Highlights
-
•
Radiation can be considered on an individual basis for recurrent/metastatic disease.
-
•
Definitive radiation trended towards improved PFS but not statistically significant.
-
•
Any radiation associated with improved OS (statistically significant)
-
•
Increased adverse events seen with any radiation use.
Keywords: Radiation, Stage 4B cervical cancer
Abstract
Objective
Platinum-based chemotherapy and bevacizumab is the standard treatment for stage IVB cervical cancer. When metastases resolve, the benefit of radiating the primary tumor is unclear. We investigate the effect of pelvic radiation on PFS following chemotherapy and bevacizumab in stage IVB cervical cancer.
Methods
This is a retrospective series of 29 patients with stage IVB cervical cancer treated with platinum-based chemotherapy and bevacizumab. 3 subgroups were evaluated: definitive pelvic radiation, palliative radiation, and no radiation. The primary outcome was the mean PFS. Progression was determined radiographically. Kaplan-Meier method and the log-rank test for equality analyzed OS and PFS.
Results
The median OS was 38.4 months. 11 patients (38%) received definitive radiation, 9 (31%) received palliative and 9 (31%) received no radiation. 7/8 in the palliative group, 7/10 who received no radiation and all in the definitive group experienced progression. The median PFS was 7.5 months and not statistically different (p = 0.62). The median OS was not attained in the definitive group, was 23 months [19.6, -] for the palliative group and 19 months [24.9–45.4] for the no radiation group (p = 0.13). OS was higher in patients receiving definitive radiation vs all others (median OS survival not reached vs 6.6 months, p = 0.04). No difference in PFS between those receiving definitive radiation vs others (12 months vs 5.1 months p = 0.32).
Conclusion
Definitive radiation is associated with improved survival among in stage IVB cervical cancer treated with chemotherapy and bevacizumab. This association could be due to treatment, patient, or disease factors associated with improved oncologic outcomes. In absence of higher-level data, shared decision-making with consideration for comorbidities and performance status should be employed.
1. Introduction
Cervical cancer is the fourth most common gynecologic cancer worldwide, with an estimated annual 570,000 cases and 311,000 deaths (Bray et al., 2018). While this disease disproportionately affects developing countries, cervical cancer remains an ongoing public health concern in the US with an estimated 13,800 new cases and 4,290 deaths expected in 2020 (Howlader et al., 2019). The stage at presentation affects the treatment course of cervical cancer, with the majority of patients presenting with potentially curable early stage or locally advanced disease treatable with surgery or chemoradiation. However, for patients presenting with distant metastases the prognosis is poor, historically surviving a mean of 7 months (Bhatla et al., 2018). More recently, however, possibly somewhat related to selection bias, a median overall survival of 16.8 months was noted with addition of bevacizumab vs 13.3 months with chemotherapy alone (Tewari et al., 2014, Tewari et al., 2017).
Following current guidelines, the preferred first line therapy for metastatic cervical cancer includes a platinum-based chemotherapy doublet (i.e., cisplatin/paclitaxel, carboplatin/paclitaxel) in combination with bevacizumab (Long et al., 2005, Kim et al., 2012, Lorusso et al., 2014, NCCN Guidelines - Cervical Cancer Version 1., 2020). In clinical practice, palliative radiation therapy, and sometimes definitive pelvic radiation, are selectively used in stage IVB patients due to significant pelvic disease burden resulting in pain and/or bleeding (Spanos et al., 1993, Im et al., 2015;10:77.). In a retrospective study of patients with metastatic cervical cancer, Perkins et al. found a statistically significant improvement in overall survival by the addition of whole pelvic radiation vs palliative radiation to standard of care chemotherapy during primary treatment. (Perkins et al., 2020). To the best of our knowledge, there are no studies in the literature examining this relationship in the setting of current treatment guidelines which include bevacizumab. As metastases often resolve with systemic treatment and leave significant residual disease in the cervix the question is raised as to what dose of radiation to the primary tumor will provide the best clinical benefit. Whether it is more beneficial to give 10 days of palliative radiation vs 25 days of definitive radiation is unclear and the decision potentially impacts quality of life as well as patient and medical resources. This retrospective study aims to investigate the role of definitive vs palliative pelvic radiation on progression free survival (PFS) as determined by radiographic findings on women with stage IVB cervical cancer treated with chemotherapy regimens containing bevacizumab.
