Abstract
Frontal corticostriatal circuits (FCSC) are involved in self-regulation of cognition, emotion, and motor function. While these circuits are implicated in attention-deficit/hyperactivity disorder (ADHD), the literature establishing FCSC associations with ADHD is inconsistent. This may be due to study variability in considerations of how fMRI motion regression was handled between groups, or study specific differences in age, sex, or the striatal subregions under investigation. Given the importance of these domains in ADHD it is crucial to consider the complex interactions of age, sex, striatal subregions and FCSC in ADHD presentation and diagnosis. In this large-scale study of 362 8–12 year-old children with ADHD (n = 165) and typically developing (TD; n = 197) children, we investigate associations between FCSC with ADHD diagnosis and symptoms, sex, and go/no-go (GNG) task performance. Results include: (1) increased striatal connectivity with age across striatal subregions with most of the frontal cortex, (2) increased frontal-limbic striatum connectivity among boys with ADHD only, mostly in default mode network (DMN) regions not associated with age, and (3) increased frontal-motor striatum connectivity to regions of the DMN were associated with greater parent-rated inattention problems, particularly among the ADHD group. Although diagnostic group differences were no longer significant when strictly controlling for head motion, with motion possibly reflecting the phenotypic variance of ADHD itself, the spatial distribution of all symptom, age, sex, and other ADHD group effects were nearly identical to the initial results. These results demonstrate differential associations of FCSC between striatal subregions with the DMN and FPN in relation to age, ADHD, sex, and inhibitory control.
Keywords: ADHD, Corticostriatal, Fmri, Development, Sex
1. Introduction
Frontal corticostriatal circuits (FCSC) are key in the development of self-regulation and learning of behavior ranging from motor to cognitive and emotion functions (Postuma and Dagher, 2006, Graybiel, 2008, Arnsten and Rubia, 2012, Nikolaidis et al., 2014, Graybiel and Grafton, 2015). Both learning and regulatory mechanisms of the FCSC are thought to be closely tied to reward through dopaminergic pathways (Volkow, 2009; Alexander, DeLong, and Strick, 1986; Tost, Alam, and Meyer-Lindenberg, 2010; Doyon et al., 2009). Understanding the diverse roles of FCSC in relation to diagnostic, symptomatic, and behavioral variation in healthy and psychiatric populations has become an important area of research in clinical neuroscience. Researchers have recognized that atypical development of FCSC may contribute to the pathophysiology of neurodevelopmental disorders, in particular attention-deficity/hyperactivity disorder (ADHD; Mennes et al., 2012; Castellanos and Proal, 2012). ADHD is associated with deficient self-regulation of attentional/cognitive (Castellanos et al., 2006), emotional (Shaw et al., 2014, Da Fonseca et al., 2009) and motor (Mostofsky et al., 2003, Mostofsky et al., 2006, Macneil et al., 2011, Gilbert et al., 2011) responses, which contribute to core symptoms of inattention, hyperactivity, and impulsivity.
Given the crucial role that FCSC plays in behavioral regulation, researchers have long hypothesized they play a crucial role in ADHD pathophysiology (Heilman et al., 1991, Denckla, 1991). However, neuroimaging studies of the intrinsic network connectivity of FCSC have yet to converge. Of the existing studies that have probed anomalous FCSC in ADHD, results have been relatively heterogenous. Some studies have observed increases in FCSC functional connectivity with diagnosis and symptom effects Dias et al., 2015, Dias et al., 2013, Ma et al., 2016, Oldehinkel et al., 2016, Oldehinkel et al., 2016, Sanefuji et al., 2017; Damiani et al., 2021, Di Martino et al., 2013, Mennes et al., 2012, Rosch et al., 2018, Yang et al., 2018), while others have observed decreases (Hong et al., 2015; Cao et al., 2006; Posner et al., 2013), both increases and decreases in FCSC in ADHD (Tomasi and Volkow, 2012; Cao et al., 2009), or no differences compared to typically developing children (Oldehinke et al., 2016a). This inconsistency of results may reflect variability in FCSC and associations with ADHD and symptom effects due to the particular striatal subregions investigated or variability in sample age and sex composition. Rosch 2018 was the first study to simultaneously consider the impact of sex and ADHD of FCSC and found key differences by sex as well as sex by diagnosis interaction in FCSC. Furthermore, regions in the FCSC are known to exhibit significant structural developmental changes tied to pubertal timing (Raznahan et al., 2014), and developmental changes in some FCSC regions are associated with ADHD (Barber et al., 2019). Finally, striatal subregions exhibit heterogeneity in their structural connectivity and functional relationships to cortex (Elliott et al., 2021), yet few prior studies have explicitly considered the cortical connectivity of striatal subregions in their analyses. Taken together, these findings suggest that characterization of differences in age, sex, and striatal subregion are essential in the pursuit of robust FCSC based biomarkers of ADHD.
In contrast to the variable findings from studies of FCSC connectivity in ADHD, a fairly consistent finding that has emerged from studies of cortico-cortico network connectivity is that adults and children with ADHD demonstrate hyperconnectivity of the default mode network (DMN), which is preferentially activated when an individual is not actively engaged in a task (Konrad and Eickhoff, 2010, Henry and Cohen, 2019, Posner et al., 2014, Duffy et al., 2021, Sripada et al., 2014, McCarthy et al., 2013, Barber et al., 2015, Hoekzema et al., 2014, Elton et al., 2014, Zhao et al., 2021). DMN hyperconnectivity is thought to relate to lapses in attention commonly associated with ADHD (Castellanos and Aoki, 2016, Sonuga-Barke and Castellanos, 2007). Recent work has even demonstrated, relative to age-matched typically developing (TD) children, children with ADHD exhibit a characteristic increased functional interaction of DMN and task positive networks (e.g. frontal parietal network (FPN); Duffy et al., 2021), which are typically characterized as anticorrelated networks (Uddin et al., 2009). While disruptions in these networks have been implicated in ADHD, a more detailed characterization of functional connectivity between the task positive networks (e.g. FPN and DMN) with the striatal subregions of FCSC may inform our understanding of the heterogeneity in the behavioral expression (e.g., symptoms, cognitive/motor/emotional deficits) of ADHD and in relation to recent findings of sex differences.
The goal of the current study was to address the lack of clarity regarding FCSC in ADHD by examining FCSC in a large sample of both boys and girls with and without a diagnosis of ADHD, with an emphasis on probing distinct striatal subregions relevant for cognitive, emotional and motor control. Our aims were to: (1) Characterize age-related changes in FCSC among a cross-sectional sample of 8–12 year-old children with and without ADHD, (2) Compare FCSC connectivity among girls and boys with ADHD compared to same sex TD children and in relation to ADHD symptoms, and (3) Examine effects of diagnosis and sex on cognitive task performance and associations with FCSC connectivity. Towards this end, we investigate how FCSC connectivity in the frontal lobe is associated with age, diagnosis, sex, inattentive and hyperactive behavioral symptoms of ADHD, and cognitive control deficits associated with ADHD (response inhibition and variability). Through permutation testing of FCSC using FSL’s Randomize tool, we assess how the spatial distribution of associations of individual variability in FCSC map onto canonical intrinsic connectivity of task positive and task negative networks.
Consistent with the longstanding recognition of frontal-striatal networks being crucial to the development of self-regulation of cognition/attention, emotion, and motor functions, our first hypothesis was that age would be associated with increases in FCSC reflecting the development of these networks. We also hypothesized that patterns of age-related change in FCSC connectivity may differ among children with and without ADHD, given prior findings of maturational lag in functional connectivity of cortical networks in ADHD (Sripada et al., 2014).
Regarding effects of ADHD diagnosis and symptoms, our second hypothesis was that ADHD diagnosis and symptom severity would be associated with increases in FCSC with DMN and decreases in FCSC with task-positive networks (TPNs), particularly the FPN, with variation across striatal subregions involved in cognitive, emotional, and motor control (see below). ADHD-associated increased in FCSC connectivity with the DMN may be reflective of poorer segregation between the DMN and FPN (Duffy at el, 2021). Furthermore, given multi-level evidence for sex effects in ADHD, including differences in both behavioral/cognitive (Cole et al., 2008, Hasson and Fine, 2012, Seymour et al., 2016) and neuroimaging biomarkers (Dirlikov et al., 2015, Jacobson et al., 2015, Mahone, 2012, Qiu et al., 2009, Seymour et al., 2017, Rosch et al., 2018), we hypothesized that girls and boys with ADHD would show different patterns of atypical FCSC connectivity. ADHD-related sex differences in FCSC remain understudied with only two studies to date addressing this question directly (Rosch et al., 2018, Chai et al., 2021). Rosch (2018) found that FCSC is associated with ADHD and symptom effects but differentially so depending on sex (Rosch, Mostofsky, and Nebel, 2018), such that girls with ADHD showed stronger positive striatal connectivity to the anterior cingulate cortex and negative striatal connectivity to the dorsolateral prefrontal cortex than boys with ADHD. In contrast, Chai et al., (2021) reported increased FCSC in the ventromedial prefrontal cortex and anterior cingulate cortex in boys with ADHD relative to girls using a ventral striatum seed (Chai et al., 2021). Given these contrasting prior results and the overlap of our sample with Rosch 2018, we hypothesized that girls with ADHD would show increased FCSC connectivity.
Finally, our third hypothesis was that, regarding ADHD-associated measures of cognitive control, go/no-go (GNG) response inhibition errors would be negatively associated with striatal connectivity to FPN (Hong et al., 2015), while GNG response time variability, thought to reflect lapses in attention (Kofler et al., 2013), would be associated with differences in striatal connectivity to DMN. Furthermore, these associations may differ among girls and boys given prior findings of ADHD-related sex differences in response control (Seymour, Mostofsky, and Rosch, 2016).
