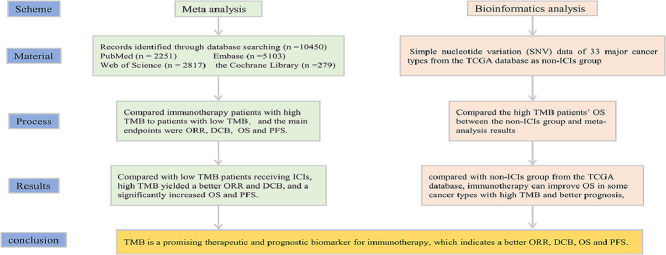
Highlights
-
•
The FDA has approved pembrolizumab in all cancers with TMB > 10Mut/Mb based on the findings from the phase 2 KEYNOTE-158 study. However, predictive efficacy of TMB is greeted with many skepticisms.
-
•
Cancer patients with high TMB have a better prognosis. Notably, there is no evidence shown whether the good prognosis is caused by the tumor itself or by immunotherapy.
-
•
We compared the meta subgroup analysis by tumor types with non-ICIs from TCGA database and found that immunotherapy can increase, not reduce, cancer patients’ OS with high TMB.
-
•
TMB is a promising therapeutic and prognostic biomarker for immunotherapy, which indicates a better ORR, DCB, OS and PFS.
Keywords: Tumor mutation burden, Immunotherapy, Immune checkpoint inhibitors, Meta-analysis, Bioinformatics
Abstract
Purpose
To explore the predictive efficacy of tumor mutation burden (TMB) as a potential biomarker for cancer patients treated with Immune checkpoint inhibitors (ICIs).
Methods
We systematically searched PubMed, Cochrane Library, Embase and Web of Science for clinical studies (published between Jan 1, 2014 and Aug 30, 2021) comparing immunotherapy patients with high TMB to patients with low TMB. Our main endpoints were objective response rate (ORR), durable clinical benefit (DCB), overall survival (OS) and progress-free Survival (PFS). Moreover, we downloaded simple nucleotide variation (SNV) data of 33 major cancer types from the TCGA database as non-ICIs group, and compared the high TMB patients’ OS between the non-ICIs group and meta-analysis results.
Results
Of 10,450 identified studies, 41 were eligible and were included in our analysis (7713 participants). Compared with low TMB patients receiving ICIs, high TMB yielded a better ORR (RR = 2.73; 95% CI: 2.31–3.22; P = 0.043) and DCB (RR = 1.93; 95% CI: 1.64–2.28; P = 0.356), and a significantly increased OS (HR =0.24; 95% CI: 0.21–0.28; P < 0.001) and PFS (HR = 0.38; 95% CI: 0.34–0.42; P < 0.001). Furthermore, compared with non-ICIs group from the TCGA database, immunotherapy can improve OS in some cancer types with high TMB and better prognosis, including colorectal cancer, gastric cancer, lung cancer, melanoma and pan-cancer.
Conclusion
TMB is a promising therapeutic and prognostic biomarker for immunotherapy, which indicates a better ORR, DCB, OS and PFS. If there is a standard for TMB assessment and cut-off, it could improve the management of different cancers.
Graphical abstract
Introduction
Cancer ranks as a leading cause of death and an important barrier to increasing life expectancy [1], and new cases continued to rise in almost all countries of the world [2]. It has long been known that surgery, radiotherapy, chemotherapy and targeted therapy are the main treatment methods for cancer. The emergence of immunotherapy has revolutionized cancer treatment. Many unprecedented advances have been made in cancer treatment with the use of immune checkpoint inhibitors (ICIs). Drugs targeting the PD-1/L1 or CTLA-4 axis have become emerging therapies for a variety of cancers, including non-small cell lung cancer (NSCLC) [3], melanoma, head and neck squamous cell carcinoma, cervical cancer and gastric cancer, etc. [4].
ICIs have been widely used in cancer clinical treatment and generated durable responses, especially in those with advanced stages [3]. Although the safety of ICIs is acceptable, the incidence of immune-related Adverse Events (irAEs) is as high as 28.6−47.4% [5], and the death rate caused by immune-related factors was also 0.6% [6]. The high cost of immunotherapy also limits the clinical application of immunotherapy. Therefore, it is urgent to identify appropriate biomarkers to select cancer patients for ICIs treatment. The earliest biomarker used to predict the clinical effect of ICIs therapy is PD-L1 expression level detected via immunohistochemical (IHC) [7,8]. Notably, PD-L1 is mainly used to predict PD-(L)1 inhibitors, and is not suitable for all ICIs, and its predictive efficacy changes when ICIs are used in combination with chemotherapy [9]. PD-L1 has also been proved to be unrelated to OS in bladder cancer receiving ICIs [7]. Several attempts have been made to identify other predictive biomarkers. One of the most intriguing and divisive is TMB. The higher TMB means the number of new antigens that can be recognized as non-self by T cells increases correspondingly and triggers anti-tumor immune response [9,10]. Many studies have revealed that high TMB is associated with a better prognosis in cancer patients receiving ICIs [11]. Recently, the FDA has approved pembrolizumab in all cancers with TMB > 10Mut/Mb based on the findings from the phase 2 KEYNOTE-158 study [11,12]. Moreover, previous studies revealed that patients with high TMB or PD-L1 positivity were found to be nearly two separate populations, even TMB was better than PD-L1 expression as a biomarker for predicting ICIs efficacy [3,13].
However, practical clinical application of TMB is greeted with many skepticisms [11]. The dominant reason may be the existence of the enormous intertumoral molecular heterogeneity, and it seems impossible to seek a uniform standard. Beside, there was a significant positive correlation between TMB and the incidence of irAEs [14]. Currently, TMB is generally considered to be an effective biomarker for immunotherapy, but different cancers have different therapy outcomes and prognoses at different TMB levels. Through reviewing the literature, we noticed an interesting problem that in patients treated without ICIs, pancreatic [15], breast [16] and colon [17] had a better OS in the low TMB group, while endometrial [18] and head and neck squamous cell carcinoma [19] run counter to the aforementioned. However, many clinical studies about immunotherapy only made a comparison among different TMB levels for prognostic indicators, such as OS and PFS, to determine the prediction effectiveness of TMB. The prognostic difference of high TMB cancer patients treated with immunotherapy or not is still unclear, and which is a clinical problem to be solved urgently.
We therefore did a meta-analysis of clinical studies to explore the clinical response and prognostic outcome of high/low TMB group of ICIs treated cancer patients. Considering that some cancers with high TMB may have better OS themselves rather than a result of immunotherapy, and for the first time, we downloaded the mutation data of 33 major cancer types from the TCGA database as the non-ICIs group, and compared the high TMB patients’ OS differences between the non-ICIs group and meta-analysis, to clarify the predictive value of TMB as a biomarker for immunotherapy.
Material and methods
Search strategy
This meta-analysis is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement [20]. The protocol was registered on the International Prospective Register of Systematic Reviews (registration number: CRD42021289605).
We selected relevant studies published between Jan 1, 2014 and August 30, 2021 by searching PubMed, Cochrane Library, Embase and Web of Science. First, the following keywords were retrieved: "mutation burden" OR "mutational burden" OR "mutation load" OR "mutational load" OR "TMB" OR "TML", and then combined with “immunotherap*” OR “immune checkpoint inhibit*” OR “ICI” OR "ICIs" OR “immune checkpoint block*” OR "ICB" OR "ICBs" OR “pembrolizumab” OR “avelumab” OR “nivolumab” OR “durvalumab” OR “tremelimumab” OR “atezolizumab” OR “Ipilimumab” OR “Cemiplimab” OR “tiragolumab” OR “Dostarlimab*” OR “Camrelizumab” OR “PD-1″ OR “programmed death 1″ OR “PD-L1” OR “programmed death-ligand 1″ OR “PD-1/PD-L1” OR “anti–PD-1/anti–PD-L1” OR “CTLA-4″ OR “Cytotoxic T-Lymphocyte Antigen 4″. Search strategies and results for each database were shown in Table S1. The primary endpoint events of this study were OS, PFS, ORR and DCB.
