Abstract
Background
HIV assays designed to detect recent infection, also known as “recency assays,” are often used to estimate HIV incidence in a specific country, region, or subpopulation, alone or as part of recent infection testing algorithms (RITAs). Recently, many countries and organizations have become interested in using recency assays within case surveillance systems and routine HIV testing services to measure other indicators beyond incidence, generally referred to as “non-incidence surveillance use cases.”
Objective
This review aims to identify published evidence that can be used to validate methodological approaches to recency-based incidence estimation and non-incidence use cases. The evidence identified through this review will be used in the forthcoming technical guidance by the World Health Organization (WHO) and United Nations Programme on HIV/AIDS (UNAIDS) on the use of HIV recency assays for identification of epidemic trends, whether for HIV incidence estimation or non-incidence indicators of recency.
Methods
To identify the best methodological and field implementation practices for the use of recency assays to estimate HIV incidence and trends in recent infections for specific populations or geographic areas, we conducted a systematic review of the literature to (1) understand the use of recency testing for surveillance in programmatic and laboratory settings, (2) review methodologies for implementing recency testing for both incidence estimation and non-incidence use cases, and (3) assess the field performance characteristics of commercially available recency assays.
Results
Among the 167 documents included in the final review, 91 (54.5%) focused on assay or algorithm performance or methodological descriptions, with high-quality evidence of accurate age- and sex-disaggregated HIV incidence estimation at national or regional levels in general population settings, but not at finer geographic levels for prevention prioritization. The remaining 76 (45.5%) described the field use of incidence assays including field-derived incidence (n=45), non-incidence (n=25), and both incidence and non-incidence use cases (n=6). The field use of incidence assays included integrating RITAs into routine surveillance and assisting with molecular genetic analyses, but evidence was generally weaker or only reported on what was done, without validation data or findings related to effectiveness of using non-incidence indicators calculated through the use of recency assays as a proxy for HIV incidence.
Conclusions
HIV recency assays have been widely validated for estimating HIV incidence in age- and sex-specific populations at national and subnational regional levels; however, there is a lack of evidence validating the accuracy and effectiveness of using recency assays to identify epidemic trends in non-incidence surveillance use cases. More research is needed to validate the use of recency assays within HIV testing services, to ensure findings can be accurately interpreted to guide prioritization of public health programming.
Keywords: HIV, recency, incidence, surveillance, recent infection
Introduction
There are many reasons to identify recently acquired HIV infections on a population level, including to (1) better understand current transmission of HIV in a country, region, or population subgroup; (2) evaluate whether specific prevention interventions are having the desired impact; and (3) focus limited resources for prevention or treatment services on groups of people or geographic locations with the greatest potential benefit (eg, reducing risk for onward transmission) [1]. HIV assays designed to detect recent infection, also known as “recency assays,” can be used to gain an understanding of these epidemic dynamics.
Recency assays discriminate recent from longstanding infection in an individual using 1 or more biomarkers, typically using an understanding of the typical patterns of immune response maturation following initial infection [2]. Individual recency assay results can be used in a cross-sectional survey to estimate incidence by building on the common epidemiological equation P = I × D (ie, prevalence = incidence × duration of infection) [3]. However, the accuracy of the incidence estimate is dependent on accurate knowledge of the performance characteristics of the recency assay or algorithm, specifically mean duration of recent infection (MDRI; ie, the average time after infection that individuals are classified as recently infected) and false recent rate (FRR; the proportion of long-infected individuals misclassified as recently infected), and the precision of the estimate is sensitive to these same parameters [4].
To date, no recency assay has fully met the target product profile for HIV incidence estimation as set out by the Foundation for Innovative Diagnostics (FIND) and the World Health Organization (WHO) in 2016 [5]. Numerous factors have been identified that adversely affect recency assay performance and lead to substantial misclassification of longstanding infections as recent (ie, raise the FRR). Factors that can affect assay performance include natural variability in individual immune responses (in particular, elite control of HIV or natural viral suppression), variability in biomarker progression for different HIV-1 subtypes, the types of specimens collected and storage methods, advanced HIV disease, and treatment with antiretroviral therapy (ART) or use of pre-exposure prophylaxis (PrEP) [6-9]. The effect of ART on increasing the FRR of recency assays appears to be more pronounced when a person receives treatment very early after initial infection [10,11], which is complicated by rapid improvements in treatment coverage worldwide, as well as uptake of PrEP. Other factors that may impact assay performance but are not yet well-characterized include sex, pregnancy status, and the presence of comorbidities [12-14].
Since the release in 2011 of technical guidance on the use of recency assays to estimate population-level HIV incidence from the WHO and Joint United Nations Programme on HIV/AIDS (UNAIDS) [1], the field has changed substantially, motivating release of interim guidance at various times [12,15-18]. Numerous examples in the peer-reviewed literature now highlight the necessity of adjustments at a local level to improve the accuracy of incidence estimates derived using recency assays within population-based surveys [13,19-28]. Beyond that primary application, however, many countries and organizations have become increasingly interested in using recency assays within HIV case surveillance systems and routine HIV testing services to measure indicators other than incidence, such as the identification of epidemiologically linked clusters of recent infections, geographic hotspots, or subpopulations with relatively high, ongoing, or emerging transmission, to inform prioritization of HIV prevention, testing, and partner notification or contact tracing interventions. These types of epidemic monitoring and evaluation strategies are generally referred to as “non-incidence surveillance use cases” for recency assays [29]. However, the nonrandom nature by which people are included in these types of surveillance systems and programs requires special attention to characterize and, ideally, mitigate the effect of selection biases on the accuracy of these non-incidence estimates.
To our knowledge, no previous systematic review has been completed of literature related to the use of HIV recency assays for surveillance purposes. We endeavored to identify published evidence that could be used to validate methodological approaches to HIV incidence estimation and other measures of recency of HIV infection using recency assays. Findings from this systematic review were designed to inform a revised technical guidance on the use of HIV recency assays for identification of epidemic trends, whether for HIV incidence estimation or for other non-incidence indicators of recency, to be released by the WHO and UNAIDS in 2022. This guidance is intended to help raise global awareness of benefits and pitfalls of the use of these assays for surveillance purposes, and set clear standards for their appropriate use.
Objectives
Our systematic review had 3 primary objectives:
Understand the use of recency testing in surveillance and programmatic and laboratory settings (to provide incidence estimates or for non-incidence surveillance use cases);
Review methodologies for implementing recency testing in population surveys, case surveillance systems, and routine monitoring and evaluation activities; and
Highlight use cases that have employed a recency assay or recent infection testing algorithm (RITA) within specific populations, with special attention to variations in assays, settings, and methods of analysis for calculating HIV incidence estimates or employing recency assays for non-incidence surveillance use cases. Within this category, one of our specific goals was to identify evidence that not only presents results of “proportion testing recent” or similar, but also reviews the methodological choice to use a simple proportion of recency or assess “factors associated with testing recent” as a proxy for HIV incidence or other indicators of ongoing HIV transmission within case surveillance systems.
Methods
Eligibility Criteria for the Systematic Review
The systematic review included 2 sets of searches, each with a different strategy. Strategy 1 involved looking for articles about recency assay performance in laboratory and field survey settings. To be eligible for inclusion in the review, articles needed to describe some aspect of performance of recency assays/methodologies (eg, MDRI, FRR, accuracy, number tested, and proportion recently infected; or correlation, R, percent agreement, or kappa related to another standard assay) and needed to address validation of method. As we were not looking to perform a meta-analysis of assay performance (ie, MDRI or FRR in various study populations) but rather review the evidence regarding validity of various methodologies for the use of these assays for surveillance purposes, simply reporting the use of a recency assay with a specific MDRI and FRR was insufficient for inclusion. Rather, to be included articles needed to compare findings with those of another standard assay, or describe in detail the methodological choices made and rationale for doing so. They also needed to use commercially available assays/methodologies used to determine recency of infection (Table 1), as the primary motivation for the review was to inform the WHO/UNAIDS technical guidance that would only cover assays that could be purchased and implemented by countries according to package inserts. Articles reviewing the use of a laboratory-developed (home-grown) assay that was not commercially available were excluded from the review.
Table 1.
List of commercially available recency assays at the time of the review.
| Product name (manufacturer) | Assay type |
| Asanté HIV-1 Rapid Recency Assay (Sedia Biosciences) | Rapid, point of care |
| HIV Swift Recent Infection Assay (Maxim Biomedical) | Rapid, point of care |
| Sedia HIV-1 Limiting Antigen Avidity (LAg-Avidity) EIA (Sedia Biosciences) | Laboratory based |
| Maxim HIV-1 LAg-Avidity EIA Kit (Maxim Biomedical) | Laboratory based |
| Genetics Systems HIV-1/HIV-2 Plus O EIA (Bio-Rad, avidity protocol) | Laboratory based |
| ARCHITECT HIV Ag/Ab Combo (Abbott, avidity protocol or unmodified protocol) | Laboratory based |
| VITROS Anti-HIV 1+2 (Ortho Diagnostics, avidity protocol) | Laboratory based |
| Geenius HIV-1/2 Confirmatory (Bio-Rad, modified protocol) | Laboratory based |
| INNO-LIA HIV I/II Score (Fujirebio, Inc.) | Laboratory based |
| Sedia BED HIV-1 Incidence EIA (Sedia Biosciences) | Laboratory based |
Strategy 2 involved looking for articles about surveillance and programmatic utilization of recency testing. To be eligible for inclusion, articles needed to describe some aspect of population-level utility (identification of “hotspots,” clusters, case surveillance, or incidence estimation), using commercially available recency assays/methodologies (eg, RITAs, adapted assay protocols) to determine recency of HIV infection. Studies could present either qualitative or quantitative data and could be descriptive studies lacking a comparator, as long as studies clearly presented outcomes specific to HIV recency testing.
Search Strategy
The literature search for the systematic review was conducted in PubMed and Web of Science, and included literature published in any language and in any indexed journal including preprint servers without peer review, from January 1, 2010, to November 11, 2021, by searching title, abstract, and MeSH terms/author keywords.
For the Strategy 1 search, search terms included HIV, recency assay, incidence assay, test for recent infection (TRI), recent infection testing algorithm (RITA), multiassay algorithm, performance, false recent rate/ratio (FRR), proportion false recent, and mean duration of recent infection (MDRI). For the Strategy 2 search, search terms included recent infection/acute infection, recent infection testing algorithm, multiassay algorithm, incidence estimates, case surveillance, hotspot identification, hotspot mapping, cluster detection, procedures and protocols, and HIV. See Multimedia Appendix 1 for search sets and terms and Multimedia Appendix 2 for the detailed search code.
Given that much of the research output in the field of HIV recency assay utilization is published in formal reports or presented in conference abstracts, we extended the search beyond traditional literature databases to include “gray literature,” that is, literature that is not formally published in peer-reviewed journals or books. We conducted a search of the gray literature through internet search engines and through websites of major international funders, subject matter conferences, and organizations involved with HIV surveillance (Multimedia Appendix 3) employing the following search terms across sites: “surveillance,” “recency testing,” “case surveillance,” “incidence estimation,” “hotspot,” and “HIV”.
We used a step-wise approach during the screening and reviewing process. After search and duplicate removal, SF screened titles and abstracts to identify papers potentially related to the focus areas and eligibility criteria. After screening was complete, full text of remaining articles was then independently reviewed by DF and SS to determine if the study met eligibility criteria; SF served as a tiebreaker for any articles for which the 2 preliminary screeners were not in agreement about inclusion. Once the full-text review was complete, SF hand-searched the references of all included articles for additional, potentially eligible articles. DF and SS then reviewed these articles and determined eligibility according to the process outlined above.
Prior to conducting our search, we developed a formal protocol and circulated it among stakeholders at the WHO and UNAIDS for approval; we have made the protocol available in unmodified form as Multimedia Appendix 4 to this article.
