Highlights
-
•
There are currently no sex-specific guidelines for evaluation and management of lipids.
-
•
Lipids are impacted by normal hormonal changes in women throughout their life cycle.
-
•
Management of lipids should incorporate sex-specific cardiovascular risk factors at each stage.
-
•
Future objectives should focus on increasing women's presence in trials of lipid-lowering therapies.
Keywords: Lipids, Cholesterol management, Women
Abstract
There are currently no sex-specific guidelines for evaluation and management of blood lipids. While previous guidelines acknowledge sex-specific risk enhancing factors for lipid management in women for CVD prevention, this review focuses on how lipids are impacted during normal hormonal changes throughout a woman's life cycle- during adolescence, pre-pregnancy, pregnancy, pre- and perimenopause, menopause, and at older ages. In this review, the authors focus on management of primary prevention of CVD by examining sex-specific cardiovascular risk factors at each stage and pay special attention to statin use, statin side effects and non-statin therapies. Women need to understand their personalized cholesterol goals and ally with their clinicians to ensure successful management. Additionally, we highlight the biases that exist when treating dyslipidemia in women and the special care clinicians should take to ensure appropriate and aggressive therapies are made available to female patients. Finally, the authors recommend future research should focus on increasing enrollment of women in lipid trials. This is of paramount importance in discovering sex-specific difference in lipid management.
Graphical abstract
Abbreviations
- TC
total cholesterol
- LDL
low-density lipoprotein
- VLDL
very low-density lipoprotein
- HDL
high-density lipoprotein
- TG
triglycerides
- Lp(a)
lipoprotein a
- ASCVD
atherosclerotic cardiovascular disease
- CVD
cardiovascular disease
- PCE
pooled cohort equations
- DM
diabetes mellitus
- FH
familial hypercholesterolemia
- CAC
coronary Artery Calcium
1. Introduction
Men are historically over-represented in clinical CVD research trials and there is often paucity of data for sex-specific guideline recommendations. There are distinct differences between the sexes, including women presenting later with CVD and often receiving less aggressive therapies [1,2]. While guidelines acknowledge sex-specific risk factors and provide general recommendations for lipid management in women [3], this manuscript focuses on how lipids are impacted during normal hormonal changes throughout a woman's life cycle. The present manuscript covers the entire spectrum of a woman's life cycle from childhood to the post-menopausal period and incorporates assessment of sex-specific cardiovascular risk factors into recommendations for management, particularly in the setting of primary prevention of CVD, which includes statin use, side effects, and non-statin therapies in women.
2. Life cycle
2.1. Childhood and adolescence
Though hyperlipidemia has not historically been thought of as a disease in children, screening is now recommended as part of routine pediatric care [4]. Increased levels of lipids have been noted even in newborns. A study from Norway [5] showed that concentrations of HDL cholesterol were significantly positively associated with maternal hyperlipidemia. Furthermore, female newborns had higher concentrations of many cholesterol metabolites compared to male newborns.
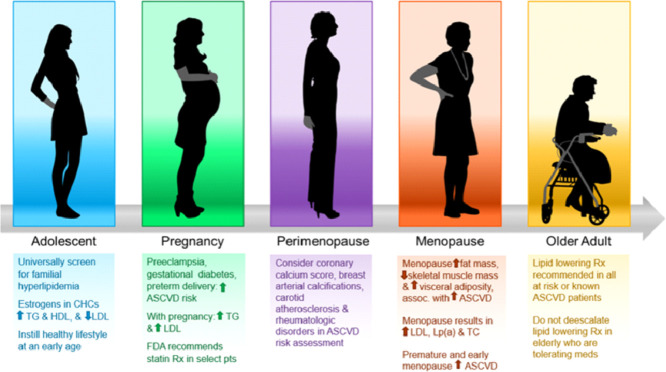
The National Heart Blood and Lung Institute's (NHLBI) Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents recommends universal screening of lipids with a non-fasting TC, HDL-C, and non-HDL cholesterol at ages 9–11 and 17–21 and then every 5 years afterwards [6]. Higher risk individuals require screening as young as 2 years old. Familial hyperlipidemia (FH) includes genetic defects resulting in severe elevations in blood cholesterol levels, increasing cardiovascular risk. The heterozygous form (HeFH) occurs in approximately 1 in 300–500 people while the homozygous form occurs in 1 out of every 1000,000 individuals [6]. FH should be suspected in children, adolescents and young adults (<20 years) if screening LDL-C ≥160 mg/dL or non-HDL cholesterol ≥190 mg/dL.
Using the National Heart Lung Blood Institute's screening guidelines in the pediatric population identifies individuals at risk of premature atherosclerosis. Evaluation for possible secondary causes of dyslipidemia should be performed. In the setting of FH, early initiation of lipid-lowering can slow the progression of atherosclerosis and reduce the risk of CVD events in adulthood [7]. Statins are preferred for initial pharmacologic treatment in children and adolescents after initiation of diet and physical activity management.
A history of early onset of menarche (at or before the age of 11) is associated with increased cardiovascular risk factors, including a more unfavorable lipid panel (specifically, higher TC, LDL and TG) [8,9] later in life at middle to older ages, as well as increased future CVD risk [10]. As elevated body mass index is one risk factor for early onset of menarche, prevention of childhood obesity and improved cardiovascular health of children and adolescents is paramount. Lifestyle habits learned at a young age focusing on healthy diet and exercise are imperative for ideal blood cholesterol levels and long-term cardiovascular health. A recently published trial showed that implementing school-based programs aimed at teaching healthy cardiovascular health habits as early as preschool can achieve lasting lifestyle changes in children, including knowledge, attitudes and habits changed towards leading a healthy lifestyle [11]. The 2018 American Heart Association (AHA)/American College of Cardiology (ACC) Guideline on the Management of Blood Cholesterol [12] and the 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease [3] recommend that patients consume a diet emphasizing vegetables, fruits, whole grains, legumes, healthy protein sources, and non-tropical vegetable oils. Intake of sweets, sugar-sweetened beverages, and red meats should be limited. All individuals should engage in at least 150 minutes per week of moderate-intensity aerobic physical activity or 75 minutes per week of vigorous-intensity aerobic physical activity [3].
