Summary
Background
Diabetes mellitus (DM) is associated with different clinical complications. The aim of this study was to explore the prevalence of RLS in people with diabetes mellitus and compare the risk of restless leg syndrome (RLS) between diabetic and non-diabetic population.
Methods
We searched for studies of RLS prevalence in DM through PubMed, Embase, and Web of Science. Two authors independently completed the literature screening, data extraction, and bias risk assessment of eligible studies. All observational studies that assessed the prevalence or risk of RLS in DM were included, where the diagnosis of RLS was based on the International Restless Legs Syndrome Study Group (IRLSSG). Percentages, odds ratio (OR) with 95% confidence intervals (CI) were used to assess pooled estimates of RLS prevalence and risk based on random-effects models. Newcastle-Ottawa-scale (NOS) or a modified NOS were used to evaluate the quality of studies.
Findings
A total of 42 studies, including 835,986 participants, met the eligibility criteria for the meta-analysis. Among them, 30 studies were included in meta-analysis to analyze the prevalence of RLS. A second meta-analysis was conducted using 31 studies to determine RLS risk between diabetes and non-diabetes. The results indicate that between 25% (95% confidence interval 21%-29%) of people with diabetes showed signs of RLS, and people with diabetes had an increased risk of developing RLS compare to people without diabetes (OR 1.98, 95%CI 1.66- 2.34, p < 0.001). However, the available evidence was limited due to potential risk of bias and variability between studies (I2>75%), all of observational design.
Interpretation
Our study suggests that the prevalence and risk of RLS might be higher in DM patients than in non-diabetes population. However, given limitations in the analysis and study design, the findings need to be corroborated in future studies.
Funding
This work was supported by the Basic Conditions Platform Construction Project of Sichuan Science and Technology Department (2019JDPT0015), and the “1・3・5 project for disciplines of excellence, West China Hospital, Sichuan University” (ZYJC18003).
Keywords: Diabetes mellitus, Diabetes, Restless legs syndrome, Diabetic peripheral neuropathy, Meta-analysis
Research in context.
Evidence before this study
Restless legs syndrome (RLS) is associated with several comorbidities, including diabetes. However, a deeper understanding of its prevalence and management is needed. We searched the PubMed, Embase, and Web of Science databases without language restriction. The last search was conducted on January 29, 2022 using terms “RLS”, “diabetes” and related. Studies were included if they focused on describing the prevalence and risk of RLS in diabetes.
Added value of this study
Our results provide to the best of our knowledge the first pooled estimate for RLS prevalence in diabetes mellitus, strengthening previous findings related to the higher risk of RLS among people with diabetes mellitus.
Implications of all the available evidence
This meta-analysis could be useful to provide more detailed strategies for screening and clinical management of diabetic patients with RLS. Given the potential risk of bias and large heterogeneity observed, more in-depth studies are urgently needed to explore the relationship between these two diseases and the pathological mechanisms of disease.
Alt-text: Unlabelled box
Introduction
Diabetes mellitus (DM) remains the emerging public health problem across the world.1,2 Restless legs syndrome (RLS) is a common neurological sensorimotor disorder characterized by uncomfortable sensations in the extremities and an overwhelming urge to move one's legs, especially in the evening and during periods of inactivity.3,4 First mentioned in 1672 by Willis, RLS was clinically described in the early 1940s by Ekbom, who coined and published the term “restless legs syndrome”.5 As a consequence, the disease has also been called “Willis-Ekbom disease”. The four minimal diagnostic criteria for RLS of the International Restless Legs Study Group (IRLSSG) were6: 1. Desire to move the limbs usually associated with paresthesias/dysesthesias. 2. Motor restlessness. 3. Symptoms are worse or exclusively present at rest (i.e. lying, sitting) with at least partial and temporary relief by activity. 4. Symptoms are worse in evening/night.
Although the pathophysiological pathways leading to RLS are still unknown, brain iron deficiency and dysfunction of the dopaminergic system appear to play a role.7 RLS can be secondary to various medical conditions, such iron deficiency, rheumatoid arthritis, end-stage renal disease, obesity, and some physiological states such as pregnancy.8, 9, 10, 11 Previous meta-analysis have shown that RLS exists in the comorbidities of a series of diseases, such as Parkinson's disease,12 migraine,13 and multiple sclerosis.9 We noticed that RLS also often exists as a comorbidity in diabetic patients.14 However, the relationship between RLS and DM has not been systematically reviewed in depth with a large sample size throughout various regions. Thereby, we performed a meta-analysis of the current observational literature in order to understand the prevalence of RLS in patients with DM.
Methods
Described as previously reported methodology,15,16 this meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.17 This meta-analysis has been registered on the INPLASY website with the registration number of INPLASY 202,150,012.
Search strategy
We searched the available literature in PubMed, Web of Science, and Embase databases. The search was limited to articles in English. The final search was conducted on January 29, 2022. The search string was (‘restless legs syndrome’, ‘Willis-Ekbom disease’, ‘RLS’) and (‘diabetes mellitus’, ‘diabetes’). The detailed strategy for searching in databases was: (((restless legs syndrome) OR (Willis-Ekbom disease)) OR (RLS)) AND ((diabetes mellitus) OR (diabetes)).
Study selection criteria
The inclusion criteria include (1) observational studies with cross-sectional, case-control or cohort designs analyzing restless legs syndrome (RLS) in diabetes mellitus patients; (2) RLS was diagnosed according to the criteria of the International Restless Legs Syndrome Study Group (IRLSSG)18; (3) reporting point prevalence of RLS or sufficient data to calculate it; (4) reporting zero prevalence among patients with DM but still meet the three inclusion criteria above.
Studies were excluded if they (1) were editorials, narrative reviews, case reports, letters, commentaries, or critiques; (2) did not report sufficient data to calculate the incidence of RLS in diabetes mellitus patients and non-diabetic population, and efforts to contact the authors were unsuccessful; (3) or did not involve patients with diabetes mellitus or RLS.
Data extraction
Two authors (NP, MX) independently assessed articles for inclusion/exclusion criteria, and discrepancies were resolved by discussion with a third reviewer (Xinglong Yang). The following data were extracted from each study: surname of the first author, publication year, country, study design, sample size, mean age, DM duration, diagnostic criteria for RLS, method of RLS evaluation, RLS prevalence in DM patients and non-diabetic population and relevant odds ratios (ORs) and 95% confidence intervals (CIs). Firstly, we gave priority to the OR value obtained through multivariate analysis, or the OR value comes from propensity score matching (PSM), which effectively adjusted for the effect of confounding factors by methods such as weighting, stratification, or regression correction. Secondly, if a separate cohort from the entire study population eliminating confounding factors artificially by the author (including age, gender, race, anemia, uremia, ferritin deficiency, peripheral neuropathy, etc.), we used the OR value calculated by a separate cohort. Finally, in the entire queue, we used the raw data to calculate the OR value based on 2 × 2 tables. If a study reported longitudinal data on RLS incidence in diabetic patients, only baseline data were extracted.