2. Materials and methods
This study is a retrospective case series of patients with radiologically confirmed stage IVB treated with platinum-based chemotherapy and bevacizumab at three institutions: MD Anderson Cancer Center (Houston, TX), Lyndon B Johnson General Hospital (Houston, TX) and Ben Taub General Hospital (Houston, TX) between April 2009 and April 2016. IRB approval was obtained from each of these institutions (HSC-MS-16–0510), and informed consent was waived given the retrospective study design.
All patients diagnosed and treated for cervical cancer in the time period were screened for stage at diagnosis. Staging was done according to the 2009 FIGO guidelines which were current at the time of diagnosis and treatment for this patient cohort. Stage IVB was defined as primary cervical cancer with distant lymphatic spread beyond the abdomen, or involvement of distant organs. The distant spread of the tumor was confirmed by imaging studies, including CT, PET or PET CT and fine needle aspiration on distant metastatic lesions. Patients were included if the histology of the tumor was squamous cell carcinoma, adenosquamous, or adenocarcinoma. Patients were excluded if the treatment regimen did not include bevacizumab as we were specifically interested in exploring the effect of palliative vs definitive radiation in the setting of the current recommended first line treatment regimen. Patients were excluded if they had recurrent cervical cancer, or if there was insufficient clinicopathologic data regarding histology, dissemination pattern, primary treatment details such as chemotherapy regimen or radiation regimen/dose, or if they were treated outside the included institutions.
Demographic variables including age, race, tobacco use, comorbidities, histology, and grade were collected. Variables pertaining to the treatment regimen were also collected including chemotherapy regimen, number of cycles, radiation therapy received and its timing with respect to chemotherapy administration.
For the analysis the patients were divided in 3 groups: definitive radiation, palliative radiation and no radiation. Patients were defined as having been included in the whole pelvic radiation arm if they received external beam radiotherapy to the whole pelvis, with or without additional radiation applied to the parametrium, para-aortic lymph nodes or other distant sites. Pelvic radiation (definitive or palliative) and for some, abbreviated brachytherapy was administered either during or after completion of systemic chemotherapy. Patients were classified as having received palliative radiation if they received a 10 day (30 Gy) course of pelvic radiation symptom control but did not receive or were not intended to receive definitive doses of radiation therapy. The radiation therapy was administered in a dose-individualized fashion, however the average dose for whole pelvic radiation was 50 Gy and for palliative radiation, the dose was 30 Gy. All patients received at least three cycles of bevacizumab and platinum-based chemotherapy, however there was significant individualization in dose and timing of the chemotherapy regimens (Table 1).
Table 1.
Demographics.
| CT + definitive RT (N = 11) | CT + palliative RT (N = 8) |
CT only (N = 10) |
p-value | |
|---|---|---|---|---|
| Age median (range) | 44.8 (35–67) | 51.2 (37–66) | 45.8 (31–71) | 0.40 |
| Race N(%) | ||||
| Non-white | 0 (0%) | 3 (37.5%) | 1 (10.0%) | 0.049 |
| White | 11 (100%) | 5 (62.5%) | 9 (90.0%) | |
| BMI (mean ± SD) | 31.1 ± 8.8 | 36.6 ± 11.2 | 31.1 ± 10.5 | 0.39 |
|
Any comorbidity N (%) |
||||
| No | 7 (63.6%) | 4 (50%) | 4 (40.0%) | 0.58 |
| Yes | 4 (36.4%) | 4 (50%) | 6 (60.0%) | |
| Histology N(%) | ||||
| Squamous cell | 10 (90.9%) | 6 (75.0%) | 7 (70.0%) | 0.78 |