2. Methods
2.1. Participants
The current sample included 362 children and adolescents with either a diagnosis of ADHD (n = 165; 47 girls) or typically developing (TD) controls (n = 197; 61 girls). All participants were between ages 8–13 (mean 10.27, SD 1.27). Summary demographics for included participants are provided in Table 1. Participants were recruited from local schools, pediatricians (electronically via MyChart), community centers using flyers and word-of-mouth. Participants with ADHD were also recruited from local outpatient clinics. Study protocols were reviewed and approved by Johns Hopkins Medicine Institutional Review Board.
Table 1.
Demographic and clinical characteristics of ADHD and TD groups overall and within sex. Cohen's d is used to measure effect size.
| TD |
ADHD |
TD vs. ADHD |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Girls |
Boys |
TD Girls vs. TD Boys | Girls |
Boys |
ADHD Girls vs. ADHD Boys | TD Girls vs. ADHD Girls | TD Boys vs. ADHD Boys | TD All vs. ADHD All | |
| (n = 61) | (n = 136) | (n = 47) | (n = 118) | ||||||
| Mean (SD) | Mean (SD) | Effect Size | Mean (SD) | Mean (SD) | Effect Size | Effect Size | Effect Size | Effect Size | |
| Age | 10.09 (1.01) | 10.43 (1.21) | - 0.30 | 10.19 (1.33) | 10.21 (1.43) | -0.01 | -0.08 | 0.17 | 0.10 |
| IQ | 113.13 (10.31) | 116.60 (12.28) | - 0.30 | 107.68 (14.25) | 105.90 (12.54) | 0.14 | 0.45* | 0.87*** | 0.73*** |
| SES Family | 54.54 (8.99) | 53.97 (9.93) | 0.06 | 51.96 (8.95) | 51.14 (9.94) | 0.08 | 0.29 | 0.29* | 0.29** |
| Inattention T-Score | 46.46 (6.37) | 45.27 (5.71) | 0.20 | 82.35 (8.56) | 72.77 (9.45) | 1.04*** | - 4.86*** | - 3.58*** | - 3.67*** |
| Hyperactive-Impulsive T-Score | 46.18 (5.02) | 46.83 (5.72) | - 0.12 | 76.98 (13.48) | 72.92 (12.58) | 0.32 | - 3.2*** | - 2.73*** | - 2.85*** |
| GNG RT variability | 105.87 (48.94) | 92.22 (48.82) | 0.28 | 128.68 (56.66) | 148.83 (99.66) | - 0.23 | - 0.43* | - 0.69*** | - 0.61*** |
| GNG ComRate | 0.35 (0.18) | 0.41 (0.18) | - 0.30 | 0.38 (0.18) | 0.51 (0.19) | - 0.70*** | - 0.13 | - 0.56*** | - 0.45*** |
| Framewise Displacement | 0.04 (0.03) | 0.04 (0.02) | 0.01 | 0.05 (0.03) | 0.05 (0.03) | 0.18 | - 0.43* | - 0.29* | - 0.33** |
| % Stim Med | 0 | 0 | n/a | 55.32% | 60.17% | - 0.10 | n/a | n/a | n/a |
| % Non-Stim Med | 0 | 0 | n/a | 6.38% | 10.17% | -0.15 | n/a | n/a | n/a |
| % ODD | 0 | 0 | n/a | 36.73% | 30.89% | 0.12 | n/a | n/a | n/a |
| % Anxiety | 1.64% | 2.21% | -0.04 | 23.40% | 19.49% | 0.09 | -0.73*** | -0.59*** | -0.63*** |
| % Depression | 0 | 0 | n/a | 0 | 4.24% | n/a | n/a | n/a | n/a |
All parents completed an initial telephone screening to determine eligibility. Children with a history of intellectual disability, seizures, traumatic brain injury, neurological illnesses, prenatal exposure to teratogens, genetic disorders, or other neurodevelopmental disorders (e.g., Autism Spectrum Disorders) were excluded from participation. Eligible participants completed two laboratory sessions for each visit. Sessions occurred within a period of six months (with nearly all occurring within 4 weeks) to maintain validity of data collected between Session 1 and Session 2. Additional inclusion criteria applied across groups following study participation include the following: 1) successful fMRI scan; (2) Full Scale Intelligence Quotient (FSIQ) above 80 using either the Weschler's Intelligence Scales for Children current at the time of testing (WISC-IV or WISC-V) or the Wechsler Abbreviated Scale for Intelligence, 2nd Edition (WASI-II) (Wechsler, 1992), and 3) mean framewise displacement (FD) (Power et al., 2012) across all fMRI scans are within 3 standard deviations from the group mean FD.
At each visit, a diagnosis of ADHD was determined using a structured or semi-structured parent interview, either the Diagnostic Interview for Children and Adolescents (DICA-IV) (Reich, 2000) or the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS) (Kaufman et al., 2016); the ADHD Rating Scale (ADHD-RS) (DuPaul et al., 1998) and the Conners Parent Rating Scale-Revised or Version 3 (Conners, 2008) were used to confirm diagnosis and to provide dimensional measures of ADHD symptom severity. Parents of all participants provided written consent, and all participants provided assent. All children taking stimulant medication (n = 97; 26 girls; see Table 1) were asked to withhold medication on the day prior to and day of testing. Children taking psychotropic medications other than stimulant medication (n = 15; 3 girls) did not discontinue their medication for study visits. Additionally, parents were instructed on both the diagnostic interview and report forms to make ratings based on their children's symptoms off of their regularly prescribed medication.
Participants were included in the ADHD group if they: (1) met criteria for an ADHD diagnosis either on the DICA-IV or K-SADS and (2) received a T-score of 60 or higher on the DSM Inattentive or DSM Hyperactive-Impulsive scales on the Conners Parent or Teacher (when available) rating scales (revised or 3rd edition), or a score of 2 or 3 (i.e., symptoms rated as occurring ‘often’ or ‘very often’) on at least 6/9 items on the Inattentive or Hyperactivity/Impulsivity scales of the ADHD-RS Home or School (when available) Version. Children with ADHD were allowed to meet criteria for comorbid psychiatric diagnoses on the DICA-IV or K-SADS including oppositional defiant disorder (ODD; n = 53; 17 girls), anxiety disorders (n = 38; 12 girls) and depressive disorders (n = 5; all boys) (Supplementary Table S1). Girls and boys with ADHD did not differ in comorbid diagnoses of ODD (p > .05), anxiety (p > .05) or depression (p > .05). Master’s level clinicians conducted all diagnostic interviews and integrated information from rating scales to inform diagnoses under the supervision of licensed doctoral level clinical psychologists.
Participants were included in the control group if they: (1) did not meet criteria for any psychiatric disorders on the DICA-IV or K-SADS, (2) had below clinically significant scores (T < 60) on the Conners Parent and Teacher (when available) rating scales, and ADHD-RS Home and School (when available) Versions, and (3) did not have immediate family with ADHD.
2.2. MRI acquisition and preprocessing
Participants completed a practice scanning session to acquaint themselves with the scanning environment. Participants entered the mock scanner room with an instructor and were guided through the sequence of events that occur on the day of their actual scan, including sliding into the scanner, wearing ear plugs, hearing loud MRI scanner noises, and being alone in the scanner for 10 min. All scanning acquisition was completed using a 3.0 T Philips 3 T ‘Achieva’ MRI scanner. MPRAGE images (Slice thickness = 1.0 mm; FOV = 26 cm; Matrix size: 256 × 256) were checked for motion prior to processing. rs-fMRI was acquired during a 6-min 30-s scan using a single-shot, partially parallel, gradient-recalled echo planar sequence with sensitivity encoding and an ascending slice order (repetition time [TR]/echo time [TE] = 2500/30 ms, flip angle = 75°, sensitivity encoding acceleration factor of 2, 47 3-mm axial slices with no slice gap, in-plane resolution of 3.05 × 3.15 mm [84 × 81 voxels]). Participants were instructed to relax, fixate on a cross-hair, and remain as still as possible.
The skull-stripped anatomical images and raw functional images were preprocessed through the Configurable Pipeline for Connectomes (CPAC; Craddock et al., 2013). Anatomical images were registered to the MNI152 template (Fonov et al., 2009) (2 mm isotropic) using ANTS (Avants et al., 2011) and segmented into gray matter (probability threshold = 0.95), white matter (probability threshold = 0.95) and cerebrospinal fluid (CSF; probability threshold = 0.95). Functional images were slice-time corrected, motion-corrected (Friston et al., 1996) and registered to the MNI152 template (3 mm isotropic). Following recommendations of Ciric et al. (2017), time series were bandpass filtered at 0.01–0.08 Hz and nuisance signal removal was performed for 24 regressors derived from the parameters estimated during motion realignment, and physiological noise was modeled using the 5 principal components with the highest variance from a CompCor decomposition of white matter and CSF time series (Behzadi et al., 2007). Mean, squared, delayed, and squared delayed regressors were also used for the global signal, and WM/CSF signal. Extracted time series were then normalized before further analysis. Ciric et al. (2017) shows that median functional connectivity associations with motion are reduced more by despiking than scrubbing. Our analyses focused on moderate distance connections between the striatum and frontal cortex, therefore we applied AFNI 3D-despike, rather than scrubbing as our motion correction approach. Furthermore, given that head motion is an known phenotypic indicator for ADHD, scrubbing would have resulted in fewer frames for our ADHD sample than the TD sample, resulting in potential biases through systematically different levels of reliability in functional connectivity estimates between groups.
