Abstract
Pollution from oil spills can seriously affect many ecosystem processes and human health. Many articles have evaluated the impact of oil spills on human health. However, most of these articles focus on occupational exposure. The effect on people living in the areas affected by oil pollution is rarely studied. Approximately 640 million people worldwide live in areas at risk of oil pollution. Thus, studying the impact of this pollution on human health should be a priority. Here, we evaluate the presence of anemia in relation to crude oil exposure in men living in areas at risk of oil contamination in the Ecuadorian Amazon (Orellana and Napo). We evaluated the hematological and biochemical parameters of 135 participants. We divided the participants into three groups according to exposure: low, medium, and high. Our results showed a significant association between exposure risk and hemoglobin and hematocrit concentration. Groups with medium- and high- contamination exposure had levels below normal values in hemoglobin and hematocrit in more than 30% and 26% of the population, respectively. In conclusion, we found that crude oil affected human health, and the prevalence of the anemia in men was dependent of the level of contamination.
Keywords: Oil exposure, Contamination, Hematological markers, Anemia, Health effects, Environmental exposure, Ecuadorian Amazon
Graphical Abstract
Highlights
-
•
Biochemical and hematological parameters were analyzed of population of Amazon of Ecuador.
-
•
A relationship between exposure to crude oil and anemia in men was observed.
-
•
GLM did not show effects on monocytes, granulocytes, lymphocytes, or red blood cells.
-
•
OR significant effects of the level of risk on hematocrit were evidenced.
1. Introduction
The Ecuadorian Amazon is located in the northeast region of the country and is one of the most biodiverse areas worldwide with a wide variety of plants, insects, amphibians, birds, and mammals [26]. Underlying this landscape of extraordinary biological and cultural diversity are large oil and gas reserves. Around 80% of the national oil extraction is concentrated in four Amazonian cities: Lago Agrio, Shushufindi, Orellana, and La Joya de los Sachas [8], [9].
Oil extraction has occurred in the Ecuadorian Amazon region since 1967 when the first oil well was drilled. Two years later, the Shushufindi-Aguarico and Sacha fields were exploited. During its early years, oil exploration was conducted with a considerable lack of environmental consideration [32]. Today, after more than 50 years of operation, the Ecuadorian pipeline system (SOTE) transports crude from the Amazonian fields to Balao Terminal located on the Pacific coast. Pipeline damage has generated spills of several million gallons of crude oil contaminating the water and soil; 1169 spills have been officially reported between 2005 and 2015 of which 81% were in Amazonia. Therefore, in 2000, Ecuador reformed its laws on the exploitation and/or extraction of oil [1], [33], [4].
Health problems have been reported from exposure to crude oil (from exposure to spills, or from living near oil fields or commercial activities) including stress and depressive symptoms, psychiatric disorders, neurological symptoms, rheumatic diseases, lupus, respiratory symptoms, cardiovascular problems, liver damage, diarrhea, asthma, skin infection, bronchitis, immunodeficiency, infertility, and anemia [42], [45]. Among the most common abnormalities associated with exposure to crude oil are hematological disorders [32], [36]: White blood cells (WBCs) tend to increase while platelets and red blood cells (RBCs) decrease [10], [2], [21], [31], [35], [39], [44], [46].
Many cases of anemia have been reported in areas affected by oil pollution in the last 10–20 years [48], [50]. Studies carried out in vulnerable groups such as women and children in Ecuadorian oil exploitation areas found symptoms of toxicity caused by oil and a frequency of anemia in schoolchildren of 16.6% this is higher than the national average (3.5%) [16], [42], [48]. The prevalence of anemia in the adult male population is generally associated with nutritional conditions [40]. However, studies in men exposed to different toxic substances including crude oil from spills/occupational exposure have linked these to an incidence of anemia [22], [23], [30], [5], [53].
Although these studies are useful in terms of population health, it is necessary to better understand the environmental, population, anthropometric and biochemical parameters that are related to liver and kidney function and health status usually [9]. Hematological, renal and hepatic functions have been shown to remain altered, over time following exposures to oil spills [12], [41]. On the other hand it is also known that alterations in liver function are accompanied by hematological alterations, and biomarkers such as aminotransferases have been observed to be increased in anemia, especially of the hemolytic type [17], which can be generated by crude oil exposure [20]. Exposure to crude oil and its derivatives also generates kidney damage [28], which can be measured in a general way by creatinine [41]. Therefore, we consider it important to perform biochemical, hematological and anthropometric parameters of men living in the Ecuadorian Amazon exposed to different levels of risks due to crude oil contamination. In addition, we established the percentage of men with anemia at three risk levels. Finally, we established the probability of anemia in men according to the risk levels for crude oil contamination.
2. Materials and methods
The present work is a causality study to evaluate the effect between the biochemical and hematological variables with respect to the risk of exposure to crude oil.
2.1. Study areas
The study included the mid basin region of the Napo River in Ecuador. This area is characterized by a large diversity of ecosystems ranging from the montane to Amazonian forests.
This region has a high risk of oil contamination that can affect human health. According to Espinosa et al. [55], 64% of population centers have different levels of risk contamination with a higher percentage (39%) of those centers exposed to a high risk of contamination. We selected locations using the categorized contamination risk map proposed by Espinosa et al. (2021) to select sampling localities. The sampling localities were located in areas where crude oil pollution is not confounded with other sources of pollution such as large crop areas, localities downstream of large cities, and densely populated centers.