Girls in their teenage years may be initiated on different forms of contraception (e.g. the “pill”, vaginal ring, transdermal patch) for a variety of reasons, including pregnancy prevention and treatment of menstrual cycle disorders [13]. The estrogen contained in oral combined hormonal contraceptives (CHC) can increase TG and HDL, but lower LDL. The magnitude of these changes is related to the potency of the estrogen and androgenicity (or lack of) of the progestin in the CHC. Similarly, the more estrogen a CHC contains, the greater impact on increasing TG [14]. These agents can cause severe hypertriglyceridemia (TG ≥500 mg/dL) in women with high baseline TG. Transdermal CHC (“the patch”) is less likely to produce clinically relevant elevations in TG. Clinicians should be aware of the potential impact of contraception on lipids. If contraception is needed, clinicians should utilize shared decision making with patients to determine which form of contraception is most appropriate for that specific individual.
2.2. Menstrual cycle
There are normal variations in the lipid profile associated with the menstrual cycle, which may explain intra-person variation in lipid profile (5–8% on average) depending on the timing of lipid profile ascertainment in relation to menses [15]. Total cholesterol and LDL-C levels increase rapidly after menses and peak during the follicular phase, followed by a decline throughout the luteal phase. The peak levels of total cholesterol and LDL-C during the follicular phase correspond to the rise and peak of estrogen, whereas their decline in the luteal phase corresponds to the risk and peak of progesterone. HDL-C levels are the highest around ovulation corresponding to the high levels of estrogen. The authors of this paper recommend that clinicians take the menstrual cycle phase into account when evaluating cholesterol levels to improve the accuracy of the clinical interpretation. Currently, standard guidelines have not been established regarding when to check lipid levels in menstruating women, but ideally levels should be monitored and compared at the same phase of the menstrual cycle.
2.3. Polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) is one of the most frequent endocrine disorders of reproductive-age women, affecting 5–13% of the general population, and is characterized by menstrual irregularity or amenorrhea, hyperandrogenism, and polycystic ovaries. Hallmarked by insulin resistance, PCOS can be associated with significant dyslipidemia [16], including increased TG, LDL-C and low HDL-C, and is also associated with increased CVD risk [17]. Lifestyle management, including weight loss if overweight/obese, can help mitigate some of the CVD-risks associated with PCOS.
2.4. Pre-pregnancy
Optimizing cardiovascular health in women of reproductive age pre-pregnancy is important to improve maternal and offspring outcomes. Pre-existing dyslipidemia poses health risks to both the woman and her fetus. Lifestyle interventions, including diet and physical activity before pregnancy, are critical. Several studies have shown that unfavorable lipid profile pre-pregnancy [18], including elevations in TG, LDL-C, total cholesterol, and non-HDL-C, are associated with increased risk of subsequently developing preeclampsia during pregnancy. The Framingham Heart Study analyzed 538 parent-offspring pairs with parental LDL-C measured in the study before the offspring's birth and concluded that maternal pre-pregnancy LDL-C levels are associated with adult offspring LDL-C levels beyond that attributable to measured lifestyle, anthropometric, and inherited genetic factors [19].
2.5. Pregnancy
In a national U.S. sample [20] of pregnant women age 20–44, only 39% of pregnant women had ideal cholesterol levels and less than 5% of women had ideal cardiovascular health overall. This is significantly lower than non-pregnant women and is particularly concerning given maternal gestational cardiovascular health is linked to their offspring's cardiovascular health [21].
Numerous changes in maternal physiology occur during pregnancy, including alterations in glucose and lipid metabolism, resulting in increases in both cholesterol and triglycerides [22]. During normal pregnancies, there is an increase in cholesterol and these levels peak near term [14]. In a trial of 8700 women, TC increased by approximately 35–37% [23] and during uncomplicated pregnancies, TC and TG did not exceed 250 mg/dL at any time during pregnancy. Thus, women with a known lipid disorder are recommended to have consultation with a lipid specialist prior to pregnancy. Higher levels of TC, TG, LDL-C, and lower levels of HDL-C during the first trimester have been associated with adverse outcomes including gestational diabetes, pulmonary embolism, and preterm birth [24]. High TG and low HDL-C levels during the third trimester are associated with increased risk of large for gestational age (LGA) and macrosomia [25]. Fortunately, a large registry from Norway [26] reported that women with FH did not appear to have a higher risk of premature delivery, preeclampsia, low birth weight infants, or congenital malformations compared to women in the general population. Of note in some countries, it is possible to test for the FH mutation in cord blood.
Clinicians should be aware of the normal lipid profile changes during pregnancy. There are currently no set lipid screening recommendations for pregnant women, but the best time to screen for dyslipidemia is prior to pregnancy. The National Lipid Association recommends that if a pre-pregnancy lipid profile has not been checked, it is reasonable to check lipids during other routine assessment during pregnancy [14]. If values are abnormal, they should be monitored during pregnancy. Otherwise, follow up should be performed about 6 weeks post-partum.
The U.S. Food and Drug Administration (FDA) recently removed its strongest recommendation that statins be considered contraindicated during pregnancy [27]. An early case series identified a small number of fetal malformations in women receiving lipophilic statins in the first trimester [12]. Subsequent cohort studies and meta-analyses have not replicated these findings, and no teratogenic effects have been identified in women receiving hydrophilic statins such as pravastatin and rosuvastatin [12,28]. Increased miscarriage rates have been reported among women receiving statins in the first trimester, though this may be confounded by medical comorbidities [12,28]. When pregnancy is planned, stopping statin therapy one to two months before pregnancy should be considered, and women of childbearing age who are on statin therapy and sexually active should use a reliable form of contraception to avoid pregnancy [12]. When pregnancy occurs, the decision to continue statin therapy should be individualized based upon the patient's risk profile [27] and shared decision making between patient and the clinician. In women with FH who need LDL-C lowering during pregnancy and wish to avoid statins, bile acid sequestrants are not systemically absorbed and felt to be safe in pregnancy. Bile acid sequestrants can lower LDL-C by 15–20%, but their use can be limited by significant GI side effects.
Interestingly, statins are currently being investigated as a potential therapy for the prevention of preeclampsia [29], given their ability to improve endothelial function and to decrease inflammation and oxidative stress. Although one clinical trial [30] failed to demonstrate a benefit for preeclampsia prevention, in that trial pravastatin (vs placebo) was started after 35 weeks gestation which was likely too late in the pathogenesis of preeclampsia to impact outcomes. Another large randomized clinical trial is on-going (NCT03944512) which is enrolling high-risk women with a prior history of preeclampsia and randomizing them to pravastatin or placebo at a much earlier stage, between 12 and 17 weeks gestation, to see if statin therapy can reduce the risk of preeclampsia, fetal loss, and maternal death.