Statistical analysis
The meta-analysis was performed using STATA version 12.0 (StataCorp, College Station, TX, USA). We evaluated RLS prevalence in DM patients according to ethnicity and sex based on the DerSimonian and Laird method for random-effects meta-analysis.19 RLS prevalence was compared between DM patients and non-diabetic individuals in terms of OR and 95% CI. p < 0.05 was considered statistically significant in all analyses.
Heterogeneity among the included studies was evaluated using the Q test and quantified using I2. An I2 value below 25% was considered as homogeneity; 25% to < 50%, low heterogeneity; 50% to < 75%, moderate heterogeneity; and at least 75%, substantial heterogeneity.20 Publication bias was assessed using Egger's and/or Begg's tests,21 and funnel plots were used to visually assess publication bias of included studies. Sensitivity analysis was used to assess the overall robustness of the included studies. Newcastle-Ottawa-scale (NOS) or a modified NOS22 was used to assess the quality and risk of bias of each study (Supplementary Table 1).
Role of funding sources
The funders of the study had no role in the study design, data collection, data analysis, data interpretation or writing of the report. The corresponding author had full access to all the data in the study and final responsibility for the decision to submit for publication.
Results
Study selection
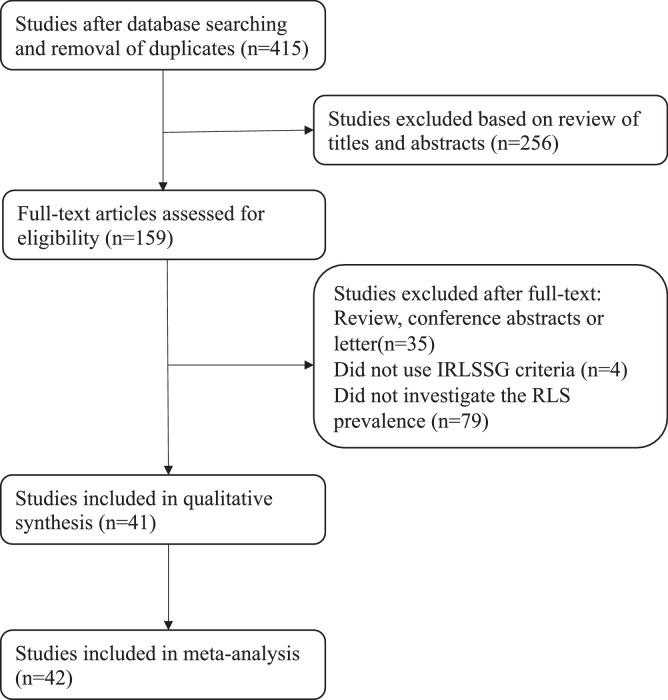
A total of 415 potentially eligible articles were identified after searching the three databases and removing duplicates (Figure 1). After eliminating 256 articles based on the title and abstract, the remaining 159 were read in full and 118 were excluded because they did not use IRLSSG diagnostic criteria (n = 4), they did not investigate RLS prevalence (n = 79), or they were conference abstracts or letters (n = 35). In the end, 41 publications containing 42 studies met all the eligibility criteria and were included in the meta-analysis.14,23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62
Figure 1.
Flow diagram of literature search. Initially, we maintain 415 results after removing duplicates. We excluded 256 literatures based on review of titles and abstract. We excluded 117 literatures based on review of full text. Eventually, 42 studies were included in this meta-analysis. IRLSSG, International Restless Legs Syndrome Study Group.
Study characteristics
The characteristics of the 42 included studies, involving 835,986 DM patients, are summarized in Table 1. All studies were observational and were published between 2001 and 2020. Sample sizes ranged from 19 to 825,442. Of the 42 studies, 18 were from Asia14,23, 24, 25,28,36,37,43,46,47,50,51,53,55,56,60, 61, 62 and 24 were from Europe and the Americas.26,27,29, 30, 31, 32, 33, 34, 35,38, 39, 40, 41, 42,44,45,48,49,52,54,57, 58, 59,63 Seven studies had a case-control design,28,40,48,50,52,56,62,63 32 had a cross-sectional design,14,23, 24, 25, 26, 27,29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39,41, 42, 43,45, 46, 47,49,51,53,55,57, 58, 59, 60, 61 and three had a cohort design.44,54
Table 1.
Characteristics of the studies included in the meta-analysis.
| Sample size |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | Year | Country | Study design | Age (years) | RLS assessment method | IRLSSG criteria | DM (M/F) | RLS (+)/ RLS (-) | Males with RLS | RLS in DM (%) | RLS in ND (%) | OR (95%CI) |
| Skomro | 2001 | Canada | Case-control | 57.5 ± 14.85 | NA | Yes | 58 (29/29) | 14/44 | 8/29 | 24.1 | 12.5 | 2.227 (0.783–6.337) |
| Happe | 2005 | Germany | Cross-sectional | 12.0 ± 3.7 | Questionnaire and interview |
Yes | 46 (21/25) | 1/45 | NA | 22.0 | NA | NA |
| Lopes | 2005 | Brazil | Cross-sectional | 58.3 ± 12.3 | NA | Yes | 100 | 27/73 | NA | 27.0 | NA | NA |
| Gemignani | 2007 | Italy | Cross-sectional | 65.4 ± 10.5 | NA | Yes | 82 | 26/56 | NA | 31.7 | NA | NA |
| Merlino | 2007 | Italy | Case-control | 65.1 ± 8.6 | Interview | Yes | 124 | 22/102 | NA | 17.7 | 5.70 | 4.65 (1.07–20.17) |
| Minai | 2007 | USA | Cross-sectional | 46.62 ± 15.36 | Questionnaire | Yes | 19 | 11/8 | NA | 57.9 | 39.1 | 2.139 (0.621–7.37) |
| Ulfberg | 2007 | Sweden | Cross-sectional | NA | Questionnaire | Yes | 52 | 6/46 | NA | 11.5 | 4.6 | 2.677 (1.085–6.603) |
| Wesstrom | 2008 | Sweden | Cross-sectional | NA | Questionnaire | Yes | 79 | 16/63 | NA | 20.2 | 15.6 | 1.37 (0.786–2.391) |
| Winkelman | 2008 | USA | Cross-sectional | 67.9 ± 10.2 | Questionnaire | Yes | 443 | 26/417 | NA | 5.9 | 5.1 | 1.156 (0.753–1.774) |
| Al-Jahdli | 2009 | Saudi Arabia | Cross-sectional | 55.7 ± 17.2 | Questionnaire | Yes | 119 | 68/51 | NA | 57.1 | 42.6 | 1.797 (1.061–3.043) |
| Juuti | 2009 | Finland | Cross-sectional | NA | Questionnaire | Yes | 88 | 42/46 | NA | 47.7 | 48.7 | 0.67 (0.31–1.47) |