Selection criteria
We conducted the study using the following inclusion criteria: (1) All the patients were diagnosed as cancer, and tumor tissue-based TMB was detected; (2) At least one of ORR, DCB, OS and PFS data could be available; (3) The sample size was greater than 20; (4) Studies on non-synonymous mutations were also included due to the consistency of the measurement principle; (5) patients treated with ICIs monotherapy. On the other hand, studies that met any of the following criteria were excluded: (1) TMB detected from circulating tumor DNA or blood; (2) The definition of high TMB and low TMB was not clear or TMB obtained by machine learning model; (3) The sample size was less than 20 or the main observed events cannot be obtained; (4) patients who had not been treated with ICIs or had received treatment other than ICIs at the same time; (5) Non-human studies, conference abstracts, reviews, meta-analyses, comments or letters.
Data extraction and quality assessment
Two independent investigators (CJL, YX) reviewed study titles and abstracts, and studies that satisfied the selection criteria were retrieved for full-text assessment. During data extraction, literature quality assessment was also completed. Some contradictions were resolved by a third investigator (CSY). The following information was extracted in an Excel spreadsheet: the first author, publication year, country of study, study type, cancer type, cancer stage, immunotherapy drug, target point of the medicine, TMB detection method, TMB cut-off value, number of patients with high/low TMB and corresponding data of DCB, ORR, OS and PFS.
Among all the included studies, the quality assessment of cohort studies used Newcastle-Ottawa Quality Assessment Scale (NOS), with 6–9 being high quality and 0–5 being low quality [21]. The single-arm clinical trials used the Methodological Index for Non-randomized Studies (MINORS) [22] scale to evaluate the selection of exposure, comparability of study groups, and outcomes. We chose the first seven of the 12 items, with each item scoring 0–2 for a total of 14 points. Studies with ≥8, and<8 were considered to have high and low risk of bias, respectively.
TCGA data download and bioinformatics analysis
We downloaded simple nucleotide variation (SNV) data of 33 major cancer types from the TCGA database (https://portal.gdc.cancer.gov/). Workflow type selected MuTect2 Variant Aggregation and masking and data format choose maf format. Then, the maftools R package was used to calculate the TMB of each sample. Next, the Kaplan-Meier analysis for overall survival was proceeded based on the TMB of 33 tumors whose cut-off level was set at the 70% value with the aid of R software and the Log-Rank was utilized to test. The K-M analysis results are presented in the form of a forest plot.
Statistical analysis
All meta-analyses were performed by Stata 16.0 software (Stata Corporation, College Station, Texas, USA) and ORR, DCB, OS and PFS were compared between high TMB and low TMB group via meta-analysis. For DCB and ORR, RR > 1 showed that the high TMB group had better therapeutic effects than the low TMB group. For combined HR and 95% CI results of OS/PFS, HR > 1 indicates that the survival rate of the high TMB group is lower than the low TMB group. The random-effect model was used when I2 value was greater than 50%, and the fixed-effect model was used when I2 value was less than 50%. In addition, we performed a subgroup analysis of OS and PFS for different tumor types, regions, and TMB detection methods.
All bioinformatics analysis, including the raw SNV data pre-processing and survival analysis, is performed using R v4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria), and maftools, survival and survminer R packages were used in our study.
Results
Study characteristics and data quality
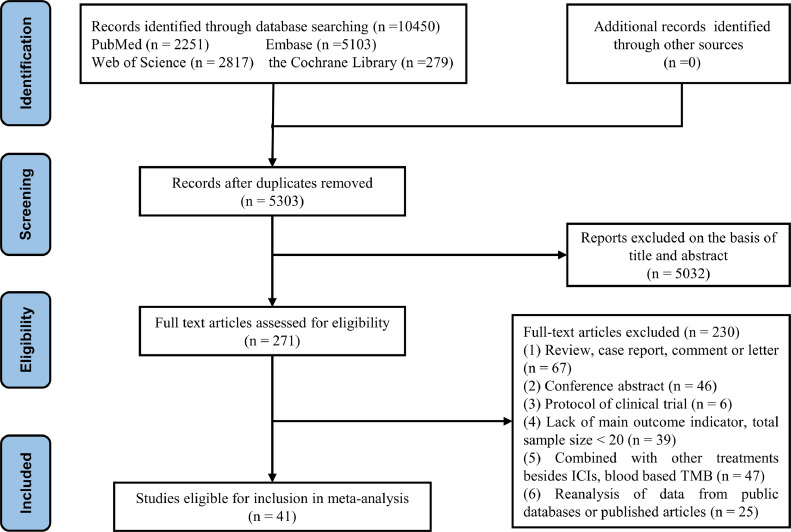
We identified 10,450 studies, of which 41 (with data for 7713 cancer participants) were included in our analysis [13,[23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. A flow diagram of the study selection process is presented in Fig. 1. Characteristics of all studies are presented in Table 1. The studies are mainly from the US, Europe and East Asia, and were published between 2014 and 2021. Most included studies chiefly concern advanced or distant metastases of NSCLC, SCLC and Melanoma. The immunotherapies used in these studies were diverse ICIs, including anti–PD-1, anti–PDL1, and anti-CTLA4 etc. The detection methods of TMB are mainly WES and NGS, and MSK-IMPAC and FoundationOne also belong to NGS. Of the 41 included studies, 32 studies used TMB and 9 used the number of non-synonymous mutations. In the literature quality evaluation, the NOS scale was used in 22 cohort studies, and the MINOS scale was used in 21 clinical single-arm studies. The results of the quality assessment are presented in Tables S2, S3.
Fig. 1.
Study selection process.
Table 1.
The baseline characteristics of included studies.
| study | Years | Country | Trail Name | Study type | Stage | Cancer type | Medicines | Target point | TMB methods | TMB cut-off | High | Low |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alborelli [23] | 2019 | Switzerland | NR | RCS | Stage Ⅰ- IV | NSCLC | Nivolumab / pembrolizumab / atezolizumab | PD-(L)1 | NGS | 9 | 25 | 51 |
| Yang [24] | 2020 | USA | NR | Clinical trial | recurrent /refractory | pan-cancer | anti–PD-(L)1/CTLA-4 | CTLA-4, PD-(L)1 | NGS | 6.88 | 9 | 94 |
| Shim [25] | 2020 | Korea | NR | PCS | advanced | NSCLC | anti-PD-(L)1 | PD-(L)1 | WES* | 272 | 47 | 151 |
| Li [26] | 2020 | China | NR | RCS | stage III | melanoma | pembrolizumab | PD-(L)1 | NR | 2 | 10 | 11 |
| Kim [27] | 2020 | Korea | NR | RCS | advanced | Gastric cancer | Nivolumab / pembrolizumab | PD-(L)1 | NGS | 14.31 | 8 | 55 |
| Huang [28] | 2020 | China | NR | RCS | advanced | NSCLC | anti-PD-(L)1 | PD-(L)1 | NGS | 10 | 14 | 20 |