Women with hypertriglyceridemia at the onset of pregnancy may develop severe hypertriglyceridemia (TG ≥500 mg/dL) during the third trimester of pregnancy, complicated by pancreatitis [12,31]. Options for management of severe hypertriglyceridemia during pregnancy include bile acid sequestrants, niacin, fibrates, omega-3 fatty acids and plasma exchange [32] in addition to strict management of blood sugar, thyroid levels and diet [12]. Omega-3 supplements have not been shown to reduce the risk of preeclampsia but may decrease the risk of pre-term delivery [33].
Statin and fibrate use during breastfeeding has not been examined in clinical trials, and there is no published data indicating that it is harmful to the infant. Rosuvastatin and pravastatin at low doses were studied in a single trial and they may be considered during breastfeeding if benefits outweigh potential risks [34]. It is important to note that while the FDA currently does not recommend statin use during breastfeeding, some clinicians are using statins in women with FH and in the setting of secondary prevention[35,36]. Bile acid sequestrants are considered safe for use during breastfeeding [14]. Similar to medication use during pregnancy, the authors of this paper recommend shared decision making between patient and clinician to determine the ideal clinical course for individual patients.
2.6. Pre & peri‑menopause
Several sex-specific cardiovascular risk enhancers emerge during the pre- and peri-menopausal periods. In general, premenopausal women have a less proatherogenic plasma lipid profile than men, with higher HDL-C and lower LDL-C and TG compared to age-matched men [37]. However, as women age, levels of LDL increase an average of 2 mg/dL per year between ages 40 and 60 years [38] and aging is associated with increases in TC, LDL, TG, and a decrease in HDL [39]. For women, while the 10-year risk for an atherosclerotic CVD (ASCVD) event may be low to intermediate, lifetime risk may be substantially elevated. The 2018 AHA/ACC guideline on the management of blood cholesterol for primary prevention recommends applying risk-enhancing factors to patients in the borderline and intermediate risk groups to identify those who may be at greater risk for ASCVD than suggested by the ACC/AHA Pooled Cohort Equations (PCE) alone [12]. The presence of a risk-enhancing factor in this group would favor the decision to initiate statin therapy for primary prevention.
Sex-specific risk-enhancing factors include premature menopause and pregnancy-related conditions including preeclampsia, gestational hypertension, gestational diabetes, and pre-term delivery [40]. Inflammatory disease risk enhancers more prevalent in women include rheumatoid arthritis and systemic lupus erythematous. High TG and elevated Lp(a) levels are also important risk factors in women. In young women, predominance of small LDL-C particles has been associated with increased risk for myocardial infarction [41].
Atherosclerosis imaging, specifically CAC scoring, can also guide decisions about cholesterol therapy in women at borderline and intermediate ASCVD risk when there remains uncertainty about risk and/or the net benefit of statin therapy [3]. Among 2684 women without diabetes in Multi-Ethnic Study of Atherosclerosis (MESA), 32% of women classified as “low risk” by Framingham Risk Score (FRS), had CAC score >0 and nearly 5% had a CAC ≥300, suggesting that women with positive CAC scoring are at potentially higher risk than their FRS classification [42]. Moreover, the presence of CAC is associated with increased risk of ASCVD events even in low-risk women [43].
In the Atherosclerosis Risk in Communities (ARIC) study, carotid plaque had a more profound effect on improving risk prediction in women than in men [44]. Breast arterial calcification (BAC), found in ∼12.7% of women undergoing breast cancer screening, is associated with CAC and coronary atherosclerotic plaque, providing an independent and incremental value to clinical risk factors for the prediction of subclinical coronary atherosclerosis [45], and has been associated with increased risk for adverse cardiac events [46].
2.7. Menopause
Menopause is defined by the final menstrual period (FMP) and heralds a decline in estrogen levels which alters the lipid profile when compared to the premenopausal stage. Menopause is associated with a more atherogenic lipid profile with increased levels of LDL-C, TC, Lp(a), with a decline in HDL-C levels. In a meta-analysis, women experienced a 1.5 mg/dL and 1.2 mg/dL annual increase in TC and LDL-C, respectively following menopause [47]. The more atherogenic profile during menopause may in part be explained by correlation of reduced estrogen levels with downregulation of the LDL receptor in the liver as well as an increase in Lp(a) levels, an independent CVD risk factor. Post-menopausal women also exhibit a rise in small dense LDL-C particles which are considered more atherogenic [48]. Data exploring the change in HDL-C levels following menopause remains controversial with most studies demonstrating no change or a slight increase in HDL-C.
Several factors including smoking, autoimmune diseases, low socioeconomic status, Black race and Hispanic ethnicity are associated with an earlier age at menopause [49,50] and may contribute to the link between premature (before age of 40) and early menopause (before age of 45) and increased cardiovascular morbidity and mortality [51]. Menopause is also associated with an increase in fat mass, loss of skeletal mass, increased waist circumference, and increased central adiposity- independent of age, BMI, parity, use of hormone therapy, physical activity, alcohol consumption, and smoking. Change in abdominal fat distribution triggered by estrogen deficiency also influences the lipid profile and increases overall cardiovascular risk [52]. Central adiposity has been shown to increase insulin resistance and levels of free fatty acid and decrease adiponectin levels. This increases the risk of development of the metabolic syndrome and produces a surge in the secretion of apo B-containing particles, as well as an amplified activity of hepatic lipase, resulting in an increase in LDL-like particles and decrease in HDL-C levels, respectively [53].
2.8. Post-Menopause & the older adult
While TC and LDL-C progressively increase during a woman's lifespan to age ∼75 years (∼10 years later than men), TC, LDL-C and HDL-C levels appear to decrease in the subsequent years, independent of weight, lifestyle, and chronic diseases [54]. Although triglyceride levels tend to decrease after age 75 years [55] (dependent on underlying metabolic factors), levels are higher in older women compared to older men 56] and independently predict CVD mortality in older women [57]. However, controversy exists in the relation between HDL-C and LDL-C to cardiovascular risk in older individuals. In the Women's Health Initiative study of a large cohort of women aged 68–81 years without CVD and not taking cholesterol-lowering medication at baseline, investigators found no association between HDL-C and LDL-C levels and survival to age 90 after adjustment for cardiovascular risk factors [58]. This suggests low cholesterol levels late in life are not directly associated with higher mortality. However, another study from Copenhagen [59] of a general population of adults age 70–100 years found that elevated LDL-C was associated with increased risk for MI and ASCVD events. The risk of CVD attributable to lipids persists in older patients, although in the INTERHEART study, CVD risk associated with ApoB cholesterol was lower in older individuals compared to the young [60].