| Tasdemir | 2009 | Turkey | Cross-sectional | 38.57 ± 15.42 | Questionnaire | Yes | 61 | 10/51 | NA | 16.4 | 3.0 | 6.278 (3.05–12.961) |
| Merlino | 2010 | Italy | Cross-sectional | 65.1 ± 8.6 | Interview | Yes | 124 | 22/102 | NA | 17.7 | NA | NA |
| Li | 2011 | China | Cross-sectional | 39.0 ± 14.8 | Questionnaire | Yes | 51 | 21/30 | NA | 41.2 | 6.4 | 10.254 (5.712–18.407) |
| Plantinga | 2012 | USA | Cross-sectional | 58.9 (57.7–60.1) | Questionnaire | No | 1424 | NA | NA | NA | NA | 1.40 (1.12–1.78) |
| Cho | 2013 | South Korea | Case-control | 62.91 ± 10.95 | Interview | Yes | 199 (92/107) | 16/183 | 7/92 | 8.0 | 3.6 | 2.56 (1.030–6.363) |
| Giannini | 2013 | Italy | Cross-sectional | 46.27 ± 16.25 | Interview | Yes | 45 | 6/39 | NA | 13.3 | 9.9 | 1.407 (0.587–3.374) |
| Lin | 2013 | China | Cross-sectional | 61.9 ± 12.6 | Questionnaire | Yes | 394 | 128/226 | NA | 32.5 | 21.5 | 3.61 (2.27–5.77) |
| Medeiros | 2013 | Brazil | Cross-sectional | 57.59 ± 11.04 | Questionnaire | Yes | 110 (38/72) | 16/94 | NA | 14.5 | NA | NA |
| Szentkiralyi (DHS) | 2013 | Germany | Cohort | 52.1 ± 13.8 | Interview | Yes | 101 | NA | NA | NA | NA | 1.57 (0.75–3.30) |
| Szentkiralyi (HSP) | 2013 | Germany | Cohort | 50.3 ± 16.4 | Interview | Yes | 349 | NA | NA | NA | NA | 1.89 (1.18–3.03) |
| Winter (W) | 2013 | France | Cross-sectional | 63.6 ± 6.9 | Questionnaire | Yes | 2230 | 340/1890 | NA | 15.2 | 11.7 | 1.19 (1.04–1.35) |
| Winter (M) | 2013 | France | Cross-sectional | 68.4 ± 9.05 | Questionnaire | Yes | 1970 | 217/1753 | NA | 11.0 | 7.2 | 1.41 (1.21–1.65) |
| Harashima | 2014 | Japan | Cross-sectional | 65.0 ± 11.2 | Questionnaire | Yes | 100 | 8/92 | NA | 8.0 | NA | NA |
| Rohani | 2014 | Iran | Cross-sectional | 61.3 ± 13.3 | Interview | Yes | 85 | 34/51 | NA | 40.0 | 34.6 | 1.259 (0.666–2.381) |
| Zobeiri | 2014 | Iran | Case-control | 46.3 ± 13.93 | Questionnaire | Yes | 140 (80/60) | 40/100 | NA | 28.6 | 7.1 | 5.20 (2.48–10.90) |
| Innes | 2015 | USA | Cross-sectional | 56.96 ± 0.49 | Questionnaire | Yes | 103 | 37/66 | NA | 35.9 | 21.5 | 2.045 (1.28–3.267) |
| Metta | 2015 | India | Cross-sectional | 51.6 ± 11.9 | NA | Yes | 100 | 17/83 | NA | 17.0 | NA | NA |
| Yildiz | 2015 | Turkey | Case-control | 50.6 ± 14.1 | Questionnaire | Yes | 27 | 15/12 | NA | 55.6 | 30.2 | 4.1 (1.2–13.7) |
| Sabic | 2016 | Bosnia and Herzegovina |
Case-control | 48.43 ± 15.37 | Questionnaire | Yes | 30 (9/21) | 7/23 | NA | 23.3 | 13.3 | 1.978 (0.513–7.635) |
| Safak | 2016 | Turkey | Cross-sectional | 71.59 ± 5.68 | Interview | Yes | 160 | 44/116 | NA | 27.5 | 12.0 | 2.68 (1.612–4.454) |
| Molnar | 2016 | USA | Cohort | 59.8 ± 14.3 | Interview | Yes | 825,442 | NA | NA | NA | NA | 1.22 (1.13–1.32) |
| Coronel | 2017 | Ecuador | Cross-sectional | 64.08 (51.99–76.17) | Questionnaire | Yes | 290 (207/83) | 134/156 | NA | 46.2 | NA | NA |
| Castillo-Torres | 2018 | Mexico | Cross-sectional | 47.85 ± 16.55 | Interview | Yes | 59 | 11/48 | NA | 18.6 | 17.4 | 1.089 (0.398–2.975) |
| Cuellar | 2018 | USA | Cross-sectional | 59.5 ± 11.6 | NA | Yes | 121 | 54/67 | NA | 44.6 | NA | NA |
| Modarresnia | 2018 | Iran | Cross-sectional | 54.89 ± 7.81 | Questionnaire | Yes | 210 (83/127) | 41/169 | NA | 19.5 | NA | NA |
| Tuo | 2018 | China | Case-control | 65.0 ± 19.0 | Questionnaire | Yes | 90 | 9/81 | NA | 10.0 | 3.1 | 3.611 (1.077–12.11) |
| Akin | 2018 | Turkey | Cross-sectional | 60.9 ± 10.3 | Interview | Yes | 318 (126/192) | 90/228 | 26/126 | 28.3 | NA | NA |
| Bhagawati | 2019 | India | Cross-sectional | 46.55 ± 15.38 | Questionnaire | Yes | 72 | 21/51 | NA | 29.2 | 17.1 | 1.995 (1.08–3.687) |
| Rafie | 2019 | Iran | Cross-sectional | 53.8 ± 12.19 | Interview | Yes | 44 | 22/22 | NA | 50.0 | 30.1 | 2.321 (1.109–4.859) |
| Sunwoo | 2019 | South Korea | Cross-sectional | 44.5 ± 15.0 | Questionnaire | Yes | 135 | 7/128 | NA | 5.2 | 2.3 | 2.281 (1.023–5.086) |
| Pinheiro | 2020 | India | Cross-sectional | 56 ± 13.5 | NA | Yes | 210 (139/71) | 17/193 | 13 | NA | NA | NA |
Abbreviations: IRLSSG, International Restless Legs Syndrome Study Group; DM, diabetes mellitus; M, males; F, females; RLS, restless legs syndrome; OR, odds ratio; CI, confidence interval; NA, not available; ND, non-diabetic population.
Risk of bias
Each study was given a Newcastle-Ottawa Scale quality score.64 The detailed scoring of each domain is in the Supplementary Table 1, and the quality score of “fair” or “good” is displayed. (Supplementary Table 1)
Overall analysis
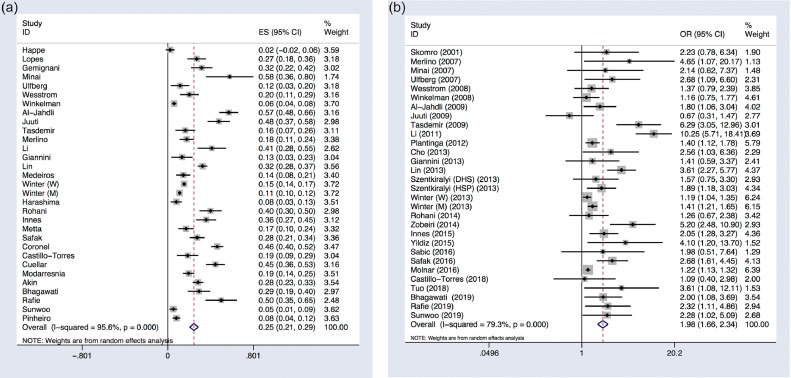
Because of the high heterogeneity observed across thirty-one studies including RLS frequency in diabetes (I2 = 95.6%, P < 0.001), the random-effect DerSimonian and Laird method was applied to analyze the prevalence of RLS among DM patients. The pooled prevalence of RLS among DM patients was 25% (95% CI 21%−29%; p < 0.001) (Table 2; Figure 2A).