Assessment of Evidence Strength
The literature included in the systematic review was rated by strength of published evidence using a 23-point rubric that we designed custom for this purpose (Figure 1). For each piece of evidence, 3 team members (SF, DF, and SS) independently rated the strength of evidence through a Microsoft Excel–based scoring rubric designed to implement the grading structure detailed in Figure 1. If there was disagreement between 2 of the team members, the third performed an assessment using the rubric and served as a tiebreaker.
Figure 1.
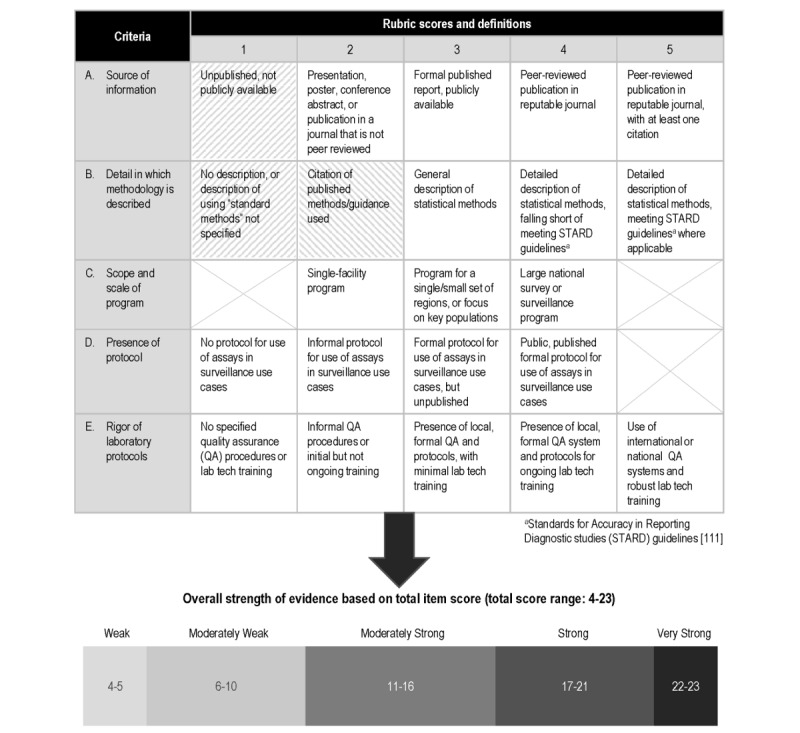
Rubric used to evaluate strength of evidence for each item reviewed. A score ranging from 1–5 was assigned to each item based on the 5 criteria in this rubric. Items with a score of 1 for source of information or detail in which methodology is described (see cells 1A and 1B with hatched shading) were automatically categorized as “weak evidence”, regardless of other criteria scores. Similarly, items with a score of 2 for detail in which methodology is described (see cell 2B with hatched shading) were automatically categorized as “moderately weak evidence” regardless of other criteria scores. Each item was then assigned an overall strength of evidence rating based on the sum of the criteria scores.
Results
Overview
The search was conducted on November 11, 2021, and resulted in 180 records identified via MEDLINE (PubMed) and 193 records identified via Web of Science. Of these, 104 were duplicates, which were removed. An additional 27 records were identified through an internet search of gray literature and 15 records were identified through a hand search of the references in previously identified records.
Literature Screening Steps
After deduplication, the remaining 311 documents from the search were initially scanned by SF for eligibility. This initial “quick screen” excluded 94 articles that very clearly did not meet inclusion criteria for the review, or did not contain sufficient detail on methods to have utility in the review. The remaining 217 documents were then subjected to a full-text review, which was conducted independently by both DF and SS. After excluding 50 full-text articles that did not meet our predefined inclusion criteria, a total of 167 studies, reports, or presentations were retained across both focus areas (Figure 2) and were graded for strength of evidence.
Figure 2.
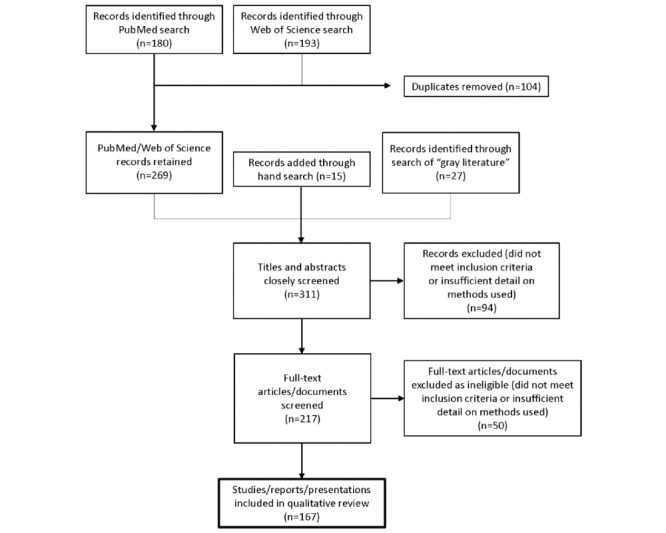
Flowchart of search process and results.
Characteristics of Included Studies
Among the 167 pieces of evidence that were identified through the systematic review and that met the inclusion criteria, 91 (54.5%) [3,7-14,18,20-28,30-100] focused on assay performance, algorithm performance, or methodological descriptions of incidence estimation. The quality of evidence was “very-strong” (58/91), “strong” (21/91), “moderately strong” (9/91), and “weak” (3/91) in these 91 articles. The remaining 76 (45.5%) pieces of evidence described field-derived incidence and non-incidence use cases or both. Of these, 45 (59%) described use for incidence estimation, 25 (33%) described non-incidence use cases, and 6 (8%) described both incidence and non-incidence use cases.
Among the 51 articles describing the use of recency assays for estimation of HIV incidence, 16 (31%) [101-116] described national surveillance in the form of population-based surveys (including 10 from the US-supported Population-based HIV Impact Assessment (PHIA) surveys). Another 12 (24%) [117-128] were also population-based surveys with a representative sampling strategy, but had a community-level (subnational) focus. Most evidence related to national or subnational incidence surveillance was judged to be “very strong” (10/28), “strong” (6/28), or “moderately strong” (8/28), with more details of strength ratings found in Multimedia Appendix 5. These population-based incidence use cases are also sometimes known as impact assessment use cases, because they are intended for repeat implementation to assess changes in incidence over time as a result of HIV prevention or care interventions. There were 3 more studies that also used recency assays to estimate incidence for intervention impact assessment, but in the more narrow context of blood donor policy implementation [129] or behavioral randomized controlled trials [130,131]. The remaining 20 articles [132-151] described calculation of incidence among key or sentinel populations, including those accessing routine HIV testing or blood donation programs. Key or sentinel population surveillance involves testing within populations that are either of specific interest because they are at higher risk for infection (key) or considered to be representative of a larger population (sentinel). Sentinel and key population surveillance may be facility based or community based. For example, needle and syringe distribution programs are a good point of contact with people who inject drugs, sexual health clinics may provide access to men who have sex with men and sex workers, and antenatal clinics are used to sample pregnant women. All evidence in this category was of “very strong” (5/20) or “strong” (15/20) quality (Multimedia Appendix 5).
Among the 31 articles describing non-incidence use cases, 24 used recency testing to assess risk factors predicting recent infection [126-128,149,150,152-170] for purposes of targeted prevention planning. A total of 6 used recency testing as part of cluster identification or analysis (including 5 that also used recency assays for determining risk factors associated with recency) [153,154,161,162,167,171], 2 used recency testing for geographic comparisons or hotspot mapping [172,173], and 5 used it for other purposes, including examining recency trends in the same population over time [166] and evaluating patterns of drug resistance [151,174-176]. One report was exploring feasibility and utility of incorporating recency testing into HIV programs, and simply reported recency proportions found through the project [151]. The quality of evidence was “very strong” (10/31) or “strong” (12/31), with the remainder (9/31, 29%) providing evidence that was “moderately weak” or “weak.”
Multimedia Appendices 5 and 6 provide details on each of the 167 pieces of evidence included in this review, including the strength rating and topic of focus for each item.
Use of Recency Assays for HIV Incidence Estimation
There were 51 documents included in this review that provided methods and findings related to the field use of recency assays for HIV incidence estimation. As detailed above, 32 studies in this review used recency assays to estimate incidence for surveillance of subnational regions or key or sentinel populations; however, these strategies have also been used extensively at a national level. In 2015 the UNAIDS and WHO released guidelines on monitoring the impact of the HIV epidemic using population-based surveys, including using recency assays for estimation of incidence [177]. Since then, 16 population-based surveys with published results have utilized this approach for national surveillance, the majority (n=11) [102-111,116] of which were part of the global PHIA [178] (including 1 that published an analysis using PHIA data, but was not an official PHIA report) [116]. These surveys involve cross-sectional, household-based, nationally representative sampling of adults and adolescents aged 15 years and older, with some surveys also including children aged 0-14 years. All PHIA countries were located in sub-Saharan Africa, except Haiti (which did not contribute evidence to this review) [179]. PHIA participants receive home-based HIV testing and counseling. Those who are HIV positive undergo a laboratory-based RITA. During the first 3 PHIA surveys in Malawi, Zimbabwe, and Lesotho, the RITA included the Sedia HIV-1 Limiting Antigen (LAg) Avidity assay in combination with viral load. The subsequent 7 surveys added antiretroviral detection to the LAg and viral load tests as an enhanced measure to distinguish recent from long-term infections. Incidence estimates were obtained from the RITA result in accordance with an established cross-sectional incidence estimator [4] and performance characteristics were consistently specified as MDRI = 130 days (95% CI 118-142), time cut-off = 1.0 year, and residual proportion false recent = 0.0%, with no uncertainty incorporated into the FRR parameter. No adjustment for subtype-related variation in MDRI was made, except in the case of Uganda, where an MDRI of 153 days was used due to Uganda’s subtype A and D–dominated epidemic [102]. Survey weights were utilized for all estimates to account for the complex sampling design. The sample size of PHIA surveys is designed to provide subnational-level (eg, provinces, regions) estimates of viral load suppression among people living with HIV aged 15-49 years with a 95% CI of ±10% or less, which typically yields reasonably precise estimates of national-level HIV incidence among people aged 15-49 years. As a result, these surveys were able to generate HIV incidence estimates disaggregated by sex and high-level region, but not estimates that could be used to target HIV prevention or care to specific districts or key populations.
In addition to the 10 PHIA studies, another 8 studies [113,117,119,134,137,140,145,147] used similar methods to calculate incidence—a published MDRI without local adaptation, and an assumed FRR of 0—and 6 used a published MDRI without reference to FRR (presumably also assuming no false recent results from the RITA) [112,118,126,130,131,136]. In each of these cases, the authors noted that by including viral load or other factors in the RITA designed to reduce FRR, further FRR adjustment was considered unnecessary. The other 27 studies used a variety of other approaches to address MDRI and FRR. Only 3 studies locally adapted both the MDRI and the FRR as part of the analysis [125,129,151]. One study locally adapted the MDRI by weighting for local subtype distribution but assumed 0 FRR [133], and 7 studies used a published MDRI but locally estimated the FRR based on internal data [114,120,121,123,132,138,143]. One study used an FRR of 0 for the main analysis, and compared incidence results with those generated assuming an FRR of 0.39% in a sensitivity analysis [124]. Two studies used a published MDRI and a published FRR (ie, from another study’s published findings of the assay’s FRR) that was different from 0 [116,148]. The remaining 13 studies estimated incidence using alternate estimators not incorporating MDRI or FRR, both with adjustments of assay performance made for the local context [128,135,139,142,149] and no local assay-based adjustments [101,115,122,127,141,144,146,150].