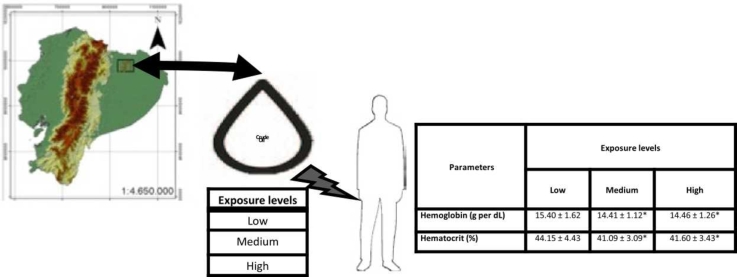
The spatial risk model defined three levels of contamination risk: low, medium, and high risk. The proposal model evaluates the pattern of the distribution of contaminants across watercourses and land uses and produces distinct results from classic models based on the distance from contamination sources. The spatial risk of contamination was based on the calculation of a friction surface and the accessibility of possible oil contamination. The friction surface calculates the cost of moving the oil under surfaces with different coverage conditions and slopes. Accessibility is measured as the expected displacement pattern of seeps and potential oil spills from wells and pipelines based on friction values. The accessibility measure accumulates the cost of the mobility of pollutants along the friction surface. High accessibility values imply a high cost of mobility, and thus this variable inverted and normalized between zero and one and was defined as risk contamination (Espinosa et al., 2021). The risk contamination variable was categorized in three levels of risk oil contamination: high ≥ 0.7, medium > 0.3 & < 0.7, and low ≤ 0.3.
We identified 26 population centers (Fig. 1) exposed exclusively to oil contamination. Of these, nine centers (El Triunfo, 11 de Julio, Cooperativa 30 de Mayo, Pre-Coopertativa Valladolid, La Primavera, Los Laureles, La Libertad, 12 de Febrero and Cooperativa 28 de Marzo) had a high risk of contamination. Eight centers (Pozo Ron, Pre-Cooperativa Huashito, Cooperativa 24 de Mayo, Pre-Cooperativa Nuevo Ecuador, Bella Unión del Napo, Agrupación Tanguila, 1 de Mayo, and Playayacu) had a medium contamination risk. Nine centers (Pre-Cooperativa Tierras Ecuatoriana, Tangay, Enokanki, Las Mercedes, Las Nieves, Conambo, Luz de America, 4 de Diciembre, and La Florida) had a low risk of contamination.
Fig. 1.
Map of sampling points in the Orellana and Napo provinces in the Ecuadorian Amazon. (A) Location of Ecuador within South America. (B) Dark-green is low exposure, light green is medium exposure, and dark red is high exposure.
2.2. Ethical consideration
This study was approved by the Ethics Committee at University San Francisco – Ecuador, 2015–056E, and Ministerio de Salud of Ecuador, MSP-DIS-2015–0078-O. All subjects provided written informed consent prior to participating.
2.3. Population characteristics
The study was performed in 2014, and 135 otherwise healthy men average 45.15 ± 14.64 years old who were exposed to oil contamination volunteered. The inclusion criteria were that the volunteers were of legal age (18 years), that they were apparently healthy without any drug regimen, and actively working. The exclusion criteria were having a genetic disorder or presenting signs of a mental or catastrophic illness. All samples were collected at the volunteers’ place of residence.
The participants were classified into three groups according to their area of residence: low exposure (n = 34), medium exposure (n = 49), and high exposure (n = 52) according to the contamination risk model. It was not possible to define a control group because most of the inhabited areas in this region are somewhat affected by hydrocarbon contamination.
To control for potential confounding factors, participants completed a questionnaire related to lifestyle (e.g., frequency of coffee and alcohol consumption, smoking habits). Trained personnel applied the questionnaire and took anthropometric measurements. We produced an adjusted generalized linear model to evaluate the effects of confounding factors.
2.4. Hematological and biochemical parameters
The blood samples were collected between 8 and 11 AM; 4 mL of blood was added to Vacutainer tubes with EDTA and 4 mL into tubes without an anticoagulant (Becton Dickinson) under aseptic conditions. The samples were then stored on ice and protected from light during the sampling and laboratory processing procedures. All samples were handled similarly and processed in under 2 h. The hematological analyses included quantifying WBCs, hemoglobin, hematocrit, and platelets. The determination of the different blood parameters was done using a Mindray BC-3200 Auto Hematology Analyzer (Shenzhen, China), which uses an electrical impedance method for cell counts and a cyanide-free method for hemoglobin. The biochemical parameters (aspartate aminotransferase, alanine aminotransferase, creatinine) were quantified using the semi-automatic Humalyzer 3000 analyzer (Wiesbaden, Germany) according to each technical specification. This equipment has a photometric system in the UV–visible range with six preset wavelengths (340, 405, 505, 545, 580, 630 nm ± 2 nm). C-reactive protein (CRP) levels were evaluated using the CRP-latex test, a rapid agglutination assay was used for the direct detection and semi-quantification of CRP in serum (Wiener Lab 2000, Rosario - Argentina). The normal range values are in Table 1.
Table 1.