Extensive prior work has showed that the length of fMRI acquisition has a significant impact on its reliability (Birn et al., 2013, Cho et al., 2021, Laumann et al., 2015, Gratton et al., 2018, Nikolaidis et al., 2020) that task-specific activity accounts for a low percentage of variance in the fMRI signal (Gratton et al., 2018), and that concatenating rest and task data together can improve the scan reliability (Cho et al., 2021). Given these findings, we used FSL to concatenate multiple resting state and task-based fMRI scans after preprocessing to aggregate the longest possible scan for each participant (Smith et al., 2014). The average total aggregate scan length per subject was 9.89 min (ranging from 5 to 22.83 min). 351 subjects had available rs-fMRI data with an average length of 6.31 min per subject (ranging from 5 to 6.5 min); 84 subjects had 4 available task-fMRI data (GNGs1, GNGs2, GNGr1, GNGr2), each had an average length of 4.07 min per subject (ranging from 3.79 to 4.08 min). ADHD participants and TD participants did not differ in rs-fMRI (p > .05), task-fMRI (p > .05) or aggregated (p > .05) scan time. Girls and Boys did not differ in rs-fMRI (p > .05) scan time, but differed in task-fMRI (p < .05) scan time and aggregated (p < .001) scan time, with girls having longer scan time than boys. The aggregated scans are then down sampled to 2 mm, and spatially smoothed at 6 mm using FSL (Smith et al., 2014).
2.3. Functional connectivity and randomise analyses
Frontal cortical and striatal regions were defined using Harvard-Oxford cortical and subcortical atlases (Kennedy et al., 2016). The frontal cortical region consisted of the following bilateral cortical regions: precentral gyrus, superior frontal gyrus, middle frontal gyrus, paracingulate gyrus, cingulate gyrus anterior division, inferior frontal gyrus, and pars triangularis, frontal pole, subcallosal cortex, juxtapositional lobule cortex (formerly supplementary motor cortex), and frontal orbital cortex. To assess spatial specificity of corticostriatal interactions in relation to self-regulation of emotion, cognition, and motor functions implicated in ADHD (Arnsten and Rubia, 2012), we created three striatal maps (limbic, executive (cognitive), and motor) based on the Harvard-Oxford probabilistic subcortical atlas. These regions were defined using the following bilateral subcortical regions: limbic striatum: ventral putamen, nucleus accumbens; executive striatum: anterior caudate, dorsal putamen; motor striatum: posterior and dorsal putamen (Kennedy et al., 2016).
An average time series was extracted from each striatal mask, and FCSC connectivity was calculated as the voxel‐wise Pearson correlation between the striatal seeds and the frontal regions using the Nilearn package (Abraham et al., 2014). Fisher Z transform (Fisher, 1921) was then applied to the correlation values. Using FSL’s Randomise (version 2.9) we applied non-parametric permutation testing to assess the relationships between frontostriatal connectivity, age, ADHD diagnosis and symptom severity, GNG performance, and sex (Nichols and Holmes, 2002, Winkler et al., 2014). We used 5000 permutations in Randomise, and statistical thresholding was performed with FSL’s threshold-free cluster enhancement (TFCE) with a family-wise error rate (FWE) of p < =.05. Temporally demeaned data before model fitting and variance smoothing for t-stats were selected.
We used Randomise to test for two-way interactions of diagnosis, age, and sex (Table 2). We also tested the main effects of diagnosis, age, and sex, as well as main effects of Inattention T-score, Hyperactivity T-score, and GNG performance. We also tested interaction effects of GNG with age, sex, and diagnosis to probe the neural correlates of ADHD-related sex differences in GNG performance (Table 1).
Table 2.
Randomise results summary across three striatal subregions. Figure numbers associated with each result are included in the final column.
| Regions |
Figure | ||||
|---|---|---|---|---|---|
| Limbic striatum | Executive striatum | Motor striatum | Figure# | ||
| Main effects | |||||
| Main Age | Positive Age effect | Positive Age effect | Positive Age effect | 1 | |
| Main Dx | – | – | – | ||
| Male Dx | ADHD>TD | – | – | 2 | |
| Female Dx | – | – | – | ||
| ADHD symptom severity effects | |||||
| Inattention T-Score | – | – | Positive effect | 2 | |
| ADHD Inattention T-Score | – | Positive effect | Positive effect | 2 | |
| Hyperactive-Impulsive T-Score | – | – | – | ||
| ADHD Hyperactive-Impulsive T-Score | – | – | – | ||
| Go/no-go main effects | |||||
| GNG RT variability | Negative effect | Negative effect | Negative effect | 3 | |
| TD GNG RT variability | – | – | – | ||
| ADHD GNG RT variability | Negative effect | Negative effect | Negative effect | 3 | |
| GNG ComRate | – | – | – | ||
| TD GNG ComRate | Positive effect | – | – | 3 | |
| ADHD GNG ComRate | – | – | – | ||
| Go/no-go interaction effects | |||||
| DX*GNG RT variability Interaction | – | – | – | ||
| Sex*GNG ComRate Interaction | – | – | F > M positive slope | 3 | |
| TD Sex*GNG ComRate Interaction | – | – | – | ||
| ADHD Sex*GNG ComRate Interaction | F > M positive slope | – | F > M positive slope | 3 | |
To investigate the spatial distribution of FCSC overlap with canonical correlation networks (Yeo et al., 2011), we compared overlap of these results with each of the networks and report percentage of each statistical map covering each cortical network (Supplemental Table 2). The frontal cortical region of interest is comprised of differing amounts of each of the 7 Yeo network, (e.g. 5% for the Dorsal Attention Network to 26% for the DMN). Therefore, we also show the amount of overlap with these networks as well as the difference in percentage of significant voxels for each analysis compared to that which would be expected due to chance (Supplemental Table 3). We assess the similarity of spatial results across analyses and report the spatial correlations of these results (Supplemental Figure 8).
2.4. Go/No-Go task
A subset of participants completed a standard GNG task (n = 298) assessing response inhibition and variability (DeRonda et al., 2021, Wodka et al., 2007). The task stimuli consisted of a green spaceship for “Go” trials (80% of trials) and a red spaceship for “No-Go” trials (20% of trials) presented on-screen for 300 ms with an interstimulus interval of 2000 ms. Participants were instructed to push the spacebar with their index finger as quickly as possible in response to green spaceships. There were 11 practice trials followed by 217 experimental trials lasting 8 min and 19 s. Responses and reaction times (RT) were recorded to calculate the commission error rate (ComRate), defined as incorrectly pressing for a red spaceship, and RT variability, using an ex-Gaussian parameter quantifying the skewed tail of the RT distribution (tau) as an index of response variability separate from response speed (Epstein et al., 2011, Kofler et al., 2013, Tamm et al., 2012).
3. Results
3.1. Demographics, ADHD symptoms, and GNG performance by sex and diagnosis
Descriptive statistics and results of t-tests comparing demographic characteristics and GNG task performance between diagnostic groups across and within sex are provided in Table 1. Cohen’s d is reported as an estimate of effect size (Cohen, 2013).
Diagnostic groups did not differ in age across or within sex, whereas intellectual reasoning ability (full scale IQ) was significantly lower in the ADHD group for both boys and girls (d=0.73, p < .001). Socioeconomic status (SES) was also significantly lower in boys with ADHD compared to TD boys (d=0.29, p < .010), whereas it did not significantly differ among girls with and without ADHD, although a similar effect size was observed. As expected, parent-rated symptoms of inattention and hyperactivity/impulsivity T-scores were much higher among children with ADHD compared to TD children (inattention d=3.67, p < .001; hyperactivity/impulsivity d=2.85, p < .001) with similar diagnostic effects among girls and boys. In addition, inattention T-scores were greater among girls compared to boys with ADHD (d=1.04, p < .001), suggesting higher severity of inattentive symptoms among ADHD females.
For the GNG task, RT variability was higher for ADHD compared to TD (d=0.61, p < .001) with significant effects among boys (d=0.69, p < .001) and girls (d=0.43, p < .05). GNG ComRate was also greater among children with ADHD compared to TD children (d=0.43, p < .001), but this significant effect was specific to boys (d=0.56, p < .001) and not observed among girls (d=0.13, p > .05); furthermore, boys with ADHD showed significantly greater GNG ComRate compared to ADHD girls (d=0.70, p < .001).
3.2. FCSC connectivity – associations with age
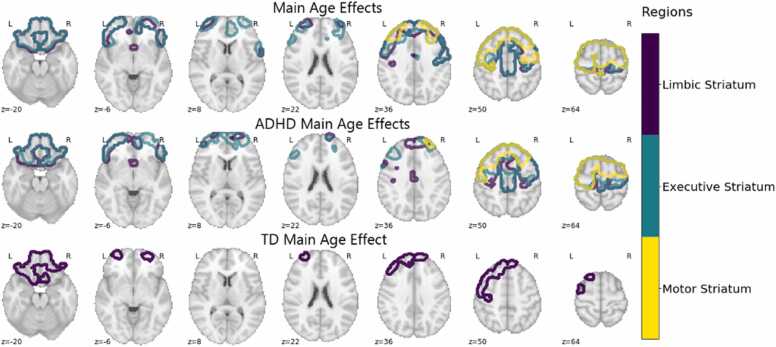
We found a significant positive main effect of age that was highly consistent across analyses using the three striatal subregions (Table 2; Pearson’s spatial correlation = 0.66–0.85; Supplemental Figure 8). By counting voxel-wise overlap between these significance maps and the Yeo 7 canonical intrinsic connectivity networks, we found that areas with significant age-associated increases in FCSC connectivity were primarily localized to the DMN (24.6%−28.4%), FPN (18.7–23.2%), and somatomotor networks (15.0–20.7%; Supplemental Table 2). These frontal cortex regions included the supplementary motor area (SMA), paracingulate gyrus, superior frontal gyrus, middle frontal gyrus, precentral gyrus, cingulate gyrus, frontal pole, and frontal orbital cortex (Fig. 1). Although there was no Diagnosis×Age interaction for FCSC connectivity across striatal subregions, we conducted an exploratory analysis examining age-related change in FCSC connectivity within our ADHD and TD groups given prior findings of maturational lag in functional connectivity of cortical networks in ADHD (Sripada et al., 2014). Interestingly, while we found significant age-associated increases with FCSC connectivity for ADHD in all three striatal subregions, for TD children significant age-associated increases with FSCS were localized to the limbic striatum (Fig. 1. Furthermore, we found notable differences in the spatial distribution of age effects between the limbic-frontal connectivity effects for TD and ADHD. ADHD children showed relatively more age-associated effects in the somatomotor, dorsal attention, and ventral attention networks while showing less in the default and limbic networks compared to TD children.