Non-incidence Surveillance Use Cases of HIV Recency Assays
One of our objectives in the review was to identify evidence that not only presents results of “proportion testing recent” or similar, but also reviews the methodological choice to use a simple proportion of recency or assess “factors associated with testing recent” as a proxy for HIV incidence or another indicator of ongoing HIV transmission within case surveillance systems. Although there were 31 documents identified across the 11-year review period that were reporting on the use of recency assays for non-incidence use cases, all of those papers reported their estimates of non-incidence recency indicators (such as “proportion recent”) without attention to whether these indicators were valid proxies of ongoing HIV transmission. As many as 19 of these studies used a recency assay as part of a RITA (along with at least one other recency assay, viral load, CD4, or similar) to help reduce misclassification rates [126,127,151-156,159,163,165,167,168,170,172-176]. Three studies adjusted their recency calculations in some other way (eg, incorporating sensitivity or specificity of the assay into estimates) [149,158,180] and the remaining 9 used the assay results according to a prespecified cut-off with no further adjustment [150,157,160-162,164,166,169,171]. Recency proportions were typically presented as [number recent]/[number tested with recency assays] × 100%, with no articles reporting original results that discussed a strategic choice of denominator to improve validity. While 10 articles compared methods for addressing misclassification or referred to the challenges of assay misclassification as a remaining limitation in their analysis, most did not include this consideration in their report [127,148,151,153,155,161,163,166,168,169].
Evidence Documenting Assay Performance, Algorithm Performance, or Incidence Estimation Methodologies
Of the 91 studies devoted to assays, algorithms, or methods of incidence estimation, 59 evaluated the performance of 1 or more assays. Among these, 46 (78%) evaluated avidity assays (eg, Maxim HIV-1 LAg-Avidity EIA), 23 (39%) evaluated BED assays (eg, Sedia BED HIV-1 Incidence EIA), 4 (7%) evaluated rapid assays (Asanté HIV-1 Rapid Recency Assay, or Maxim Swift HIV Recent Infection Assay), and 3 (5%) evaluated comparative antigen reactivity assays (eg, Geenius HIV-1/2 Confirmatory–modified protocol). These studies reported various aspects of assay performance, including FRR (38/59), MDRI (31/59), sensitivity and specificity (10/59), and correlation of results between different assays (12/59). In addition, 20 of the studies explored a range of assay cut-off thresholds, to identify a cut-off that would achieve optimal FRR and MDRI results. Details of which studies are in which categories can be found in Multimedia Appendix 6.
While 16 of the 59 studies utilized standard sample panels from the Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) or other sources, the majority evaluated assays against patients from 1 or more geographic regions, including Africa (20/59), North America (13/59, 12 from the United States), western Europe (11/59), east Asia (8/59), the Caribbean (3/59, Trinidad), eastern Europe (1/59, Estonia), and south Asia (1/59, Iran; Multimedia Appendix 6). Importantly, these studies found that key performance parameters, such as FRR, MDRI, optical density, or avidity index, were impacted by a wide range of patient characteristics, including ART treatment status (18/59); HIV viral load levels (16/59); HIV subtype (10/59); elite controllers or slow progressors (8/59); low CD4 count, advanced infection, or AIDS (6/59); sex (4/59); risk factors such as male sex, injection drug use, or sex work (2/59); postpartum status (1/59); and sample type (plasma vs dried blood spot, 1/59).
In addition to the studies examining assay performance, another 19 of the 91 examined the performance of algorithms that included 1 or more recency assays (Multimedia Appendix 6). Among these, 11/19 (58%) evaluated algorithm FRR, 9/19 (47%) evaluated MDRI or other window parameters, and 9/19 (47%) evaluated algorithm impact on incidence estimates. The studies evaluated algorithm performance among patients from a variety of regions, including Africa (12/19), North America (6/19, 5 United States and 1 Mexico), South America (1/19, Brazil), western Europe (1/19), and east Asia (1/19). Among these studies, algorithm performance was found to be impacted by patients’ ART status (4/19), HIV viral load (3/19), CD4 count or advanced infection (3/19), and HIV subtype (2/19).
While most of these studies examined only 1 or a handful of algorithms, those by Laeyendecker et al [75,76], Kassanjee et al [54], Konikoff et al [24], and Brookmeyer et al [41] explored the performance of hundreds to thousands of potential algorithm configurations, involving numerous combinations of cut-off values across several assays applied to a common set of samples, to identify optimal algorithms for the specific assays used.
Finally, 13 of the 91 studies addressed various aspects of methodologies for estimating incidence using recency assays. While these studies represented a diverse assemblage, they fell broadly into several categories. A total of 5 presented statistical methodologies for managing uncertainties in the window periods of recency assays [12,33,40,67,70]; 3 provided a comparison of the results of assay-based estimates of HIV incidence with estimates using other incidence methods such as longitudinal surveys, acute infection (RNA positive/antibody negative) staging within cohorts, and dynamic models such as UNAIDS Estimation Projection Package (EPP)/Spectrum and Thembisa [39,43,57]; 2 studies presented novel statistical methods for estimating HIV incidence from the use of recency assays in cross-sectional surveys [31,48]. Bao et al [46] adapted the UNAIDS EPP to incorporate data from incidence assays, to narrow the uncertainty intervals of estimated incidence. The 2015 meeting report from the WHO Working Group on HIV Incidence Assays reviewed various early efforts to estimate incidence through HIV case surveillance using recency assays [18]. Finally, Welte et al [3] proposed a set of optimal characteristics for recency assays as a potential guide for the future development of new assays for estimating incidence.
Discussion
Principal Findings
Despite the widespread use of HIV recency assays for both HIV incidence estimation and non-incidence surveillance use cases, evidence on validated and accurate uses of recency assays for non-incidence surveillance remains weak. Based on the evidence identified through this review, there is a clear rationale for the use of recency assays for population-level HIV incidence estimation, and convincing evidence regarding best practices for this use.
In the meantime, while already in wide use, use of recency assays for non-incidence use cases remains questionable. Godin and colleagues [181] recently presented results of a simulation analysis to compare the accuracy of various HIV recency indicators as a proxy for incidence, using different denominators for the proportions calculated. (As they did not report any original recency testing results, this paper was not eligible for inclusion in this review.) In this comparison, the authors found that recency indicators calculated as the [number of recent results]/[number of HIV-positive tests]—as is commonly used among the studies contained in this review—was not, in fact, a satisfactory proxy for HIV incidence, and in some cases even resulted in identifying temporal trends in an opposite direction from the incidence trend. Godin et al [181] suggested that estimating the proportion recent as the [number of recent results]/[number of people at risk for HIV acquisition] was more indicative of incidence trends; however, this method of calculating recency in non-incidence use cases was not reported by any of the studies or programs found in our review.
There were 24 analyses included in this review that assessed predictors or correlates of recent infection. Implied in these analyses is an assumption that subgroups with significantly greater odds of recent infection are currently experiencing more HIV transmission than other subgroups, and that the disparity could be intervened upon by targeting public health prevention efforts to these subgroups. Our analysis, which identified scant evidence validating this methodological assumption, highlights the wasteful expenditures in the public health response to HIV. Misidentification of clusters, hotspots, and other imprecisely defined proxy indicators of incidence through recency testing may result in misdirected or poorly designed prevention plans and missed opportunities for targeting limited resources. Simple calculation of a “proportion recent” in an HIV testing setting may be difficult to interpret, and is affected by both the denominator used (ie, new HIV diagnoses versus people at risk for HIV) and changes in testing coverage and frequency of diagnostic testing in the population. An unexpectedly high or rising proportion of new diagnoses being classified as recent infections may indicate either (1) ongoing transmission or (2) that the testing program is capturing more recent infections because most older infections have already been diagnosed. More evidence about the appropriate interpretation and use of these types of indicators is necessary.
More reports of countries or studies using HIV recency assays for identification and mapping of geographic hotspots will likely emerge as a result of the US President’s Emergency Plan for AIDS Relief (PEPFAR) “TRACE” initiative (Tracking with Recency Assays to Control the Epidemic) in the near future. Beginning in fiscal year 2019, PEPFAR funded 16 countries (El Salvador, Eswatini, Ethiopia, Guatemala, Kenya, Lesotho, Malawi, Namibia, Nicaragua, Panama, Rwanda, Tanzania, Uganda, Vietnam, Zambia, and Zimbabwe) who are nearing the 90-90-90 targets to introduce the TRACE initiative [182]. Through TRACE, a lateral flow rapid recency assay is conducted as a supplementary test in routine HIV testing services or within HIV case surveillance—combined with viral load results where possible—to detect recent infection among people newly diagnosed with HIV in all (or most) facility- and community-based testing sites in a country to drive prevention and care planning. Hopefully findings from these efforts will be forthcoming in the literature, along with further evidence validating the use of recency assays for this purpose.
Limitations
There are several limitations to our systematic review. First, given our search strategy many of the articles included in this review involved findings relevant to the performance of specific commercially available recency assays. However, some of those assays (eg, the Sedia BED HIV-1 Incidence EIA) are technically available but no longer in wide use, due to inferior performance for HIV incidence estimation compared with other available assays. Further, some assays included in this review are not available in all countries globally. Second, as with all systematic reviews, our review was time limited. Therefore, it is possible that some relevant literature that has been recently published or that was missed by our choice of search terms in the prespecified protocol is not included in this review.
Conclusions
Surveillance strategies to accurately estimate HIV incidence or detect patterns of recent transmission are critical to global efforts to end the HIV epidemic. However, these calculations are only useful if they are timely and accurate, with potential biases clearly defined. Calculations that are considerably higher or lower than reality may result in incorrect interpretations of the data, and misalignment of resources as a result. This review found ample evidence to guide the use of recency assays in population-based surveys to accurately estimate HIV incidence. However, more research is needed to validate their use within HIV testing services and to explore best practices for calculating HIV recency indicators other than incidence to ensure that findings from recency testing can be accurately interpreted to guide prioritization of public health programming.
Abbreviations
- ART
antiretroviral therapy
- CEPHIA
Consortium for the Evaluation and Performance of HIV Incidence Assays
- EPP
Estimation Projection Package
- FIND
Foundation for Innovative Diagnostics
- FRR
false recent rate
- LAg
limiting antigen
- MDRI
mean duration of recent infection
- PHIA
Population-based HIV Impact Assessment
- RITA
recent infection testing algorithm
- TRI
test for recent infection
- UNAIDS
United Nations Programme on HIV/AIDS
- WHO
World Health Organization
Search sets and terms used for title, abstract, and MeSH terms/author keyword searches.
Search code.
Websites searched for eligible grey literature during the review.
Assessing the utility of HIV recency assays for surveillance purposes: systematic review protocol.
Sources identified during a systematic review of the literature (as described in the 'Methods' section) are organized below. Sources are ordered by (1) literature type (peer-reviewed vs gray), then (2) strength of evidence (highest to lowest), and then (3) last name of the first author (alphabetical).
Evidence included in this review, including the strength rating and topic of focus for each item.
Footnotes
Conflicts of Interest: SNF and EG have received consulting income and research support from Sedia Biosciences Corporation, Gilead Sciences, and through the CDC-funded TRACE program, as a subcontract from the University of California, San Francisco.