Normal range values for various indices.
| Parameter | Normal Range value (male) |
|---|---|
| White blood Cells (×103 per mL)a | 4.5–11.5 |
| Lymphocytes (%)a | 20–40 |
| Granulocytes (%)a | 40–60 |
| Monocytes (%)a | 2–8 |
| Hemoglobin (g per dL)b | 14.0–17.0 |
| Hematocrit (%)b | 40–50 |
| Platelet (×103 per mL)a | 150–450 |
| RBC (×106 per μL)a | 4.5–6 |
| AST (IU/L)c | 10–37 |
| ALT (IU/L)c | 10–42 |
| Creatinine (mg per L) | 0.4–1 |
| CRP (mg per L)d | < 5.9 |
RBC = red blood cells, AST = alanine aminotransferase, ALT = aspartate aminotransferase, CRP = C-reactive protein.
Reference values according to Indiana University Medical Center Health Partners, Indianapolis, IN.
Reference values according to National Heart, Lung and Blood Institute.
Reference values according to the technique used.
Semi-quantitative method values less than 6 considered negative.
2.5. Statistical analysis
We adjusted a generalized linear model with a binomial family to evaluate the effect of contamination risk on the occurrence of altered biochemical and hematological parameters. We further calculated the odds ratio from the abnormalities using the low risk of contamination as a reference and then tested the results for statistical significance with Fisher’s exact test. Age and lifestyle factors (e.g., alcohol consumption, tobacco use) were used as adjustment variables. The analysis was implemented in R using the base [43] and epitools [3] packages.
3. Results
The sample population’s self-reported demographic and lifestyle characteristics are shown in Table 2 and are classified according to exposure risk. Overall, 59.3% of the general population self-identified as Mestizo, 18.5% as Caucasian, 17.8% as Native, and 2.2% as Afro-Ecuadorian. The questionnaire data also indicated that 36.8% were smokers, 72.3% frequent drinkers, 27.7% occasional drinkers, and 64.4% drank coffee. From the physical examination, 14.1% were obese (BMI ≥ 30), and 28.9% had high blood pressure (SBP or DBP ≥ 140/90 mmHg).
Table 2.
Characteristics and habits of the study population.
| General Population n = 135 | Low Level n = 34 | Medium Level n = 49 | High Level n = 52 | |
|---|---|---|---|---|
| Age | 45.15 ± 14.64 | 46.74 ± 16.09 | 44.24 ± 13.63 | 44.96 ± 14.79 |
| BMI | 26.04 ± 3.59 | 25.98 ± 3.50 | 26.16 ± 3.67 | 25.97 ± 3.63 |
| Normal weight (%) | 43.0 | 44.1 | 40.8 | 44.2 |
| Overweight (%) | 43.0 | 44.1 | 42.9 | 42.3 |
| Obesity (%) | 14.1 | 11.8 | 16.3 | 13.5 |
| SBP (mmHg) | 131.95 ± 21.75 | 137.26 ± 26.29 | 125.84 ± 16.28 | 134.23 ± 22.05 |
| DPB (mmHg) | 72.44 ± 10.60 | 76.50 ± 10.99 | 69.94 ± 9.48 * | 72.15 ± 10.72 |
| Hypertension (%) | 28.9 | 41.2 | 22.4 | 26.9 |
| Frequent drinker (%) | 72.3 | 58.82 | 78.52 | 74.07 |
| Occasional drinker (%) | 27.7 | 41.18 | 21.48 | 25.95 |
| Smoker (%) | 36.8 | 21.2 | 44.9 | 39.2 |
| Not Smoker (%) | 63.2 | 78.8 | 55.1 | 60.8 |
| Drink coffee (%) | 64.4 | 52.9 | 65.3 | 71.2 |
| Not drink coffee (%) | 35.6 | 47.1 | 34.7 | 28.8 |
| Afro-Ecuadorian (%) | 2.2 | 5.9 | – | 1.9 |
| Caucasian (%) | 18.5 | 23.5 | 4.1 | 28.8 |
| Native (%) | 17.8 | – | 49 | – |
| Mestizo (%) | 59.3 | 70.6 | 44.9 | 65.4 |
Values represent mean ± SD or percentage.
BMI = Body mass index, DBP = Diastolic blood pressure, SBP = Systolic blood pressure * P < 0.05. between low and medium levels, analyzed using the ANOVA test.
Table 3 presents the mean plus standard deviation for the values obtained from hematological and biochemical assays. Creatinine levels were within the normal parameters in all three groups, and there was no difference between groups. Hepatic functioning was reflected by AST and ALT levels; no differences were observed between the exposure groups, but the values neared the upper normal limit. Inflammation was assessed using CRP levels, which were high in the medium- (12.8%) and high-exposure (14.3%) groups.
Table 3.
Mean of hematologic and biochemical parameters according to exposure groups.