Fig. 1.
ADHD Age Effects. Dark blue, teal, and yellow correspond to the limbic, executive, and motor striatum respectively. Main age effects include significant voxels in the superior frontal gyrus, middle frontal gyrus, supplementary motor cortex, precentral gyrus, frontal orbital cortex, frontal pole, frontal medial cortex, and subcallosal cortex. For the ADHD group effects include significant voxels in the superior frontal gyrus, supplementary motor cortex, middle frontal gyrus, precentral gyrus, frontal orbital cortex, frontal pole. TD age effects were relatively restricted, including significant voxels in the subcallosal cortex, frontal medial cortex, and frontal pole. At least 50% of the voxels in each region had significant voxels in order to be mentioned here.
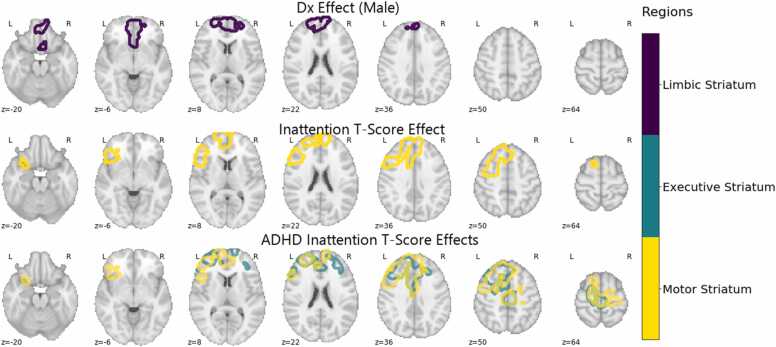
3.3. FCSC connectivity – associations with ADHD diagnosis and symptom severity
The effect of diagnosis on FCSC connectivity was specific to boys, such that boys with ADHD showed greater FCSC connectivity selectively with the limbic striatum and regions of the DMN (70%), with nearly no significant regions in the FPN (3.5%; Fig. 2). To assess the robustness of all Randomise results, we also repeated all analyses while controlling for both total scan length (which differed between boys and girls) and mean framewise displacement, which differed among ADHD and TD groups (Table 1). Although the ADHD>TD results in males were no longer significant when including scan length and FD as a covariate, we found high concordance in the spatial maps of our other analyses and those corrected for motion (Pearson’s spatial correlation; average = 0.97; range 0.88–0.99; See Supplemental Table S1 and Supplemental Fig. S5). Correcting for both scan length and motion also showed high spatial similarity with our original analyses (Pearson’s spatial correlation; average= 0.92; range 0.82–0.98). We chose to not match the groups on mean FD given evidence that head motion is correlated with ADHD symptomatology and that head motion and ADHD may have similar genetic loadings (Couvy-Duchesne et al., 2016), suggesting that covarying for FD accounts for variance attributable to ADHD. We include spatial maps of significant results controlling for only motion (Supplemental Figs. 1–3) as well as both motion and scan length (Supplemental Figures 4–6).
Fig. 2.
ADHD Diagnosis and Symptom Severity Effects. Dark blue, teal, and yellow correspond to the limbic, executive, and motor striatum respectively. Diagnosis effects in males were found in the subcallosal cortex, and frontal medial cortex. Inattention T-Score effect was found in the inferior frontal gyrus, pars triangularis, inferior frontal gyrus, pars opercularis, paracingulate gyrus, and superior frontal gyrus. Inattention T-score effects for the ADHD sample were found in the paracingulate gyrus, superior frontal gyrus, anterior cingulate gyrus, and middle frontal gyrus. At least 50% of the voxels in each region had significant voxels in order to be mentioned here.
Analysis of ADHD symptom associations across the sample revealed a significant positive relationship between parent-rated inattention (T-score) and FCSC connectivity between the motor striatum and regions of the DMN and FPN (54.9%; 22.9%) including the frontal pole, superior frontal gyrus, middle frontal gyrus, and paracingulate cortex (Fig. 2). These effects were spatially segregated from the effects of diagnosis as evidenced by the relatively low spatial overlap of the inattention T-score effects and the diagnosis effects (Pearson’s r = 0.23). The significant inattention effects seemed to be driven mainly by the ADHD group, with a broader range of areas demonstrating a significant positive relationships between inattention T-score and FCSC connectivity in the motor and executive striatum and regions of the DMN (26.8%−34.6%) and FPN (21.9%−23.9%) including the superior frontal gyrus, middle frontal gyrus, paracingulate gyrus, cingulate gyrus, precentral gyrus, and frontal pole (Fig. 2). The frontal regions showing significant associations were highly consistent across striatal subregions (Pearson’s spatial correlation = 0.61). We found no relationship between FCSC connectivity and the hyperactivity/impulsivity T-score.
3.4. FCSC connectivity – associations with GNG task performance
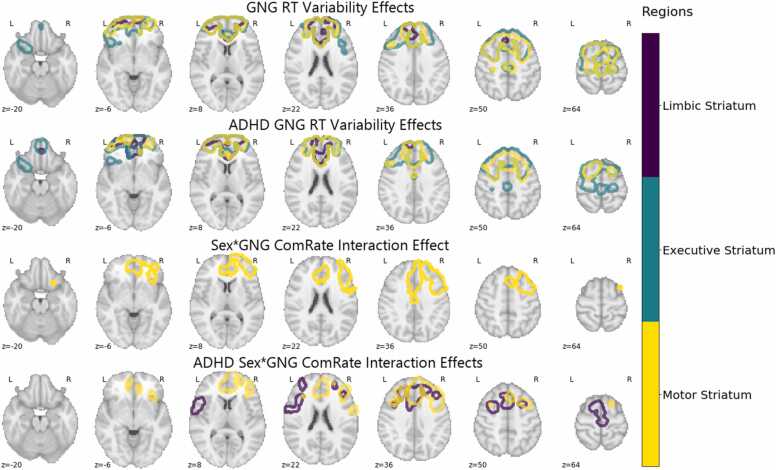
Analysis of GNG task associations with FCSC connectivity showed a negative relationship with GNG RT variability (tau) that reproduced across all striatal regions and regions of the frontal cortex in the DMN (42.7%−43.0%) and FPN (21.3%−22.9%) including the paracingulate gyrus, superior frontal gyrus, and frontal pole (Pearson’s correlation = 0.50–0.82). In other words, greater RT variability was associated with less FCSC connectivity. The executive striatum and motor striatum showed a tight similarity of findings which also included the middle frontal gyrus, cingulate gyrus, frontal orbital cortex and frontal medial cortex (Pearson’s correlation = 0.82 Fig. 3). The limbic striatum showed a moderately different relationship to FCSC connectivity, with a reduced spatial overlap with the executive and motor striatum effects (Pearson’s correlation 0.50 and 0.58 respectively), and more notably nearly all regions significantly associated with RT variability were located in the DMN (72.6%). This significant negative relationship between FCSC connectivity and RT variability seemed to be mostly driven by the ADHD group, who showed largely a similar spatial significance pattern, while the TD group did not show a significant relationship between FCSC connectivity and RT variability. Spatial correlation between the full sample maps and ADHD sample alone calculated pairwise for each striatal seed shows these maps to be highly consistent (Pearson’s correlation = 0.69–0.92; Fig. 3).
Fig. 3.
Randomise results associated with GNG task performance. Dark blue, teal, and yellow correspond to the limbic, executive, and motor striatum respectively. GNG RT effects were observed in the paracingulate gyrus, superior frontal gyrus, frontal pole, and middle frontal gyrus. ADHD GNG RT effects were found in the paracingulate gyrus, superior frontal gyrus, frontal pole, anterior cingulate gyrus, and middle frontal gyrus. Sex*GNG commission error rate interaction effects were observed in the paracingulate gyrus, anterior cingulate gyrus, and middle frontal gyrus. ADHD sample Sex*GNG commission error rate interaction effects were observed in the paracingulate gyrus and anterior cingulate gyrus. At least 50% of the voxels in each region had significant voxels in order to be mentioned here.
Although there was no evidence of FCSC connectivity in relation to GNG ComRate across the ADHD and TD groups (Table 2), given the behavioral finding of ADHD-related sex differences for GNG ComRate, such that only boys with ADHD showed increased ComRate compared to TD boys and girls with ADHD, we tested whether sex moderated the relationship between GNG ComRate and FCSC connectivity. Results indicated a significant sex*GNG ComRate interaction in the motor subregion of the striatum, with girls having a significantly stronger positive relationship between FCSC connectivity and GNG ComRate than boys in both the full sample and within the ADHD sample (Fig. 3). Significant regions for the full sample were highly consistent with the ADHD sample (Fig. 3) indicating FCSC connectivity of the limbic and motor striatum with subregions of the FPN (31.3–45.9%) and DMN (13.9–34.8%) including the frontal pole, middle frontal gyrus, paracingulate gyrus, superior frontal gyrus (Pearson’s correlation = 0.68).