| Fang A [29] | 2019 | China | NR | Clinical trial | Advanced /recurrent | NSCLC | Anti–PD-(L)1 | PD-(L)1 | WES* | 157 | 25 | 48 |
| Fang B [29] | NGS* | 10 | 26 | 49 | ||||||||
| Ricciuti [30] | 2019 | USA | NR | RCS | Limited | SCLC | anti–PD-1 / CTLA-4 | PD-1/CTLA-4 | NGS | 9.68 | 26 | 26 |
| Chae [31] | 2019 | USA | NR | RCS | 75.6% metastasis | NSCLC | anti-PD-(L)1 | PD-(L)1 | FoundationOne | 15 | 11 | 23 |
| Heeke A [32] | 2019 | France | NR | RCS | NR | NSCLC | anti-PD-(L)1 | PD-(L)1 | FoundationOne | 15 | 15 | 21 |
| Heeke B [32] | melanoma | anti-PD-(L)1 | PD-(L)1 | FoundationOne | 18 | 15 | 17 | |||||
| Ready [33] | 2019 | USA | CheckMate-568 | Clinical trial | IV/recurrent IIIB | NSCLC | anti-PD-(L)1 / CTLA-4 | PD-(L)1, CTLA-4 | FoundationOne | 10 | 48 | 50 |
| Singnal [34] | 2019 | NR | RCS | NR | NSCLC | Anti-PD-(L)1 | PD-(L)1 | FoundationOne | 20 | 161 | 1116 | |
| Samsteina [35] | 2019 | USA | NR | Clinical trial | advanced | pan-cancer | ICIs | ICP | MSK-IMPACT | 52.2 | 合计1662 | |
| Cristescu A [36] | 2018 | USA | KEYNOTE-028/ KEYNOTE-012 | Clinical trial | NR | pan-cancer | pembrolizumab | PD-(L)1 | WES* | 102.5 | 37 | 82 |
| Cristescu B [36] | 2018 | USA | KEYNOTE-012 B1/B2 | Clinical trial | NR | HNSCC | pembrolizumab | PD-(L)1 | WES* | 86 | 54 | 53 |
| Cristescu C [36] | 2018 | USA | KEYNOTE-001/ KEYNOTE-006 | Clinical trial | NR | melanoma | pembrolizumab | PD-(L)1 | WES* | 191.5 | 59 | 30 |
| Rizvia [37] | 2018 | USA | NR | RCS | advanced | NSCLC | anti–PD-(L)1 / CTLA-4 | PD-(L)1, CTLA-4 | MSK-IMPACT | 7.4 | 380 | 379 |
| Hellmann 1 [38] | 2018 | Europe and America | CheckMate-012 (NCT01454102) | Clinical trial | Stage IIIB/IV | NSCLC | Nivolumab / ipilimumab | PD-1, CTLA-4 | WES* | 158 | 47 | 48 |
| Hellmann 2A [39] | 2018 | Europe and America | CheckMate-032 (NCT01928394) | Clinical trial | NR | SCLC | Nivolumab | PD-1 | FoundationOne* | tertile | 42 | 47 |
| Hellmann 2B [39] | Nivolumab + Ipilimumab | PD-1, CTLA-4 | FoundationOne* | tertile | 26 | 27 | ||||||
| Janjigiana [40] | 2018 | NR | PCS | advanced | esophagogastric cancer | ICIs | ICP | MSK-IMPACT | 9.7 | 10 | 30 | |
| Goodman [41] | 2017 | USA | NR | RCS | locally/advanced/metastatic | pan-cancer | immunotherapy | PD-(L)1, CTLA4, IL2, Other | FoundationOne | 20 | 38 | 113 |
| Johnson [42] | 2016 | USA | NR | RCS | Stage IV | melanoma | Nivolumab / pembrolizumab / atezolizumab | PD-(L)1 | NGS | 23.1 | 27 | 14 |
| Synder A [43] | 2014 | USA | Not reported | Clinical trial | metastatic | melanoma | Ipilimumab / tremelimumab | CTLA4 | WES* | 100 | 17 | 8 |
| Synder B [43] | 30 | 9 | ||||||||||
| Daniel [44] | 2021 | Canada | NR | RCS | metastatic | solid tumor | investigational immunotherapy | NR | NGS | 12 | 7 | 15 |
| Chen [45] | 2021 | China | NR | PCS | IIIB-IV | NSCLC | anti-PD-1 / PD-L1 therapy | PD-(L)1 | OncoScreen | 7 | 14 | 18 |
| Hana [46] | 2021 | Korea | NR | PCS | advanced | pan-cancer | ICIs | ICP | NGS | 10 | 58 | 443 |
| Gogas [47] | 2020 | Europe and America | NCT03273153 | Clinical trial | advanced or metastatic | melanoma | pembrolizumab | PD-(L)1 | FoundationOne | 10 | Total 224 | |
| Hyojin [48] | 2021 | Korea | NR | RCS | surgically resected | NSCLC | ICIs | ICP | NGS | 5.29 | 15 | 15 |
| Wang [49] | 2021 | china | NCT02915432 | Clinical trial | recurrent or Metastatic | nasopharyngeal cancer | Toripalimab | PD-(L)1 | WES | 2.9 | 17 | 157 |
| Alexandra [50] | 2021 | Canada | NCT02155621 | Clinical trial | advanced or metastatic | pan-cancer | ICIs | ICP | NGS | 10 | 19 | 63 |
| Michele [51] | 2021 | UK | NCT02563002 | Clinical trial | surgical resected | colorectal cancer | Nivolumab / pembrolizumab / ipilimumab | PD-(L)1, CTLA4 | WES | 12 | 26 | 6 |
| Keigo [52] | 2021 | Japan | NR | PCS | Advanced/metastatic | gastrointestinal cancer | Pembrolizumab / Nivolumab | PD-(L)1 | WES/NGS | 10 | 41 | 4 |
| Goodman [53] | 2020 | USA | NR | RCS | NR | pan-cancer | anti-PD-1/L1 | PD-(L)1 | NGS | 10 | 39 | 38 |
| Goodman [54] | 2019 | USA | NCT02478931 | RCS | NR | pan-cancer | ICIs | ICP | FoundationOne | 20 | 15 | 45 |
| Yelena [55] | 2021 | USA | NCT01693562 | Clinical trial | Advanced /metastatic | pan-cancer | Durvalumab | PD-(L)1 | NGS | tertile | 13 | 24 |
| Schrock [56] | 2019 | USA | NR | RCS | Stage Ⅱ- IV | colorectal cancer | anti-PD-(L)1 | PD-(L)1 | NGS | 37.4 | 13 | 9 |
| Carl [57] | 2018 | USA | NR | Clinical trial | metastatic | melanoma | Nivolumab / pembrolizumab / ipilimumab | PD-(L)1, CTLA4 | WES | 7.1 | 39 | 119 |
| Chester [58] | 2021 | USA | NR | RCS | advanced | NSCLC | Nivolumab / pembrolizumab / atezolizumab | PD-(L)1, CTLA4 | FoundationOne | 10 | 35 | 53 |
| Tang [59] | 2019 | China | NCT02836795 | Clinical trial | metastatic | melanoma or urologic cancers | Toripalimab | PD-(L)1 | NGS | 6 | 11 | 12 |
| Wang [13] | 2019 | China | NCT02915432 | Clinical trial | chemorefractory | gastric cancer | Toripalimab | PD-(L)1 | WES | 12(20%) | 12 | 42 |
| Hodi A [60] | 2021 | Europe and America | checkmate067 | Clinical trial | advanced | melanoma | Nivolumab | PD-(L)1 | WES* | 203.5 | 87 | 89 |
| Hodi B [60] | Nivolumab + ipilimumab | PD-(L)1, CTLA-4 | 203.5 | 88 | 96 | |||||||
| Hodi C [60] | Nivolumab | PD-(L)1 | 203.5 | 94 | 84 | |||||||
| Hugo [61] | 2016 | USA | NR | RCS | metastatic | melanoma | Pembrolizumab / Nivolumab | PD-(L)1 | WES* | top third | 12 | 27 |
| Rizvia [62] | 2015 | USA | NR | RCS | NR | NSCLC | pembrolizumab | PD-(L)1 | WES* | 200 | 17 | 17 |
A, B and C represent different datasets from the same study; 1 and 2 represent different studies from the same first author; * represents that non-synonymous mutations were used to replace TMB in the study. RCS retrospective cohort study; PCS prospective cohort study, NR not report, ICIs immune checkpoint inhibitors, ICP Immune checkpoint, NES next generation sequencing, WES whole exome sequencing.