| Exposure levels |
||||||
|---|---|---|---|---|---|---|
| Total | Low | Medium | High Level | p -value | ||
| WBC (×103 per μL) | ||||||
| Mean | 6.91 ± 1.63 | 6.92 ± 1.90 | 6.67 ± 1.57 | 7.08 ± 1.57 | 0.7053 | |
| Lymphocytes (%) | ||||||
| Mean | 32.64 ± 7.92 | 32.19 ± 6.59 | 31.34 ± 7.63 | 34.35 ± 8.69 | 0.3292 | |
| Granulocytes (%) | ||||||
| Mean | 57.57 ± 8.14 | 58.43 ± 6.28 | 59.06 ± 7.88 | 55.43 ± 8.94 | 0.4014 | |
| Monocytes (%) | ||||||
| Mean | 9.82 ± 2.23 | 9.52 ± 1.96 | 9.60 ± 1.97 | 10.32 ± 2.60 | 0.5125 | |
| Platelet (×109 per μL) | ||||||
| Mean | 219.56 ± 52.16 | 221.14 ± 52.24 | 222.40 ± 51.62 | 215.52 ± 53.71 | 0.7427 | |
| RBC | ||||||
| Mean | 4.99 ± 0.46 | 5.31 ± 0.49 | 4.86 ± 0.44 | 4.97 ± 0.41 | 0.003 | ** |
| Hemoglobin (g per dL) | ||||||
| Mean | 14.62 ± 1.33 | 15.40 ± 1.62 | 14.41 ± 1.12 | 14.46 ± 1.26 | 0.0328 | * |
| Hematocrit (%) | ||||||
| Mean | 41.86 ± 3.65 | 44.15 ± 4.43 | 41.09 ± 3.09 | 41.60 ± 3.43 | 0.0176 | * |
| AST (IU/L) | ||||||
| Mean | 38.74 ± 18.71 | 37.75 ± 16.72 | 36.53 ± 16.40 | 41.71 ± 21.85 | 0.9385 | |
| ALT (IU/L) | ||||||
| Mean | 37.81 ± 18.24 | 42.14 ± 18.09 | 34.64 ± 14.24 | 39.19 ± 21.77 | 0.9556 | |
| Creatinine (mg/dL) | ||||||
| 0.83 ± 0.12 | 0.82 ± 0.14 | 0.83 ± 0.11 | 0.85 ± 0.14 | 0.3640 | ||
| CRP (mg/dL) | ||||||
| High (%) | 10.9 | 0 | 12.8 | 14.3 | 0.4007 | |
Values represent mean ± SD and percentages. RBC = red blood cells, WBC = white blood cells, ALP = alkaline phosphatase; ALT = alanine amino transferase; AST = aspartate amino transferase; CRP = C-reactive protein. Analysis used an ordinary ANOVA test.
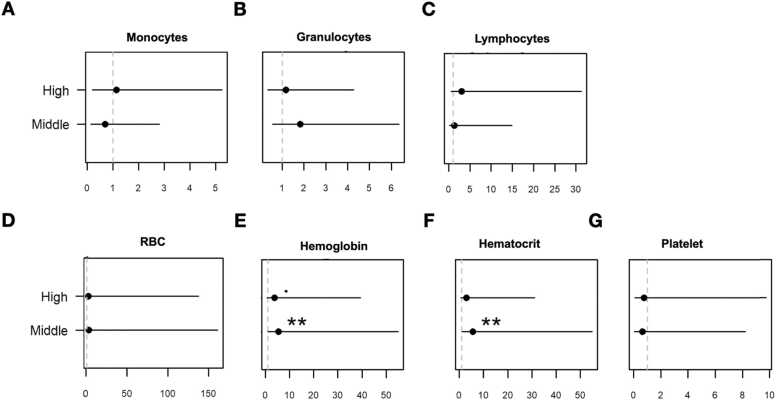
There were no significant differences in the WBC, lymphocyte, monocyte, granulocyte, or platelet values between groups. However, monocyte levels in all cases exceeded the normal range and were the highest in the high-exposure group (Table 3). RBCs, hemoglobin, and hematocrit levels were within the established normal ranges for all groups. The low-exposure group had significantly higher values than the medium- and high-exposure groups (p < 0.05, in both cases). A significant percentage of individuals had monocytes and granulocytes above normal values at all exposure levels (Fig. 2).
Fig. 2.
Percentage of individual with normal, low or high hematological parameters according to exposure group. (A) White blood cells (WBCs), (B) monocytes, (C) granulocytes, (D) lymphocytes, (E) red blood cells (RBCs), (F) hemoglobin, (G) hematocrit, and (H) platelets.
The World Health Organization defines anemia in men as hemoglobin levels below 13.0 g/dL [54] while this criterion in the literature is as high as 14.2 g/dL [7]. Here, anemia was defined by a hemoglobin concentration under 14.0 g/dL as recommended by the National Heart, Lung, and Blood Institute (Institute National Heart Lung and Blood 2011). By this definition, men in the medium- and high-exposure to oil exploration groups were more likely to have anemia (38% and 30%, respectively) than their low-exposure counterparts (10%). Similarly, more individuals in the medium- and high-exposure groups (36%, and 22%, respectively) had hematocrit values below the normal limit than in the low-exposure group (10%) (Fig. 2).
Generalized linear models did not show effects on monocytes, granulocytes, lymphocytes, or red blood cells. However, hemoglobin and hematocrit levels had obvious and significant effects. The medium and high contamination risk groups showed an increase in abnormal hemoglobin and hematocrit levels (Table 4).
Table 4.