| Adenocarcinoma | 0 | 1 (12.5%) | 3 (30.0%) | |
| Adenosquamous | 1 (9.1%) | 1 (12.5%) | 0 | |
| Grade N(%) | ||||
| Grade 2 | 2 (18.2%) | 2 (25.0%) | 3 (30.0%) | 0.96 |
| Grade 3 | 6 (54.5%) | 5 (62.5%) | 7 (70.0%) | |
| Unknown | 3 (27.3%) | 1 (12.5%) | 0 | |
| GOG Performance status N (%) | ||||
| 0 | 4 (36.3%) | 5 (62.5%) | 6 (60.0%) | 0.31 |
| 1 | 7 (63.7%) | 2 (25.0%) | 4 (40.0%) | |
| 2 | 0 | 1 (12.5%) | 0 | |
| Hydronephrosis | 1.00 | |||
| No | 7 (63.7%) | 6 (75.0%) | 7 (70.0%) | |
| Yes | 4 (36.3%) | 2 (25.0%) | 3 (30.0%) | |
| Number metastases | ||||
| 1 | 1 (9.1%) | 0 | 2 (20.0%) | 0.56 |
| 2–5 | 2 (18.2%) | 1 (12.5%) | 1 (10.0%) | |
| 6+ | 8 (72.7%) | 7 (87.5%) | 7 (70.0%) | |
| CT cycles (mean) | 6.7 (3–9) | 7.8 (6–12) | 8.4 (6–9) | 0.23 |
Legend: BMI – body mass index, CT – chemotherapy, RT – radiation therapy.
Our primary objective was determining progression free survival (PFS), with overall survival as a secondary outcome. Progression free survival (PFS) was defined as the time from date of diagnosis to the first recorded evidence of progression or recurrence, whether local or distant. Without progression, survivors were censored at last follow-up and non-survivors were censored at the date of death. Overall survival (OS) was defined as the time from date of diagnosis to date of death or to the date of last known follow up. Two analyses were performed, first comparing OS and PFS among the three groups (definitive, palliative and no radiation) and again among definitive radiation versus others (palliative + no radiation).
Descriptive statistics were used to summarize patient demographics and clinical characteristics. Categorical variables were analyzed using the Chi-square test and Fisher’s exact test. Continuous variables were compared using the Kruskal-Wallis test for skewed and non-normally distributed data. Survival curves were calculated using the Kaplan-Meier method. All data was analyzed using the STATA/IC software version 15.1 (StataCorp, College Station, TX). P values < 0.05 were considered statistically significant.
3. Results
Of the 29 patients identified for this case series, 11 (37.9%) received chemotherapy and definitive pelvic radiation. 8 patients (27.6%) received chemotherapy and palliative pelvic radiation. 10 patients (34.5%) received chemotherapy only. The mean age in the cohort was 47 years (SD = 10.6). 25 patients (86.2%) were Caucasian. 21 patients (72.4%) had squamous cell carcinomas, 4 (13.7%) had adenocarcinoma and 2 (6.8%) had adenosquamous tumors. There were no statistically significant differences between groups with respect to demographic or disease characteristics, although there were several clinically meaningful demographic differences (Table 1). Notably, all women who received definitive radiation were white (p = 0.049).
With respect to the toxicity profile 2 (25%) patients in the palliative radiation group experienced GI fistulas, 1 (12.5%) patient in the palliative group and 1 (9.1%) patient in the definitive group experienced neutropenia and 1 (12.5%) patient in the palliative group experienced CNS toxicity (Table 2). The rate of toxicities was not statistically different between groups (p = 0.91) however the results were limited by the small sample size.
Table 2.
Toxicity.
| CT + definitive RT (N = 11) | CT + palliative RT (N = 8) |
CT only (N = 10) |
p-value | |
|---|---|---|---|---|
| GI Fistula | 0 | 2 (25.0%) | 0 | 0.91 |
| Neutropenia | 1 (9.1%) | 1 (12.5%) | 0 | |
| CNS toxicity | 0 | 1 (12.5%) | 0 | |
| Other | 1 (9.1%) | 0 | 1 (10.0) |
Legend: BMI – body mass index, CT – chemotherapy, RT – radiation therapy.
All the patients experienced recurrence or progression of their disease in the cohort.