3.5. Intrinsic network specificity in frontal striatal correlates of ADHD and development
The cortical networks with the greatest overlap with the regions with significant cortico-striatal interactions with Inattention, ADHD, and the GNG task were the DMN and FPN. Interestingly, the loadings on each of these two networks are strongly negatively related (Pearson’s correlation = −0.72), when frontal-striatal connectivity shows significant loadings in the FPN it tends not to do so in the DMN and vice versa. For example, the GNG tau and Inattention T-score results show a significant relationship between striatal connectivity and the DMN but very little connectivity to the FPN, while the sex by GNG interaction effects load significantly onto the FPN but not the DMN.
4. Discussion
4.1. Overview
ADHD is a heterogeneous disorder in terms of symptom presentation and associated deficits in cognitive, emotional, and motor control. Understanding the full range brain-behavioral pattern can provide a crucial roadmap for unraveling ADHD heterogeneity. Therefore, the current study investigated associations between fMRI-based measurement of FCSC connectivity and ADHD in a large sample of 362 school-age children and considered effects of sex and age. Addressing our first hypothesis, we found that, across the entire population (both ADHD and TD children), FCSC connectivity increased with age across much of the frontal cortex, including regions in the somatomotor network (SMN), FPN and DMN. Importantly, these age-related patterns of FCSC connectivity were largely distinct from those FCSC connectivity associated with diagnosis, symptom severity, and GNG performance, showing uniquely significant FCSC connectivity patterns in the subcallosal cortex, supplementary motor cortex, frontal orbital cortex, and precentral gyrus. Addressing our second hypotheses, we found that ADHD diagnosis was associated with greater FCSC connectivity but this was specific to boys with ADHD and connectivity between the limbic striatum with the DMN, extending prior work demonstrating cortico-cortico hyperconnectivity of the DMN in ADHD (Konrad and Eickhoff, 2010, Henry and Cohen, 2019, Posner et al., 2014, Sripada et al., 2014, Duffy et al., 2021, McCarthy et al., 2013, Barber et al., 2015, Hoekzema et al., 2014, Elton et al., 2014, Zhao et al., 2021). Interestingly, we found that parent-rated inattentive symptom ratings were associated with increased connectivity of the executive and motor striatum with regions of the DMN and FPN cortical networks, suggesting spatially distinct effects from the observed diagnostic group difference in boys. Finally, RT variability during a GNG task (which was elevated among children with ADHD, regardless of sex) was associated with decreased FCSC connectivity with DMN and FPN across striatal subregions In contrast, sex differences were observed for associations between FCSC connectivity and GNG inhibition errors (which were elevated among boys with ADHD only), such that the positive association was stronger among girls. Overall, our results provide evidence that heterogeneity in ADHD related to sex differences, symptom presentation, and cognitive task performance may be linked to dysregulation of task positive and task negative network connectivity with the striatum.
4.2. FCSC and age
Regarding our first hypothesis, FCSC connectivity increased with age across striatal subregions when examined in the full sample of ADHD and TD children. These age-related increases were observed across a large set of regions covering the SMN, DMN, FPN, and Dorsal Attention networks. Although the Diagnosis×Age interaction was not significant, there were notable differences in the spatial distribution of age-associations for ADHD vs. TD children. ADHD children showed an expanded set of FCSC regions with significant positive associations with age compared to TD children, with localized effects involving the limbic striatum, suggesting that changes in FCSC connectivity may be more widespread among children ADHD in this developmental period. Consistent with these findings, one previous investigation of FCSC connectivity age-effects in children with (and without) ADHD found similar evidence for a much more extensive set of FCSC patterns associated with age in children with ADHD and found limited overlap of FCSC patterns associated with age versus those associated with ADHD severity (Barber et.al, 2019). The findings thereby suggest that while age associations with FCSC connectivity are quite extensive, they are also sensitive to ADHD and should be considered whenever investigating FCSC connectivity links to ADHD diagnosis and symptom severity.
4.3. FCSC connectivity and ADHD
Our second hypothesis, that ADHD diagnosis and symptom severity would be associated with increased striatal-DMN connectivity and decreased striatal-FPN connectivity, and that these associations may be stronger for girls with ADHD, was only partially supported. Consistent with this hypothesis, we found an effect of ADHD diagnosis for connectivity between the limbic striatum and DMN cortical regions, but only for males. These findings are inconsistent with our previous study (Rosch et al., 2018), reporting greater FCSC in girls with ADHD. However, we did not examine striatal subregions with different functional roles in our previous analyses and focused instead on connectivity with select cortical regions, which may have contributed to the discrepant results. It is also important to note that there were no Diagnosis×Sex interactions in our current analyses, suggesting that the diagnosis effects may be similar, but weaker in girls and not significant due to the smaller sample. These inconsistent findings may otherwise relate to variability in the methods (e.g., the use of group ICA and analysis of specific cortical regions in our prior study) and sample (e.g., elimination of comorbid mood and anxiety disorders in our prior study), highlighting the need for further research to understand ADHD-related sex differences in brain structure, function, and behavior. In addition to diagnostic group differences in FCSC connectivity, executive and motor striatal subregions both demonstrated a significant positive association between functional connectivity to DMN and FPN regions inattention t-score in ADHD children. Thus, although girls with ADHD did not significantly differ from TD girls in FCSC connectivity, they do display greater levels of inattention symptom severity, that relates to greater FCSC connectivity between the motor and executive striatum with regions of the DMN and FPN.
Our findings of atypical limbic FCSC in ADHD is consistent with prior studies implicating this circuit in ADHD. Increasing evidence from neuroimaging studies for anomalous limbic circuitry in ADHD, including ventromedial/orbitofrontal cortical regions and subcortical regions including the ventral striatum and amygdala (Dias et al., 2013, Tomasi and Volkow, 2012, Mennes et al., 2012; Cao et al., 2006, Cao et al., 2009; Posner et al., 2013). Our results suggest that limbic FCSC connectivity may be particularly sensitive to group-level diagnostic differences in boys, which are primarily examined in the ADHD literature as most studies include predominantly or exclusively male samples thereby limiting examination of sex differences. Our findings indicate, for the first time, novel dysregulation between DMN regions and the striatum in ADHD (specifically, the limbic striatum), something that has not been well characterized previously. Collectively, these findings suggest that disruptions in FCSC connectivity with functionally distinct striatal subregions may differentially relate to overall diagnostic group differences (involving the limbic striatum) and heterogeneity in symptom presentation (involving the motor and executive striatum).
ADHD has been widely regarded as a neurodevelopmental disorder involving dysregulation of FCSC connectivity as well as task-positive/task negative network integration (Barber et al., 2015, Castellanos et al., 2008, Sripada et al., 2014, Hoekzema et al., 2014, Kessler et al., 2014, Elton et al., 2014). Our findings show that FCSC connectivity associations with ADHD, sex, symptom severity and cognitive control are mostly located in the FPN and DMN. Furthermore, we found across all analyses that the FPN and DMN FCSC connectivity associations were themselves negatively correlated. These findings suggest that FCSC connectivity may contribute to the task-positive/task-negative dysregulation often characterized in ADHD. DMN dysregulation is often purported to contribute to ADHD-associated difficulties with sustaining attention. Our findings suggest that DMN-striatum integration may play a role in DMN dysregulation associated with ADHD. FCSC connectivity are also closely involved in the emergence and integration of motor, cognitive, and affective skills over development. Given the key role of these circuits in child and adolescent cognitive maturation, and the neurodevelopmental nature of ADHD, dysregulation in FCSC connectivity involved in task positive/negative integration may contribute to the development of previously-reported differences in cortico-cortico dysregulation. Furthermore, it may be possible that alterations in corticostriatal circuits may be an early hallmark of emergent dysfunction in cortico-cortico DMN-related alterations in ADHD. Future longitudinal work may be able to clarify the emergence of task-positive task negative dysregulation in cortico-cortico and corticostriatal networks.
4.4. FCSC connectivity and response control
Our third hypothesis, that GNG response inhibition errors would be associated with differences in striatal connectivity to FPN, whereas GNG RT variability would be associated with differences in striatal connectivity to DMN, and that this may differ among girls and boys, was partially supported. We found that across the sample (ADHD and TD), increased GNG RT variability associated with decreased striatal-DMN and FPN connectivity with similar effects across striatal subregions and this effect was driven by the ADHD group. In addition, a significant Sex×GNG ComRate interaction for FCSC connectivity was observed across the sample and within the ADHD group, suggesting ADHD-related sex differences in this relationship. Specifically, girls with ADHD showed a stronger positive relationship between functional connectivity of the striatum and FPN frontal cortical regions and GNG ComRate. Thus, although as a group, girls with ADHD did not show anomalous FCSC connectivity or response inhibition, within group heterogeneity did reveal associations between GNG ComRate and FCSC connectivity. Recent work by Duffy et al. (2021) found no association between ComRate and a graph-theory based participation coefficient metric of connectivity between the DMN and subcortical seeds taken from the Power atlas (Power et al., 2011). Differences in the seed regions used, sample size, and the analytical approach may have contributed to these findings contrasting the current work.
4.5. Limitations
While the current study aimed to investigate corticostriatal associations with ADHD in a large sample of school aged children, some limitations are worth noting. First and foremost, the cross-sectional nature of this sample limits our ability to conduct a thorough mapping of the development of corticostriatal circuits and their association with the emergence of ADHD symptoms. Future work collecting longitudinal data (with three time points) is currently ongoing and will be critical for re-evaluating the consistency of corticostriatal associations with age, sex, diagnosis, and symptom severity. Another important limitation worth mentioning is that the amount of fMRI data collected was also limited to ten minutes on average, whereas reliable sampling of individual differences in resting state connectivity (ICC > 0.8) requires functional data acquisitions of over 25 min (Gordon et al., 2017, Laumann et al., 2015, O’Connor et al., 2017, Cho et al., 2021). Collecting this much data in a young sample can be challenging, though it may be possible that as this sample gets into middle and late adolescence that we are able to acquire more high quality functional imaging data.