Generalized linear models with binomial adjustment of the occurrence of abnormal cases in relation to contamination risk levels.
| Monocytes | Granulocytes | ||||||||||
| Estimate | SE | Z-value | p-value | Estimate | SE | Z-value | p-value | ||||
| (Intercept) | 1.4469 | 0.5557 | 2.6037 | 0.0092 | (Intercept) | -0.6931 | 0.4629 | -1.4974 | 0.1343 | ||
| Medium level | -0.3483 | 0.648 | -0.5375 | 0.5909 | Medium level | 0.6098 | 0.5457 | 1.1174 | 0.2638 | ||
| High level | 0.1335 | 0.6936 | 0.1925 | 0.8473 | High level | 0.1542 | 0.5722 | 0.2694 | 0.7876 | ||
| Lymphocytes | |||||||||||
| Estimate | SE | Z-value | p-value | ||||||||
| (Intercept) | -2.2513 | 0.7433 | -3.0288 | 0.0025 | |||||||
| Medium level | 0.3054 | 0.862 | 0.3543 | 0.7231 | |||||||
| High level | 1.1199 | 0.8275 | 1.3534 | 0.1759 | |||||||
| RBC (Red blood cells) | Hemoglobin | ||||||||||
| Estimate | SE | Z-value | p-value | Estimate | SE | Z-value | p-value | ||||
| (Intercept) | -2.9957 | 1.0246 | -2.9238 | 0.0035 | (Intercept) | -2.2513 | 0.7433 | -3.0288 | 0.0025 | ||
| Medium level | 1.2281 | 1.1032 | 1.1132 | 0.2656 | Medium level | 1.7405 | 0.8009 | 2.1733 | 0.0298 | * | |
| High level | 1.0217 | 1.1303 | 0.9039 | 0.3661 | High level | 1.3689 | 0.8187 | 1.672 | 0.0945 | ||
| Hematocrit | Platelet | ||||||||||
| Estimate | SE | Z-value | p-value | Estimate | SE | Z-value | p-value | ||||
| (Intercept) | -2.2513 | 0.7433 | -3.0288 | 0.0025 | (Intercept)) | -2,2513 | 0,7434 | -3,0284 | 0,0025 | ||
| Medium level | 1.7405 | 0.8009 | 2.1733 | 0.0298 | * | Medium level | -0,4568 | 0,953 | -0,4793 | 0,6317 | |
| High level | 1.1199 | 0.8275 | 1.3534 | 0.1759 | High level | -0,2877 | 0,9551 | -0,3012 | 0,7633 | ||
P < 0.05. Age and lifestyle metrics like smoking and drinking were used as adjusting variables.
The risk of hematological parameters being affected by the level of risk to exposure to crude oil was estimated and adjusted for age, smoking, and drinking. The increased risk of anemia occurrence in the medium- (OR of 5.57, 95% CI: 1.12–55.00) and high-exposure (OR of 3.8, 95% CI: 0.73–39, 32) groups approached the limit of statistical significance. The ORs for hematocrit were 5.58 (95% CI: 1.12–55.00) and 3.0162 (0.55–31.25 CI) for the medium and high-exposure level groups, respectively (Fig. 3).
Fig. 3.
Crude odds ratios for association of hematological parameters with level of risk: (A) monocytes, (b) granulocytes, (c) lymphocytes, (d) red blood cells (rbcs), (e) hemoglobin, (f) hematocrit and (g) platelet. * P < 0.05; ** P < 0.01.
4. Discussion and conclusions
Aminotransferase levels were close to the upper limit of the normal range for the three exposure groups, which may be due to the higher incidence of hepatitis in this region. This could also be related to the metabolic syndrome (considering the high percentage of overweight and obesity found in the population). This reaches a frequency between 18% and 25% in men living in urban and rural areas of the Ecuadorian Amazon [16], [51].
Exposure to oil crude affects the hematological system in humans and is especially seen in increased incidence of nonlymphocytic leukemia, acute lymphocytic leukemia, chronic myelocytic leukemia, and chronic lymphocytic leukemia [11], [29], [45], [49]. In this study population, we found no individuals with WBC increases related to leukemic processes probably due to the size of the sample. However, we did see an increase in the percentage of monocytosis at all three exposure levels. Monocytosis is extremely nonspecific and is indicative of several conditions including acute and chronic inflammation as well as parasitic and bacterial infections [47], [52]. A large increase in granulocytes (not significant) was observed in the population especially in men in the medium-exposure group, also had a higher percentage of smokers (around 50%); this would suggest that the granulocyte increase in this population was likely due to smoking and was in response to the inflammatory process induced by smoking [15], [19].
The anemia prevalence found in this study (24.4%) is higher than reported for the entire Ecuadorian male population, which is 4.1% for men between 40 and 59 years according to the National Health and Nutrition Survey ENSANUT-ECU 2012 [16]. The anemia increase is 5.9-fold higher considering the criteria for the diagnosis of anemia according to the National Heart, Lung, and Blood Institute (hemoglobin < 14 g/dL) [25] and 2.1-fold higher than the criteria established by the WHO (hemoglobin <13 g/dL) with which the ENSANUT-ECU 2012 was performed [16]. The highest percentage of anemia was found (38% and 31%, respectively) in the medium- and high-exposure groups. A significant association was observed with increased risk of developing the disease in response to crude oil exposure. While it is difficult to find studies that directly link anemia and oil exposure in humans, this is not the case in animal species. Contact with crude oil can be hemotoxic in seabirds and other avian species [27], [34], [38], [39].