There is no established age cutoff above which screening for lipid abnormalities should not be performed for primary CVD prevention, but current guidelines give little specific guidance for those > 75 years old. As such, the National Lipid Association recommends cholesterol screening for adults >75 years of age in the context of clinician-patient discussion regarding the possible benefits of preventive therapy in the context of CVD risk factors, comorbidities and life expectancy [61]. The PCE to estimate lifetime and 10-year ASCVD risk was not validated in those ≥80 years [62], and studies have suggested that the performance of the PCE is suboptimal in those ≥80 years and may overestimate risk [63]. Additionally, CAC and carotid plaque burden may better discriminate between lower and higher coronary heart disease risk in older adults [64,65]. Women have lower CAC scores than men across the age deciles from post-menopause to age 80, but women have disproportionately higher CVD mortality risk then men when CAC >0 [66]. Knowledge gaps remain regarding the optimal risk stratification for guiding lipid-lowering therapy in older women and ethnic minority women.
There are many factors that should be considered when discussing a cholesterol lowering agent in older women, such as patient preference, cost, polypharmacy, comorbidities, adverse effects, frailty and life expectancy. The higher likelihood of drug-drug interactions and age-related pharmacodynamic and pharmacokinetic changes may predispose elderly women to higher medication side effects [67]. For older women who are tolerating lipid-lowering therapy for primary or secondary prevention, the authors of this manuscript do not recommend de-prescribing pharmacologic lipid-lowering therapy based on age. In patients with known CVD or at high CVD risk without established CVD, cholesterol lowering with statins consistently demonstrates benefit in CVD risk reduction even in the very old [68], however, few randomized lipid-lowering primary prevention trials have focused on older individuals [69,70]. The Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) compared pravastatin 40 mg versus placebo and found a 15% reduction in incidence of composite coronary death, myocardial infarction and stroke in older individuals after a mean of 3 years (range 70–82 years, 52% women),although the treatment benefit was not statistically significant in women [69]. Older participants > 70 years in the JUPITER and HOPE-3 trials demonstrated high CVD event rates as well as large absolute rate reductions associated with statin treatment and no sex differences reported, supporting the use of statins in primary prevention among older women and men [70]. Currently two ongoing clinical trials, PREVENTABLE (NCT04262206) and STAREE (NCT02099123), are evaluating the effects of statins for primary prevention in older patients >75 and >70 years, respectively. Sex-specific analyses are needed.
3. Other considerations
3.1. Approaches to prevention and response to treatment
Meta-analyses have shown that similar risk women and men benefit equally from statin therapy in both primary and secondary prevention, without interaction by sex [71]. Women also benefit similarly from ezetimibe [72] and PCSK9 inhibitor therapy [73]. Nevertheless, women are less likely than men to be prescribed any statin therapy or to receive a statin at the guideline-recommended intensity [74]. One major barrier is lack of appreciation of CVD risk by clinicians. In the CASCADE registry, women with FH were less likely to achieve LDL-C goals and to receive statin therapy, and in particular, high intensity statin therapy. Black and Asian women were least likely to achieve LDL goals [75]. When compared to men, women with established CVD are less likely to receive a statin or ezetimibe [76]. PCSK9 inhibitor prescription rejections are higher in women, racial minorities and lower income groups. Patients with rejected PCSK9 inhibitor prescriptions had significantly increased cardiovascular event rates when compared with those in the paid cohort [77].
Additionally, women are more likely to discontinue statin therapy due to perceived side effects compared to men [78]. Approximately 10% of patients discontinue statins secondary to subjective complaints [78], many of which are amplified by disinformation in the lay press. Muscle complaints, including statin-associated muscle symptoms (SAMS), occur in 31% of women compared with 26% of men with more women switching or stopping a statin due to side-effects [79]. Data from a meta-analysis comparing low dose (10 mg) vs. high dose (80 mg) atorvastatin found no difference in incidence of myopathy or rhabdomyolysis [80]. Risk factors for myopathy include older age, female gender, hypothyroidism, pre-existing muscle disease, chronic kidney disease and use of concomitant drug therapy with statin interactions [81]. SAMS have not been proven to be linked to a direct physiologic statin effect and management of symptoms requires a patient-centered approach. More women report dissatisfaction with how their provider explains personalized cholesterol management, which may contribute to statin non-adherence [81].
Lastly, despite the well-known benefits of cardiac rehabilitation programs, services are underutilized, especially among women [82]. Studies have shown that women of various ages who participate in cardiac rehabilitation programs have significant improvement in quality of life measures, exercise tolerance, and HDL (+5%) [83]. The lack of female participation is a lost opportunity to improve lipid profiles and long-term CV outcomes in women and should remain a focus for clinicians in the setting of secondary prevention.
3.2. Socioeconomic disparities
Disparities in treatment by gender are compounded further by socioeconomic disparities. It has been well documented that factors like reduced access to care, low income, and lack of social support impact cardiovascular care and outcomes- including lipid management [84]. While many socioeconomic barriers remain to be overcome, several changes have been proposed to combat disparities. These interventions include changes at the level of health policy and health care systems, but also at the level of individual clinicians and researchers who can advocate for and institute changes in their practices to overcome some of these barriers.
3.3. Representation in clinical trials
Finally, underrepresentation in research and clinical trials for cholesterol lowering agents remains a large barrier to effective lipid management in women [85]. A review of randomized clinical trials of CVD prevention showed that while the enrollment of women increased significantly over time, women's overall representation remained low relative to their disease burden [86]. In this particular review, representation of women was lowest for trials focusing on hyperlipidemia (28%) while women accounted for 49% of all individuals with hyperlipidemia. As another example, IMPROVE-IT enrolled patients after acute coronary syndrome and randomized them to statin versus statin plus ezetimibe. The study demonstrated a lower rate of the primary composite cardiovascular outcome (composite of cardiovascular death, nonfatal myocardial infarction, unstable angina requiring hospitalization, coronary revascularization ≥30 days after randomization, or nonfatal stroke), but only 24% of participants were women [87]. Sex specific outcomes from IMPROVE-IT suggest that women might benefit even more from ezetimibe when considering the total number of primary events (p = 0.08), but the study may have been underpowered [72]. Bempedoic acid, a newer LDL-C lowering agent, has been evaluated in two randomized controlled trials for patients with established ASCVD or HeFH on maximally tolerated statin therapy: both trials underrepresented women (73% men in CLEAR Harmony and 63.7% men in CLEAR Wisdom) [88,89]. However, in the on-going CLEAR OUTCOMES cardiovascular outcome trial evaluating bempedoic acid among statin-intolerant patients, women represent approximately half of the study population [90]. In landmark trials supporting the use of commercially available PCSK9 inhibitors for LDL-C lowering, representation of women was again low. In the FOURIER and ODYSSEY OUTCOMES studies, the benefits of evolocumab and alirocumab were examined, respectively; women represented only approximately 25% of patients in both trials [91,92].