Relationship between TMB and main endpoints
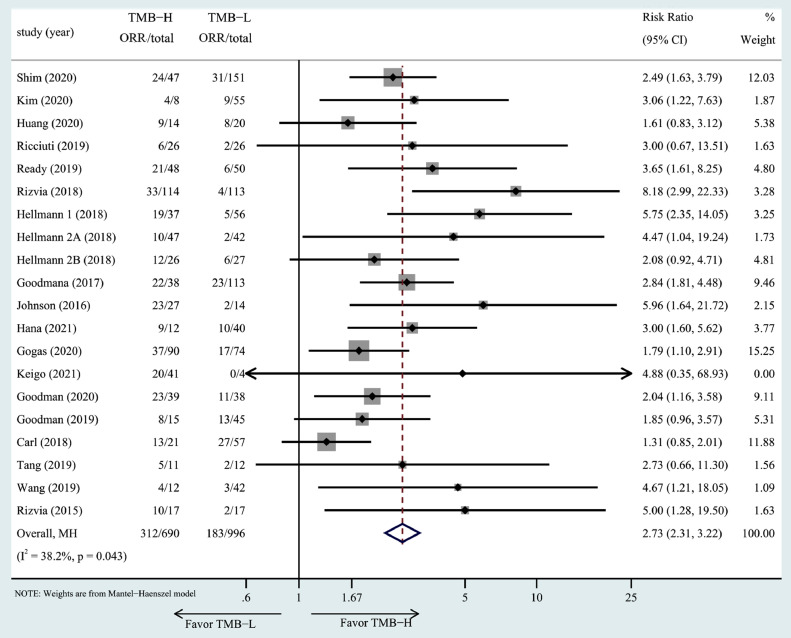
TMB and ORR. The association of high TMB with ORR was investigated in 19 studies including 20 cohorts. Patients in the high TMB group had a higher ORR in all included studies. Meta-analysis indicated that high TMB was associated with a better ORR (RR = 2.73, 95% CI: 2.31–3.22, P= 0.043, Fig. 2).
Fig. 2.
The forest plot of ORR in patients with high TMB compared to those with low TMB.
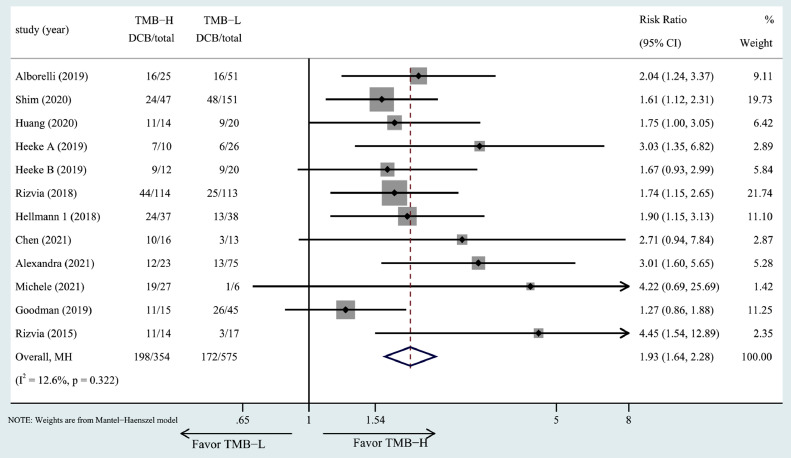
TMB and DCB. The association of high TMB with DCB was investigated in 11 studies including 12 cohorts. Patients in the high TMB group had a higher DCB in all included studies. The RR of DCB for the high TMB group versus low TMB group was 1.93 (RR = 1.93, 95% CI: 1.64–2.28, P= 0.356, Fig. 3).
Fig. 3.
The forest plot of DCB in patients with high TMB compared to those with low TMB.
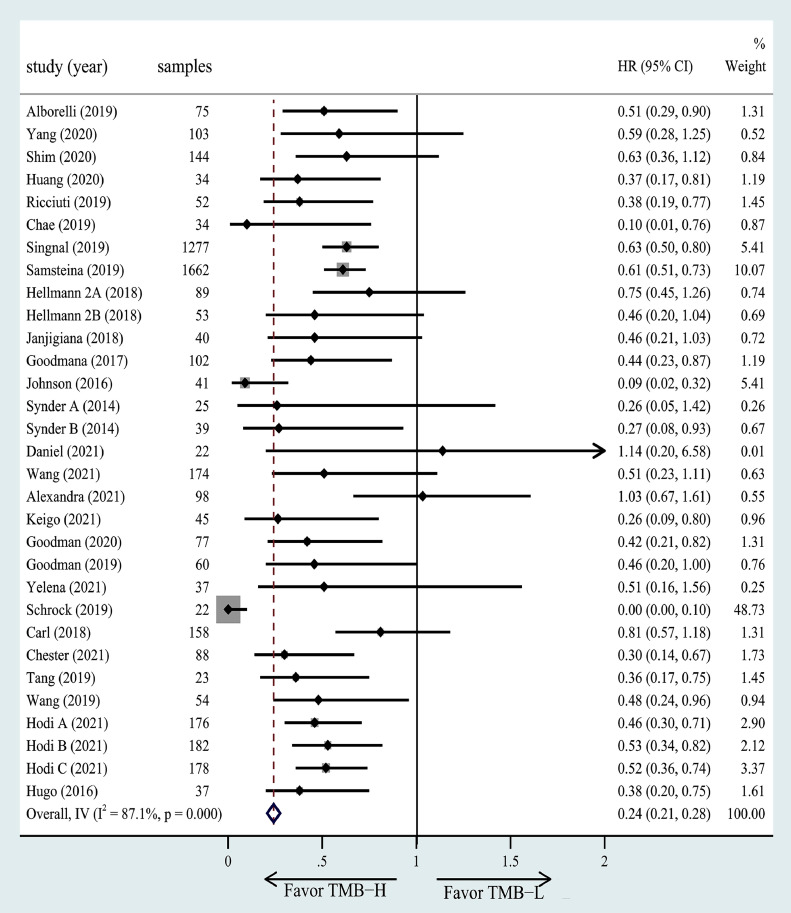
TMB and OS. The association of high TMB with OS was investigated in 27 studies including 31 cohorts. Two studies of all those had better OS in the low TMB group. The results of the meta-analysis showed significantly greater benefits for the high TMB group receiving ICIs as compared to the low TMB group (HR =0.24, 95% CI = 0.21–0.28, P< 0.001, Fig. 4).
Fig. 4.
The forest plot of OS in patients with high TMB compared to those with low TMB.
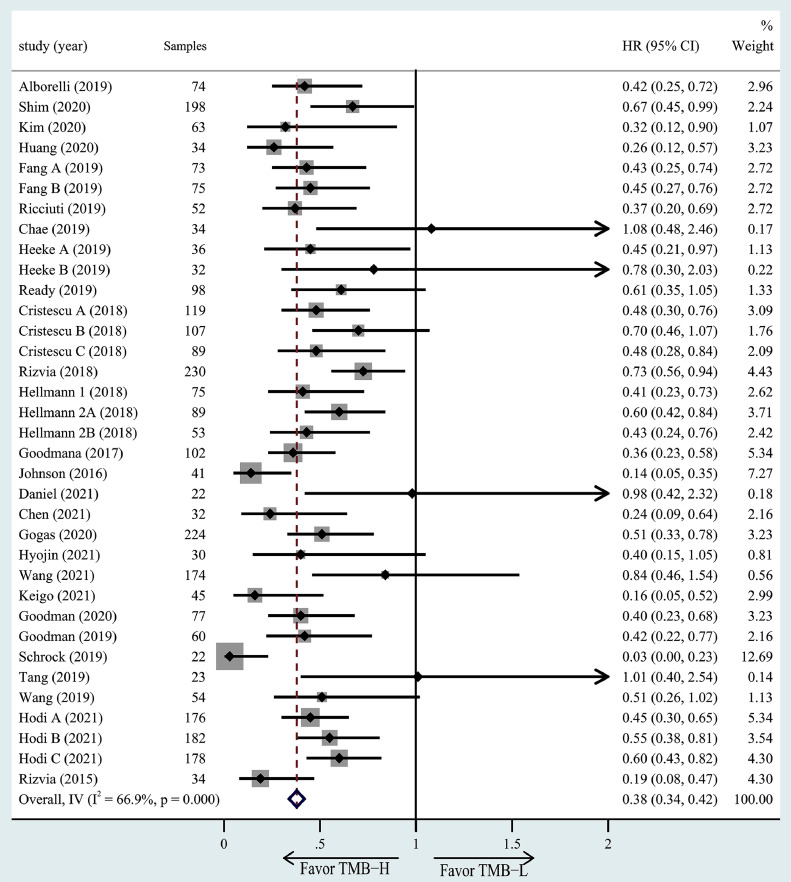
TMB and PFS. The association of high TMB with OS was investigated in 28 studies including 35 cohorts. 33 cohorts of all those had better OS in the high TMB group. Meta-analysis showed significantly greater benefits for the high TMB group receiving ICIs as compared to the low TMB group (HR =0.38, 95% CI = 0.34–0.42, P< 0.001, Fig. 5).