References
- 1.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance . WHO. Geneva: 2011. [2022-01-21]. When and How to Use Assays for Recent Infection to Estimate HIV Incidence at a Population Level. https://www.who.int/diagnostics_laboratory/hiv_incidence_may13_final.pdf . [Google Scholar]
- 2.World Health Organization . Geneva: World Health Organization; 2009. Oct 05, [2022-01-22]. Meeting Report: WHO Technical Working Group on HIV Incidence Assays (Cape Town, South Africa, 16 and 17 July) https://www.who.int/diagnostics_laboratory/links/hiviwg_capetown_07_09.pdf . [Google Scholar]
- 3.Welte A, McWalter T A, Laeyendecker O, Hallett T B. Using tests for recent infection to estimate incidence: problems and prospects for HIV. Euro Surveill. 2010 Jun 17;15(24) http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19589 . [PMC free article] [PubMed] [Google Scholar]
- 4.Kassanjee Reshma, McWalter Thomas A, Bärnighausen Till, Welte Alex. A new general biomarker-based incidence estimator. Epidemiology. 2012 Sep;23(5):721–8. doi: 10.1097/EDE.0b013e3182576c07. http://europepmc.org/abstract/MED/22627902 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FIND Target Product Profile for Tests for Recent HIV Infection. Find. 2017. Feb, [2022-01-21]. https://www.finddx.org/wp-content/uploads/2019/03/HIV-Incidence-TPP-FIND-2017.pdf .
- 6.Chaillon Antoine, Le Vu Stéphane, Brunet Sylvie, Gras Guillaume, Bastides Frédéric, Bernard Louis, Meyer Laurence, Barin Francis. Decreased specificity of an assay for recent infection in HIV-1-infected patients on highly active antiretroviral treatment: implications for incidence estimates. Clin Vaccine Immunol. 2012 Aug;19(8):1248–53. doi: 10.1128/CVI.00120-12. https://journals.asm.org/doi/10.1128/CVI.00120-12?url_ver=Z39.88-2003=ori:rid:crossref.org=cr_pub%3dpubmed .CVI.00120-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Longosz Andrew F, Serwadda David, Nalugoda Fred, Kigozi Godfrey, Franco Veronica, Gray Ronald H, Quinn Thomas C, Eshleman Susan H, Laeyendecker Oliver. Impact of HIV subtype on performance of the limiting antigen-avidity enzyme immunoassay, the bio-rad avidity assay, and the BED capture immunoassay in Rakai, Uganda. AIDS Res Hum Retroviruses. 2014 Apr;30(4):339–44. doi: 10.1089/aid.2013.0169. http://europepmc.org/abstract/MED/24083837 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Keating Sheila M, Hanson Debra, Lebedeva Mila, Laeyendecker Oliver, Ali-Napo N'ko L, Owen S Michele, Stramer Susan L, Moore Richard D, Norris Philip J, Busch Michael P. Lower-sensitivity and avidity modifications of the vitros anti-HIV 1+2 assay for detection of recent HIV infections and incidence estimation. J Clin Microbiol. 2012 Dec;50(12):3968–76. doi: 10.1128/JCM.01454-12. http://europepmc.org/abstract/MED/23035182 .JCM.01454-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laeyendecker Oliver, Brookmeyer Ron, Oliver Amy E, Mullis Caroline E, Eaton Kevin P, Mueller Amy C, Jacobson Lisa P, Margolick Joseph B, Brown Joelle, Rinaldo Charles R, Quinn Thomas C, Eshleman Susan H, Multicenter Aids Cohort Study Macs Factors associated with incorrect identification of recent HIV infection using the BED capture immunoassay. AIDS Res Hum Retroviruses. 2012 Aug;28(8):816–22. doi: 10.1089/aid.2011.0258. http://europepmc.org/abstract/MED/22014036 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel Jessica M, Piwowar-Manning Estelle, Debevec Barbara, Walsky Tamara, Schlusser Katherine, Laeyendecker Oliver, Wilson Ethan A, McCauley Marybeth, Gamble Theresa, Tegha Gerald, Soko Dean, Kumwenda Johnstone, Hosseinipour Mina C, Chen Ying Q, Cohen Myron S, Eshleman Susan H. Brief Report: Impact of Early Antiretroviral Therapy on the Performance of HIV Rapid Tests and HIV Incidence Assays. J Acquir Immune Defic Syndr. 2017 Aug 01;75(4):426–430. doi: 10.1097/QAI.0000000000001421. http://europepmc.org/abstract/MED/28471839 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klock Ethan, Mwinnya George, Eller Leigh Anne, Fernandez Reinaldo E, Kibuuka Hannah, Nitayaphan Sorachai, Kosgei Josphat, Moore Richard D, Robb Merlin, Eshleman Susan H, Laeyendecker Oliver. Impact of Early Antiretroviral Treatment Initiation on Performance of Cross-Sectional Incidence Assays. AIDS Res Hum Retroviruses. 2020 Jul;36(7):583–589. doi: 10.1089/AID.2019.0286. http://europepmc.org/abstract/MED/32295382 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . WHO. Geneva: 2018. [2022-01-22]. Meeting Report: WHO Working Group on HIV Incidence Measurement and Data Use. https://apps.who.int/iris/bitstream/handle/10665/272940/WHO-CDS-HIV-18.9-eng.pdf . [Google Scholar]
- 13.Gonese Elizabeth, Kilmarx Peter H, van Schalkwyk Cari, Grebe Eduard, Mutasa Kuda, Ntozini Robert, Parekh Bharat, Dobbs Trudy, Duong Pottinger Yen, Masciotra Silvina, Owen Michele, Nachega Jean B, van Zyl Gert, Hargrove John W. Evaluation of the Performance of Three Biomarker Assays for Recent HIV Infection Using a Well-Characterized HIV-1 Subtype C Incidence Cohort. AIDS Res Hum Retroviruses. 2019 Jul;35(7):615–627. doi: 10.1089/AID.2019.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grebe E, Murphy G, Keating SM, Hampton D, Busch MP, Facente SN, Marson K, Longosz A, Eshleman SH, Welte A, Parkin N, Busch MP, Quinn TC, Laeyendecker O. Impact of HIV-1 Subtype and Sex on Sedia Limiting Antigen Avidity Assay Performance. Conference on Retroviruses and Opportunistic Infections (CROI); 2019; Seattle, WA. 2019. https://www.croiconference.org/wp-content/uploads/sites/2/posters/2019/1430_Grebe_0942.pdf . [Google Scholar]
- 15.World Health Organization. Joint United Nations Programme on HIV/AIDS (UNAIDS) Geneva, Switzerland: WHO; 2013. May 30, [2022-01-21]. WHO/UNAIDS Technical Update on HIV Incidence Assays for Surveillance and Epidemic Monitoring. https://www.unaids.org/sites/default/files/sub_landing/files/2013_TechnicalUpdate_WHO_UNAIDS_HIVincidenceAssays.pdf . [Google Scholar]
- 16.Joint United Nations Programme on HIV/AIDS (UNAIDS) World Health Organization . WHO. Geneva, Switzerland: WHO; 2015. [2022-01-21]. Technical Update on HIV Incidence Assays for Surveillance and Monitoring Purposes. https://www.unaids.org/sites/default/files/media_asset/HIVincidenceassayssurveillancemonitoring_en.pdf . [Google Scholar]
- 17.Global HIV Strategic Information Working Group . UNAIDS. Geneva, Switzerland: WHO; 2018. [2022-01-21]. Recent Infection Testing Algorithm Technical Update: Applications for HIV surveillance and programme monitoring. https://www.unaids.org/sites/default/files/media_asset/infection_testing_algorithm_en.pdf . [Google Scholar]
- 18.World Health Organization. UNAIDS . Geneva, Switzerland: WHO; 2017. [2022-01-22]. WHO Working Group on HIV incidence assays: Estimating HIV incidence using HIV case surveillance: Meeting report, Glion, Switzerland, 10-11 December 2015. https://www.who.int/diagnostics_laboratory/links/170321_who_working_group_on_hiv_incidence_assays_meeting_report_06.pdf . [Google Scholar]
- 19.Mammone Alessia, Pezzotti Patrizio, Angeletti Claudio, Orchi Nicoletta, Carboni Angela, Navarra Assunta, Sciarrone Maria R, Sias Catia, Puro Vincenzo, Guasticchi Gabriella, Ippolito Giuseppe, Borgia Piero, Girardi Enrico, SENDIH Study Group HIV incidence estimate combining HIV/AIDS surveillance, testing history information and HIV test to identify recent infections in Lazio, Italy. BMC Infect Dis. 2012 Mar 20;12:65. doi: 10.1186/1471-2334-12-65. https://bmcinfectdis.biomedcentral.com/articles/10.1186/1471-2334-12-65 .1471-2334-12-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Qiyu, Wang Yikui, Liu Jing, Duan Xing, Chen Meibin, Yang Jin, Yang Tao, Yang Shijiang, Guan Peng, Jiang Yan, Duan Song, Wang Jibao, Jin Cong. Identifying major drivers of incident HIV infection using recent infection testing algorithms (RITAs) to precisely inform targeted prevention. Int J Infect Dis. 2020 Dec;101:131–137. doi: 10.1016/j.ijid.2020.09.1421. https://linkinghub.elsevier.com/retrieve/pii/S1201-9712(20)32137-8 .S1201-9712(20)32137-8 [DOI] [PubMed] [Google Scholar]
- 21.Laeyendecker Oliver, Brookmeyer Ron, Mullis Caroline E, Donnell Deborah, Lingappa Jairam, Celum Connie, Baeten Jared M, Campbell Mary S, Essex Max, de Bruyn Guy, Farquhar Carey, Quinn Thomas C, Eshleman Susan H, Partners in Prevention HSV/HIV Transmission Study Team Specificity of four laboratory approaches for cross-sectional HIV incidence determination: analysis of samples from adults with known nonrecent HIV infection from five African countries. AIDS Res Hum Retroviruses. 2012 Oct;28(10):1177–83. doi: 10.1089/aid.2011.0341. http://europepmc.org/abstract/MED/22283149 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cousins Matthew M, Konikoff Jacob, Sabin Devin, Khaki Leila, Longosz Andrew F, Laeyendecker Oliver, Celum Connie, Buchbinder Susan P, Seage George R, Kirk Gregory D, Moore Richard D, Mehta Shruti H, Margolick Joseph B, Brown Joelle, Mayer Kenneth H, Kobin Beryl A, Wheeler Darrell, Justman Jessica E, Hodder Sally L, Quinn Thomas C, Brookmeyer Ron, Eshleman Susan H. A comparison of two measures of HIV diversity in multi-assay algorithms for HIV incidence estimation. PLoS One. 2014;9(6):e101043. doi: 10.1371/journal.pone.0101043. https://dx.plos.org/10.1371/journal.pone.0101043 .PONE-D-14-04683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karatzas-Delgado Eli F, Ruiz-González Verónica, García-Cisneros Santa, Olamendi-Portugal María L, Herrera-Ortiz Antonia, López-Gatell Hugo, González-Rodríguez Andrea, Sánchez-Alemán Miguel A. Evaluation of an HIV recent infection testing algorithm with serological assays among men who have sex with men in Mexico. J Infect Public Health. 2020 Apr;13(4):509–513. doi: 10.1016/j.jiph.2019.11.002. https://linkinghub.elsevier.com/retrieve/pii/S1876-0341(19)30339-9 .S1876-0341(19)30339-9 [DOI] [PubMed] [Google Scholar]
- 24.Konikoff J, Brookmeyer R, Longosz AF, Cousins MM, Celum C, Buchbinder SP, Seage GR, Kirk GD, Moore RD, Mehta SH, Margolick JB, Brown J, Mayer KH, Koblin BA, Justman JE, Hodder SL, Quinn TC, Eshleman SH, Laeyendecker O. Performance of a limiting-antigen avidity enzyme immunoassay for cross-sectional estimation of HIV incidence in the United States. PLoS One. 2013 Dec 27;8(12):e82772. doi: 10.1371/journal.pone.0082772. https://dx.plos.org/10.1371/journal.pone.0082772 .PONE-D-13-35607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim Andrea A, Rehle Thomas. Short Communication: Assessing Estimates of HIV Incidence with a Recent Infection Testing Algorithm That Includes Viral Load Testing and Exposure to Antiretroviral Therapy. AIDS Res Hum Retroviruses. 2018 Oct;34(10):863–866. doi: 10.1089/AID.2017.0316. [DOI] [PubMed] [Google Scholar]
- 26.Shah Neha S, Duong Yen T, Le Linh-Vi, Tuan Nguyen Anh, Parekh Bharat S, Ha Hoang Thi Thanh, Pham Quang Duy, Cuc Cao Thi Thu, Dobbs Trudy, Tram Tran Hong, Lien Truong Thi Xuan, Wagar Nick, Yang Chunfu, Martin Amy, Wolfe Mitchell, Hien Nguyen Tran, Kim Andrea A. Estimating False-Recent Classification for the Limiting-Antigen Avidity EIA and BED-Capture Enzyme Immunoassay in Vietnam: Implications for HIV-1 Incidence Estimates. AIDS Res Hum Retroviruses. 2017 Jun;33(6):546–554. doi: 10.1089/AID.2016.0203. http://europepmc.org/abstract/MED/28193090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Keating Sheila M, Kassanjee Reshma, Lebedeva Mila, Facente Shelley N, MacArthur Jeffrey C, Grebe Eduard, Murphy Gary, Welte Alex, Martin Jeffrey N, Little Susan, Price Matthew A, Kallas Esper G, Busch Michael P, Pilcher Christopher D. Performance of the Bio-Rad Geenius HIV1/2 Supplemental Assay in Detecting Recent HIV Infection and Calculating Population Incidence. J Acquir Immune Defic Syndr. 2016 Dec 15;73(5):581–588. doi: 10.1097/QAI.0000000000001146. http://europepmc.org/abstract/MED/27509247 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duong Yen T, Kassanjee Reshma, Welte Alex, Morgan Meade, De Anindya, Dobbs Trudy, Rottinghaus Erin, Nkengasong John, Curlin Marcel E, Kittinunvorakoon Chonticha, Raengsakulrach Boonyos, Martin Michael, Choopanya Kachit, Vanichseni Suphak, Jiang Yan, Qiu Maofeng, Yu Haiying, Hao Yan, Shah Neha, Le Linh-Vi, Kim Andrea A, Nguyen Tuan Anh, Ampofo William, Parekh Bharat S. Recalibration of the limiting antigen avidity EIA to determine mean duration of recent infection in divergent HIV-1 subtypes. PLoS One. 2015;10(2):e0114947. doi: 10.1371/journal.pone.0114947. https://dx.plos.org/10.1371/journal.pone.0114947 .PONE-D-14-30403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.FIND. World Health Organization WHO. 2016. [2022-01-21]. https://apps.who.int/iris/bitstream/handle/10665/254868/WHO-HIV-2017.04-eng.pdf .