Table 2.
Meta-analysis of the prevalence of restless legs syndrome (RLS) in diabetes mellitus (DM) and the risk of RLS in patients with DM.
| Outcome measures | Number of studies | Sample size | Heterogeneity (I2, P) | Model | Estimates with 95%CI | p-value | Conclusion |
|---|---|---|---|---|---|---|---|
| Overall analyses | |||||||
| Prevalence of RLS in DM | 31 | 8020 | I2 = 95.6%, P < 0.001 | Random | 25% (21%−29%) | < 0.001 | Significant |
| Comparison of RLS prevalence between DM patients and non-diabetic population | 30 | 834,193 | I2 = 79.3%, P < 0.001 | Random | 1.98 (1.66–2.34) | < 0.001 | Significant |
| Subgroup analyses of the prevalence of RLS in DM | |||||||
| Subgroups stratified by ethnicity | |||||||
| Asian | 14 | 2059 | I2 = 95.2%, P < 0.001 | Random | 26% (19–34%) | < 0.001 | Significant |
| non-Asian | 17 | 5961 | I2 = 95.7%, P < 0.001 | Random | 23% (18%−28%) | ||
| Subgroups stratified by sex | |||||||
| Male | 2 | 528 | I2 = 99.2%, P < 0.001 | Random | 49% (0%−103%) | 0.004 | Significant |
| Female | 2 | 528 | I2 = 60.7%, P = 0.111 | Random | 29% (20%−39%) | ||
| Subgroup analyses of the comparison of RLS prevalence between DM patients and non-diabetic population | |||||||
| Subgroups stratified by population | |||||||
| Asian | 13 | 1577 | I2 = 68.2%, P < 0.001 | Random | 3.10 (2.21–4.35) | < 0.001 | Significant |
| non-Asian | 17 | 832, 616 | I2 = 28.0%, P = 0.136 | Random | 1.34 (1.21–1.48) | ||
| Subgroups stratified by methods | |||||||
| Interview | 10 | 826, 608 | I2 = 55.7%, P = 0.016 | Random | 1.71 (1.30–2.25) | < 0.001 | Significant |
| Questionnaire | 19 | 7527 | I2 = 83.7%, P < 0.001 | Random | 2.16 (1.68–2.78) | ||
| Subgroups stratified by OR extraction | <0.001 | Significant | |||||
| Reported | 12 | 832,850 | I2 = 76.1%, P < 0.001 | Random | 1.59 (1.33–1.90) | ||
| DDE | 18 | 1685 | I2 = 71.1%, P < 0.001 | Random | 2.31 (1.68–3.16) | ||
| Subgroups stratified according to whether the sample size is greater than ten thousand | |||||||
| More than ten thousand | 1 | 825,442 | Random | 1.22 (1.13–1.32) | < 0.001 | Significant | |
| Less than ten thousand | 29 | 8751 | I2 = 77.1%, P < 0.001 | Random | 2.07 (1.70–2.53) |
Abbreviations: CI, confidence interval; RLS, restless legs syndrome; DM, diabetes mellitus; DDE, demographic data extrapolated.
Figure 2.
Forest plots of overall analysis. (A) Forest plot of the prevalence of restless leg syndrome (RLS) among patients with diabetes mellitus (DM). (B) Forest plot comparing the prevalence of restless leg syndrome (RLS) between diabetes mellitus (DM) patients and non-diabetic population. Each study corresponds to a line segment parallel to the X-axis; the black dots represent the estimated value of the effect of each study; the square represents the weight of each research, the larger the weight, the larger the area of the square; the line segment represents the 95% confidence interval of the effect size of each study; the diamond represents the summary results of the Meta-analysis synthesis of each study; the center of the diamond represents the point estimate of the summary result effect size, and is marked with a dashed line perpendicular to the X axis; the width of the diamond represents the summary result effect size 95%CI. X = 0 is invalid line, which means no statistical significance if 95% CI intersect it.
For the comparison of RLS prevalence between DM patients and non-diabetic population, the random-effects model was again adopted because of the significant heterogeneity (I2 = 79.3%, P < 0.001). The pooled data revealed that patients with diabetes had a higher risk of RLS (OR 1.98, 95% CI 1.66–2.34; p < 0.001) than non-diabetic population (Table 2; Figure 2B).
Subgroup analysis
Prevalence of RLS among DM patients
In subgroups divided by ethnicity, 14 studies included Asian patients and 17 studies included non-Asian patients. The pooled estimates showed that the prevalence of RLS among diabetic patients was higher in Asia (26%, 95% CI 19%−34%; P < 0.001; I2 = 95.2%, p < 0.001) than outside Asia (23%, 95% CI 18%−28%; P < 0.001; I2 = 95.7%, p < 0.001). (Table 2, Supplementary Figure 1)
Two studies evaluated the prevalence of RLS in both male and female diabetes patients. The prevalence of RLS was higher in male patients (49%, 95% CI 0%−103%; P < 0.001; I2 = 99.2%, p < 0.001) than in female patients (29%, 95% CI 20%−39%; P = 0.001; I2 = 60.7%, p = 0.004) (Table 2, Supplementary Figure 2).
Comparison of RLS prevalence between DM patients and non-diabetic population
In subgroups classified by populations, 13 studies were performed in Asians23,25,28,36,37,46,47,50,51,53,55,56,62 and 17 in non-Asians.26,27,32,34,35,40,42,44,45,48,49,52,54,57, 58, 59 Risk of RLS was higher among diabetic patients than in non-diabetic population, and the risk was higher in Asian populations (OR 3.10, 95% CI 2.21 - 4.35; P < 0.001; I2 = 68.2%, p < 0.001) than in non-Asian populations (OR 1.34, 95% CI 1.22 – 1.48; P = 0.136; I2 = 28%, p < 0.001) (Table 2, Supplementary Figure 3).
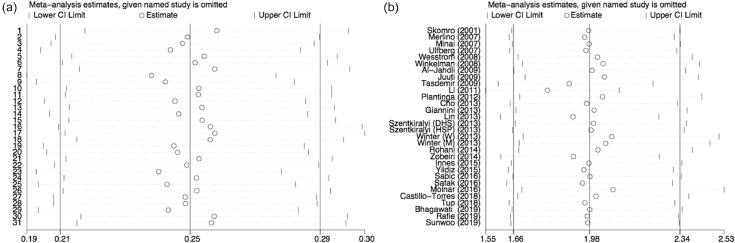
Figure 3.