4. Conclusion
Clinicians should be aware of variations in the lipid profile during a women's life cycle in relationship to hormonal factors and sex-specific CVD risk factor enhancers should be recognized when treating dyslipidemia in women. Updates to cholesterol management guidelines should include acknowledgement and management strategies that take these risk factors into consideration. Clinicians should be aware of the biases that exist when treating dyslipidemia in women and take special care to ensure that appropriate and aggressive therapies are made available to their female patients. Finally, despite some progress as noted, future objectives should focus on increasing women's presence in cardiovascular clinical trials including those studying new lipid lowering therapeutics.
CRediT authorship contribution statement
Jyoti Sharma: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Jan McAlister: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Niti R. Aggarwal: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Janet Wei: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Puja K. Mehta: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Odayme Quesada: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Deirdre Mattina: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Nandita S. Scott: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Erin D. Michos: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Zainab Mahmoud: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Karla Kurrelmeyer: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Glaucia Maria Moraes De Oliveira: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. Kathryn J. Lindley: Project administration, Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
All authors have no disclosures to make regarding funding or other relationships unless specifically noted. Dr. Odayme Quesada reports NIH K23HL1518677; Dr. Michos reports advisory boards for Novartis, Novo Nordisk, Amarin, Esperion, Bayer, Astra Zeneca, and Boehringer Ingeleheim. Given her role as Co-Editor of the American Journal of Preventive Cardiology, Dr. Michos had no input in the peer review process or decision regarding acceptance.
References
- 1.Hao Y., Liu J., Liu J., et al. Sex differences in in-hospital management and outcomes of patients with acute coronary syndrome. Circulation. 2019;139:1776–1785. doi: 10.1161/CIRCULATIONAHA.118.037655. [DOI] [PubMed] [Google Scholar]
- 2.Khan S.U., Khan M.Z., Raghu Subramanian C., et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnett D.K., Blumenthal R.S., Albert M.A., et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease. J Am Coll Cardiol. 2019;74:e177–e232. doi: 10.1016/j.jacc.2019.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Daniels S.R., Benuck I., Christakis D.A., et al. Expert panel on integrated guidelines for cardiovascular health & risk reduction in children and adolescents: summary report. Pediatrics. 2011;128(suppl 5):S1–S44. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Øyri L.K.L., Bogsrud M.P., Christensen J.J., et al. Novel associations between parental and newborn cord blood metabolic profiles in the Norwegian mother, father and child cohort study. BMC Med. 2021;19:91. doi: 10.1186/s12916-021-01959-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goldberg A.C., Hopkins P.N., et al. Familial hypercholesterolemia: screening, diagnosis and management of pediatric and adult patients: guidance from the national lipid association expert panel on familial hypercholesterolemia. J Clin Lipidol. 2011;5:133–140. doi: 10.1016/j.jacl.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Luirink I.K., Wiegman A., Kusters D.M., et al. 20-year follow-up of statins in children with familial hypercholesterolemia. N Engl J Med. 2019;381:1547–1556. doi: 10.1056/NEJMoa1816454. [DOI] [PubMed] [Google Scholar]
- 8.Feng Y., Hong X., Wilker E., et al. Effects of age at menarche, reproductive years, and menopause on metabolic risk factors for cardiovascular diseases. Atherosclerosis. 2008;196:590–597. doi: 10.1016/j.atherosclerosis.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bubach Horta BL, Goncalves H., et al. Early age at menarche and metabolic cardiovascular risk factors: mediation by body composition in adulthood. Sci Rep. 2021;11(1):148. doi: 10.1038/s41598-020-80496-7. Jan 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peters S.A., Woodward M. Women's reproductive factors and incident cardiovascular disease in the UK biobank. Heart. 2018;104:1069–1075. doi: 10.1136/heartjnl-2017-312289. [DOI] [PubMed] [Google Scholar]
- 11.Santos-Beneit G., Fernández-Jiménez R., de Cos-Gandoy A., et al. Lessons learned from 10 years of preschool intervention for health promotion: JACC state-of-the-art review. J Am Coll Cardiol. 2022;79(3):283–298. doi: 10.1016/j.jacc.2021.10.046. Jan. [DOI] [PubMed] [Google Scholar]
- 12.Grundy S.M., Stone N.J., Bailey A.L., et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Committee on Adolescence- American Academy of Pediatrics Policy statement- contraception for adolescents. Pediatrics. 2014;134(4):e1244–e1256. doi: 10.1542/peds.2014-2299. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson T.A., Maki K.C., Orringer C.E., et al. National lipid association recommendations for patient-centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9:S1–S122. doi: 10.1016/j.jacl.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Mumford S.L., Dasharathy S., Pollack A.Z., Schisterman E.F. Variations in lipid levels according to menstrual cycle phase: clinical implications. Clin Lipidol. 2011;6:225–234. doi: 10.2217/clp.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osibogun O., Ogunmoroti O., Michos E.D. Polycystic ovary syndrome and cardiometabolic risk: opportunities for cardiovascular disease prevention. Trends Cardiovasc Med. 2020;30:399–404. doi: 10.1016/j.tcm.2019.08.010. [DOI] [PubMed] [Google Scholar]