- 30.McNicholl Janet M, McDougal J Steven, Wasinrapee Punneeporn, Branson Bernard M, Martin Michael, Tappero Jordan W, Mock Philip A, Green Timothy A, Hu Dale J, Parekh Bharat, Thai-U.S. BED Assay Validation Working Group Assessment of BED HIV-1 incidence assay in seroconverter cohorts: effect of individuals with long-term infection and importance of stable incidence. PLoS One. 2011 Mar 04;6(3):e14748. doi: 10.1371/journal.pone.0014748. https://dx.plos.org/10.1371/journal.pone.0014748 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hargrove John, van Schalkwyk Cari, Eastwood Hayden. BED estimates of HIV incidence: resolving the differences, making things simpler. PLoS One. 2012;7(1):e29736. doi: 10.1371/journal.pone.0029736. https://dx.plos.org/10.1371/journal.pone.0029736 .PONE-D-11-13230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schlusser Katherine E, Pilcher Christopher, Kallas Esper G, Santos Breno R, Deeks Steven G, Facente Shelley, Keating Sheila M, Busch Michael P, Murphy Gary, Welte Alex, Quinn Thomas, Eshleman Susan H, Laeyendecker Oliver. Comparison of cross-sectional HIV incidence assay results from dried blood spots and plasma. PLoS One. 2017;12(2):e0172283. doi: 10.1371/journal.pone.0172283. https://dx.plos.org/10.1371/journal.pone.0172283 .PONE-D-16-25285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Yuejia, Laeyendecker Oliver, Wang Rui. Cross-sectional human immunodeficiency virus incidence estimation accounting for heterogeneity across communities. Biometrics. 2019 Sep;75(3):1017–1028. doi: 10.1111/biom.13046. http://europepmc.org/abstract/MED/30746695 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duong Yen T, Qiu Maofeng, De Anindya K, Jackson Keisha, Dobbs Trudy, Kim Andrea A, Nkengasong John N, Parekh Bharat S. Detection of recent HIV-1 infection using a new limiting-antigen avidity assay: potential for HIV-1 incidence estimates and avidity maturation studies. PLoS One. 2012;7(3):e33328. doi: 10.1371/journal.pone.0033328. https://dx.plos.org/10.1371/journal.pone.0033328 .PONE-D-11-24174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parekh Bharat S, Hanson Debra L, Hargrove John, Branson Bernard, Green Timothy, Dobbs Trudy, Constantine Niel, Overbaugh Julie, McDougal J Steven. Determination of mean recency period for estimation of HIV type 1 Incidence with the BED-capture EIA in persons infected with diverse subtypes. AIDS Res Hum Retroviruses. 2011 Mar;27(3):265–73. doi: 10.1089/aid.2010.0159. [DOI] [PubMed] [Google Scholar]
- 36.Kirkpatrick Allison R, Patel Eshan U, Celum Connie L, Moore Richard D, Blankson Joel N, Mehta Shruti H, Kirk Gregory D, Margolick Joseph B, Quinn Thomas C, Eshleman Susan H, Laeyendecker Oliver. Development and Evaluation of a Modified Fourth-Generation Human Immunodeficiency Virus Enzyme Immunoassay for Cross-Sectional Incidence Estimation in Clade B Populations. AIDS Res Hum Retroviruses. 2016 Aug;32(8):756–62. doi: 10.1089/AID.2015.0198. http://europepmc.org/abstract/MED/26988426 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Keating Sheila M, Rountree Wes, Grebe Eduard, Pappas Andrea L, Stone Mars, Hampton Dylan, Todd Christopher A, Poniewierski Marek S, Sanchez Ana, Porth Cassandra G, Denny Thomas N, Busch Michael P, EQAPOL Limiting Antigen (LAg) Incidence Assay External Quality Assurance (EQA) Program Development of an international external quality assurance program for HIV-1 incidence using the Limiting Antigen Avidity assay. PLoS One. 2019;14(9):e0222290. doi: 10.1371/journal.pone.0222290. https://dx.plos.org/10.1371/journal.pone.0222290 .PONE-D-19-14662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Braunstein Sarah L, Nash Denis, Kim Andrea A, Ford Ken, Mwambarangwe Lambert, Ingabire Chantal M, Vyankandondera Joseph, van de Wijgert Janneke H H M. Dual testing algorithm of BED-CEIA and AxSYM Avidity Index assays performs best in identifying recent HIV infection in a sample of Rwandan sex workers. PLoS One. 2011 Apr 12;6(4):e18402. doi: 10.1371/journal.pone.0018402. https://dx.plos.org/10.1371/journal.pone.0018402 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim Andrea A, Hallett Timothy, Stover John, Gouws Eleanor, Musinguzi Joshua, Mureithi Patrick K, Bunnell Rebecca, Hargrove John, Mermin Jonathan, Kaiser Reinhard K, Barsigo Anne, Ghys Peter D. Estimating HIV incidence among adults in Kenya and Uganda: a systematic comparison of multiple methods. PLoS One. 2011 Mar 07;6(3):e17535. doi: 10.1371/journal.pone.0017535. https://dx.plos.org/10.1371/journal.pone.0017535 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mastro Timothy D, Kim Andrea A, Hallett Timothy, Rehle Thomas, Welte Alex, Laeyendecker Oliver, Oluoch Tom, Garcia-Calleja Jesus M. Estimating HIV Incidence in Populations Using Tests for Recent Infection: Issues, Challenges and the Way Forward. J HIV AIDS Surveill Epidemiol. 2010 Jan 01;2(1):1–14. http://europepmc.org/abstract/MED/21743821 . [PMC free article] [PubMed] [Google Scholar]
- 41.Brookmeyer Ron, Konikoff Jacob, Laeyendecker Oliver, Eshleman Susan H. Estimation of HIV incidence using multiple biomarkers. Am J Epidemiol. 2013 Feb 01;177(3):264–72. doi: 10.1093/aje/kws436. http://europepmc.org/abstract/MED/23302151 .kws436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laeyendecker Oliver, Brookmeyer Ron, Cousins Matthew M, Mullis Caroline E, Konikoff Jacob, Donnell Deborah, Celum Connie, Buchbinder Susan P, Seage George R, Kirk Gregory D, Mehta Shruti H, Astemborski Jacquie, Jacobson Lisa P, Margolick Joseph B, Brown Joelle, Quinn Thomas C, Eshleman Susan H. HIV incidence determination in the United States: a multiassay approach. J Infect Dis. 2013 Jan 15;207(2):232–9. doi: 10.1093/infdis/jis659. http://europepmc.org/abstract/MED/23129760 .jis659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vermeulen Marion, Chowdhury Dhuly, Swanevelder Ronel, Grebe Eduard, Brambilla Donald, Jentsch Ute, Busch Michael, Van Zyl Gert, Murphy Edward L, REDS-III International Program South Africa HIV incidence in South African blood donors from 2012 to 2016: a comparison of estimation methods. Vox Sang. 2021 Jan;116(1):71–80. doi: 10.1111/vox.12987. http://europepmc.org/abstract/MED/32762088 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Longosz Andrew F, Morrison Charles S, Chen Pai-Lien, Arts Eric, Nankya Immaculate, Salata Robert A, Franco Veronica, Quinn Thomas C, Eshleman Susan H, Laeyendecker Oliver. Immune responses in Ugandan women infected with subtypes A and D HIV using the BED capture immunoassay and an antibody avidity assay. J Acquir Immune Defic Syndr. 2014 Apr 01;65(4):390–6. doi: 10.1097/QAI.0000000000000006. http://europepmc.org/abstract/MED/24583613 .00126334-201404010-00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hauser Andrea, Santos-Hoevener Claudia, Meixenberger Karolin, Zimmermann Ruth, Somogyi Sybille, Fiedler Stefan, Hofmann Alexandra, Bartmeyer Barbara, Jansen Klaus, Hamouda Osamah, Bannert Norbert, Kuecherer Claudia. Improved testing of recent HIV-1 infections with the BioRad avidity assay compared to the limiting antigen avidity assay and BED Capture enzyme immunoassay: evaluation using reference sample panels from the German Seroconverter Cohort. PLoS One. 2014;9(6):e98038. doi: 10.1371/journal.pone.0098038. https://dx.plos.org/10.1371/journal.pone.0098038 .PONE-D-14-02780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bao Le, Ye Jingyi, Hallett Timothy B. Incorporating incidence information within the UNAIDS Estimation and Projection Package framework: a study based on simulated incidence assay data. AIDS. 2014 Nov;28 Suppl 4:S515–22. doi: 10.1097/QAD.0000000000000434. http://europepmc.org/abstract/MED/25406754 .00002030-201411004-00013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grebe Eduard, Welte Alex, Hall Jake, Keating Sheila M, Facente Shelley N, Marson Kara, Martin Jeffrey N, Little Susan J, Price Matthew A, Kallas Esper G, Busch Michael P, Pilcher Christopher D, Murphy Gary. Infection Staging and Incidence Surveillance Applications of High Dynamic Range Diagnostic Immuno-Assay Platforms. J Acquir Immune Defic Syndr. 2017 Dec 15;76(5):547–555. doi: 10.1097/QAI.0000000000001537. http://europepmc.org/abstract/MED/28914669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mahiane Severin Guy, Fiamma Agnès, Auvert Bertran. Mixture models for calibrating the BED for HIV incidence testing. Stat Med. 2014 May 10;33(10):1767–83. doi: 10.1002/sim.6059. http://europepmc.org/abstract/MED/24834521 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sempa Joseph B, Welte Alex, Busch Michael P, Hall Jake, Hampton Dylan, Facente Shelley N, Keating Sheila M, Marson Kara, Parkin Neil, Pilcher Christopher D, Murphy Gary, Grebe Eduard, Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) Performance comparison of the Maxim and Sedia Limiting Antigen Avidity assays for HIV incidence surveillance. PLoS One. 2019;14(7):e0220345. doi: 10.1371/journal.pone.0220345. https://dx.plos.org/10.1371/journal.pone.0220345 .PONE-D-19-11719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serhir Bouchra, Hamel Denis, Doualla-Bell Florence, Routy Jean Pierre, Beaulac Sylvie-Nancy, Legault Mario, Fauvel Micheline, Tremblay Cécile, Quebec Primary HIV infection study group Performance of Bio-Rad and Limiting Antigen Avidity Assays in Detecting Recent HIV Infections Using the Quebec Primary HIV-1 Infection Cohort. PLoS One. 2016;11(5):e0156023. doi: 10.1371/journal.pone.0156023. https://dx.plos.org/10.1371/journal.pone.0156023 .PONE-D-16-00643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schlusser Katherine E, Konikoff Jacob, Kirkpatrick Allison R, Morrison Charles, Chipato Tsungai, Chen Pai-Lien, Munjoma Marshall, Eshleman Susan H, Laeyendecker Oliver. Short Communication: Comparison of Maxim and Sedia Limiting Antigen Assay Performance for Measuring HIV Incidence. AIDS Res Hum Retroviruses. 