Fig. 5.
The forest plot of PFS in patients with high TMB compared to those with low TMB.
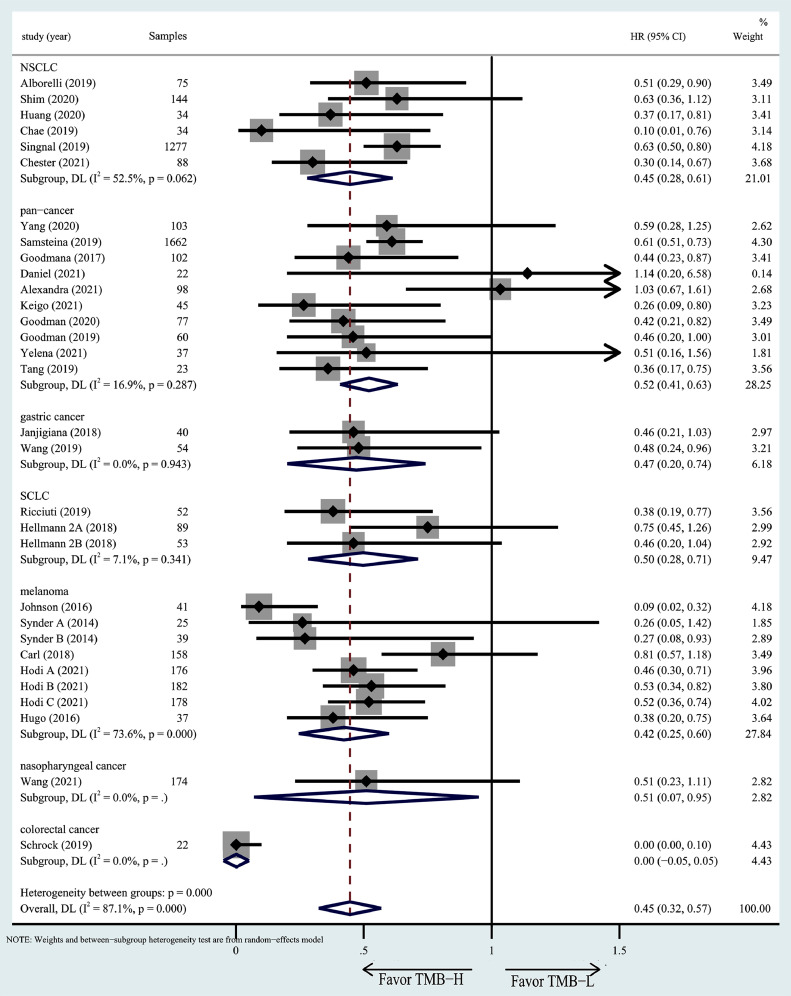
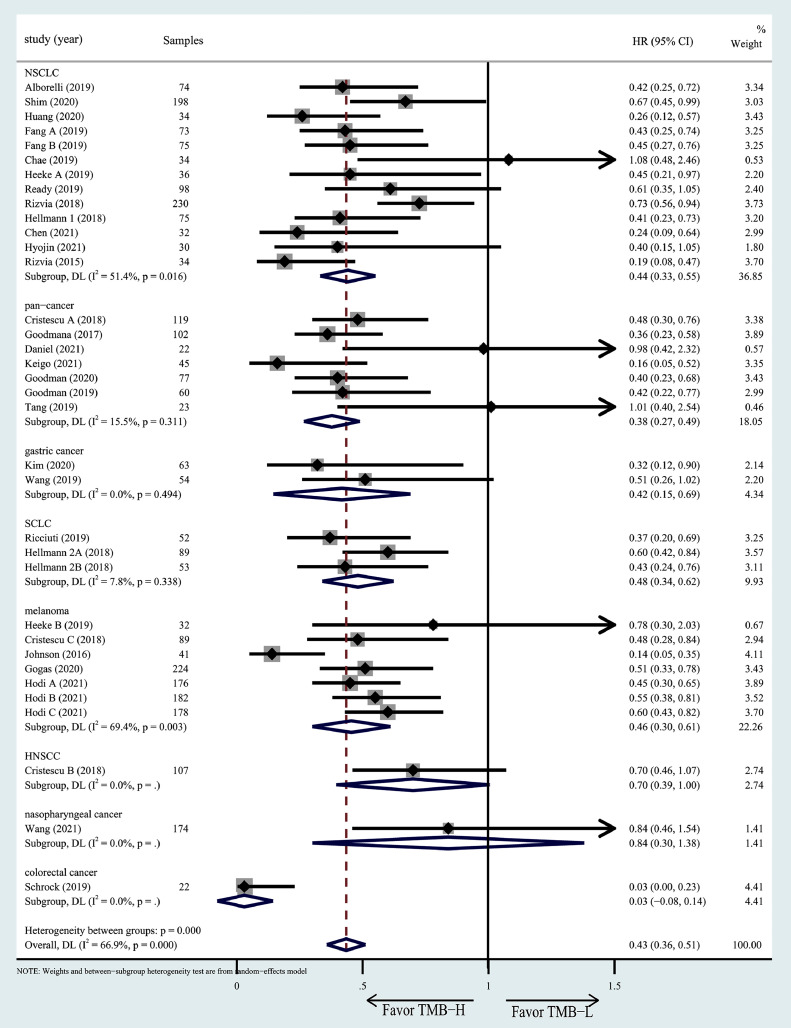
Subgroup analyses for OS/PFS
To explore the influence of different tumor types, regions and TMB detection methods on the relationship between TMB and prognosis, and to test whether the heterogeneity was caused by these factors, we conducted a subgroup analysis. We found that different tumor types appeared to have a different OS (Fig. 6) and PFS (Fig. 7). Colorectal cancer with high TMB appeared to have better OS than other cancer patients, while HNSCC and nasopharyngeal cancer have better PFS than others. However, there is no significant difference between TMB detection methods in different regions (Figs. S1 and S2) and different TMB detection methods (Figs. S3 and S4).
Fig. 6.
Subgroup analysis in OS of patients with high/low TMB based on different cancer types.
Fig. 7.
Subgroup analysis in OS of patients with high/low TMB based on different cancer types.
Prognosis comparison of high TMB ICIs versus no-ICIs
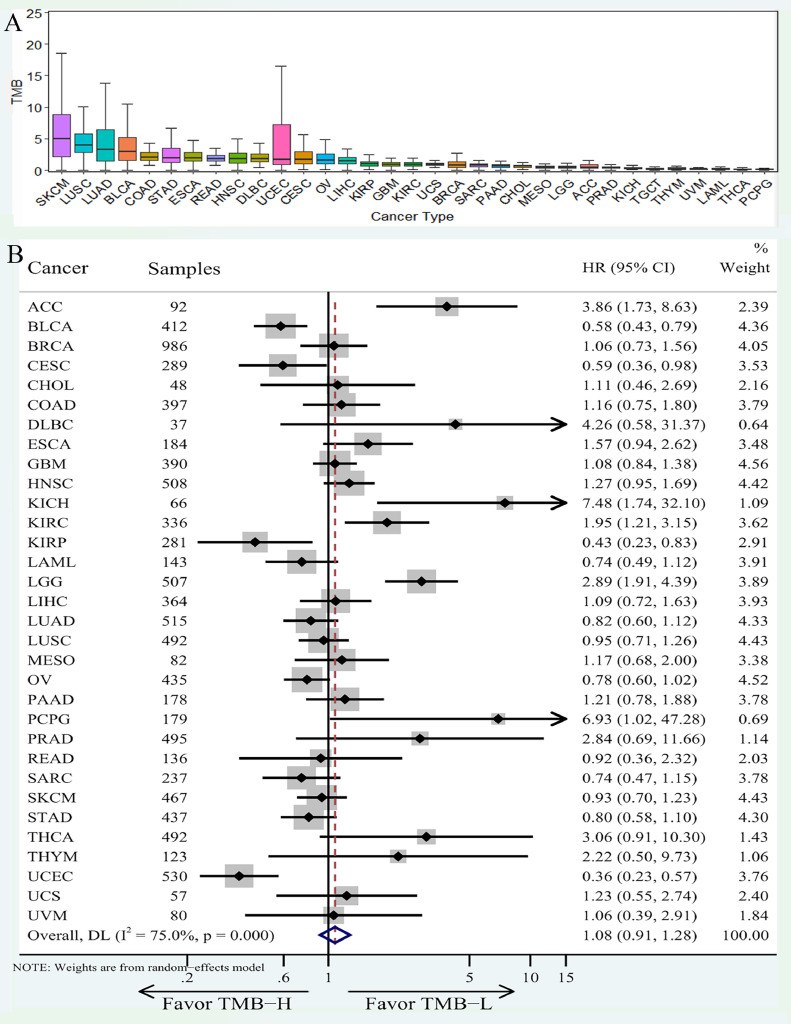
We analyzed the TMB of 33 tumors downloaded from TCGA, and found TMB varies greatly among patients with different tumors (Fig. 8A), among which SKCM, LUSC, LUAD and BLCA have a high level of TMB, while the TMB of UVM, LAML, THCA and PCPG are relatively low and have poor immunogenicity. The k-M analysis for OS was conducted and the results were presented in the form of a forest plot (Fig. 8B). According to the results, KICH, PCPG, DLBC, ACC and LGG had a better prognosis in the low TMB group, while UCEC, CESC and BLCA patients showed better prognosis in the high TMB group. It suggests that we should consider the OS changes of the high TMB group before and after immunotherapy when exploring the immunotherapy efficacy in patients with a better prognosis of the high TMB group.