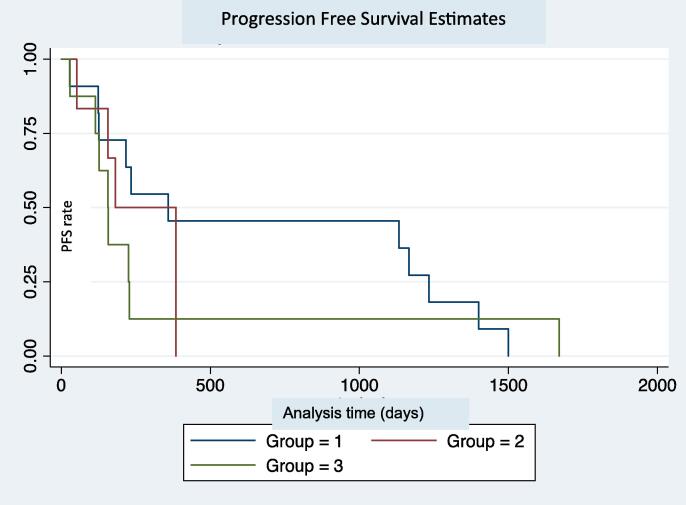
The median progression free survival (PFS) was 7.5 months [4.2–12.8] for the cohort. PFS was 12 months [4.2–41.1] for the definitive radiation group, 6 months [5.2–7.5] for the palliative radiation group and 5.2 months [3.8–7.5] for the chemotherapy only group. The progression free survival (PFS) was not significantly different between the three groups (p = 0.62) (Fig. 1A).
Fig. 1A.
Progression free survival estimate in days. Group 1 - Definitive RT and chemotherapy. Group 2 - Palliative RT and chemotherapy. Group 3 - No RT, chemotherapy only.
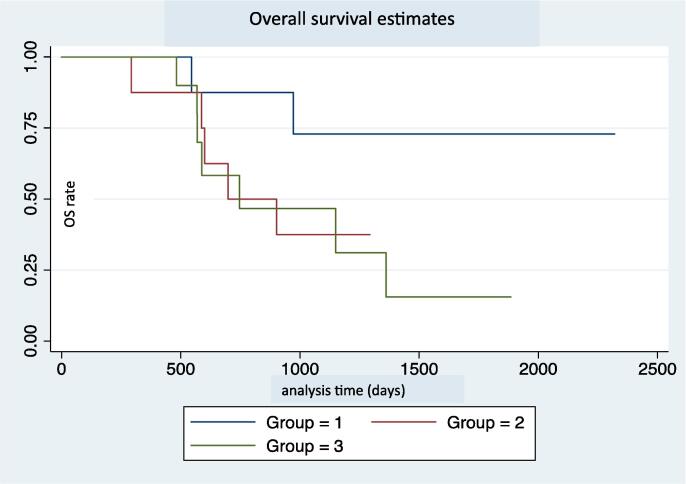
The median overall survival (OS) was 38 months [19.6-] for the cohort.
Median OS was not attained in the definitive radiation group. The median OS was 23 months [19.6, -] for the palliative radiation group and 19 months [24.9–45.4] for the no radiation group. The difference in overall survival was not statistically significant between groups (P = 0.13) (Fig. 2A).
Fig. 2A.
Overall survival estimate in days. Group 1 - Definitive RT and chemotherapy. Group 2 - Palliative RT and chemotherapy. Group 3 - No RT, chemotherapy only.