2017 Jun;33(6):555–557. doi: 10.1089/aid.2016.0245. http://europepmc.org/abstract/MED/28318310 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu Li, Laeyendecker Oliver, Wendel Sarah K, Liang Fuxiong, Liu Wei, Wang Xueyan, Wang Lu, Pang Xianwu, Fang Zhongliao. Short Communication: Low False Recent Rate of Limiting-Antigen Avidity Assay Among Long-Term Infected Subjects from Guangxi, China. AIDS Res Hum Retroviruses. 2015 Dec;31(12):1247–9. doi: 10.1089/aid.2015.0097. http://europepmc.org/abstract/MED/26331573 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Huik Kristi, Soodla Pilleriin, Pauskar Merit, Owen S Michele, Luo Wei, Murphy Gary, Jõgeda Ene-Ly, Kallas Eveli, Rajasaar Heli, Avi Radko, Masciotra Silvina, Lutsar Irja, CASCADE Collaboration in the EuroCoord The concordance of the limiting antigen and the Bio-Rad avidity assays in persons from Estonia infected mainly with HIV-1 CRF06_cpx. PLoS One. 2019;14(5):e0217048. doi: 10.1371/journal.pone.0217048. https://dx.plos.org/10.1371/journal.pone.0217048 .PONE-D-18-14395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kassanjee Reshma, Pilcher Christopher D, Busch Michael P, Murphy Gary, Facente Shelley N, Keating Sheila M, Mckinney Elaine, Marson Kara, Price Matthew A, Martin Jeffrey N, Little Susan J, Hecht Frederick M, Kallas Esper G, Welte Alex, Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) Viral load criteria and threshold optimization to improve HIV incidence assay characteristics. AIDS. 2016 Sep 24;30(15):2361–71. doi: 10.1097/QAD.0000000000001209. http://europepmc.org/abstract/MED/27454561 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kassanjee Reshma, Pilcher Christopher D, Keating Sheila M, Facente Shelley N, McKinney Elaine, Price Matthew A, Martin Jeffrey N, Little Susan, Hecht Frederick M, Kallas Esper G, Welte Alex, Busch Michael P, Murphy Gary, Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) Independent assessment of candidate HIV incidence assays on specimens in the CEPHIA repository. AIDS. 2014 Oct 23;28(16):2439–49. doi: 10.1097/QAD.0000000000000429. http://europepmc.org/abstract/MED/25144218 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huerga Helena, Shiferie Fisseha, Grebe Eduard, Giuliani Ruggero, Farhat Jihane Ben, Van-Cutsem Gilles, Cohen Karen. A comparison of self-report and antiretroviral detection to inform estimates of antiretroviral therapy coverage, viral load suppression and HIV incidence in Kwazulu-Natal, South Africa. BMC Infect Dis. 2017 Sep 29;17(1):653. doi: 10.1186/s12879-017-2740-y. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2740-y .10.1186/s12879-017-2740-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rehle Thomas, Johnson Leigh, Hallett Timothy, Mahy Mary, Kim Andrea, Odido Helen, Onoya Dorina, Jooste Sean, Shisana Olive, Puren Adrian, Parekh Bharat, Stover John. A Comparison of South African National HIV Incidence Estimates: A Critical Appraisal of Different Methods. PLoS One. 2015;10(7):e0133255. doi: 10.1371/journal.pone.0133255. https://dx.plos.org/10.1371/journal.pone.0133255 .PONE-D-14-55153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Grebe E, Welte A, Hall J, Busch MP, Facente SN, Keating S. Recency staging of HIV infections through routine diagnostic testing [Poster]. Conference on Retroviruses and Opportunistic Infections (CROI); 2017; Boston, MA. 2017. [Google Scholar]
- 59.Ramos E, Ortega J, Daza G, Namking Y, Harb S, Dragavon J, Coombs RW. Use of the Sample-to-Cutoff Ratio (S/CO) to Identify Recency of HIV-1 Infection [Poster]. Conference on Retroviruses and Opportunistic Infections (CROI); 2015; Seattle, WA. 2015. [Google Scholar]
- 60.Grebe E, Vermeulen M, Brits T, Swanevelder R, Jacobs G, Busch MP, Welte A. Performance Validation of the Sedia HIV-1 Limiting Antigen (LAg)-Avidity EIA in South African Blood Donors [Poster]. Conference on Retroviruses and Opportunistic Infections (CROI); 2018; Boston, MA. 2018. [Google Scholar]
- 61.Laeyendecker Oliver, Gray Ronald H, Grabowski M Kate, Reynolds Steven J, Ndyanabo Anthony, Ssekasanvu Joseph, Fernandez Reinaldo E, Wawer Maria J, Serwadda David, Quinn Thomas C. Validation of the Limiting Antigen Avidity Assay to Estimate Level and Trends in HIV Incidence in an A/D Epidemic in Rakai, Uganda. AIDS Res Hum Retroviruses. 2019 Apr;35(4):364–367. doi: 10.1089/AID.2018.0207. http://europepmc.org/abstract/MED/30560723 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verhofstede Chris, Fransen Katrien, Van Den Heuvel Annelies, Van Laethem Kristel, Ruelle Jean, Vancutsem Ellen, Stoffels Karolien, Van den Wijngaert Sigi, Delforge Marie-Luce, Vaira Dolores, Hebberecht Laura, Schauvliege Marlies, Mortier Virginie, Dauwe Kenny, Callens Steven. Decision tree for accurate infection timing in individuals newly diagnosed with HIV-1 infection. BMC Infect Dis. 2017 Nov 29;17(1):738. doi: 10.1186/s12879-017-2850-6. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-017-2850-6 .10.1186/s12879-017-2850-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hassan Jaythoon, Moran Joanne, Murphy Gary, Mason Olivia, Connell Jeff, De Gascun Cillian. Discrimination between recent and non-recent HIV infections using routine diagnostic serological assays. Med Microbiol Immunol. 2019 Oct;208(5):693–702. doi: 10.1007/s00430-019-00590-0.10.1007/s00430-019-00590-0 [DOI] [PubMed] [Google Scholar]
- 64.CEPHIA. Grebe E, Facente SN, Hampton D, Cheng C, Owen R, Keating SM, Pilcher CD, Welte A, Busch MP, Murphy G. Zenodo. San Francisco, CA: 2019. [2021-01-23]. Evaluation of the Asante HIV-1 Rapid Recency Assay. [DOI] [Google Scholar]
- 65.Duong Yen T, Dobbs Trudy, Mavengere Yvonne, Manjengwa Julius, Rottinghaus Erin, Saito Suzue, Bock Naomi, Philip Neena, Justman Jessica, Bicego George, Nkengasong John N, Parekh Bharat S. Field Validation of Limiting-Antigen Avidity Enzyme Immunoassay to Estimate HIV-1 Incidence in Cross-Sectional Survey in Swaziland. AIDS Res Hum Retroviruses. 2019 Oct;35(10):896–905. doi: 10.1089/AID.2018.0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yufenyuy E, Detorio M, Tan X, Shanmugam V, Dobbs T, Kim A. Evaluation of Rapid Tests for Recent HIV Infection: Implications for Real-time Surveillance and Epidemic Control [Poster]. The 10th IAS Conference on HIV Science; 2019; Mexico City, Mexico. 2019. [Google Scholar]
- 67.Carnegie NB. Bootstrap confidence intervals and bias correction in the estimation of HIV incidence from surveillance data with testing for recent infection. Stat Med. 2011 Apr 15;30(8):854–65. doi: 10.1002/sim.4134. [DOI] [PubMed] [Google Scholar]
- 68.Curtis Kelly A, Price Krystin Ambrose, Niedzwiedz Philip, Masciotra Silvina, Owen Michele. Short Communication: Persistence of HIV Antibody Avidity in the Presence of Antiretroviral Therapy. AIDS Res Hum Retroviruses. 2016 Jun;32(6):561–3. doi: 10.1089/AID.2015.0247. [DOI] [PubMed] [Google Scholar]
- 69.Hanson Debra L, Song Ruiguang, Masciotra Silvina, Hernandez Angela, Dobbs Trudy L, Parekh Bharat S, Owen S Michele, Green Timothy A. Mean Recency Period for Estimation of HIV-1 Incidence with the BED-Capture EIA and Bio-Rad Avidity in Persons Diagnosed in the United States with Subtype B. PLoS One. 2016;11(4):e0152327. doi: 10.1371/journal.pone.0152327. https://dx.plos.org/10.1371/journal.pone.0152327 .PONE-D-15-51274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hargrove John, Eastwood Hayden, Mahiane Guy, van Schalkwyk Cari. How should we best estimate the mean recency duration for the BED method? PLoS One. 2012;7(11):e49661. doi: 10.1371/journal.pone.0049661. https://dx.plos.org/10.1371/journal.pone.0049661 .PONE-D-12-07590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hargrove John, Humphrey Jean, ZVITAMBO Study Group Short communication: Simplified estimation of the long-term specificity of the BED assay to improve estimates of HIV incidence. AIDS Res Hum Retroviruses. 2010 Sep;26(9):977–9. doi: 10.1089/aid.2010.0009. [DOI] [PubMed] [Google Scholar]
- 72.Hauser Andrea, Heiden Matthias An der, Meixenberger Karolin, Han Orjin, Fiedler Stefan, Hanke Kirsten, Koppe Uwe, Hofmann Alexandra, Bremer Viviane, Bartmeyer Barbara, Kuecherer Claudia, Bannert Norbert. Evaluation of a BioRad Avidity assay for identification of recent HIV-1 infections using dried serum or plasma spots. J Virol Methods. 2019 Apr;266:114–120. doi: 10.1016/j.jviromet.2019.02.002.S0166-0934(18)30560-3 [DOI] [PubMed] [Google Scholar]
- 73.Hladik Wolfgang, Olara Dennis, Mermin Jonathan, Moore David, Were Willy, Alexander Lorraine, Downing Robert. Effect of CD4+ T cell count and antiretroviral treatment on two serological HIV incidence assays. AIDS Res Hum Retroviruses. 2012 Jan;28(1):95–9. doi: 10.1089/AID.2010.0347. [DOI] [PubMed] [Google Scholar]