Fig. 8.
TMB distribution and its relationship with prognosis in 33 major cancer types from TCGA. (A) The TMB distribution of 33 tumors from TCGA database was presented in the form of boxplot; (B) The results of K-M survival analysis for 33 cancer types were presented in the form of forest map.
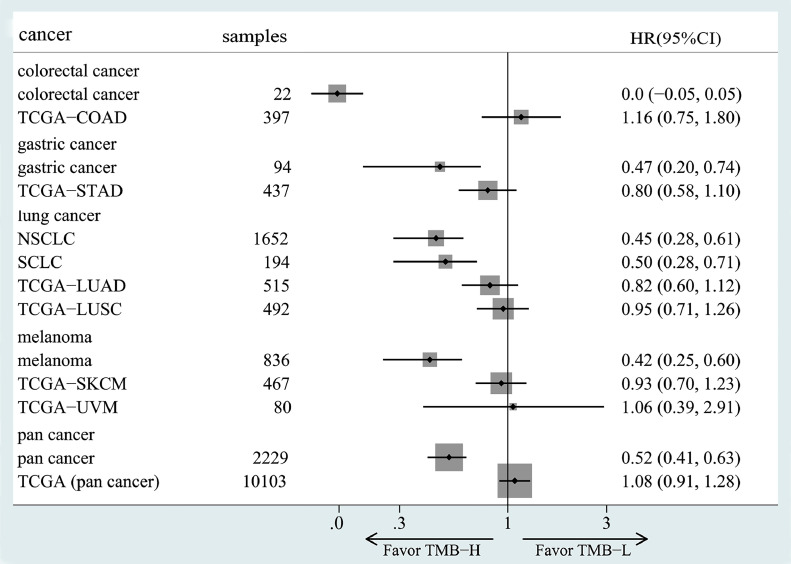
We compared the OS subgroups analysis results for different tumor types in meta-analysis with the K-M analysis results for TCGA-derived cancers, and the results were shown in Fig. 9. We found that after immunotherapy for colorectal cancer, gastric cancer, lung cancer, melanoma and pan-cancer, the OS improvement in the high TMB group was significantly better than non-ICIs. Moreover, the overall results of pan-cancer data showed that high TMB patients had a high response rate to immunotherapy and a better prognosis. TMB is an effective biomarker, but further studies are needed in specific cancers.
Fig. 9.
Meta-analysis results compared with non-ICIs group from the TCGA database. Among them, names begin with TCGA were no-ICIs group, and the others were ICIs-treated results obtained by meta-analysis.
Discussion
Our results show that, compared with the low TMB group, high TMB cancer patients with immunotherapy have a higher ORR, DCB and better OS, PFS, which was in agreement with previous studies [63,64]. In addition, TCGA-derived data were used as the no-ICIs group for comparison, and it was found that the HR of high TMB patients who received ICIs was significantly reduced, indicating that high TMB patients with immunotherapy had better OS than without it. These results indicate that TMB is a promising biomarker for response to immunotherapy, with higher TMB score predicting better clinical response and longer overall survival. These results thus lend support to high TMB as a therapeutic biomarker that can improve the management of cancer immunotherapy.
TMB is one of the important predictive biomarkers for the companion diagnosis of immunotherapy. Many studies have shown that TMB can predict the clinical response of cancer patients to ICIs [65]. The results of our study showed that compared with low TMB, almost all tumors with high TMB had better ORR(RR=2.73) and DCB(RR=1.93). In terms of prognosis, high TMB had better OS (HR=0.24) and PFS (HR=0.38). Although TMB is associated with improved survival in patients receiving ICIs across many cancer types, there may not be one universal definition of high TMB, which is closely related to tumor heterogeneity [40]. The TMB cut-off values of included study are also diverse, with some being 10 mutations/mb and some by a specific percentage. In lung cancer, it was found no significant difference in the ORR when TMB cut-off level was set at the 50% value, and ORR increased with higher TMB, plateauing at 10 or more mutations/mb [38,39]. In several included studies involving melanoma, the TMB thresholds are highly variable between 2 and 23.1. Also, TMB was not sufficient to predict OS in patients receiving anti-CTLA-4 therapy [25] and benefit from dual checkpoint blockade [28]. Almost all studies try to find a more applicable TMB threshold, so as to make the research results more meaningful, which is also a factor that cannot be corrected in this study. Moreover, because TMB and non-synonym mutations are both used to detect mutations in tumor tissues, they were both included in this study.
To explore the influence of different tumor types, regions and TMB detection methods on the relationship between TMB and prognosis, we made a subgroup analysis. Among different cancer types, we found that patients with high TMB had a better prognosis than low. Compared with other tumors, patients with colorectal cancer have a better prognosis after immunotherapy [41], which indicates that colorectal cancer patients can benefit greatly from immunotherapy. In earlier studies, WES was the main detection method of TMB. WES is also considered to be the gold standard method for assessing tissue TMB [53]. With the development of medicine and technology, NGS sequencing has been gradually applied in TMB detection due to its more timely and economic advantages. Our study showed that there was no significant difference between the two TMB values measured using WES and NGS in predicting the OS and PFS of patients receiving ICI. In addition, all included studies are mainly from European, American and East Asian countries. No significant differences were found among the different regions.
Considering that some tumors with high TMB may have a better prognosis, it is not reasonable just to compare the OS/PFS between high TMB and low TMB to make a conclusion. We adopted data from the TCGA database as no-ICIs for comparison (a small part also underwent immunotherapy, which can be ignored). Through the analysis of 33 TCGA tumors, it was found that SKCM, LUSC, LUAD and BLCA have higher TMB and immunogenicity, which may be more suitable for immunotherapy [66]. The TMB of UVM, LAML, THCA and PCPG are relatively low and have poor immunogenicity. Improving the immunogenicity of these low TMB cancers can improve the response rate to clinical immunotherapy [67]. In terms of OS, there are significant differences between tumors. Among them, UCEC, CESC, BLCA and other tumor patients showed better prognosis in the high TMB group, while KICH, PCPG, DLBC, ACC, LGG and other tumor patients showed better prognosis in the low TMB group. However, when compared with the results of meta-analysis, we found that compared with no-ICIs, high TMB tumor patients had a higher OS improvement after immunotherapy.