- 17.Okoth K., Chandan J.S., Marshall T., Thangaratinam S., Thomas G.N., Nirantharakumar K., Adderley N.J. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. doi: 10.1136/bmj.m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnussen E.B., Vatten L.J., Lund-Nilsen T.I., et al. Prepregnancy cardiovascular risk factors as predictors of pre-eclampsia: population based cohort study. BMJ. 2007;335:978. doi: 10.1136/bmj.39366.416817.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mendelson M.M., Lyass A., O'Donnell C.J., et al. Association of maternal pre-pregnancy dyslipidemia with adult offspring dyslipidemia in excess of anthropometric, lifestyle, and genetic factors in the Framingham Heart Study. JAMA Cardiol. 2016;1(1):26–35. doi: 10.1001/jamacardio.2015.0304. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perak A.M., Ning H., Khan S.S., et al. Cardiovascular health among pregnant women, aged 20 to 44 years, in the United States. J Am Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perak A.M., Lancki N., Kuang A., et al. Hyperglycemia, adverse pregnancy outcome study cooperative research G. Associations of gestational cardiovascular health with pregnancy outcomes: the hyperglycemia and adverse pregnancy outcome study. Am J Obstet Gynecol. 2021;224(2):210.e1–210.e17. doi: 10.1016/j.ajog.2020.07.053. e211-210 e217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regitz-Zagrosek V., Roos-Hesselink J.W., Bauersachs J., et al. 2018 ESC guidelines for the management of cardiovascular diseases during pregnancy. Eur Heart J. 2018;39:3165–3241. doi: 10.1093/eurheartj/ehy340. [DOI] [PubMed] [Google Scholar]
- 23.Wiznitzer A., Mayer A., Novack V., et al. Association of lipid levels during gestation with preeclampsia and gestational diabetes mellitus: a population-based study. Am J Obstet Gynecol. 2009;201:482. doi: 10.1016/j.ajog.2009.05.032. e1–482.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang C., Zhu W., Wei Y., et al. The associations between early pregnancy lipid profiles and pregnancy outcomes. J Perinatol. 2017;37(2):127–133. doi: 10.1038/jp.2016.191. [DOI] [PubMed] [Google Scholar]
- 25.Xi F., Chen H., Chen Q., et al. Second-trimester and third-trimester maternal lipid profiles significantly correlated to LGA and macrosomia. Arch Gynecol Obstet. 2021;304(4):885–894. doi: 10.1007/s00404-021-06010-0. Mar 2. [DOI] [PubMed] [Google Scholar]
- 26.Toleikyte I., Retterstol K., Leren T.P., Iversen P.O. Pregnancy outcomes in familial hypercholesterolemia: a registry-based study. Circulation. 2011;124:1606–1614. doi: 10.1161/CIRCULATIONAHA.110.990929. [DOI] [PubMed] [Google Scholar]
- 27.FDA . US Food and Drug Administration; 2022. Requests removal of strongest warning against using cholesterol-lowering statins during pregnancy; still advises most pregnant patients should stop taking statins. https://www.fda.gov/drugs/drug-safety-and-availability/fda-requests-removal-strongest-warning-against-using-cholesterol-lowering-statins-during-pregnancy [Accessed 17 Mar 2022] [Google Scholar]
- 28.Zarek J., Koren G. The fetal safety of statins: a systematic review and meta-analysis. J Obstet Gynaecol Can. 2014;36:506–509. doi: 10.1016/S1701-2163(15)30565-X. [DOI] [PubMed] [Google Scholar]
- 29.Smith D.D., Costantine M.M. The role of statins in the prevention of preeclampsia. Am J Obstet Gynecol. 2020;S0002-9378(20):30868–30871. doi: 10.1016/j.ajog.2020.08.040. Aug 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dobert M., Varouxaki A.N., Mu A.C., et al. Pravastatin versus placebo in pregnancies at high risk of term preeclampsia. Circulation. 2021;144:670–679. doi: 10.1161/CIRCULATIONAHA.121.053963. [DOI] [PubMed] [Google Scholar]
- 31.Toleikyte I., Retterstol K., Leren T.P., et al. Pregnancy outcomes in familial hypercholesterolemia: a registry-based study. Circulation. 2011;124:1606–1614. doi: 10.1161/CIRCULATIONAHA.110.990929. [DOI] [PubMed] [Google Scholar]
- 32.Perrone S., Brunelli R., Perrone G., et al. A successful term pregnancy with severe hypertriglyceridaemia and acute pancreatitis. Clinical management and review of the literature. Atheroscler Suppl. 2019;40:117–121. doi: 10.1016/j.atherosclerosissup.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 33.Middleton P., Gomersall J.C., Gould J.F., et al. Omega-3 fatty acid addition during pregnancy. Cochrane Database Syst Rev. 2018;11 doi: 10.1002/14651858.CD003402.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anderson P.O. Treating hyperlipidemia during breastfeeding. Breastfeed Med. 2020;15(3):1–3. doi: 10.1089/bfm.2019.0300. [DOI] [PubMed] [Google Scholar]
- 35.Holmsen S.T., Bakkebø T., Seferowicz M., Retterstøl K. Statins and breastfeeding in familial hypercholesterolaemia. Tidsskr Nor Laegeforen. 2017 May 23;137(10):686–687. doi: 10.4045/tidsskr.16.0838. [DOI] [PubMed] [Google Scholar]
- 36.Lwin E.M.P., Leggett C., Ritchie U., et al. Transfer of rosuvastatin into breast milk: liquid chromatography-mass spectrometry methodology and clinical recommendations. Drug Des Dev Ther. 2018;12:3645–3651. doi: 10.2147/DDDT.S184053. Oct 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang X., Magkos F., Mittendorfer B. Sex differences in lipid and lipoprotein metabolism: it's not just about sex hormones. J Clin Endocrinol Metab. 2011;96:885–893. doi: 10.1210/jc.2010-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnson C.L., Rifkind B.M., Sempos C.T., et al. Declining serum total cholesterol levels among US adults: the national health and nutrition examination surveys. JAMA. 1993;269:3002–3008. [PubMed] [Google Scholar]
- 39.Feng L., Nian S., Tong Z., et al. Age-related trends in lipid levels: a large-scale cross-sectional study of the general Chinese population. BMJ Open. 2020;10(3) doi: 10.1136/bmjopen-2019-034226. Mar 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agarwala A., Liu J., Ballantyne C.M., Virani S.S. The use of risk enhancing factors to personalize ASCVD risk assessment: evidence and recommendations from the 2018 AHA/ACC multi-society cholesterol guidelines. Curr Cardiovasc Risk Rep. 2019;13(7):18. doi: 10.1007/s12170-019-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kamigaki A.S., Siscovick D.S., Schwartz S.M., et al. Low density lipoprotein particle size and risk of early-onset myocardial infarction in women. Am J Epidemiol. 2001;153:939–945. doi: 10.1093/aje/153.10.939. [DOI] [PubMed] [Google Scholar]
- 42.Lakoski S., Greenland P., Wong N.D., et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as "low risk" based on Framingham risk score: the multi-ethnic study of atherosclerosis (MESA) Arch Intern Med. 2007;167:2437–2442. doi: 10.1001/archinte.167.22.2437. [DOI] [PubMed] [Google Scholar]