Sensitivity analysis. (A) Sensitivity analysis of the overall prevalence of restless leg syndrome (RLS) in diabetes mellitus (DM). (B) Sensitivity analysis of the risk of RLS in patients with DM. The sphere represents the combined estimate of the meta-analysis after omitting a particular study. It can be roughly seen that the balls fall in the middle of the two short vertical lines, which means that the overall stability is good.
Next we stratified the studies based on the method of RLS assessment. There are two common methods for RLS assessment. One is to issue questionnaires to participants, and they conduct self-assessment. The other is that doctors conduct face-to-face interviews with participants.65,66 Except for six studies that cannot obtain RLS evaluation methods from the text. Ten studies based on interviews27,28,32,40,44,46,47,51,54 showed that the risk of RLS was higher in diabetic patients than in non-diabetic population (OR 1.71, 95% CI 1.30–2.25; P = 0.016; I2 = 55.7%, p < 0.001). Similarly, 19 studies based on questionnaires23,25,26,34, 35, 36, 37,42,45,48, 49, 50,53,55, 56, 57, 58, 59,62 also showed that higher risk of RLS in diabetic patients than in non-diabetic population (OR 2.16, 95% CI 1.68–2.78; P < 0.001; I2 = 83.7%, p < 0.001). (Table 2, Supplementary Figure 4)
We conduct subgroup analysis according to the extraction method of OR value. Whether it is a statistical analysis of OR values reported in the direct literature (OR 1.59, 95% CI 1.33–1.90; P < 0.001; I2 = 76.1%, p < 0.001) or a statistical analysis of OR values that we extrapolated from demographic data (OR 2.31, 95% CI 1.68–3.16; P < 0.001; I2 = 71.1%, p < 0.001), they are consistent with the overall results. (Table 2, Supplementary Figure 5)
We also conduct subgroup analysis of the study based on whether the sample size is greater than 10,000. The results show that the results of the sample size greater than 10,000 (OR 1.22, 95% CI 1.13–1.32; p < 0.001) and the results of the sample size less than 10,000 (OR 2.07, 95% CI 1.65–2.4; P < 0.001; I2 = 77.1%, p < 0.001) are consistent with the overall research results (Table 2, Supplementary Figure 6).
Sensitivity analysis
We performed a sensitivity analysis by omitting the individual studies one at a time to test the robustness of our findings. We found that the sequential removal of each study did not alter the results of primary overall analyses (Figure 3A and B).
Publication bias
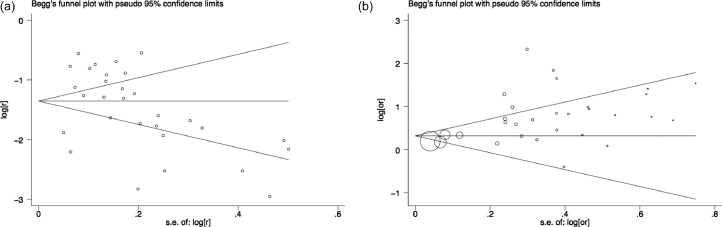
Begg's funnel plots estimating the publication bias in the included studies analyzing risk of RLS in DM patients (Figure 4A) and in included studies comparing RLS risk between patients and the non-diabetic population (Figure 4B) appeared symmetrical with respective P values of 0.007 and 0.276. Egger's test showed that the P values are 0.834 and 0.049, respectively. The funnel plots seen by the naked eye showed that a potential publication bias exists in our research.
Figure 4.
Funnel plots. (A) Funnel plot to assess bias in the overall prevalence of restless leg syndrome (RLS) in diabetes mellitus (DM). (B) Funnel plot to assess bias in the comparison of RLS prevalence between DM patients and non-diabetic population. The naked eye can see that not all the balls fall in the middle of the funnel, suggesting that there may be potential bias.
Discussion
Conclusion was reached from our analysis of 31 studies involving 8020 individuals, namely, a pooled RLS prevalence (based on IRLSSG diagnostic criteria) of 25% among DM patients, much higher than the prevalence of 5–10% reported in the general population.67 The prevalence appears to be slightly whereas significantly higher in Asian countries (26%) than non-Asian countries (23%). Moreover, the prevalence of RLS in male patients was higher than in female patients (49% vs. 29%). In addition, results were found that nearly two-fold higher risk of RLS in diabetic patients than in non-diabetic population (OR 1.98, 95% CI 1.66–2.34; p < 0.001). This increased risk was stable across ethnic subgroup, OR extraction subgroup, and sample size subgroup.
While the reasons behind an association between DM and RLS remain unclear, we speculate that the association may be explained by one or more of the following pathophysiological mechanisms. Up to 96% of patients with RLS and type 2 diabetes have diabetic neuropathy,68 and type 2 diabetic patients are at 7.9-fold higher risk of RLS if they also have diabetic neuropathy.40 Abnormal peripheral inputs due to small-fiber neuropathy associated with diabetes can trigger spinal generators underlying RLS phenomena.31 Indeed, results from rats were found that show reduced dopamine content in the striatum and midbrain, which are important parts of RLS circuitry.69 Additionally, individuals with diabetes are at increased risk of sleep disorders, particularly excessive daytime sleepiness,29 and RLS patients are with significantly higher levels of hemoglobin A1C and fasting plasma glucose than healthy controls.70
Results were gotten that the prevalence of RLS was higher in Asian diabetic patients than in non-Asian patients, which is contrary to the previous higher risk of RLS in multiple sclerosis patients and Parkinson's disease in non-Asian populations. This change may reflect the difference in RLS assessment methods, that is, the threshold used to determine whether symptoms occur frequently to ensure the diagnosis of RLS.71 The differences in patient's age, disease severity and peripheral nerve involvement also contribute to the difference. In addition, results were also reported that differential expression of genes related to DM risk among various ethnic groups.72 Asians may be less sensitive to insulin than non-Asians, potentially leaving them more susceptible to complications related to DM.73 Results from our work were found namely, RLS prevalence was higher among male patients than among female patients, according to two of 31 studies that reported sex information. This contrasts with the higher frequency of women with RLS in other comorbidities, such as Parkinson's disease and multiple sclerosis. This may be related to the different comorbidities of the patients and the limited sample size we included. The underlying mechanism needs further study.
RLS is often accompanied by many diseases, such as neurological diseases, sleep-related diseases and so on. Because many patients have mild symptoms, it is difficult to identify.
Our meta-analysis presents several limitations. First, the retrospective nature of the study meant that data on several potentially important variables were missing. Second, moderate statistical heterogeneity and potential bias was showed. This might be explained by differences in demographic characteristics and study designs. Indeed, studies differed in the method of RLS assessment, the threshold of symptom frequency for diagnosing RLS, the age of patients, as well as the severity and duration of DM, all of which may have confounded our analyses. Nevertheless, our sensitivity analysis suggested robust overall findings. Third, most studies includes both type 1 DM (T1DM) and type 2 DM (T2DM), which caused the difficulty to analyze the diabetes mellitus type. Fourth, only articles in English were included, which may cause some selection bias. Additional eligible studies may be identified by including other languages. Fifth, although we performed some subgroups analysis and showed stable results. Our research will still be affected by some untreated confounding factors. Sixth, the 825,442 subjects came from one study among the 835,986 subjects included in the meta-analysis, whereas the process of inclusion/exclusion is in conformity with the specification of Cochrane Collaboration. Thereby, final number of included studies are adhered to statistical methodology and we have added discussion in related parts. However, it needs to be considered that this paper may have a disproportionate weight during the analysis process, which may affect the results. In addition, although the studies we included are based on the criteria of the International Restless Legs Syndrome Research Study Group (IRLSSG), the significance of polysomnography for restless legs syndrome (RLS) should still be considered. Our results need to be verified in future large prospective studies due to the lack of sleep physiology examination. Also, the impact of diabetes treatment on the prevalence of restless legs syndrome should also be considered in future studies.