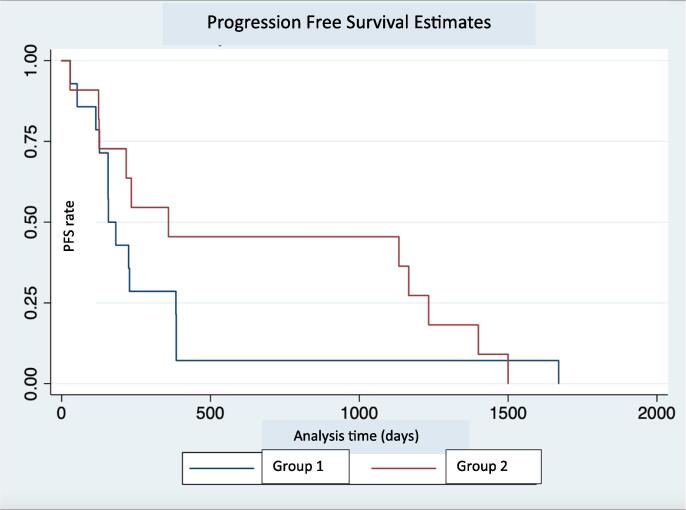
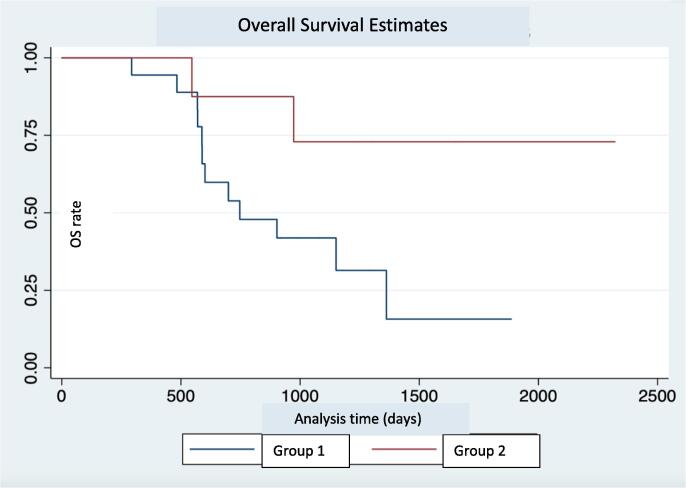
We performed an additional analysis comparing the definitive radiation group with the other patients in our cohort (palliative radiation group + chemotherapy only group). This showed a statistically significant advantage in the overall survival of the definitive radiation group (p = 0.04) (Fig. 1B). The median survival for the palliative radiation and chemotherapy only composite group was 6.6 months. Due to our sample size limitation the median survival was not reached for the definitive radiation group. There was no statistically significant difference in the PFS between patients receiving definitive radiation and others (11.1 months vs 5.1 months, p = 0.32), although the trend appears to favor the definitive radiation group. (Fig. 2B).
Fig. 1B.
Progression free survival estimate in days. Group 1 – No Definitive RT. Group 2 – Definitive RT.
Fig. 2B.
Overall survival estimate in days. Group 1 – No Definitive RT. Group 2 – Definitive RT.
4. Discussion
In this study, we examined a retrospective cohort to determine if definitive radiation or palliative radiation influenced progression free survival in stage IVB cervical cancer patients treated with bevacizumab. The present study did not show a statistically significant difference in OS or PFS between the groups treated with definitive radiation, palliative radiation or bevacizumab containing chemotherapy alone. However, this may have been underestimated due to study size as there was a trend towards improved survival with definitive radiation therapy. While not statistically significant, we noted a clinically significant 6 months difference between the PFS favoring the definitive group compared to the palliative group. Despite our small sample size, the mean OS and PFS noted in our chemotherapy only group was similar to previous results from larger scale studies (Schefter, 2014). In our chemotherapy only group the mean OS was 19 months and the mean PFS was 5.2 months compared to 17 months and 8.2 months respectively noted by Tewari et al. (Tewari et al., 2014) In our cohort, the group receiving definitive radiation trended towards longer PFS compared to the other groups without higher rates of severe GI toxicities in setting of radiation and bevacizumab. While this trend suggests the possibility of benefit from definitive pelvic radiation, consideration of this treatment option should be balanced against the possibility of exposure to additional toxicities, time commitment, resource use when there appears to be no long-term survival benefit. Recurrence and progression rates, whether local or distant, were not statistically different between groups (data not shown).
Previous studies have investigated the role of pelvic radiation in stage IVB cervical cancer patients treated with chemotherapy. Perkins et al demonstrated a 7 month increase in PFS and a 24 month increase in OS in patients with stage IVB disease treated with definitive pelvic radiation and chemotherapy when compared to chemotherapy only. (Im et al., 2015). However, in their study only 20 of the 95 patients in their cohort (21.0%) received bevacizumab in addition to platinum-based chemotherapy as the standard of care changed during their follow-up period. Additionally, Kim et al examined if stage IVB cervical cancer patients had improved clinical outcomes with combined chemoradiation vs. systemic chemotherapy alone and found that combined chemotherapy and radiation did have a progression free survival benefit and overall survival benefit in patients with lymphatic metastasis (Kim et al., 2013). Our study did not find a statistically significant survival benefit. With respect to progression free survival, a definite trend is noted in favor of definitive pelvic therapy in combination with bevacizumab containing chemotherapy, however the relationship failed to reach significance.
