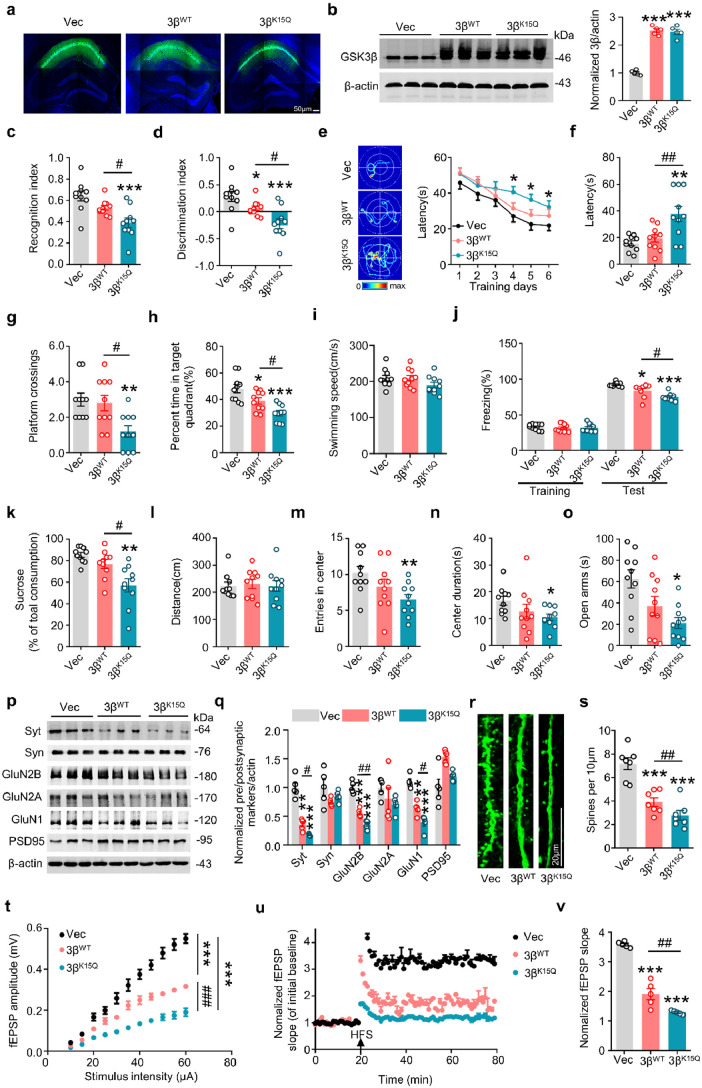
Figure 4.
GSK-3β K15-acetylation causes memory impairment and synaptic dysfunction. (a) Representative image showing virus expression in hippocampal CA1 of 2-month-old C57 mice after stereotactic infusion for 1 month. (b) Infusion of AAV-GSK-3βWT or AAV-GSK-3βK15Q upregulated GSK-3β protein level compared with the AAV-Vector measured by Western blotting. (n = 5 for each group, one-way ANOVA, ***p < 0.001 vs Vec, bar = 50 μm). (c,d) Mice expressing GSK-3βK15Q had memory deficits shown by the decreased recognition index (c) and the discrimination index (d) recorded at 24 h after training during novel object recognition test. (n = 10 for each group, one-way ANOVA, *p < 0.05, ***p < 0.001 vs Vec, #p < 0.05 vs GSK-3βWT). (e–i) Mice expressing GSK-3βK15Q had spatial learning deficit shown by the increased latency to find the platform at days 4, 5, and 6 during Morris water maze training trial (e), and memory deficit shown by the increased latency to reach the target quadrant (f), decreased crossings in the platform site (g), and percent time in target quadrant (h) during probe trial done at day 8 by removed the platform; and the GSK-3βK15Q mice did not show difference in swimming speed (i). (n = 10 for each group, one-way ANOVA, *p < 0.05, **p < 0.01, ***p < 0.001 vs Vec, #p < 0.05, ##p < 0.01 vs GSK-3βWT). (j) The memory deficit in GSK-3βK15Q mice was also detected by fear conditioning test shown by the lowest percent of freezing time. (n = 10 for each group, one-way ANOVA, *p < 0.05, ***p < 0.001 vs Vec, #p < 0.05 vs GSK-3βWT). (k–o) Mice expressing GSK-3βK15Q showed anxiety-like behavior evidenced by the decreased sugar intake in water preference test (k), reduced entries and time in center with unchanged total moved distance in open field test (l-n), and decreased time spent in open arms during elevated plus-maze test (o). (n = 10 for each group, one-way ANOVA, *p < 0.05, **p < 0.01 vs Vec, #p < 0.05 vs GSK-3βWT). (p,q) Mice expressing GSK-3βK15Q showed significantly decreased levels of GluN2B, GluN1 and Syt with no change of GluN2A, PSD95 and Syn compared with GSK-3βWT in the hippocampus. (n = 5 for each group, one-way ANOVA, **p < 0.01, ***p < 0.001 vs Vec, #p < 0.05, ##p < 0.01 vs GSK-3βWT). (r,s) Mice expressing GSK-3βK15Q showed significant spine loss in GFP-positive neurons at hippocampal CA1. (n = 7 for each group, one-way ANOVA, ***p < 0.001 vs Vec, ##p < 0.01 vs GSK-3βWT, bar = 20 μm). (t–v) Mice expressing GSK-3βK15Q showed synaptic dysfunction demonstrated by the decreased input–output curve (t) and the decreased fEPSP slope induced by applying 3 trains of high-frequency stimulation (HFS) (u,v). (n = 5 mice for each group, one-way ANOVA, ***p < 0.001 vs Vec, ##p < 0.01, ###p < 0.001 vs GSK-3βWT). Data were presented as mean ± SEM.