In conclusion, to the best of our knowledge this is the first pooled estimate for RLS prevalence in DM, strengthening previous suggestions that RLS risk is higher among DM patients than among non-diabetic population. However, more studies are needed to determine whether this is true for both types of diabetes. The available evidence justifies further exploration of the potential link between RLS and DM and efforts to develop effective treatments against both disorders.
Data sharing statement
This meta-analysis of secondary analysis of raw data from published original articles. All the data used for the study are included in the manuscript and supplementary material.
Author contributions
Conceptualization: NP, MX, YXL, XYM, LT; Date curation: NP, MX, YXL; Formal analysis: NPP, MX, YX; Funding acquisition: XY; Investigation: MX, YXL; Methodology: NP; Project administration: XY, LT; Resources: NP; Software: NP; Xin Mu; YXL; Supervision: XY; Validation: XY; Visualization: NP; XY; Writing-original draft preparation: NP, MX; Writing-review and editing: YXL, XYM, LT.
Funding
This work was supported by the Basic Conditions Platform Construction Project of Sichuan Science and Technology Department (2019JDPT0015), and the “1・3・5 project for disciplines of excellence, West China Hospital, Sichuan University” (ZYJC18003).
Declaration of interests
The authors declare that they have no potential conflicts of interest.
Acknowledgments
None.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2022.101357.
Contributor Information
Tian Li, Email: tian@fmmu.edu.cn.
Yanming Xu, Email: neuroxym999@163.com.
Appendix. Supplementary materials
References
- 1.Delanerolle G., Phiri P., Zeng Y., et al. A systematic review and meta-analysis of gestational diabetes mellitus and mental health among BAME populations. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li T., Providencia R., Mu N., et al. Association of metformin monotherapy or combined therapy with cardiovascular risks in patients with type 2 diabetes mellitus. Cardiovasc Diabetol. 2021;20(1):30. doi: 10.1186/s12933-020-01202-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burman D. Sleep disorders: restless legs syndrome. FP Essent. 2017;460:29–32. [PubMed] [Google Scholar]
- 4.Allen R.P., Picchietti D., Hening W.A., et al. Restless legs syndrome: diagnostic criteria, special considerations, and epidemiology. A report from the restless legs syndrome diagnosis and epidemiology workshop at the National Institutes of Health. Sleep Med. 2003;4(2):101–119. doi: 10.1016/s1389-9457(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 5.Odin P., Mrowka M., Shing M. Restless legs syndrome. Eur J Neurol. 2002;9(Suppl 3):59–67. doi: 10.1046/j.1468-1331.9.s3.8.x. [DOI] [PubMed] [Google Scholar]
- 6.Allen R.P., Picchietti D.L., Garcia-Borreguero D., et al. Restless legs syndrome/Willis-Ekbom disease diagnostic criteria: updated International Restless Legs Syndrome Study Group (IRLSSG) consensus criteria–history, rationale, description, and significance. Sleep Med. 2014;15(8):860–873. doi: 10.1016/j.sleep.2014.03.025. [DOI] [PubMed] [Google Scholar]
- 7.Allen R. Dopamine and iron in the pathophysiology of restless legs syndrome (RLS) Sleep Med. 2004;5(4):385–391. doi: 10.1016/j.sleep.2004.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Bogan R.K., Cheray J.A. Restless legs syndrome: a review of diagnosis and management in primary care. Postgrad Med. 2013;125(3):99–111. doi: 10.3810/pgm.2013.05.2636. [DOI] [PubMed] [Google Scholar]
- 9.Ning P., Hu F., Yang B., et al. Systematic review and meta-analysis of observational studies to understand the prevalence of restless legs syndrome in multiple sclerosis: an update. Sleep Med. 2018;50:97–104. doi: 10.1016/j.sleep.2018.05.039. [DOI] [PubMed] [Google Scholar]
- 10.Trenkwalder C., Paulus W., Walters A.S. The restless legs syndrome. Lancet Neurol. 2005;4(8):465–475. doi: 10.1016/S1474-4422(05)70139-3. [DOI] [PubMed] [Google Scholar]
- 11.Michopoulos I., Ferentinos P., Oulis P., Gournellis R. Restless legs syndrome associated with the combined use of quetiapine and venlafaxine. J Clin Psychopharmacol. 2014;34(1):159–161. doi: 10.1097/JCP.0b013e3182a95af2. [DOI] [PubMed] [Google Scholar]
- 12.Yang X., Liu B., Shen H., et al. Prevalence of restless legs syndrome in Parkinson's disease: a systematic review and meta-analysis of observational studies. Sleep Med. 2018;43:40–46. doi: 10.1016/j.sleep.2017.11.1146. [DOI] [PubMed] [Google Scholar]
- 13.Yang X., Liu B., Yang B., et al. Prevalence of restless legs syndrome in individuals with migraine: a systematic review and meta-analysis of observational studies. Neurol Sci. 2018;39(11):1927–1934. doi: 10.1007/s10072-018-3527-7. [DOI] [PubMed] [Google Scholar]
- 14.Harashima S., Nishimura A., Osugi T., et al. Restless legs syndrome in patients with type 2 diabetes: effectiveness of pramipexole therapy. BMJ Support Palliat Care. 2016;6(1):89–93. doi: 10.1136/bmjspcare-2014-000691. [DOI] [PubMed] [Google Scholar]
- 15.Zhang F., Wang K., Du P., et al. Risk of stroke in cancer survivors: a meta-analysis of population-based cohort studies. Neurology. 2021;96(4):e513–ee26. doi: 10.1212/WNL.0000000000011264. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F., Liu L., Zhang C., Ji S., Mei Z., Li T. Association of metabolic syndrome and its components with risk of stroke recurrence and mortality: a meta-analysis. Neurology. 2021;97(7):e695–e705. doi: 10.1212/WNL.0000000000012415. [DOI] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walters A.S. Toward a better definition of the restless legs syndrome. The international restless legs syndrome study group. Mov Disord. 1995;10(5):634–642. doi: 10.1002/mds.870100517. [DOI] [PubMed] [Google Scholar]