4.6. Conclusions
In conclusion, we found support for our hypotheses that FCSC connectivity would be sensitive to age, sex, ADHD diagnosis and symptom severity, and cognitive task performance. In a large sample of 362 school aged children we found experimental evidence to support theoretical accounts that ADHD would be reflected in dysregulated FCSC connectivity of task positive and task negative networks, reflecting poor segregation of these networks and lapses in attention typically associated with ADHD. We found evidence that while striatal subregions associated with motor, executive, and limbic cortical areas are all highly developmentally sensitive, these striatal subregions show specific sensitivity for ADHD and symptom effects that are also important to consider.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the NIH (R21 MH118556-01, R01 MH078160, R01 MH085328, K23 MH101322, K23 MH107734, P50 HD103538, P41EB015909, P41EB031771, 1S10OD021648) Brain and Behavior Research Foundation, NARSAD Young Investigator Grant.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101101.
Appendix A. Supplementary material
Supplementary material
.
Data availability statement
The data used in the current manuscript are not yet available for public release because they are part of a longitudinal study for which data collection is currently ongoing.
References
- Abraham A., Pedregosa F., Eickenberg M., Gervais P., Mueller A., Kossaifi J., Gramfort A., Thirion B., Varoquaux G. Machine learning for neuroimaging with scikit-learn. Front. Neuroinform. 2014;8:14. doi: 10.3389/fninf.2014.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., DeLong M.R., Strick P.L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu. Rev. Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Arnsten A.F.T., Rubia K. Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J. Am. Acad. Child Adolesc. Psychiatry. 2012;51(4):356–367. doi: 10.1016/j.jaac.2012.01.008. [DOI] [PubMed] [Google Scholar]
- Avants B.B., Tustison N.J., Song G., Cook P.A., Klein A., Gee J.C. A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage. 2011;54(3):2033–2044. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Jacobson L.A., Wexler J.L., Nebel M.B., Caffo B.S., Pekar J.J., Mostofsky S.H. Connectivity supporting attention in children with attention deficit hyperactivity disorder. NeuroImage. Clin. 2015;7:68–81. doi: 10.1016/j.nicl.2014.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber A.D., Sarpal D.K., John M., Fales C.L., Mostofsky S.H., Malhotra A.K., Karlsgodt K.H., Lencz T., Pediatric Imaging, Neurocognition, Genetics (PING) Study Consortium Age-normative pathways of striatal connectivity related to clinical symptoms in the general population. Biol. Psychiatry. 2019;85(11):966–976. doi: 10.1016/j.biopsych.2019.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y., Restom K., Liau J., Liu T.T. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37(1):90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. NeuroImage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Zang Y., Sun L., Sui M., Long X., Zou Q., Wang Y. Abnormal neural activity in children with attention deficit hyperactivity disorder: a resting-state functional magnetic resonance imaging study. NeuroReport. 2006;17(10):1033–1036. doi: 10.1097/01.wnr.0000224769.92454.5d. [DOI] [PubMed] [Google Scholar]
- Cao X., Cao Q., Long X., Sun L., Sui M., Zhu C., Zuo X., Zang Y., Wang Y. Abnormal resting-state functional connectivity patterns of the putamen in medication-naïve children with attention deficit hyperactivity disorder. Brain Res. 2009;1303:195–206. doi: 10.1016/j.brainres.2009.08.029. [DOI] [PubMed] [Google Scholar]
- Castellanos F.X., Aoki Y. Intrinsic functional connectivity in attention-deficit/hyperactivity disorder: a science in development. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(3):253–261. doi: 10.1016/j.bpsc.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Margulies D.S., Kelly C., Uddin L.Q., Ghaffari M., Kirsch A., Shaw D., Shehzad Z., Di Martino A., Biswal B., Sonuga-Barke E.J.S., Rotrosen J., Adler L.A., Milham M.P. Cingulate-precuneus interactions: a new locus of dysfunction in adult attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2008;63(3):332–337. doi: 10.1016/j.biopsych.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Proal E. Large-scale brain systems in ADHD: beyond the prefrontal–striatal model. Trends Cogn. Sci. 2012;16(1):17–26. doi: 10.1016/j.tics.2011.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos F.X., Sonuga-Barke E.J.S., Milham M.P., Tannock R. Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn. Sci. 2006;10(3):117–123. doi: 10.1016/j.tics.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Chai Y., Chimelis Santiago J.R., Bixler K.A., Aalsma M., Yu M., Hulvershorn L.A. Sex-specific frontal-striatal connectivity differences among adolescents with externalizing disorders. NeuroImage Clin. 2021;32 doi: 10.1016/j.nicl.2021.102789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J.W., Korchmaros A., Vogelstein J.T., Milham M.P., Xu T. Impact of concatenating fMRI data on reliability for functional connectomics. NeuroImage. 2021;226(117549) doi: 10.1016/j.neuroimage.2020.117549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciric R., Nomi J.S., Uddin L.Q., Satpute A.B. Contextual connectivity: a framework for understanding the intrinsic dynamic architecture of large-scale functional brain networks. Sci. Rep. 2017;7(1):6537. doi: 10.1038/s41598-017-06866-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Routledge; 2013. Statistical Power Analysis for the Behavioral Sciences.〈https://play.google.com/store/books/details?id=cIJH0lR33bgC〉 [Google Scholar]
- Cole W.R., Mostofsky S.H., Larson J.C.G., Denckla M.B., Mahone E.M. Age-related changes in motor subtle signs among girls and boys with ADHD. Neurology. 2008;71(19):1514–1520. doi: 10.1212/01.wnl.0000334275.57734.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners, C.K. , 2008. Conners 3. 〈https://www.cognitivecentre.com/wp-content/uploads/Conners3_Brochure_2017_Insequence.pdf〉.
- Couvy-Duchesne B., Ebejer J.L., Gillespie N.A., Duffy D.L., Hickie I.B., Thompson P.M., Martin N.G., de Zubicaray G.I., McMahon K.L., Medland S.E., Wright M.J. Head motion and inattention/hyperactivity share common genetic influences: implications for fMRI studies of ADHD. PLoS One. 2016;11(1) doi: 10.1371/journal.pone.0146271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock, C., Sikka, S., Cheung, B., Khanuja, R., Ghosh, S.S., Yan, C., Li, Q., Lurie, D., Vogelstein, J., Burns, R., Colcombe, S., Mennes, M., Kelly, C., Di Martino, A., Fx, C., Milham, M. , 2013. Towards automated analysis of connectomes: the configurable pipeline for the analysis of connectomes (C-PAC. In: Proceedings of the Front. Neuroinform. Conference Abstract: Neuroinformatics. 〈https://doi.org/10〉.
- Da Fonseca D., Seguier V., Santos A., Poinso F., Deruelle C. Emotion understanding in children with ADHD. Child Psychiatry Hum. Dev. 2009;40(1):111–121. doi: 10.1007/s10578-008-0114-9. [DOI] [PubMed] [Google Scholar]
- Damiani S., Tarchi L., Scalabrini A., Marini S., Provenzani U., Rocchetti M., Oliva F., Politi P. Beneath the surface: hyper-connectivity between caudate and salience regions in ADHD fMRI at rest. Eur. Child Adolesc. Psychiatry. 2021;30(4):619–631. doi: 10.1007/s00787-020-01545-0. [DOI] [PubMed] [Google Scholar]
- Denckla M.B. Attention deficit hyperactivity disorder-residual type. J. Child Neurol. 1991;6(Suppl):S44–S50. doi: 10.1177/0883073891006001s06. [DOI] [PubMed] [Google Scholar]
- DeRonda A., Zhao Y., Seymour K.E., Mostofsky S.H., Rosch K.S. Distinct patterns of impaired cognitive control among boys and girls with ADHD across development. Res. Child Adolesc. Psychopathol. 2021;49(7):835–848. doi: 10.1007/s10802-021-00792-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino A., Zuo X.-N., Kelly C., Grzadzinski R., Mennes M., Schvarcz A., Rodman J., Lord C., Castellanos F.X., Milham M.P. Shared and Distinct intrinsic functional network centrality in autism and attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2013;74(8):623–632. doi: 10.1016/j.biopsych.2013.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias T.G.C., Iyer S.P., Carpenter S.D., Cary R.P., Wilson V.B., Mitchell S.H., Nigg J.T., Fair D.A. Characterizing heterogeneity in children with and without ADHD based on reward system connectivity. Dev. Cogn. Neurosci. 2015;11:155–174. doi: 10.1016/j.dcn.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias T.G.C., Wilson V.B., Bathula D.R., Iyer S.P., Mills K.L., Thurlow B.L., Stevens C.A., Musser E.D., Carpenter S.D., Grayson D.S., Mitchell S.H., Nigg J.T., Fair D.A. Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2013;23(1):33–45. doi: 10.1016/j.euroneuro.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirlikov B., Shiels Rosch K., Crocetti D., Denckla M.B., Mahone E.M., Mostofsky S.H. Distinct frontal lobe morphology in girls and boys with ADHD. NeuroImage Clin. 2015;7:222–229. doi: 10.1016/j.nicl.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J., Bellec P., Amsel R., Penhune V., Monchi O., Carrier J., Lehéricy S., Benali H. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res. 2009;199(1):61–75. doi: 10.1016/j.bbr.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Duffy K.A., Rosch K.S., Nebel M.B., Seymour K.E., Lindquist M.A., Pekar J.J., Mostofsky S.H., Cohen J.R. Increased integration between default mode and task-relevant networks in children with ADHD is associated with impaired response control. Dev. Cogn. Neurosci. 2021;50 doi: 10.1016/j.dcn.2021.100980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul, G.J., Power, T.J., Anastopoulos, A.D., & Reid, R. (1998). ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation. 79. 〈https://psycnet.apa.org/fulltext/1998–06605-000.pdf〉.