- 43.Kavousi M., Desai C.S., Ayers C., et al. Prevalence and prognostic implications of coronary artery calcification in low-risk women: a meta-analysis. JAMA. 2016;316:2126–2134. doi: 10.1001/jama.2016.17020. [DOI] [PubMed] [Google Scholar]
- 44.Nambi V., Chambless L., Folsom A.R., et al. Carotid intima-media thickness and presence or absence of plaque improves prediction of coronary heart disease risk: the ARIC (Atherosclerosis Risk In Communities) study. J Am Coll Cardiol. 2010;55:1600–1607. doi: 10.1016/j.jacc.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Margolies L., Salvatore M., Hecht H.S., et al. Digital Mammography and screening for coronary artery disease. JACC Cardiovasc Imaging. 2016;9:350–360. doi: 10.1016/j.jcmg.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 46.Suh J.W., Yun B. Breast arterial calcification: a potential surrogate marker for cardiovascular disease. J Cardiovasc Imaging. 2018;26:125–134. doi: 10.4250/jcvi.2018.26.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ambikairajah A., Walsh E., Cherbuin N. Lipid profile differences during menopause: a review with meta-analysis. Menopause. 2019;26(11):1327–1333. doi: 10.1097/GME.0000000000001403. [DOI] [PubMed] [Google Scholar]
- 48.Campos H., McNamara J.R., Wilson P.W.F., et al. Differences in low density lipoprotein subfractions and apolipoproteins in premenopausal and postmenopausal women. J Clin Endocrinol Metab. 1988;67(1):30–35. doi: 10.1210/jcem-67-1-30. [DOI] [PubMed] [Google Scholar]
- 49.Henderson K.D.L., Bernstein L., Henderson B., Kolonel L., Pike M.C. Predictors of the timing of natural menopause in the multiethnic cohort study. Am J Epidemiol. 2008;167(11):1287–1294. doi: 10.1093/aje/kwn046. [DOI] [PubMed] [Google Scholar]
- 50.Sammaritano L.R. Menopause in patients with autoimmune diseases. Autoimmunity reviews. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 51.Honigberg M.C., Zekavat S.M., Aragam K., et al. Association of premature natural and surgical menopause with incident cardiovascular disease. JAMA. 2019;322:2411–2421. doi: 10.1001/jama.2019.19191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donato G.B., Fuchs S.C., Oppermann K., Bastos C., Spritzer P.M. Association between menopause status and central adiposity measured at different cutoffs of waist circumference and waist-to-hip ratio. Menopause. 2006;13(2):280–285. doi: 10.1097/01.gme.0000177907.32634.ae. Mar. [DOI] [PubMed] [Google Scholar]
- 53.Carr M.C. The emergence of the metabolic syndrome with menopause. J Clin Endocrinol Metab. 2003;88(6):2404–2411. doi: 10.1210/jc.2003-030242. [DOI] [PubMed] [Google Scholar]
- 54.Ferrara A., Barrett-Connor E., Shan J. Total, LDL, and HDL cholesterol decrease with age in older men and women. The Rancho Bernardo study 1984-1994. Circulation. 1997;96:37–43. doi: 10.1161/01.cir.96.1.37. [DOI] [PubMed] [Google Scholar]
- 55.Streja E, Streja DA.Endotext. Management of Dyslipidemia in the Elderly. 2022. South Dartmouth (MA): MDText.com, Inc. www.ncbi.nlm.nih.gov/books/NBK279133/ [Google Scholar]
- 56.Miller M., Stone N.J., Ballantyne C., et al. American heart association clinical lipidology, thrombosis, and prevention committee of the council on nutrition, physical activity, and metabolism; council on arteriosclerosis, thrombosis and vascular biology; council on cardiovascular nursing; council on the kidney in cardiovascular disease. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2011;123(20):2292–2333. doi: 10.1161/CIR.0b013e3182160726. May 24. [DOI] [PubMed] [Google Scholar]
- 57.Mazza A., Tikhonoff V., Schiavon L., et al. Triglycerides+high-density-lipoprotein-cholesterol dyslipidaemia, a coronary risk factor in elderly women: the cardiovascular study in the elderly. Intern Med J. 2005;35:604–610. doi: 10.1111/j.1445-5994.2005.00940.x. [DOI] [PubMed] [Google Scholar]
- 58.Maihofer A.X., Shadyab A.H., Wild R.A., LaCroix A.Z. Associations between serum levels of cholesterol and survival to Age 90 in postmenopausal women. J Am Geriatr Soc. 2020;68:288–296. doi: 10.1111/jgs.16306. [DOI] [PubMed] [Google Scholar]
- 59.Mortensen M.B., Nordestgaard B.G. Elevated LDL cholesterol and increased risk of myocardial infarction and atherosclerotic cardiovascular disease in individuals aged 70-100 years: a contemporary primary prevention cohort. Lancet. 2020;396:1644–1652. doi: 10.1016/S0140-6736(20)32233-9. [DOI] [PubMed] [Google Scholar]
- 60.Sniderman A.D., Islam S., McQueen M., et al. Age and cardiovascular risk attributable to apolipoprotein B, low-density lipoprotein cholesterol or non-high-density lipoprotein cholesterol. J Am Heart Assoc. 2016 Oct 13;5(10) doi: 10.1161/JAHA.116.003665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Brown W.V., Bittner V.A., McKenney J.M., Ziajka P.E. JCL roundtable: lipid-lowering drugs in those older than 75 years of age. J Clin Lipidol. 2014;8:533–541. doi: 10.1016/j.jacl.2014.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Goff D.C., Lloyd-Jones D.M., Bennett G., et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2935–2959. doi: 10.1016/j.jacc.2013.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nanna M.G., Peterson E.D., Wojdyla D., Navar A.M. The accuracy of cardiovascular pooled cohort risk estimates in U.S. Older adults. J Gen Intern Med. 2020;35:1701–1708. doi: 10.1007/s11606-019-05361-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yano Y., O'Donnell C.J., Kuller L., et al. Association of coronary artery calcium score vs age with cardiovascular risk in older adults: an analysis of pooled population-based studies. JAMA Cardiol. 2017;2:986–994. doi: 10.1001/jamacardio.2017.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mortensen M.B., Fuster V., Muntendam P., et al. A simple disease-guided approach to personalize ACC/AHA-recommended statin allocation in elderly people: the bioimage study. J Am Coll Cardiol. 2016;68:881–891. doi: 10.1016/j.jacc.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 66.Shaw L.J., Min J.K., Nasir K., et al. Sex differences in calcified plaque and long-term cardiovascular mortality: observations from the CAC Consortium. Eur Heart J. 2018;39:3727–3735. doi: 10.1093/eurheartj/ehy534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alexander K.P., Blazing M.A., Rosenson R.S., et al. Management of hyperlipidemia in older adults. J Cardiovasc Pharmacol Ther. 2009;14:49–58. doi: 10.1177/1074248408328927. [DOI] [PubMed] [Google Scholar]