Our study contributes to the literature by examining a population of stage IVB only patients with distant metastasis who received radiation to the pelvis, either palliative or whole pelvic radiation. The strength of this study is the fact that all patients received platinum-based chemotherapy and bevacizumab, the current preferred first line treatment in metastatic or recurrent cervical cancer. To the best of our knowledge, no other studies have investigated the effect of type of radiation therapy in patients with Stage IV B disease.
Aside from the small sample size and retrospective data collection, our study has several other limitations. The reason for the decision to treat patients with either definitive or palliative radiation versus no radiation is not always clearly documented in the medical record. Patient selection bias, such as choosing to give definitive treatment to patients with more limited disease extent may contribute to this observed difference between the groups. Treatment decisions could certainly be affected by provider and/or institutional preferences, availability of resources, or other clinical factors not captured in the data collection process. The rate of toxicities could be inaccurate due to limitations in the follow-up period.
Additionally, there were demographic differences between the definitive and the palliative groups which suggest the patients in the palliative group were younger and had less comorbidities. We also acknowledge the racial difference between the treatment groups, specifically that all women who received definitve radiation were white. While we cannot draw any definitive conclusions, we acknowledge that this finding could be a marker of racial disparity and/or inequity with respect to access to oncologic care. While these findings were not statistically different, they do suggest that demographic factors were important considerations by the provider in the decision to offer palliative versus definitive radiation. This highlights the importance of larger randomized studies in the future. Despite these notable differences, our patient groups were similar with respect to clinical variables that could potentially affect the oncologic outcomes, including performance status, number of metastases, number of chemotherapy cycles received, rates of renal obstruction, metastases to lungs, para-aortic and supraclavicular nodes involvement. The only exception we noted was that patients with bony metastases were less likely to receive definitive radiation.
Another limitation we acknowledge in our study is the measurement of the OS and PFS from the time of diagnosis as opposed to from the time from the completion of treatment. This may introduce a time bias seeing as patients would have to be alive and not experience progression through the chemotherapy received. This was in part from limitations in the data included in the medical record. Given the small sample size and exploratory nature of this study we consider the trends obtained from this case series to provide a meaningful future direction of larger prospective trials.
5. Conclusion
The addition of definitive pelvic radiation to patients with stage IVB cervical cancer treated with platinum-based chemotherapy and bevacizumab trended towards improvement in PFS compared to the groups receiving palliative radiation only or chemotherapy alone. Patients treated with definitive radiation did experience a statistically significant improvement in OS when compared to all other patients in our case series. While not all our findings were statistically significant, the trends are consistent with previously published data investigating the role of pelvic radiation in patients with metastatic cervical cancer which show a potential benefit to the use radiation therapy in this patient population. Likewise, our findings which did reach statistical significance should not be interpreted as evidence of causality but rather introduce a meaningful consideration when formulating individualized treatment plans for patients with metastatic cervical cancer. Larger scale adequately powered studies, specifically a propensity matched observational study or a randomized controlled trial, are needed to confirm and further explore these results. In absence of higher-level data guiding clinical practice, shared decision making between patient and physician with consideration for patient comorbidities and performance status should be employed. Further directions include, investigating side effects and toxicity profile of palliative vs definitive vs no radiation in this patient population and in the era of immunotherapy.