Petroleum is a complex mixture of different elements and chemical compounds including heavy metals such as Cd, Pb, Zn, Cr, As, and Hg [29], [37], [45]. Of these, at least Pb, As, Cd, and Hg have been related to anemia incidence [22], [23], [24], [53]. In the Ecuadorian Amazon, studies on heavy metal contamination—specifically in the provinces of Orellana and Sucumbíos—show concentrations within the permitted limit except for Ba in soil, which exceeded the Ecuadorian limit of 200 mg/kg. The concentration of Mo was higher than urban areas of Ecuador [33], [6]. The presence of barium and molybdenum could be related to the extraction of crude oil in the area [33], [6]. Paradoxically, the molybdenum-iron complex increases hemoglobin [13], [18]. Another focus of heavy metal contamination could come from consumption of foods such as cocoa and cassava, which revealed the presence of metals (Cd, Pb) in studies of the area that exceeded the limits recommended by FAO/WHO [33], [6].
One of the limitations of this study is that there is a high incidence of hepatitis [14] and exposure to wood or coal burning in this area. These can be risk factors for the development of anemia. It would be important in later studies to relate the effect of chronic infections and consider more variables as other possible pollutants in the area generated by both anthropogenic activity and from natural sources.
In conclusion, our results showed that men residing in areas with medium and high levels of exposure to crude oil had lower levels of hemoglobin and hematocrit and an increased risk of developing anemia versus men in low-level exposed areas. However, more studies are needed to fully understand the causes and effects with extrapolation to other people living in the area.
Funding
This work was supported by Secretaria de Educación Superior, Tecnología e Innovación del Ecuador (SENESCYT) (CPC-SENESCYT-03-2014), and Universidad Técnica Particular de Loja – Ecuador (PROY_FIN_CCNN_0034).
CRediT authorship contribution statement
MIR, APA and NCBM data collection and data analysis and data interpretation. CIE and NCBM study conception and design. CIE statistical analyzed. All authors interpreted the results and prepared the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Ebba Malmqvist for revisions to this manuscript and all the technical staff that contributed to this work: Gabriela Gonzalez, Santiago Sotomayor, Sandra Cuenca, and Wilson Sanchez. We are also very grateful for the volunteers from the Orellana and Napo provinces who made this work possible.
Handling Editor: Dr. Aristidis Tsatsakis
Contributor Information
María Isabel Ramírez, Email: miramirez@utpl.edu.ec.
Ana Paulina Arévalo-Jaramillo, Email: aparevalo1@utpl.edu.ec.
Carlos Iván Espinosa, Email: ciespinosa@utpl.edu.ec.
Natalia Bailon-Moscoso, Email: ncbailon@utpl.edu.ec.
References
- 1.Amazon frontlines , Impactos ambientales, 2021.〈https://www.amazonfrontlines.org/es/nuestro-trabajo/territorios/impactos/〉 (Accessed 7 Jan 2021).
- 2.Anderson D., Hughes J.A., Cebulska-Wasilewska A., et al. Biological monitoring of workers exposed to emissions from petroleum plants. Environ. Health Perspect. 1996;104:609–613. doi: 10.1289/ehp.96104s3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.T.J. Aragon, epitools: Epidemiology Tools, 2020.
- 4.Arellano P., Tansey K., Balzter H., Tellkamp M. Plant family-specific impacts of petroleum pollution on biodiversity and leaf chlorophyll content in the Amazon rainforest of Ecuador. PLoS One. 2017;12:1–18. doi: 10.1371/journal.pone.0169867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baak Mann Y., Byoung Y.A., Sc H., et al. In environmental medicine GRAND ROUNDS aplastic anemia in a petrochemical factory worker. Gd Rounds Environ. Med. 1999;107:1998–2000. doi: 10.1289/ehp.99107851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barraza F., Maurice L., Uzu G., et al. Science of the Total Environment Distribution, contents and health risk assessment of metal ( loid) s in small-scale farms in the Ecuadorian Amazon: an insight into impacts of oil activities. Sci. Total Environ. 2018;622–623:106–120. doi: 10.1016/j.scitotenv.2017.11.246. [DOI] [PubMed] [Google Scholar]
- 7.Beutler E., Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046.BLOOD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bustamante T., Jarrín M.C. Impactos sociales de la actividad petrolera en Ecuador: un análisis de los indicadores. Íconos - Rev. Ciencias Soc. 2015;0:19. doi: 10.17141/iconos.21.2005.77. [DOI] [Google Scholar]
- 9.Coronel Vargas G., Au W.W., Izzotti A. Public health issues from crude-oil production in the Ecuadorian Amazon territories. Sci. Total Environ. 2020;719 doi: 10.1016/j.scitotenv.2019.134647. [DOI] [PubMed] [Google Scholar]
- 10.D’Andrea M. a, Reddy G.K. Health consequences among subjects involved in gulf oil spill clean-up activities. Am. J. Med. 2013;126:966–974. doi: 10.1016/j.amjmed.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 11.D’Andrea M. a, Reddy G.K. Health risks associated with crude oil spill exposure. Am. J. Med. 2014;127:886.e9–886.e13. doi: 10.1016/j.amjmed.2014.04.035. [DOI] [PubMed] [Google Scholar]
- 12.D’Andrea M.A., Reddy G.K. The development of long-term adverse health effects in oil spill cleanup workers of the Deepwater Horizon offshore drilling rig disaster. Front. Public Health. 2018;6:117. doi: 10.3389/fpubh.2018.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieckmann W.J., Priddle H.D. Anemia of pregnancy treated with molybdenum-iron complex. Am. J. Obstet. Gynecol. 1949;57:541–546. doi: 10.1016/0002-9378(49)90239-2. [DOI] [PubMed] [Google Scholar]
- 14.EC Ministerio de Salud Pública (MSP), Gaceta Epidemiológica Semanal No 51 Semana Epidemiológica No. 1–52, 2014.