- Elliott B.L., D’Ardenne K., Mukherjee P., Schweitzer J.B., McClure S.M. Limbic and executive meso- and nigrostriatal tracts predict impulsivity differences in attention-deficit/hyperactivity disorder. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2021 doi: 10.1016/j.bpsc.2021.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elton A., Alcauter S., Gao W. Network connectivity abnormality profile supports a categorical-dimensional hybrid model of ADHD. Hum. Brain Mapp. 2014;35(9):4531–4543. doi: 10.1002/hbm.22492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein, J.N., Langberg, J.M., Rosen, P.J., Graham, A., Narad, M.E., Antonini, T.N., Brinkman, W.B., Froehlich, T., Simon, J.O., & Altaye, M. , 2011. Evidence for higher reaction time variability for children with ADHD on a range of cognitive tasks including reward and event rate manipulations. In: Neuropsychology, 25 (4) 427–441. 〈 10.1037/a0022155〉. [DOI] [PMC free article] [PubMed]
- Fisher R.A. On the’probable error’of a coefficient of correlation deduced from a small sample. Metron Int. J. Stat. 1921;1:1–32. 〈https://ci.nii.ac.jp/naid/10012392243/〉 [Google Scholar]
- Fonov V.S., Evans A.C., McKinstry R.C., Almli C.R., Collins D.L. Unbiased nonlinear average age-appropriate brain templates from birth to adulthood. NeuroImage. 2009;47(Supplement 1):S102. doi: 10.1016/S1053-8119(09)70884-5. [DOI] [Google Scholar]
- Friston, K.J., Williams, S., Howard, R., Frackowiak, R.S. J., Turner, R. , 1996. Movement-related effects in fMRI time-series. In: Proceedings of the Magnetic Resonance in Medicine, 35 (3) 346–355. 〈 10.1002/mrm.1910350312〉. [DOI] [PubMed]
- Gilbert D.L., Isaacs K.M., Augusta M., Macneil L.K., Mostofsky S.H. Motor cortex inhibition: a marker of ADHD behavior and motor development in children. Neurology. 2011;76(7):615–621. doi: 10.1212/WNL.0b013e31820c2ebd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon E.M., Laumann T.O., Gilmore A.W., Newbold D.J., Greene D.J., Berg J.J., Ortega M., Hoyt-Drazen C., Gratton C., Sun H., Hampton J.M., Coalson R.S., Nguyen A.L., McDermott K.B., Shimony J.S., Snyder A.Z., Schlaggar B.L., Petersen S.E., Nelson S.M., Dosenbach N.U.F. Precision functional mapping of individual human brains. Neuron. 2017;95(4):791–807.e7. doi: 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton C., Laumann T.O., Nielsen A.N., Greene D.J., Gordon E.M., Gilmore A.W., Nelson S.M., Coalson R.S., Snyder A.Z., Schlaggar B.L., Dosenbach N.U.F., Petersen S.E. Functional brain networks are dominated by stable group and individual factors, not cognitive or daily variation. Neuron. 2018;98(2):439–452.e5. doi: 10.1016/j.neuron.2018.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graybiel A.M. Habits, rituals, and the evaluative brain. Annu. Rev. Neurosci. 2008;31:359–387. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- Graybiel, A.M., Grafton, S.T. , 2015. The striatum: where skills and habits meet. In: Cold Spring Harbor Perspectives in Biology, 7 (8) a021691. 〈 10.1101/cshperspect.a021691〉. [DOI] [PMC free article] [PubMed]
- Hasson R., Fine J.G. Gender differences among children with ADHD on continuous performance tests: a meta-analytic review. J. Atten. Disord. 2012;16(3):190–198. doi: 10.1177/1087054711427398. [DOI] [PubMed] [Google Scholar]
- Heilman K.M., Voeller K.K., Nadeau S.E. A possible pathophysiologic substrate of attention deficit hyperactivity disorder. J. Child Neurol. 1991;6(Suppl):S76–S81. doi: 10.1177/0883073891006001s09. [DOI] [PubMed] [Google Scholar]
- Henry, T.R., Cohen, J.R. , 2019. Dysfunctional brain network organization in neurodevelopmental disorders. In: Connectomics, 83–100. 〈https://doi.org/10.1016/b978-0-12–813838-0.00005-4〉.
- Hoekzema E., Carmona S., Ramos-Quiroga J.A., Richarte Fernández V., Bosch R., Soliva J.C., Rovira M., Bulbena A., Tobeña A., Casas M., Vilarroya O. An independent components and functional connectivity analysis of resting state fMRI data points to neural network dysregulation in adult ADHD. Hum. Brain Mapp. 2014;35(4):1261–1272. doi: 10.1002/hbm.22250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.-B., Harrison B.J., Fornito A., Sohn C.-H., Song I.-C., Kim J.-W. Functional dysconnectivity of corticostriatal circuitry and differential response to methylphenidate in youth with attention-deficit/hyperactivity disorder. J. Psychiatry Neurosci. JPN. 2015;40(1):46–57. doi: 10.1503/jpn.130290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson L.A., Peterson D.J., Rosch K.S., Crocetti D., Mori S., Mostofsky S.H. Sex-based dissociation of white matter microstructure in children with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry. 2015;54(11):938–946. doi: 10.1016/j.jaac.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman, J., Birmaher, B., Axelson, D., Perepletchikova, F., Brent, D., Ryan, N. , 2016. Kiddie Schedule for Affective Disorders and Schizophrenia for School-Aged Children--Present and Lifetime Version (Kiddie-SADS-PL 2016). Pittsburgh Pennsylvania: Western Psychiatric Institute and Clinic and Yale University.
- Kennedy, D., Haselgrove, C., Fischl, B., Breeze, J., Frazier, J., Seidman, L., Goldstein, J., Kosofsky, B. , 2016. Harvard Oxford Cortical and Subcortical Structural Atlases. Harvard Center for Morphometric Analysis.
- Kessler D., Angstadt M., Welsh R.C., Sripada C. Modality-spanning deficits in attention-deficit/hyperactivity disorder in functional networks, gray matter, and white matter. J. Neurosci. Off. J. Soc. Neurosci. 2014;34(50):16555–16566. doi: 10.1523/JNEUROSCI.3156-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofler M.J., Rapport M.D., Sarver D.E., Raiker J.S., Orban S.A., Friedman L.M., Kolomeyer E.G. Reaction time variability in ADHD: a meta-analytic review of 319 studies. Clin. Psychol. Rev. 2013;33(6):795–811. doi: 10.1016/j.cpr.2013.06.001. [DOI] [PubMed] [Google Scholar]
- Konrad K., Eickhoff S.B. Is the ADHD brain wired differently? A review on structural and functional connectivity in attention deficit hyperactivity disorder. Hum. Brain Mapp. 2010;31(6):904–916. doi: 10.1002/hbm.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laumann T.O., Gordon E.M., Adeyemo B., Snyder A.Z., Joo S.J., Chen M.-Y., Gilmore A.W., McDermott K.B., Nelson S.M., Dosenbach N.U.F., Schlaggar B.L., Mumford J.A., Poldrack R.A., Petersen S.E. Functional system and areal organization of a highly sampled individual human brain. Neuron. 2015;87(3):657–670. doi: 10.1016/j.neuron.2015.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma I., van Holstein M., Mies G.W., Mennes M., Buitelaar J., Cools R., Cillessen A.H.N., Krebs R.M., Scheres A. Ventral striatal hyperconnectivity during rewarded interference control in adolescents with ADHD. Cortex J. Devoted Study Nerv. Syst. Behav. 2016;82:225–236. doi: 10.1016/j.cortex.2016.05.021. [DOI] [PubMed] [Google Scholar]
- Macneil L.K., Xavier P., Garvey M.A., Gilbert D.L., Ranta M.E., Denckla M.B., Mostofsky S.H. Quantifying excessive mirror overflow in children with attention-deficit/hyperactivity disorder. Neurology. 2011;76(7):622–628. doi: 10.1212/WNL.0b013e31820c3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahone, E.M. , 2012, October. Neuropsychiatric Differences Between Boys and Girls with ADHD. 29, 34. 〈https://go.gale.com/ps/i.do?id=GALE%7CA332893499&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=08932905&p=AONE&sw=w〉.