Our study is the first time to explore the effect of immunotherapy or not on the prognosis of high TMB patients, and to fill in the potential defect that may exist in comparison of high TMB and low TMB. In addition, our research method combines meta-analysis and bioinformatics. This study also has some limitations. First, there are not enough studies on the relationship between cancer ICIs therapy and TMB, so it is not possible to obtain completely credible meta-analysis results for all cancer types. Second, TMB cut-off values vary from study to study, which may result in imprecise deviation. Third, every single outcome was not reported in every study, reducing the credibility of the analysis results. More in-depth, on the premise of more relevant studies, comprehensive analysis of a larger sample can be carried out to obtain an accurate conclusion on the relationship between ICIs and TMB in all tumors. To increase the utility of TMB, it is urgent to coordinate the consistency of TMB assessment and threshold value in the future.
Conclusion
Although further studies are needed to establish the optimal approach to the application of TMB in practice, these results support that TMB is a promising predictive biomarker, which can predict clinical response and prognostic outcome of immunotherapy. Our findings thus lend support to high TMB as a therapeutic biomarker that can improve the management of cancer immunotherapy.
Funding
This work was supported by the regulatory mechanism of AMPK in ischemic-reperfusion injury and fibrosis in renal transplantation (CY2015-YJRC08); Gansu Provincial Education Department outstanding graduate “innovation star” project (2021CXZX-154); the Open Foundation of Gansu Key Laboratory of Functional Genomics and Molecular Diagnostics; Gansu Province Intellectual Property Planning project (21ZSCQ012); the Second Hospital of Lanzhou University "Cuiying Science and Technology Innovation" project (CY2021-QN-A20).
Data availability
All data and material analyzed during this study are included in this article.
CRediT authorship contribution statement
Jinlong Cao: Conceptualization, Methodology, Formal analysis, Writing – original draft. Xin Yang: Conceptualization, Methodology, Formal analysis, Writing – original draft. Siyu Chen: Methodology, Investigation. Jirong Wang: Formal analysis, Investigation, Writing – review & editing. Xinpeng Fan: Formal analysis, Investigation, Writing – review & editing. Shengjun Fu: Supervision, Data curation. Li Yang: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Funding acquisition.
Declaration of Competing Interest
All the authors declare no conflicts of interest.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.tranon.2022.101375.
Contributor Information
Jinlong Cao, Email: caojl18@lzu.edu.cn.
Li Yang, Email: ery_yangli@lzu.edu.cn.
Appendix. Supplementary materials
Table S1 Search strategies and results of 4 databases.
Table S2 The NOS tool for assessing the risk of bias of cohort studies.
Table S3 The MINORS tool for assessing the risk of bias of single-arm clinical trials.
Fig. S1 Subgroup analysis in OS of patients with high/low TMB based on different regions.
Fig. S2 Subgroup analysis in OS of patients with high/low TMB based on different TMB detection methods.
Fig. S3 Subgroup analysis in PFS of patients with high/low TMB based on different regions.
Fig. S4 Subgroup analysis in PFS of patients with high/low TMB based on different TMB detection methods.
Reference
- 1.Bray F.A.O., et al. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127(16):3029–3030. doi: 10.1002/cncr.33587. [DOI] [PubMed] [Google Scholar]
- 2.Sung H.A.O., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Carbone D.P., et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N. Engl. J. Med. 2017;376(25):2415–2426. doi: 10.1056/NEJMoa1613493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cristescu R.A.O., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi: 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dolladille C., et al. Immune checkpoint inhibitor rechallenge after immune-related adverse events in patients with cancer. JAMA Oncol. 2020;6(6):865–871. doi: 10.1001/jamaoncol.2020.0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang D.Y., et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghate K., et al. PD-L1 expression and clinical outcomes in patients with advanced urothelial carcinoma treated with checkpoint inhibitors: a meta-analysis. Cancer Treat. Rev. 2019;76:51–56. doi: 10.1016/j.ctrv.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Onuma A.E., et al. Immune checkpoint inhibitors in hepatocellular cancer: current understanding on mechanisms of resistance and biomarkers of response to treatment. Gene Express. 2020;20(1):53–65. doi: 10.3727/105221620X15880179864121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Addeo A., et al. TMB or not TMB as a biomarker: that is the question. Crit. Rev. Oncol. Hematol. 2021;163 doi: 10.1016/j.critrevonc.2021.103374. [DOI] [PubMed] [Google Scholar]
- 10.Rizvi N.A., et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prasad V., Addeo A. The FDA approval of pembrolizumab for patients with TMB >10 mut/Mb: was it a wise decision? No. Ann. Oncol. 2020;31(9):1112–1114. doi: 10.1016/j.annonc.2020.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Marcus L., et al. FDA approval summary: pembrolizumab for the treatment of tumor mutational burden-high solid tumors. Clin. Cancer Res. 2021;27(17):4685–4689. doi: 10.1158/1078-0432.CCR-21-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F., et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann. Oncol. 2019;30(9):1479–1486. doi: 10.1093/annonc/mdz197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bomze D., et al. Association between immune-related adverse events during anti-PD-1 therapy and tumor mutational burden. JAMA Oncol. 2019;5(11):1633–1635. doi: 10.1001/jamaoncol.2019.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo D., et al. Characterization of the immune cell infiltration profile in pancreatic carcinoma to aid in immunotherapy. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.677609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J., et al. ADRB1 was identified as a potential biomarker for breast cancer by the co-analysis of tumor mutational burden and immune infiltration. Aging (Albany NY) 2020;13(1):351–363. doi: 10.18632/aging.104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Z., et al. Correlations between tumor mutation burden and immunocyte infiltration and their prognostic value in colon cancer. Front. Genet. 2021;12 doi: 10.3389/fgene.2021.623424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou H., et al. Integrated analysis of tumor mutation burden and immune infiltrates in endometrial cancer. Curr. Probl. Cancer. 2021;45(2) doi: 10.1016/j.currproblcancer.2020.100660. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., et al. Age and mutations as predictors of the response to immunotherapy in head and neck squamous cell cancer. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.608969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moher D., et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 22.Slim K., et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J. Surg. 2003;73(9):712–716. doi: 10.1046/j.1445-2197.2003.02748.x. [DOI] [PubMed] [Google Scholar]
- 23.Hugo W., et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell. 2016;165(1):35–44. doi: 10.1016/j.cell.2016.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janjigian Y.Y., et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov. 2018;8(1):49–58. doi: 10.1158/2159-8290.CD-17-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Snyder A., et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014;371(23):2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rizvi N.A., et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348(6230):124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson D.B., et al. Targeted next generation sequencing identifies markers of response to PD-1 blockade. Cancer Immunol. Res. 2016;4(11):959–967. doi: 10.1158/2326-6066.CIR-16-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goodman A.M., et al. Tumor mutational burden as an independent predictor of response to immunotherapy in diverse cancers. Mol. Cancer Ther. 2017;16(11):2598–2608. doi: 10.1158/1535-7163.MCT-17-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cristescu R., et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science. 2018;362(6411):eaar3593. doi: 10.1126/science.aar3593. pp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellmann M.D., et al. Tumor mutational burden and efficacy of nivolumab monotherapy and in combination with ipilimumab in small-cell lung cancer. Cancer Cell. 2018;33(5):853–861. doi: 10.1016/j.ccell.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hellmann M.D., et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell. 2018;33(5):843–852. doi: 10.1016/j.ccell.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morrison C., et al. Predicting response to checkpoint inhibitors in melanoma beyond PD-L1 and mutational burden. J. Immunother. Cancer. 2018;6(1):32. doi: 10.1186/s40425-018-0344-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rizvi H., et al. Molecular determinants of response to anti-programmed cell death (PD)-1 and anti-programmed death-ligand 1 (PD-L1) blockade in patients with non-small-cell lung cancer profiled with targeted next-generation sequencing. J. Clin. Oncol. 2018;36(7):633–641. doi: 10.1200/JCO.2017.75.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chae Y.K., et al. Association of tumor mutational burden with DNA repair mutations and response to anti-PD-1/PD-L1 therapy in non-small-cell lung cancer. Clin. Lung Cancer. 2019;20(2):88–96. doi: 10.1016/j.cllc.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Fang W., et al. Comprehensive genomic profiling identifies novel genetic predictors of response to anti-PD-(L)1 therapies in non-small cell lung cancer. Clin. Cancer Res. 2019;25(16):5015–5026. doi: 10.1158/1078-0432.CCR-19-0585. [DOI] [PubMed] [Google Scholar]