- 15.Flouris A.D., Poulianiti K.P., Chorti M.S., et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food Chem. Toxicol. 2012;50:3600–3603. doi: 10.1016/j.fct.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 16.Freire W., Ramírez-Luzuriaga MJ, Belmont P.,W. Freire, M.J. Ramírez-Luzuriaga, P. Belmont et al., Encuesta Nacional de Salud y Nutrición. ENSANUT-ECU 2012, Primera Ed. Quito- Ecuador, 2012.
- 17.Gkamprela E., Deutsch M., Pectasides D. Iron deficiency anemia in chronic liver disease: etiopathogenesis, diagnosis and treatment. Ann. Gastroenterol. 2017;30:405. doi: 10.20524/aog.2017.0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grech B.J. Mechanistic insights into the treatment of iron-deficiency anemia and arthritis in humans with dietary molybdenum. Eur. J. Clin. Nutr. 2021 doi: 10.1038/s41430-020-00845-7. [DOI] [PubMed] [Google Scholar]
- 19.Gutiérrez Maydata A. Oxidantes en el humo del cigarrillo y enfermedades cardiopulmonares. Rev. Cubana Med. 2018;10400 [Google Scholar]
- 20.Harr K.E., Cunningham F.L., Pritsos C.A., et al. Weathered MC252 crude oil-induced anemia and abnormal erythroid morphology in double-crested cormorants (Phalacrocorax auritus) with light microscopic and ultrastructural description of Heinz bodies. Ecotoxicol. Environ. Saf. 2017;146:29–39. doi: 10.1016/j.ecoenv.2017.07.030. [DOI] [PubMed] [Google Scholar]
- 21.Harr K.E., Rishniw M., Rupp T.L., et al. Dermal exposure to weathered MC252 crude oil results in echocardiographically identifiable systolic myocardial dysfunction in double-crested cormorants (Phalacrocorax auritus) Ecotoxicol. Environ. Saf. 2017;146:76–82. doi: 10.1016/j.ecoenv.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Heck J.E., Chen Y., Grann V.R., et al. Arsenic exposure and anemia in Bangladesh: a population-based study. J. Occup. Environ. Med. 2008;50:80–87. doi: 10.1097/jom.0b013e31815ae9d4. [DOI] [PubMed] [Google Scholar]
- 23.Hegazy A.A., Zaher M.M., Abd M.A., et al. Relation between anemia and blood levels of lead, copper, zinc and iron among children. BMC Res.Not. 2010;3:9. doi: 10.1186/1756-0500-3-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horiguchi H., Oguma E., Kayama F. Cadmium induces anemia through interdependent progress of hemolysis, body iron accumulation, and insufficient erythropoietin production in rats. Toxicol. Sci. 2011;122:198–210. doi: 10.1093/toxsci/kfr100. [DOI] [PubMed] [Google Scholar]
- 25.Insitute National Heart Lung and Blood Guia breve sobre la anemia. Natl. Hear. Lung Blood Inst. 2011 〈https://www.nhlbi.nih.gov/files/docs/public/blood/anemia-inbrief_yg_sp.pdf〉 [Google Scholar]
- 26.Joppa L.N., Visconti P., Jenkins C.N., Pimm S.L. Achieving the convention on biological diversity’s goals for plant conservation. Science. 2013;341:1100–1103. doi: 10.1126/science.1241706. [DOI] [PubMed] [Google Scholar]
- 27.King M.D., Elliott J.E., Williams T.D. Effects of petroleum exposure on birds: a review. Sci. Total Environ. 2021;755 doi: 10.1016/j.scitotenv.2020.142834. [DOI] [PubMed] [Google Scholar]
- 28.Kuppusamy S., Maddela N.R., Megharaj M., Venkateswarlu K. Springer; 2020. Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation. [Google Scholar]
- 29.Kuppusamy S., Maddela N.R., Megharaj M., Venkateswarlu K. Springer; 2019. Total Petroleum Hydrocarbons: Environmental Fate, Toxicity, and Remediation. [Google Scholar]
- 30.Le C.H.H. The prevalence of anemia and moderate-severe anemia in the US population (NHANES 2003-2012) PLoS One. 2016;11 doi: 10.1371/journal.pone.0166635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linet M.S., Yin S.-N., Gilbert E.S., et al. A retrospective cohort study of cause-specific mortality and incidence of hematopoietic malignancies in Chinese benzene-exposed workers. Int. J. Cancer. 2015;00 doi: 10.1002/ijc.29591. [DOI] [PubMed] [Google Scholar]
- 32.A. Maldonado, A. Narváez, ECUADOR NI ES NI SERÁ YA PAÍS AMAZÓNICO. Inventario de impactos petroleros-1, Primera. Quito, 2003.