- McCarthy H., Skokauskas N., Mulligan A., Donohoe G., Mullins D., Kelly J., Johnson K., Fagan A., Gill M., Meaney J., Frodl T. Attention network hypoconnectivity with default and affective network hyperconnectivity in adults diagnosed with attention-deficit/hyperactivity disorder in childhood. JAMA Psychiatry. 2013;70(12):1329–1337. doi: 10.1001/jamapsychiatry.2013.2174. [DOI] [PubMed] [Google Scholar]
- Mennes, M., Potler, N.V., Kelly, C., Di Martino, A., Xavier Castellanos, F., Milham, M.P. (2012). Resting State Functional Connectivity Correlates of Inhibitory Control in Children with Attention-Deficit/Hyperactivity Disorder. In: Frontiers in Psychiatry, 2. 〈 10.3389/fpsyt.2011.00083〉. [DOI] [PMC free article] [PubMed]
- Mostofsky S.H., Newschaffer C.J., Denckla M.B. Overflow movements predict impaired response inhibition in children with ADHD. Percept. Mot. Skills. 2003;97(3 Pt 2):1315–1331. doi: 10.2466/pms.2003.97.3f.1315. [DOI] [PubMed] [Google Scholar]
- Mostofsky S.H., Rimrodt S.L., Schafer J.G.B., Boyce A., Goldberg M.C., Pekar J.J., Denckla M.B. Atypical motor and sensory cortex activation in attention-deficit/hyperactivity disorder: a functional magnetic resonance imaging study of simple sequential finger tapping. Biol. Psychiatry. 2006;59(1):48–56. doi: 10.1016/j.biopsych.2005.06.011. [DOI] [PubMed] [Google Scholar]
- Nichols T.E., Holmes A.P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15(1):1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis A., Solon Heinsfeld A., Xu T., Bellec P., Vogelstein J., Milham M. Bagging improves reproducibility of functional parcellation of the human brain. NeuroImage. 2020;214(116678) doi: 10.1016/j.neuroimage.2020.116678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidis A., Voss M.W., Lee H., Vo L.T.K., Kramer A.F. Parietal plasticity after training with a complex video game is associated with individual differences in improvements in an untrained working memory task. Front. Hum. Neurosci. 2014;8:169. doi: 10.3389/fnhum.2014.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor D., Potler N.V., Kovacs M., Xu T., Ai L., Pellman J., Vanderwal T., Parra L.C., Cohen S., Ghosh S., Escalera J., Grant-Villegas N., Osman Y., Bui A., Craddock R.C., Milham M.P. The Healthy Brain Network Serial Scanning Initiative: a resource for evaluating inter-individual differences and their reliabilities across scan conditions and sessions. GigaScience. 2017;6(2):1–14. doi: 10.1093/gigascience/giw011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M., Beckmann C.F., Franke B., Hartman C.A., Hoekstra P.J., Oosterlaan J., Heslenfeld D., Buitelaar J.K., Mennes M. Functional connectivity in cortico-subcortical brain networks underlying reward processing in attention-deficit/hyperactivity disorder. NeuroImage. Clin. 2016;12:796–805. doi: 10.1016/j.nicl.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldehinkel M., Beckmann C.F., Pruim R.H.R., van Oort E.S.B., Franke B., Hartman C.A., Hoekstra P.J., Oosterlaan J., Heslenfeld D., Buitelaar J.K., Mennes M. Attention-Deficit/Hyperactivity Disorder symptoms coincide with altered striatal connectivity. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2016;1(4):353–363. doi: 10.1016/j.bpsc.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Park C., Wang Z. Connecting the dots: a review of resting connectivity MRI studies in attention-deficit/hyperactivity disorder. Neuropsychol. Rev. 2014;24(1):3–15. doi: 10.1007/s11065-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner J., Rauh V., Gruber A., Gat I., Wang Z., Peterson B.S. Dissociable attentional and affective circuits in medication-naïve children with attention-deficit/hyperactivity disorder. Psychiatry Res. 2013;213(1):24–30. doi: 10.1016/j.pscychresns.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma R.B., Dagher A. Basal ganglia functional connectivity based on a meta-analysis of 126 positron emission tomography and functional magnetic resonance imaging publications. Cereb. Cortex. 2006;16(10):1508–1521. doi: 10.1093/cercor/bhj088. [DOI] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. NeuroImage. 2012;59(3):2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Cohen A.L., Nelson S.M., Wig G.S., Barnes K.A., Church J.A., Vogel A.C., Laumann T.O., Miezin F.M., Schlaggar B.L., Petersen S.E. Functional network organization of the human brain. Neuron. 2011;72(4):665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu A., Crocetti D., Adler M., Mahone E.M., Denckla M.B., Miller M.I., Mostofsky S.H. Basal ganglia volume and shape in children with attention deficit hyperactivity disorder. Am. J. Psychiatry. 2009;166(1):74–82. doi: 10.1176/appi.ajp.2008.08030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raznahan A., Shaw P.W., Lerch J.P., Clasen L.S., Greenstein D., Berman R., Pipitone J., Chakravarty M.M., Giedd J.N. Longitudinal four-dimensional mapping of subcortical anatomy in human development. Proc. Natl. Acad. Sci. USA. 2014;111(4):1592–1597. doi: 10.1073/pnas.1316911111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich W. Diagnostic interview for children and adolescents (DICA) J. Am. Acad. Child Adolesc. Psychiatry. 2000;39(1):59–66. doi: 10.1097/00004583-200001000-00017. [DOI] [PubMed] [Google Scholar]
- Rosch K.S., Mostofsky S.H., Nebel M.B. ADHD-related sex differences in fronto-subcortical intrinsic functional connectivity and associations with delay discounting. J. Neurodev. Disord. 2018;10(1):34. doi: 10.1186/s11689-018-9254-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanefuji, M., Craig, M., Parlatini, V., Mehta, M.A., Murphy, D.G., Catani, M., Cerliani, L., de Schotten, M.T. , 2017. Double-dissociation between the mechanism leading to impulsivity and inattention in Attention Deficit Hyperactivity Disorder: A resting-state functional connectivity study. In: Cortex 86, 290–302. 〈 10.1016/j.cortex.2016.06.005〉. [DOI] [PubMed]
- Seymour K.E., Mostofsky S.H., Rosch K.S. Cognitive load differentially impacts response control in girls and boys with ADHD. J. Abnorm. Child Psychol. 2016;44(1):141–154. doi: 10.1007/s10802-015-9976-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour K.E., Tang X., Crocetti D., Mostofsky S.H., Miller M.I., Rosch K.S. Anomalous subcortical morphology in boys, but not girls, with ADHD compared to typically developing controls and correlates with emotion dysregulation. Psychiatry Res. Neuroimaging. 2017;261:20–28. doi: 10.1016/j.pscychresns.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P., Stringaris A., Nigg J., Leibenluft E. Emotion dysregulation in attention deficit hyperactivity disorder. Am. J. Psychiatry. 2014;171(3):276–293. doi: 10.1176/appi.ajp.2013.13070966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S., Woolrich, M., Behrens, T., Beckmann, C.F., Flitney, D., Jenkinson, M., Bannister, P., Clare, S., De Luca, M., Hansen, P., et al. , 2014. Fmrib software library. Big Healthcare Challenges Chronic Disease, Oxford Centre Funct. Magn. Reson. Imag. Brain Softw. Library, Oxford, UK, Tech. Rep. 〈https://innovation.ox.ac.uk/wp-content/uploads/2014/08/FMRIB-Steve-Smith.pdf〉.
- Sonuga-Barke E.J.S., Castellanos F.X. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neurosci. Biobehav. Rev. 2007;31(7):977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Sripada C., Kessler D., Fang Y., Welsh R.C., Prem Kumar K., Angstadt M. Disrupted network architecture of the resting brain in attention-deficit/hyperactivity disorder. Hum. Brain Mapp. 2014;35(9):4693–4705. doi: 10.1002/hbm.22504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L., Narad M.E., Antonini T.N., O’Brien K.M., Hawk L.W., Jr., Epstein J.N. Reaction time variability in ADHD: a review. Neurother. J. Am. Soc. Exp. Neurother. 2012;9(3):500–508. doi: 10.1007/s13311-012-0138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D., Volkow N.D. Abnormal functional connectivity in children with attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2012;71(5):443–450. doi: 10.1016/j.biopsych.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H., Alam T., Meyer-Lindenberg A. Dopamine and psychosis: theory, pathomechanisms and intermediate phenotypes. Neurosci. Biobehav. Rev. 2010;34(5):689–700. doi: 10.1016/j.neubiorev.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin L.Q., Kelly A.M., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of default mode network components: correlation, anticorrelation, and causality. Hum. Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow N.D., Wang G.-J., Kollins S.H., Wigal T.L., Newcorn J.H., Telang F., Fowler J.S., Zhu W., Logan J., Ma Y., Pradhan K., Wong C., Swanson J.M. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA: J. Am. Med. Assoc. 2009;302(10):1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1992. Wechsler Individual Achievement Test. [Google Scholar]
- Winkler A.M., Ridgway G.R., Webster M.A., Smith S.M., Nichols T.E. Permutation inference for the general linear model. NeuroImage. 2014;92:381–397. doi: 10.1016/j.neuroimage.2014.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodka E.L., Mahone E.M., Blankner J.G., Larson J.C.G., Fotedar S., Denckla M.B., Mostofsky S.H. Evidence that response inhibition is a primary deficit in ADHD. J. Clin. Exp. Neuropsychol. 2007;29(4):345–356. doi: 10.1080/13803390600678046. [DOI] [PubMed] [Google Scholar]
- Yang Z., Li H., Tu W., Wang S., Ren Y., Yi Y., Wu T., Jiang K., Shen H., Wu J., Dong X. Altered patterns of resting-state functional connectivity between the caudate and other brain regions in medication-naïve children with attention deficit hyperactivity disorder. Clin. Imaging. 2018;47:47–51. doi: 10.1016/j.clinimag.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Yeo B.T.T., Krienen F.M., Sepulcre J., Sabuncu M.R., Lashkari D., Hollinshead M., Roffman J.L., Smoller J.W., Zöllei L., Polimeni J.R., Fischl B., Liu H., Buckner R.L. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J. Neurophysiol. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Nebel M.B., Caffo B.S., Mostofsky S.H., Rosch K.S. Beyond massive univariate tests: Covariance regression reveals complex patterns of functional connectivity related to attention-deficit/hyperactivity disorder, age, sex, and response control. Biol. Psychiatry Glob. Open Sci. 2021 doi: 10.1016/j.bpsgos.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Data Availability Statement
The data used in the current manuscript are not yet available for public release because they are part of a longitudinal study for which data collection is currently ongoing.