- 74.Huang Jiegang, Wang Minlian, Huang Chunyuan, Liang Bingyu, Jiang Junjun, Ning Chuanyi, Zang Ning, Chen Hui, Liu Jie, Chen Rongfeng, Liao Yanyan, Ye Li, Liang Hao. Western Blot-Based Logistic Regression Model for the Identification of Recent HIV-1 Infection: A Promising HIV-1 Surveillance Approach for Resource-Limited Regions. Biomed Res Int. 2018;2018:4390318. doi: 10.1155/2018/4390318. doi: 10.1155/2018/4390318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Laeyendecker Oliver, Konikoff Jacob, Morrison Douglas E, Brookmeyer Ronald, Wang Jing, Celum Connie, Morrison Charles S, Abdool Karim Quarraisha, Pettifor Audrey E, Eshleman Susan H. Identification and validation of a multi-assay algorithm for cross-sectional HIV incidence estimation in populations with subtype C infection. J Int AIDS Soc. 2018 Feb;21(2) doi: 10.1002/jia2.25082. http://europepmc.org/abstract/MED/29489059 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laeyendecker Oliver, Kulich Michal, Donnell Deborah, Komárek Arnošt, Omelka Marek, Mullis Caroline E, Szekeres Greg, Piwowar-Manning Estelle, Fiamma Agnes, Gray Ronald H, Lutalo Tom, Morrison Charles S, Salata Robert A, Chipato Tsungai, Celum Connie, Kahle Erin M, Taha Taha E, Kumwenda Newton I, Karim Quarraisha Abdool, Naranbhai Vivek, Lingappa Jairam R, Sweat Michael D, Coates Thomas, Eshleman Susan H. Development of methods for cross-sectional HIV incidence estimation in a large, community randomized trial. PLoS One. 2013;8(11):e78818. doi: 10.1371/journal.pone.0078818. https://dx.plos.org/10.1371/journal.pone.0078818 .PONE-D-13-25906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Longosz Andrew F, Morrison Charles S, Chen Pai-Lien, Brand Hilmarie H, Arts Eric, Nankya Immaculate, Salata Robert A, Quinn Thomas C, Eshleman Susan H, Laeyendecker Oliver. Comparison of antibody responses to HIV infection in Ugandan women infected with HIV subtypes A and D. AIDS Res Hum Retroviruses. 2015 Apr;31(4):421–7. doi: 10.1089/AID.2014.0081. http://europepmc.org/abstract/MED/25317854 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Moyo Sikhulile, Vandormael Alain, Wilkinson Eduan, Engelbrecht Susan, Gaseitsiwe Simani, Kotokwe Kenanao P, Musonda Rosemary, Tanser Frank, Essex Max, Novitsky Vladimir, de Oliveira Tulio. Analysis of Viral Diversity in Relation to the Recency of HIV-1C Infection in Botswana. PLoS One. 2016;11(8):e0160649. doi: 10.1371/journal.pone.0160649. https://dx.plos.org/10.1371/journal.pone.0160649 .PONE-D-16-11405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moyo Sikhulile, LeCuyer Tessa, Wang Rui, Gaseitsiwe Simani, Weng Jia, Musonda Rosemary, Bussmann Hermann, Mine Madisa, Engelbrecht Susan, Makhema Joseph, Marlink Richard, Baum Marianna K, Novitsky Vladimir, Essex M. Evaluation of the false recent classification rates of multiassay algorithms in estimating HIV type 1 subtype C incidence. AIDS Res Hum Retroviruses. 2014 Jan;30(1):29–36. doi: 10.1089/aid.2013.0055. http://europepmc.org/abstract/MED/23937344 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nikolopoulos GK, Katsoulidou A, Kantzanou M, Rokka C, Tsiara C, Sypsa V, Paraskevis D, PSICHOGIOU M, FRIEDMAN S, HATZAKIS A. Evaluation of the limiting antigen avidity EIA (LAg) in people who inject drugs in Greece. Epidemiol. Infect. 2016 Oct 26;145(2):401–412. doi: 10.1017/s0950268816002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schüpbach Jörg, Gebhardt MD, Scherrer AU, Bisset LR, Niederhauser C, Regenass S, Yerly S, Aubert V, Suter F, Pfister S, Martinetti G, Andreutti C, Klimkait T, Brandenberger M, Günthard Huldrych F, Swiss HIV Cohort Study Simple estimation of incident HIV infection rates in notification cohorts based on window periods of algorithms for evaluation of line-immunoassay result patterns. PLoS One. 2013 Aug 26;8(8):e71662. doi: 10.1371/journal.pone.0071662. https://dx.plos.org/10.1371/journal.pone.0071662 .PONE-D-12-37732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Suligoi Barbara, Regine Vincenza, Raimondo Mariangela, Rodella Anna, Terlenghi Luigina, Caruso Arnaldo, Bagnarelli Patrizia, Capobianchi Maria Rosaria, Zanchetta Nadia, Ghisetti Valeria, Galli Claudio. HIV avidity index performance using a modified fourth-generation immunoassay to detect recent HIV infections. Clin Chem Lab Med. 2017 Oct 26;55(12):2010–2019. doi: 10.1515/cclm-2016-1192./j/cclm.ahead-of-print/cclm-2016-1192/cclm-2016-1192.xml [DOI] [PubMed] [Google Scholar]
- 83.Voetsch Andrew C, Duong Yen T, Stupp Paul, Saito Suzue, McCracken Stephen, Dobbs Trudy, Winterhalter Frieda S, Williams Daniel B, Mengistu Assegid, Mugurungi Owen, Chikwanda Prisca, Musuka Godfrey, Ndongmo Clement B, Dlamini Sindisiwe, Nuwagaba-Biribonwoha Harriet, Pasipamire Munyaradzi, Tegbaru Belete, Eshetu Frehywot, Biraro Samuel, Ward Jennifer, Aibo Dorothy, Kabala Andrew, Mgomella George S, Malewo Optatus, Mushi Jeremiah, Payne Danielle, Mengistu Yohannes, Asiimwe Fred, Shang Judith D, Dokubo Emily K, Eno Laura T, Zoung-Kanyi Bissek Anne-Cécile, Kingwara Leonard, Junghae Muthoni, Kiiru John N, Mwesigwa Richard C N, Balachandra Shirish, Lobognon Roger, Kampira Elizabeth, Detorio Mervi, Yufenyuy Ernest L, Brown Kristin, Patel Hetal K, Parekh Bharat S. HIV-1 Recent Infection Testing Algorithm With Antiretroviral Drug Detection to Improve Accuracy of Incidence Estimates. J Acquir Immune Defic Syndr. 2021 Aug 01;87(Suppl 1):S73–S80. doi: 10.1097/QAI.0000000000002707.00126334-202108011-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Curtis Kelly A, Rudolph Donna L, Pan Yi, Delaney Kevin, Anastos Kathryn, DeHovitz Jack, Kassaye Seble G, Hanson Carl V, French Audrey L, Golub Elizabeth, Adimora Adaora A, Ofotokun Igho, Bolivar Hector, Kempf Mirjam-Colette, Peters Philip J, Switzer William M. Evaluation of the Abbott ARCHITECT HIV Ag/Ab combo assay for determining recent HIV-1 infection. PLoS One. 2021;16(7):e0242641. doi: 10.1371/journal.pone.0242641. https://dx.plos.org/10.1371/journal.pone.0242641 .PONE-D-20-34878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernández Gema, Manzardo C, Montoliu A, Campbell C, Fernández Gregorio, Casabona J, Miró José Maria, Matas L, Rivaya B, González Victoria. Evaluation of an antibody avidity index method for detecting recent human immunodeficiency virus type 1 infection using an automated chemiluminescence immunoassay. Enferm Infecc Microbiol Clin. 2015 Apr;33(4):238–42. doi: 10.1016/j.eimc.2014.04.014.S0213-005X(14)00200-6 [DOI] [PubMed] [Google Scholar]
- 86.Galiwango Ronald Moses, Ssuuna Charles, Kaleebu Pontiano, Kigozi Godfrey, Kagaayi Joseph, Nakigozi Gertrude, Reynolds Steven James, Lutalo Tom, Kankaka Edward Nelson, Wasswa John Bosco, Kalibbala Sarah N, Kigozi Aminah N, Watera Christine, Ejang Julia, Ndyanabo Anthony, Anok Aggrey J, Ssemwanga Deogratius, Kibengo Freddie M, Quinn Thomas C, Grabowski Mary, Chang Larry W, Wawer Maria, Gray Ronald, Laeyendecker Oliver, Serwadda David. Short Communication: Validation of the Asante HIV-1 Rapid Recency Assay for Detection of Recent HIV-1 Infections in Uganda. AIDS Res Hum Retroviruses. 2021 Dec;37(12):893–896. doi: 10.1089/AID.2020.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hargrove John W, van Schalkwyk Cari, Humphrey Jean H, Mutasa Kuda, Ntozini Robert, Owen Sherry Michele, Masciotra Silvina, Parekh Bharat S, Duong Yen T, Dobbs Trudy, Kilmarx Peter H, Gonese Elizabeth. Short Communication: Heightened HIV Antibody Responses in Postpartum Women as Exemplified by Recent Infection Assays: Implications for Incidence Estimates. AIDS Res Hum Retroviruses. 2017 Sep;33(9):902–904. doi: 10.1089/AID.2016.0319. http://europepmc.org/abstract/MED/28443672 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marinda Edmore T, Hargrove John, Preiser Wolfgang, Slabbert Hannes, van Zyl Gert, Levin Jonathan, Moulton Lawrence H, Welte Alex, Humphrey Jean. Significantly diminished long-term specificity of the BED capture enzyme immunoassay among patients with HIV-1 with very low CD4 counts and those on antiretroviral therapy. J Acquir Immune Defic Syndr. 2010 Apr 01;53(4):496–9. doi: 10.1097/qai.0b013e3181b61938. [DOI] [PubMed] [Google Scholar]
- 89.Mullis Caroline E, Munshaw Supriya, Grabowski Mary K, Eshleman Susan H, Serwadda David, Brookmeyer Ronald, Nalugoda Fred, Kigozi Godfrey, Kagaayi Joseph, Tobian Aaron A R, Wawer Maria, Gray Ronald H, Quinn Thomas C, Laeyendecker Oliver. Differential specificity of HIV incidence assays in HIV subtypes A and D-infected individuals from Rakai, Uganda. AIDS Res Hum Retroviruses. 2013 Aug;29(8):1146–50. doi: 10.1089/aid.2012.0105. http://europepmc.org/abstract/MED/23641870 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murphy G, Pilcher C D, Keating S M, Kassanjee R, Facente S N, Welte A, Grebe E, Marson K, Busch M P, Dailey P, Parkin N, Osborn J, Ongarello S, Marsh K, Garcia-Calleja J M, Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) Moving towards a reliable HIV incidence test - current status, resources available, future directions and challenges ahead. Epidemiol Infect. 2017 Apr;145(5):925–941. doi: 10.1017/S0950268816002910.S0950268816002910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Suligoi Barbara, Rodella Anna, Raimondo Mariangela, Regine Vincenza, Terlenghi Luigina, Manca Nino, Casari Salvatore, Camoni Laura, Salfa Maria Cristina, Galli Claudio. Avidity Index for anti-HIV antibodies: comparison between third- and fourth-generation automated immunoassays. J Clin Microbiol. 2011 Jul;49(7):2610–3. doi: 10.1128/JCM.02115-10. http://europepmc.org/abstract/MED/21543577 .JCM.02115-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rikhtegaran Tehrani Z, Azadmanesh K, Mostafavi E, Gharibzadeh S, Soori S, Azizi M, Khabiri A. High avidity anti-integrase antibodies discriminate recent and non-recent HIV infection: Implications for HIV incidence assay. J Virol Methods. 2018 Mar;253:5–10. doi: 10.1016/j.jviromet.2017.12.003.S0166-0934(17)30509-8 [DOI] [PubMed] [Google Scholar]