- 19.DerSimonian R., Laird N. Meta-analysis in clinical trials revisited. Contemp Clin Trials. 2015;45:139–145. doi: 10.1016/j.cct.2015.09.002. Pt A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Egger M., Smith G.D., Phillips A.N. Meta-analysis: principles and procedures. BMJ. 1997;315(7121):1533–1537. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fralick M., Sy E., Hassan A., Burke M.J., Mostofsky E., Karsies T. Association of concussion with the risk of suicide: a systematic review and meta-analysis. JAMA Neurol. 2019;76(2):144–151. doi: 10.1001/jamaneurol.2018.3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bhagawati J., Kumar S., Agrawal A.K., Acharya S., Wanjari A.K., Kamble T.K. Impact of different stages of chronic kidney disease on the severity of Willis-Ekbom disease. J Family Med Prim Care. 2019;8(2):432–436. doi: 10.4103/jfmpc.jfmpc_418_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akin S., Boluk C., Turk Boru U., et al. Restless legs syndrome in type 2 diabetes mellitus. Prim Care Diabetes. 2019;13(1):87–91. doi: 10.1016/j.pcd.2018.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Al-Jahdali H.H., Al-Qadhi W.A., Khogeer H.A., Al-Hejaili F.F., Al-Ghamdi S.M., Al Sayyari A.A. Restless legs syndrome in patients on dialysis. Saudi J Kidney Dis Transpl. 2009;20(3):378–385. [PubMed] [Google Scholar]
- 26.Winter A.C., Berger K., Glynn R.J., et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in men. Am J Med. 2013;126(3):228–235. doi: 10.1016/j.amjmed.2012.06.039. 35 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castillo-Torres S.A., Ibarra-Sifuentes H.R., Sanchez-Teran H., Sanchez-Martinez C., Chavez-Luevanos B., Estrada-Bellmann I. Restless legs syndrome in end-stage renal disease patients undergoing hemodialysis. Arq Neuropsiquiatr. 2018;76(12):827–830. doi: 10.1590/0004-282X20180133. [DOI] [PubMed] [Google Scholar]
- 28.Cho Y.W., Na G.Y., Lim J.G., et al. Prevalence and clinical characteristics of restless legs syndrome in diabetic peripheral neuropathy: comparison with chronic osteoarthritis. Sleep Med. 2013;14(12):1387–1392. doi: 10.1016/j.sleep.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 29.Arosemena Coronel M., Sanchez Armijos J., Tettamanti Miranda D., et al. Excessive daytime somnolence is associated with hypoglycemia in adult Latinos with type 2 diabetes mellitus. Sleep Med. 2017;36:6–9. doi: 10.1016/j.sleep.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Cuellar N.G., Ratcliffe S.J. Restless legs syndrome in type 2 diabetes: implications to diabetes educators. Diabetes Educ. 2008;34(2):218–234. doi: 10.1177/0145721708314180. [DOI] [PubMed] [Google Scholar]
- 31.Gemignani F., Brindani F., Vitetta F., Marbini A., Calzetti S. Restless legs syndrome in diabetic neuropathy: a frequent manifestation of small fiber neuropathy. J Peripher Nerv Syst. 2007;12(1):50–53. doi: 10.1111/j.1529-8027.2007.00116.x. [DOI] [PubMed] [Google Scholar]
- 32.Giannini G., Zanigni S., Melotti R., et al. Association between restless legs syndrome and hypertension: a preliminary population-based study in South Tyrol, Italy. Eur J Neurol. 2014;21(1):72–78. doi: 10.1111/ene.12244. [DOI] [PubMed] [Google Scholar]
- 33.Happe S., Treptau N., Ziegler R., Harms E. Restless legs syndrome and sleep problems in children and adolescents with insulin-dependent diabetes mellitus type 1. Neuropediatrics. 2005;36(2):98–103. doi: 10.1055/s-2005-837685. [DOI] [PubMed] [Google Scholar]
- 34.Innes K.E., Kandati S., Flack K.L., Agarwal P., Selfe T.K. The association of restless legs syndrome to history of gestational diabetes in an appalachian primary care population. J Clin Sleep Med. 2015;11(10):1121–1130. doi: 10.5664/jcsm.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juuti A.K., Laara E., Rajala U., et al. Prevalence and associated factors of restless legs in a 57-year-old urban population in northern Finland. Acta Neurol Scand. 2010;122(1):63–69. doi: 10.1111/j.1600-0404.2009.01262.x. [DOI] [PubMed] [Google Scholar]
- 36.Li L.H., Chen H.B., Zhang L.P., Wang Z.W., Wang C.P. A community-based investigation on restless legs syndrome in a town in China. Sleep Med. 2012;13(4):342–345. doi: 10.1016/j.sleep.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 37.Lin C.H., Wu V.C., Li W.Y., et al. Restless legs syndrome in end-stage renal disease: a multicenter study in Taiwan. Eur J Neurol. 2013;20(7):1025–1031. doi: 10.1111/ene.12095. [DOI] [PubMed] [Google Scholar]
- 38.Lopes L.A., Lins Cde M., Adeodato V.G., et al. Restless legs syndrome and quality of sleep in type 2 diabetes. Diabetes Care. 2005;28(11):2633–2636. doi: 10.2337/diacare.28.11.2633. [DOI] [PubMed] [Google Scholar]
- 39.Medeiros C., Bruin V., Ferrer D., et al. Excessive daytime sleepiness in type 2 diabetes. Arq Bras Endocrinol Metabol. 2013;57(6):425–430. doi: 10.1590/s0004-27302013000600003. [DOI] [PubMed] [Google Scholar]
- 40.Merlino G., Fratticci L., Valente M., et al. Association of restless legs syndrome in type 2 diabetes: a case-control study. Sleep. 2007;30(7):866–871. doi: 10.1093/sleep/30.7.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merlino G., Valente M., Serafini A., et al. Effects of restless legs syndrome on quality of life and psychological status in patients with type 2 diabetes. Diabetes Educ. 2010;36(1):79–87. doi: 10.1177/0145721709351252. [DOI] [PubMed] [Google Scholar]
- 42.Minai O.A., Golish J.A., Yataco J.C., Budev M.M., Blazey H., Giannini C. Restless legs syndrome in lung transplant recipients. J Heart Lung Transplant. 2007;26(1):24–29. doi: 10.1016/j.healun.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Modarresnia L., Golgiri F., Madani N.H., Emami Z., Tanha K. Restless Legs syndrome in iranian people with type 2 diabetes mellitus: the role in quality of life and quality of sleep. J Clin Sleep Med. 2018;14(2):223–228. doi: 10.5664/jcsm.6938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Molnar M.Z., Lu J.L., Kalantar-Zadeh K., Kovesdy C.P. Association of incident restless legs syndrome with outcomes in a large cohort of US veterans. J Sleep Res. 2016;25(1):47–56. doi: 10.1111/jsr.12335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plantinga L., Rao M.N., Schillinger D. Prevalence of self-reported sleep problems among people with diabetes in the United States, 2005-2008. Prev Chronic Dis. 2012;9:E76. [PMC free article] [PubMed] [Google Scholar]
- 46.Rafie S., Jafari M., Azizi M., Bahadoram M., Jafari S. Restless legs syndrome in hemodialysis patients. Saudi J Kidney Dis Transpl. 2016;27(2):326–330. doi: 10.4103/1319-2442.178553. [DOI] [PubMed] [Google Scholar]
- 47.Rohani M., Aghaei M., Jenabi A., Yazdanfar S., Mousavi D., Miri S. Restless legs syndrome in hemodialysis patients in Iran. Neurol Sci. 2015;36(5):723–727. doi: 10.1007/s10072-014-2026-8. [DOI] [PubMed] [Google Scholar]