- 33.Maurice L., López F., Becerra S., et al. Drinking water quality in areas impacted by oil activities in Ecuador: associated health risks and social perception of human exposure. Sci. Total Environ. 2019;690:1203–1217. doi: 10.1016/j.scitotenv.2019.07.089. [DOI] [PubMed] [Google Scholar]
- 34.Mazet J.K., Gardner I.A., Jessup D.A., et al. Evaluation of changes in hematologic and clinical biochemical values after exposure to petroleum products in mink (Mustela vison) as a model for assessment of sea otters (Enhydra lutris) Am. J. Vet. Res. 2000;61:1197–1203. doi: 10.2460/ajvr.2000.61.1197. [DOI] [PubMed] [Google Scholar]
- 35.McLoone P., Dyussupov O., Nurtlessov Z., et al. The effect of exposure to crude oil on the immune system. Health implications for people living near oil exploration activities. Int. J. Environ. Health Res. 2019:1–26. doi: 10.1080/09603123.2019.1689232. [DOI] [PubMed] [Google Scholar]
- 36.Moñino Aguilera N., Galdos Balzategui A., Exposición a la contaminación por actividad petrolera y estado de salud de la comuna Yamanunka (Sucumbíos, Ecuador), 2008.
- 37.Nelson W.L. McGraw-Hill; 2018. Petroleum Refinery Engineering. [Google Scholar]
- 38.Newman S.H., Mazet J.K., Ziccardi M.H., et al. Haematological changes and anaemia associated with captivity and petroleum exposure in seabirds. Comp. Haematol. Int. 1999;9:60–67. doi: 10.1007/BF02585537. [DOI] [Google Scholar]
- 39.Ordinioha B., Brisibe S. The human health implications of crude oil spills in the Niger delta, Nigeria: An interpretation of published studies. Niger. Med. J. 2013;54:10–16. doi: 10.4103/0300-1652.108887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Organización Mundial de la Salud, OMS | Prevalencia mundial de la anemia y número de personas afectadas. In: Who, 2016. 〈http://www.who.int/vmnis/database/anaemia/anaemia_data_status_t2/es/〉.
- 41.Owusu B.A., Lim A., Intawong C., et al. Haematological, renal, and hepatic function changes among Rayong oil spill clean-up workers: a longitudinal study. Int. Arch. Occup. Environ. Health. 2022:1–9. doi: 10.1007/s00420-022-01834-y. [DOI] [PubMed] [Google Scholar]
- 42.Quizhpe E., Sebastián M.S., Hurtig A.K. Prevalencia de anemia en escolares de la zona amazónica de Ecuador. Rev. Panam Salud Publica. 2003;13:355–361. doi: 10.1590/s1020-49892003000500003. [DOI] [PubMed] [Google Scholar]
- 43.R Core Team, A language and enviromental for statistical computing. R foundation for statistical compunting, 2020.
- 44.Raabe G.K., Collingwood K.W., Wong O. An updated mortality study of workers at a petroleum refinery in Beaumont, Texas. Am. J. Ind. Med. 1998;33:61–81. doi: 10.1002/(SICI)1097-0274(199801)33:1<61::AID-AJIM8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 45.Ramirez M.I., Arevalo A.P., Sotomayor S., Bailon-Moscoso N. Contamination by oil crude extraction – refinement and their effects on human health. Environ. Pollut. 2017;231:415–425. doi: 10.1016/j.envpol.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez M.I., Arevalo A.P., Sotomayor S., Bailon-Moscoso N. Contamination by oil crude extraction – refinement and their effects on human health. Environ. Pollut. 2017;231:415–425. doi: 10.1016/j.envpol.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 47.Rice L., Jung M. Hematology. Seventh ed. Elsevier Inc; 2018. Neutrophilic leukocytosis, neutropenia, monocytosis, and monocytopenia; pp. 675–681. [Google Scholar]
- 48.San Sebastián M., Armstrong B., Córdoba J. a, Stephens C. Exposures and cancer incidence near oil fields in the Amazon basin of Ecuador. Occup. Environ. Med. 2001;58:517–522. doi: 10.1136/oem.58.8.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.San Sebastian M., Armstrong B., Stephens C. Health of women living near oil wells and oil production stations in the Amazon region of Ecuador. Rev. Panam Salud Publica. 2001;9:375–384. doi: 10.1590/S1020-49892001000600004. [DOI] [PubMed] [Google Scholar]
- 50.San Sebastián M., Tanguila A., Santi S., et al. INFORME YANA CURI Impacto de la actividad petrolera en la salud de poblaciones rurales de la Amazonía ecuatoriana. Quito-Ecuador. 2004 [Google Scholar]
- 51.Strauss R.S., Barlow S.E., Dietz W.H. Prevelance of abnormal serum aminotransferase values in overweight and obese adolescents. J. Pediatr. 2000;136:727–733. doi: 10.1016/S0022-3476(00)24645-3. [DOI] [PubMed] [Google Scholar]
- 52.Thachil J., Owusu-Ogori S., Bates I. In: Manson’s Tropical Diseases. Twenty Third. Farrar J., Hotez P.J., Junghanss T., editors. Elsevier Saunders; 2013. Haematological diseases in the tropics; pp. 894–932.e7. [Google Scholar]
- 53.Westhoff D.D., Samaha R.J., Barnes A. Arsenic intoxication as a cause of megaloblastic anemia. Blood. 1975;45:241–246. doi: 10.1182/blood.V45.2.241.241. [DOI] [PubMed] [Google Scholar]
- 54.WHO . World Health Organization; 2011. Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. [Google Scholar]
- 55.Espinosa C.I., Reyes-Bueno F., Ramírez M.I., Arévalo A.P., Bailon-Moscoso N., Duncan D.H. Vulnerability of Human Populations to Contamination from Petroleum Exploitation in the Napo River Basin: An Approach for Spatially Explicit Risk Assessment. Sustainability. 2021;13 doi: 10.3390/su13169230. [DOI] [Google Scholar]