- 68.Savarese G., Gotto A.M., Paolillo S., et al. Benefits of statins in elderly subjects without established cardiovascular disease: a meta-analysis. J Am Coll Cardiol. 2013;62:2090–2099. doi: 10.1016/j.jacc.2013.07.069. [DOI] [PubMed] [Google Scholar]
- 69.Shepherd J., Blauw G.J., Murphy M.B., et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 70.Ridker P.M., Lonn E., Paynter N.P., Glynn R., Yusuf S. Primary prevention with statin therapy in the elderly: new meta-analyses from the contemporary JUPITER and HOPE-3 randomized trials. Circulation. 2017;135:1979–1981. doi: 10.1161/CIRCULATIONAHA.117.028271. [DOI] [PubMed] [Google Scholar]
- 71.Kostis W.J., Cheng J.Q., Dobrzynski J.M., Cabrera J., Kostis J.B. Meta-analysis of statin effects in women versus men. J Am Coll Cardiol. 2012;59:572–582. doi: 10.1016/j.jacc.2011.09.067. [DOI] [PubMed] [Google Scholar]
- 72.Kato E.T., Cannon C.P., Blazing M.A., Bohula E., Guneri S., White J.A., et al. Efficacy and safety of adding ezetimibe to statin therapy among women and men: insight from IMPROVE-IT (improved reduction of outcomes: vytorin efficacy international trial) J Am Heart Assoc. 2017 Nov 18;6(11):e006901. doi: 10.1161/JAHA.117.006901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sever P., Gouni-Berthold I., Keech A., Giugliano R., Pedersen T.R., Im K., et al. LDL-cholesterol lowering with evolocumab, and outcomes according to age and sex in patients in the FOURIER Trial. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320902750. [DOI] [PubMed] [Google Scholar]
- 74.Nanna M.G., Wang T.Y., Xiang Q., et al. Sex differences in the use of statins in community practice. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Amrock S.M., Duell P.B., Knickelbine T., et al. Health disparities among adult patients with a phenotypic diagnosis of familial hypercholesterolemia in the CASCADE-FH™ patient registry. Atherosclerosis. 2017;267:19–26. doi: 10.1016/j.atherosclerosis.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 76.Shen X., DiMario S., Philip K. Gender disparities in health resource utilization in patients with atherosclerotic cardiovascular disease: a retrospective cross-sectional study. Adv Ther. 2019;36:3424–3434. doi: 10.1007/s12325-019-01107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Myers K.D., Farboodi N., Mwamburi M., Howard W., Staszak D., Gidding S., Baum S.J., Wilemon K., Rader D.J. Effect of access to prescribed PCSK9 inhibitors on cardiovascular outcomes. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Newman C.B., Preiss D., et al. Statin Safety and associated adverse events: a scientific statement from the American Heart Association. Arterioscler Thromb Vasc Biol. 2019;39:e38–e81. doi: 10.1161/ATV.0000000000000073. [DOI] [PubMed] [Google Scholar]
- 79.Karlis D., Wild R.A., et al. Gender differences in side effects and attitudes regarding statin use in the understanding statin use in America and gaps in patient education (USAGE) study. J Clin Lipidol. 2016;10:833–841. doi: 10.1016/j.jacl.2016.02.016. Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 80.Newman C., Tsai J., et al. Comparative safety of atorvastatin 80mg versus 10mg derived from analysis of 49 completed trials in 14,236 patients. Am J Cardiol. 2006;97:61–67. doi: 10.1016/j.amjcard.2005.07.108. [DOI] [PubMed] [Google Scholar]
- 81.Tobert J.A. Efficacy and long-term adverse effect pattern of lovastatin. Am J Cardiol. 1988;62:28J–34J. doi: 10.1016/0002-9149(88)90004-5. [DOI] [PubMed] [Google Scholar]
- 82.Vidal-Almela S., Czajkowski B., Prince S.A., et al. Lessons learned from community- and home-based physical activity programs: a narrative review of factors influencing women’s participation in cardiac rehabilitation. Eur J Prev Cardiol. 2020 doi: 10.1177/2047487320907748. Mar 72047487320907748. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 83.Kennedy M.D., Haykowsky M., Daub B., et al. Effects of a comprehensive cardiac rehabilitation program on quality of life and exercise tolerance in women: a retrospective analysis. Curr Control Trials Cardiovasc Med. 2003;4(1):1. doi: 10.1186/1468-6708-4-1. Apr 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lindley K.J., Aggarwal N.R., Briller J.E., et al. Socioeconomic determinants of health and cardiovascular outcomes in women: JACC review topic of the week. J Am Coll Cardiol. 2021;(19):1919–1929. doi: 10.1016/j.jacc.2021.09.011. Nov, 78. [DOI] [PubMed] [Google Scholar]
- 85.Khan S.U., Khan M.Z., Raghu Subramanian C., et al. Participation of women and older participants in randomized clinical trials of lipid-lowering therapies: a systematic review. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.5202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melloni C., Berger J.S., Wang T.Y., et al. Representation of women in randomized clinical trials of cardiovascular disease prevention. Circ Cardiovasc Qual Outcomes. 2010;3(2):135–142. doi: 10.1161/CIRCOUTCOMES.110.868307. Mar. [DOI] [PubMed] [Google Scholar]
- 87.Cannon C.P., Blazing M.A., Giugliano R.P., et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. doi: 10.1056/NEJMoa1410489. [DOI] [PubMed] [Google Scholar]
- 88.Ray K.K., Bays H.E., Catapano A.L., Lalwani N.D., Bloedon L.T., Sterling L.R., Robinson P.L. Ballantyne CM; CLEAR harmony trial. Safety and efficacy of bempedoic acid to reduce LDL cholesterol. N Engl J Med. 2019;380:1022–1032. doi: 10.1056/NEJMoa1803917. [DOI] [PubMed] [Google Scholar]
- 89.Goldberg A.C., Leiter L.A., Stroes E.S.G., et al. Effect of bempedoic acid vs placebo added to maximally tolerated statins on low-density lipoprotein cholesterol in patients at high risk for cardiovascular disease: the CLEAR wisdom randomized clinical trial. JAMA. 2019;322(18):1780–1788. doi: 10.1001/jama.2019.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicholls S., Lincoff A.M., Bays H.E., et al. Rationale and design of the CLEAR-outcomes trial: evaluating the effect of bempedoic acid on cardiovascular events in patients with statin intolerance. Am Heart J. 2021;235:104–112. doi: 10.1016/j.ahj.2020.10.060. [DOI] [PubMed] [Google Scholar]
- 91.Sabatine M.S., Giugliano R.P., Keech A.C., et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 92.Schwartz G.G., Steg P.G., Szarek M., et al. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379(22):2097–2107. doi: 10.1056/NEJMoa1801174. [DOI] [PubMed] [Google Scholar]