CRediT authorship contribution statement
Rachel L. Wiley: Conceptualization, Methodology, Data curation, Investigation, Writing – review & editing. Ioana L. Bondre: Conceptualization, Formal analysis, Investigation, Writing – original draft. Randa Jalloul: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing – review & editing, Visualization, Supervision. Ann H. Klopp: Conceptualization, Writing – review & editing. Jolyn S. Taylor: Conceptualization, Writing – review & editing. Lois M. Ramondetta: Conceptualization, Methodology, Investigation, Resources, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gore.2022.100963.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Bhatla N., Aoki D., Sharma D.N., Sankaranarayanan R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018;143:22–36. doi: 10.1002/ijgo.12611. [DOI] [PubMed] [Google Scholar]
- Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Howlader, N., Noone, A.M., Krapcho, M., Miller, D., Brest, A., Yu, M., Ruhl, J., Tatalovich, Z., Mariotto, A., Lewis, D.R., Chen, H.S., Feuer, E.J., Cronin, K.A. (Eds.), 2020. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April.
- Im J.H., Yoon H.I., Kim S., et al. Tailored radiotherapeutic strategies for disseminated uterine cervical cancer patients. Radiat Oncol. 2015;10:77. doi: 10.1186/s13014-015-0373-0. Published 2015 Apr 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.Y., Kim J.Y., Kim J.H., Yoon M.S., Kim J., Kim Y.S. Curative chemoradiotherapy in patients with stage IVB cervical cancer presenting with paraortic and left supraclavicular lymph node metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(3):741–747. doi: 10.1016/j.ijrobp.2012.01.070. [DOI] [PubMed] [Google Scholar]
- Kim H.S., Kim T., Lee E.S., Kim H.J., Chung H.H., Kim J.W., Song Y.S., Park N.H. Impact of Chemoradiation on Prognosis in Stage IVB Cervical Cancer with Distant Lymphatic Metastasis. Cancer Res. Treatment: Official J. Korean Cancer Assoc. 2013;45(3):193–201. doi: 10.4143/crt.2013.45.3.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long H.J., 3rd, Bundy B.N., Grendys E.C., Jr, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group Study. J. Clin. Oncol. 2005;23(21):4626–4633. doi: 10.1200/JCO.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Lorusso D., Petrelli F., Coinu A., Raspagliesi F., Barni S. A systematic review comparing cisplatin and carboplatin plus paclitaxel-based chemotherapy for recurrent or metastatic cervical cancer. Gynecol. Oncol. 2014;133(1):117–123. doi: 10.1016/j.ygyno.2014.01.042. [DOI] [PubMed] [Google Scholar]
- “NCCN Guidelines - Cervical Cancer Version 1.2020.” NCCN Clinical Practice Guidelines in Oncology, NCCN, 28 Feb. 2020, www.nccn.org/professionals/physician_gls/pdf/cervical_blocks.pdf.
- Perkins V., Moore K., Vesely S., et al. Incorporation of whole pelvic radiation into treatment of stage IVB cervical cancer: A novel treatment strategy. Gynecol. Oncol. 2020;156(1):100–106. doi: 10.1016/j.ygyno.2019.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schefter, T., 2014. Radiation Therapy Oncology Group (RTOG). RTOG 0417: efficacy of bevacizumab in combination with definitive radiation therapy and cisplatin chemotherapy in untreated patients with locally advanced cervical carcinoma. Int. J. Radiat. Oncol. Biol. Phys. Jan 1;88(1):101-5. doi: 10.1016/j.ijrobp.2013.10.022. [DOI] [PubMed]
- Spanos W.J., Jr, Perez C.A., Marcus S., et al. Effect of rest interval on tumor and normal tissue response–a report of phase III study of accelerated split course palliative radiation for advanced pelvic malignancies (RTOG-8502) Int. J. Radiat. Oncol. Biol. Phys. 1993;25(3):399–403. doi: 10.1016/0360-3016(93)90059-5. [DOI] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Long H.J., 3rd, Penson R.T., Huang H., Ramondetta L.M., Landrum L.M., Oaknin A., Reid T.J., Leitao M.M., Michael H.E., Monk B.J. Improved survival with bevacizumab in advanced cervical cancer. N. Engl. J. Med. 2014;370(8):734 doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tewari K.S., Sill M.W., Penson R.T., et al. Bevacizumab for advanced cervical cancer: final overall survival and adverse event analysis of a randomised, controlled, open-label, phase 3 trial (Gynecologic Oncology Group 240) Lancet. 2017;390(10103):1654–1663. doi: 10.1016/S0140-6736(17)31607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.