- 36.Goodman A.M., et al. Microsatellite-stable tumors with high mutational burden benefit from immunotherapy. Cancer Immunol. Res. 2019;7(10):1570–1573. doi: 10.1158/2326-6066.CIR-19-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heeke S., et al. In-house implementation of tumor mutational burden testing to predict durable clinical benefit in non-small cell lung cancer and melanoma patients. Cancers. 2019;11(9):1271. doi: 10.3390/cancers11091271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ready N., et al. First-line nivolumab plus ipilimumab in advanced non-small-cell lung cancer (CheckMate 568): outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J. Clin. Oncol. 2019;37(12):992–1000. doi: 10.1200/JCO.18.01042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ricciuti B., et al. Use of targeted next generation sequencing to characterize tumor mutational burden and efficacy of immune checkpoint inhibition in small cell lung cancer. J. Immunother. Cancer. 2019;7(1):87. doi: 10.1186/s40425-019-0572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samstein R.M., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrock A.B., et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann. Oncol. 2019;30(7):1096–1103. doi: 10.1093/annonc/mdz134. [DOI] [PubMed] [Google Scholar]
- 42.Singal G., et al. Association of patient characteristics and tumor genomics with clinical outcomes among patients with non-small cell lung cancer using a clinicogenomic database. JAMA. 2019;321(14):1391–1399. doi: 10.1001/jama.2019.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tang B., et al. Safety and clinical activity with an anti-PD-1 antibody JS001 in advanced melanoma or urologic cancer patients. J. Hematol. Oncol. 2019;12(1):7. doi: 10.1186/s13045-018-0693-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alborelli I., et al. Tumor mutational burden assessed by targeted NGS predicts clinical benefit from immune checkpoint inhibitors in non-small cell lung cancer. J. Pathol. 2020;250(1):19–29. doi: 10.1002/path.5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goodman A.M., et al. MHC-I genotype and tumor mutational burden predict response to immunotherapy. Genome Med. 2020;12(1):45. doi: 10.1186/s13073-020-00743-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang D., et al. Tumor mutation burden as a potential biomarker for PD-1/PD-L1 inhibition in advanced non-small cell lung cancer. Target Oncol. 2020;15(1):93–100. doi: 10.1007/s11523-020-00703-3. [DOI] [PubMed] [Google Scholar]
- 47.Kim J., et al. Tumor mutational burden determined by panel sequencing predicts survival after immunotherapy in patients with advanced gastric cancer. Front. Oncol. 2020;10:314. doi: 10.3389/fonc.2020.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T., Jia D.D., Teng L.S. Adjuvant pembrolizumab versus high-dose interferon α-2b for Chinese patients with resected stage III melanoma: a retrospective cohort study. Invest. New Drugs. 2020;38(5):1334–1341. doi: 10.1007/s10637-020-00913-6. [DOI] [PubMed] [Google Scholar]
- 49.Shim J.H., et al. HLA-corrected tumor mutation burden and homologous recombination deficiency for the prediction of response to PD-(L)1 blockade in advanced non-small-cell lung cancer patients. Ann. Oncol. 2020;31(7):902–911. doi: 10.1016/j.annonc.2020.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Yang R.K., et al. Identification of biomarkers of immune checkpoint blockade efficacy in recurrent or refractory solid tumor malignancies. Oncotarget. 2020;11(6):600–618. doi: 10.18632/oncotarget.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Araujo D.V., et al. Applications of circulating tumor DNA in a cohort of phase i solid tumor patients treated with immunotherapy. JNCI Cancer Spectr. 2021;5(3) doi: 10.1093/jncics/pkaa122. pp.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bortolomeazzi M., et al. Immunogenomics of colorectal cancer response to checkpoint blockade: analysis of the KEYNOTE 177 trial and validation cohorts. Gastroenterology. 2021;161(4):1179–1193. doi: 10.1053/j.gastro.2021.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen X., et al. Blood tumor mutation burden can predict the clinical response to immune checkpoint inhibitors in advanced non-small cell lung cancer patients. Cancer Immunol. Immunother. 2021;70(12):3513–3524. doi: 10.1007/s00262-021-02943-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chida K., et al. A low tumor mutational burden and PTEN mutations are predictors of a negative response to PD-1 blockade in MSI-H/dMMR gastrointestinal tumors. Clin. Cancer Res. 2021;27(13):3714–3724. doi: 10.1158/1078-0432.CCR-21-0401. [DOI] [PubMed] [Google Scholar]
- 55.Gogas H., et al. Cobimetinib plus atezolizumab in BRAFV600 wild-type melanoma: primary results from the randomized phase III IMspire170 study. Ann. Oncol. 2021;32(3):384–394. doi: 10.1016/j.annonc.2020.12.004. official journal of the european society for medical oncology. [DOI] [PubMed] [Google Scholar]
- 56.Hodi F.S., et al. TMB and inflammatory gene expression associated with clinical outcomes following immunotherapy in advanced melanoma. Cancer Immunol. Res. 2021;9(10):1202–1213. doi: 10.1158/2326-6066.CIR-20-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kao C., et al. Predictive value of combining biomarkers for clinical outcomes in advanced non-small cell lung cancer patients receiving immune checkpoint inhibitors. Clin. Lung Cancer. 2021;22(6):500–509. doi: 10.1016/j.cllc.2021.03.017. [DOI] [PubMed] [Google Scholar]
- 58.Kim H., et al. Clinical sequencing to assess tumor mutational burden as a useful biomarker to immunotherapy in various solid tumors. Ther .Adv. Med. Oncol. 2021;13 doi: 10.1177/1758835921992992. pp.1758835921992992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kim H., et al. Comparison of the predictive power of a combination versus individual biomarker testing in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Cancer Res. Treat. 2021 doi: 10.4143/crt.2021.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazdun Y., et al. A new pipeline to predict and confirm tumor neoantigens predict better response to immune checkpoint blockade. Mol. Cancer Res. 2021;19(3):498–506. doi: 10.1158/1541-7786.MCR-19-1118. [DOI] [PubMed] [Google Scholar]
- 61.Pender A., et al. Genome and transcriptome biomarkers of response to immune checkpoint inhibitors in advanced solid tumors. Clin. Cancer Res. 2021;27(1):202–212. doi: 10.1158/1078-0432.CCR-20-1163. [DOI] [PubMed] [Google Scholar]
- 62.Wang F.H., et al. Efficacy, safety, and correlative biomarkers of toripalimab in previously treated recurrent or metastatic nasopharyngeal carcinoma: a phase II clinical trial (POLARIS-02) J. Clin. Oncol. 2021;39(7):704–712. doi: 10.1200/JCO.20.02712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Huang T., et al. Prognostic role of tumor mutational burden in cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. Front. Oncol. 2021;11:2937. doi: 10.3389/fonc.2021.706652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yang Z., et al. Clinical significance of tumour mutation burden in immunotherapy across multiple cancer types: an individual meta-analysis. Jpn. J. Clin. Oncol. 2020;50(9):1023–1031. doi: 10.1093/jjco/hyaa076. [DOI] [PubMed] [Google Scholar]
- 65.Samstein R.M., et al. Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat. Genet. 2019;51(2):202–206. doi: 10.1038/s41588-018-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weinstein J., et al. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang J., et al. ADRB1 was identified as a potential biomarker for breast cancer by the co-analysis of tumor mutational burden and immune infiltration. Aging. 2020;13(1):351–363. doi: 10.18632/aging.104204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Search strategies and results of 4 databases.
Table S2 The NOS tool for assessing the risk of bias of cohort studies.
Table S3 The MINORS tool for assessing the risk of bias of single-arm clinical trials.
Fig. S1 Subgroup analysis in OS of patients with high/low TMB based on different regions.
Fig. S2 Subgroup analysis in OS of patients with high/low TMB based on different TMB detection methods.
Fig. S3 Subgroup analysis in PFS of patients with high/low TMB based on different regions.
Fig. S4 Subgroup analysis in PFS of patients with high/low TMB based on different TMB detection methods.
Data Availability Statement
All data and material analyzed during this study are included in this article.