. 2021;11(1):107. doi: 10.1186/s13568-021-01268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murugesan G.R., et al. Phytogenic feed additives as an alternative to antibiotic growth Promoters in broiler chickens. Front. Vet. Sci. 2015;2:21. doi: 10.3389/fvets.2015.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Qiagen H. 2021. Germany. Ingenuity Knowledge Base.https://digitalinsights.qiagen.com/products-overview/ Available from. [Google Scholar]
- 61.Information, N.C.f.B. 2021. https://www.ncbi.nlm.nih.gov/gene Available from. [Google Scholar]
- 62.Costa D.A., et al. Anticonvulsant and antioxidant effects of cyano-carvone and its action on acetylcholinesterase activity in mice hippocampus. Cell. Mol. Neurobiol. 2012;32(4):633–640. doi: 10.1007/s10571-012-9812-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang W., et al. L-menthol exhibits antidepressive-like effects mediated by the modification of 5-HTergic, GABAergic and DAergic systems. Cogn. Neurodyn. 2019;13(2):191–200. doi: 10.1007/s11571-018-9513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Franco R., Reyes-Resina I., Navarro G. Dopamine in health and disease: much more than a neurotransmitter. Biomedicines. 2021;9(2) doi: 10.3390/biomedicines9020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24(1):9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Antunes F., et al. Autophagy and intermittent fasting: the connection for cancer therapy? Clinics. 2018;73(suppl 1):e814s. doi: 10.6061/clinics/2018/e814s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannan M.A., et al. Intermittent fasting, a possible priming tool for host defense against SARS-CoV-2 infection: crosstalk among calorie restriction, autophagy and immune response. Immunol. Lett. 2020;226:38–45. doi: 10.1016/j.imlet.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bagherniya M., et al. The effect of fasting or calorie restriction on autophagy induction: a review of the literature. Ageing Res. Rev. 2018;47:183–197. doi: 10.1016/j.arr.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 69.Wang Y., et al. Impact of different durations of fasting on intestinal autophagy and serum metabolome in broiler chicken. Animals (Basel) 2021;11(8) doi: 10.3390/ani11082183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study has been deposited at NCBI SRA database under the accession number PRJNA809869.