- 48.Sabic A., Sinanovic O., Sabic D., Galic G. Restless legs syndrome in patients with hypertension and diabetes mellitus. Med Arch. 2016;70(2):116–118. doi: 10.5455/medarh.2016.70.116-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winter A.C., Schurks M., Glynn R.J., et al. Vascular risk factors, cardiovascular disease, and restless legs syndrome in women. Am J Med. 2013;126(3):220–227. doi: 10.1016/j.amjmed.2012.06.040. 7 e1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yildiz D., Kahvecioglu S., Buyukkoyuncu N., et al. Restless-legs syndrome and insomnia in hemodialysis patients. Ren Fail. 2016;38(2):194–197. doi: 10.3109/0886022X.2015.1111118. [DOI] [PubMed] [Google Scholar]
- 51.Safak E.D., Gocer S., Mucuk S., et al. The prevalence and related factors of restless leg syndrome in the community dwelling elderly; in Kayseri, Turkey: a cross-sectional study. Arch Gerontol Geriatr. 2016;65:29–35. doi: 10.1016/j.archger.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 52.Skomro R.P., Ludwig S., Salamon E., Kryger M.H. Sleep complaints and restless legs syndrome in adult type 2 diabetics. Sleep Med. 2001;2(5):417–422. doi: 10.1016/s1389-9457(01)00110-1. [DOI] [PubMed] [Google Scholar]
- 53.Sunwoo J.S., Kim W.J., Chu M.K., Yang K.I. Association between restless legs syndrome symptoms and self-reported hypertension: a nationwide questionnaire study in Korea. J Korean Med Sci. 2019;34(16):e130. doi: 10.3346/jkms.2019.34.e130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Szentkiralyi A., Volzke H., Hoffmann W., Happe S., Berger K. A time sequence analysis of the relationship between cardiovascular risk factors, vascular diseases and restless legs syndrome in the general population. J Sleep Res. 2013;22(4):434–442. doi: 10.1111/jsr.12040. [DOI] [PubMed] [Google Scholar]
- 55.Tasdemir M., Erdogan H., Boru U.T., Dilaver E., Kumas A. Epidemiology of restless legs syndrome in Turkish adults on the western Black Sea coast of Turkey: a door-to-door study in a rural area. Sleep Med. 2010;11(1):82–86. doi: 10.1016/j.sleep.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 56.Tuo H., Tian Z., Ma X., et al. Clinical and radiological characteristics of restless legs syndrome following acute lacunar infarction. Sleep Med. 2019;53:81–87. doi: 10.1016/j.sleep.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 57.Ulfberg J., Bjorvatn B., Leissner L., et al. Comorbidity in restless legs syndrome among a sample of Swedish adults. Sleep Med. 2007;8(7–8):768–772. doi: 10.1016/j.sleep.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 58.Wesstrom J., Nilsson S., Sundstrom-Poromaa I., Ulfberg J. Restless legs syndrome among women: prevalence, co-morbidity and possible relationship to menopause. Climacteric. 2008;11(5):422–428. doi: 10.1080/13697130802359683. [DOI] [PubMed] [Google Scholar]
- 59.Winkelman J.W., Shahar E., Sharief I., Gottlieb D.J. Association of restless legs syndrome and cardiovascular disease in the Sleep Heart Health Study. Neurology. 2008;70(1):35–42. doi: 10.1212/01.wnl.0000287072.93277.c9. [DOI] [PubMed] [Google Scholar]
- 60.Wu M., Guo J., Guo L., Zuo Q. The C-reactive protein/albumin ratio predicts overall survival of patients with advanced pancreatic cancer. Tumour Biol. 2016;37(9):12525–12533. doi: 10.1007/s13277-016-5122-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pinheiro T., Thomas T., Devaraj U., Ramachandran P., Krishnaswamy U.M. Prevalence of restless legs syndrome and quality of sleep in type 2 diabetics. J Diabetes Complications. 2020;34(12) doi: 10.1016/j.jdiacomp.2020.107727. [DOI] [PubMed] [Google Scholar]
- 62.Zobeiri M., Shokoohi A. Restless leg syndrome in diabetics compared with normal controls. Sleep Disord. 2014;2014 doi: 10.1155/2014/871751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Daniele T.M., de Bruin V.M., e Forte A.C., de Oliveira D.S., Pompeu C.M., de Bruin P.F. The relationship between physical activity, restless legs syndrome, and health-related quality of life in type 2 diabetes. Endocrine. 2013;44(1):125–131. doi: 10.1007/s12020-012-9841-6. [DOI] [PubMed] [Google Scholar]
- 64.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 65.Hogl B., Kiechl S., Willeit J., et al. Restless legs syndrome: a community-based study of prevalence, severity, and risk factors. Neurology. 2005;64(11):1920–1924. doi: 10.1212/01.WNL.0000163996.64461.A3. [DOI] [PubMed] [Google Scholar]
- 66.Berger K., von Eckardstein A., Trenkwalder C., Rothdach A., Junker R., Weiland S.K. Iron metabolism and the risk of restless legs syndrome in an elderly general population-the MEMO-study. J Neurol. 2002;249(9):1195–1199. doi: 10.1007/s00415-002-0805-2. [DOI] [PubMed] [Google Scholar]
- 67.Zucconi M., Ferini-Strambi L. Epidemiology and clinical findings of restless legs syndrome. Sleep Med. 2004;5(3):293–299. doi: 10.1016/j.sleep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 68.Greco D., Gambina F., Pisciotta M., Abrignani M., Maggio F. Clinical characteristics and associated comorbidities in diabetic patients with restless legs syndrome. Exp Clin Endocrinol Diabetes. 2009;117(9):496–499. doi: 10.1055/s-0029-1220739. [DOI] [PubMed] [Google Scholar]
- 69.Gallego M., Setien R., Izquierdo M.J., Casis O., Casis E. Diabetes-induced biochemical changes in central and peripheral catecholaminergic systems. Physiol Res. 2003;52(6):735–741. [PubMed] [Google Scholar]
- 70.Keckeis M., Lattova Z., Maurovich-Horvat E., et al. Impaired glucose tolerance in sleep disorders. PLoS ONE. 2010;5(3):e9444. doi: 10.1371/journal.pone.0009444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Italian R.S.G., Manconi M., Ferini-Strambi L., et al. Multicenter case-control study on restless legs syndrome in multiple sclerosis: the REMS study. Sleep. 2008;31(7):944–952. [PMC free article] [PubMed] [Google Scholar]
- 72.Li S.J., Wang Z.Q., Li Y.J., et al. Diabetes mellitus and risk of anastomotic leakage after esophagectomy: a systematic review and meta-analysis. Dis Esophagus. 2017;30(6):1–12. doi: 10.1093/dote/dox006. [DOI] [PubMed] [Google Scholar]
- 73.Chiu K.C., Cohan P., Lee N.P., Chuang L.M. Insulin sensitivity differs among ethnic groups with a compensatory response in beta-cell function. Diabetes Care. 2000;23(9):1353–1358. doi: 10.2337/diacare.23.9.1353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.