- 93.Wei X, Smith AJ, Forrest DW, Cardenas GA, Beck DW, LaLota M, Metsch LR, Sionean C, Owen SM, Johnson JA. Incident Infection and Resistance Mutation Analysis of Dried Blood Spots Collected in a Field Study of HIV Risk Groups, 2007-2010. PLoS One. 2016 Jul 14;11(7):e0159266. doi: 10.1371/journal.pone.0159266. https://dx.plos.org/10.1371/journal.pone.0159266 .PONE-D-16-10418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lynch Briana A, Patel Eshan U, Courtney Colleen R, Nanfack Aubin J, Bimela Jude, Wang Xiaohong, Eid Issa, Quinn Thomas C, Laeyendecker Oliver, Nyambi Phillipe N, Duerr Ralf, Redd Andrew D. Short Communication: False Recent Ratio of the Limiting-Antigen Avidity Assay and Viral Load Testing Algorithm Among Cameroonians with Long-Term HIV Infection. AIDS Res Hum Retroviruses. 2017 Nov;33(11):1114–1116. doi: 10.1089/AID.2017.0084. http://europepmc.org/abstract/MED/28670965 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Moyo Sikhulile, Kotokwe Kenanao P, Mohammed Terence, Boleo Corretah, Mupfumi Lucy, Chishala Samuel, Tsalaile Lesedi, Bussmann Hermann, Gaseitsiwe Simani, Musonda Rosemary, Makhema Joseph, Baum Marianna, Marlink Richard, Engelbrecht Susan, Essex Max, Novitsky Vladimir. Short Communication: Low False Recent Rate of Limiting Antigen-Avidity Assay Combined with HIV-1 RNA Data in Botswana. AIDS Res Hum Retroviruses. 2017 Jan;33(1):17–18. doi: 10.1089/AID.2016.0127. http://europepmc.org/abstract/MED/27481530 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.CEPHIA. Murphy G, Busch MP, Pilcher C, Welte A, McKinney E, Keating S, Facente SN, Kassanjee R. Zenodo. San Francisco, CA: Zenodo; 2015. [2022-01-23]. Ortho Avidity-VITROS ECi Evaluation Report. [DOI] [Google Scholar]
- 97.CEPHIA. Murphy G, Busch MP, Pilcher CD, Welte A, McKinney E, Keating S, Facente SN, Kassanjee R. Zenodo. San Francisco, CA: Zenodo; 2015. [2022-01-23]. Ortho Less Sensitive (LS)-VITROS ECi Evaluation Report. [DOI] [Google Scholar]
- 98.CEPHIA. Murphy G, Busch MP, Pilcher C, Welte A, McKinney E, Keating S, Facente SN, Kassanjee R. Zenodo. San Francisco, CA: Zenodo; 2015. [2022-01-23]. SEDIA BED HIV-1 Incidence EIA Evaluation Report. [DOI] [Google Scholar]
- 99.CEPHIA. Murphy G, Busch MP, Pilcher C, Welte A, McKinney E, Keating S, Facente SN, Kassanjee R. Zenodo. San Francisco, CA: Zenodo; 2015. [2022-01-23]. SEDIA HIV-1 LAg-Avidity EIA Evaluation Report. [DOI] [Google Scholar]
- 100.CEPHIA. Murphy G, Busch MP, Pilcher CD, Welte A, McKinney E, Keating S, Facente SN, Kassanjee R. Zenodo. San Francisco, CA: Zenodo; 2015. [2022-01-23]. Bio-Rad GS HIV-1/HIV-2 PLUS O EIA Avidity Assay Evaluation Report. [DOI] [Google Scholar]
- 101.Kim Andrea A, McDougal John S, Hargrove John, Rehle Thomas, Pillay-Van Wyk Victoria, Puren Adrian, Ekra Alexandre, Borget-Alloue Marie-Yolande, Adje-Toure Christiane, Abdullahi Ahmed Sheikh, Odawo Linus, Marum Lawrence, Parekh Bharat S. Evaluating the BED capture enzyme immunoassay to estimate HIV incidence among adults in three countries in sub-Saharan Africa. AIDS Res Hum Retroviruses. 2010 Oct;26(10):1051–61. doi: 10.1089/aid.2009.0218. [DOI] [PubMed] [Google Scholar]
- 102.Ministry of Health Uganda . Uganda Population-based HIV Impact Assessment (UPHIA) 2016-2017: Final Report. Kampala, Uganda: Ministry of Health Uganda; 2019. Jul, [Google Scholar]
- 103.Ministry of Health Malawi . Malawi Population-Based HIV Impact Assessment (MPHIA), 2015-2016: Final Report. Lilongwe, Malawi: Ministry of Health Malawi; 2018. Oct, [Google Scholar]
- 104.Ministry of Health and Child Care (MOHCC) Zimbabwe . Zimbabwe Population-based HIV Impact Assessment (ZIMPHIA) 2015-2016: Final Report. Harare, Zimbabwe: MOHCC; 2019. Aug, [Google Scholar]
- 105.Ministry of Health Lesotho. Centers for Disease Control and Prevention (CDC) ICAP at Columbia University . Lesotho Population-based HIV Impact Assessment (LePHIA) 2016-2017: Final Report. Masuru, Lesotho, Atlanta, GA, and New York, NY: Ministry of Health, CDC, and ICAP; 2019. Sep, [Google Scholar]
- 106.Government of the Kingdom of Eswatini. Swaziland HIV Incidence Measurement Survey 2 (SHIMS2) 2016-2017: Final Report. Mbabane, Kingdom of Eswatini: Government of the Kingdom of Eswatini; 2019. Apr, [Google Scholar]
- 107.Tanzania Commission for AIDS (TACAIDS) Zanzibar AIDS Commission (ZAC) Tanzania HIV Impact Survey (THIS) 2016-2017: Final Report. Dar es Salaam, Tanzania: TACAIDS, ZAC; 2018. Dec, [2022-01-20]. https://phia.icap.columbia.edu/wp-content/uploads/2019/06/FINAL_THIS-2016-2017_Final-Report__06.21.19_for-web_TS.pdf . [Google Scholar]
- 108.Ministry of Health Zambia . Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: Final Report. Lusaka, Zambia: Ministry of Health; 2019. Feb, [Google Scholar]
- 109.Ministry of Health and Social Services (MoHSS) Namibia Population-based HIV Impact Assessment (NAMPHIA) 2017: Final Report. Windhoek, Namibia: MOHSS Namibia; 2019. [2022-01-20]. https://phia.icap.columbia.edu/namphia-final-report/ [Google Scholar]
- 110.Ethiopian Public Health Institute (EPHI) Ethiopia Population-based HIV Impact Assessment (EPHIA) 2017-2018: Final Report. Addis Ababa, Ethiopia: EPHI; 2020. Aug, [2022-01-20]. https://phia.icap.columbia.edu/ethiopia-final-report/ [Google Scholar]
- 111.Rwanda Biomedical Center (RBC) Rwanda Population-based HIV Impact Assessment (RPHIA) 2018-2019: Final Report. Kigali, Rwanda: RBC; 2020. Sep, [2022-01-20]. https://phia.icap.columbia.edu/rwanda-final-report/ [Google Scholar]
- 112.de Oliveira Garcia Mateos Sheila, Preiss Liliana, Gonçalez Thelma T, Di Lorenzo Oliveira Claudia, Grebe Eduard, Di Germanio Clara, Stone Mars, Amorim Filho Luiz, Carneiro Proietti Anna Bárbara, Belisario Andre Rolim, de Almeida-Neto Cesar, Mendrone-Junior Alfredo, Loureiro Paula, Busch Michael P, Custer Brian, Cerdeira Sabino Ester, Recipient Epidemiology‚ Donor Evaluation Study (REDS-III) International Component Brazil 10-year analysis of human immunodeficiency virus incidence in first-time and repeat donors in Brazil. Vox Sang. 2021 Feb;116(2):207–216. doi: 10.1111/vox.13002. http://europepmc.org/abstract/MED/32996602 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Soodla P, Simmons R, Huik K, Pauskar M, Jõgeda EL, Rajasaar H, Kallaste E, Maimets M, Avi R, Murphy G, Porter K, Lutsar I, Concerted Action on SeroConversion to AIDS and Death in Europe (CASCADE) Collaboration in EuroCoord HIV incidence in the Estonian population in 2013 determined using the HIV-1 limiting antigen avidity assay. HIV Med. 2018 Jan;19(1):33–41. doi: 10.1111/hiv.12535. doi: 10.1111/hiv.12535. [DOI] [PubMed] [Google Scholar]
- 114.Tsertsvadze T, Chkhartishvili N, Dvali N, Karchava M, Chokoshvili O, Tavadze L, Gamkrelidze A, Zohrabyan L. Estimating HIV incidence in eastern European country of Georgia: 2010-2012. Int J STD AIDS. 2014 Nov 26;25(13):913–20. doi: 10.1177/0956462414525939.0956462414525939 [DOI] [PubMed] [Google Scholar]
- 115.Matsuoka S, Nagashima M, Sadamasu K, Mori H, Kawahata T, Zaitsu S, Nakamura A, de Souza MS, Matano T. Estimating HIV-1 incidence in Japan from the proportion of recent infections. Prev Med Rep. 2019 Dec;16:100994. doi: 10.1016/j.pmedr.2019.100994. https://linkinghub.elsevier.com/retrieve/pii/S2211-3355(19)30165-2 .S2211-3355(19)30165-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonese E, Musuka G, Ruangtragool L, Hakim A, Parekh B, Dobbs T, Duong YT, Patel H, Mhangara M, Mugurungi O, Mapingure M, Saito S, Herman-Roloff A, Gwanzura L, Tippett-Barr B, Kilmarx PH, Justman J. Comparison of HIV Incidence in the Zimbabwe Population-Based HIV Impact Assessment Survey (2015–2016) with Modeled Estimates: Progress Toward Epidemic Control. AIDS Research and Human Retroviruses. 2020 Aug 01;36(8):656–662. doi: 10.1089/aid.2020.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Moyo S, Gaseitsiwe S, Boleo C, Huesa J, Kotokwe K, Pretorius Holme M, Gaolathe T, Bennett K, Leidner J, Wirth KE, Moore J, Lockman S, Makhema JM, Essex M, Novitsky V. Low Cross-Sectional HIV-1 Incidence at end of Botswana "Ya Tsie" Prevention Study [Poster]. CROI; 2019; Seattle, WA. 2019. Mar 04, [Google Scholar]
- 118.Klock E, Wilson E, Fernandez RE, Piwowar-Manning Estelle, Moore A, Kosloff B, Bwalya J, Bell-Mandla Nomtha, James A, Ayles H, Bock P, Donnell D, Fidler S, Hayes R, Eshleman SH, Laeyendecker O, HPTN071 (PopART) study team Validation of population-level HIV-1 incidence estimation by cross-sectional incidence assays in the HPTN 071 (PopART) trial. J Int AIDS Soc. 2021 Dec 12;24(12):e25830. doi: 10.1002/jia2.25830. http://europepmc.org/abstract/MED/34897992 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Negedu-Momoh Olubunmi R, Balogun Oluseyi, Dafa Ibrahim, Etuk Akan, Oladele Edward Adekola, Adedokun Oluwasanmi, James Ezekiel, Pandey Satish R, Khamofu Hadiza, Badru Titi, Robinson Janet, Mastro Timothy D, Torpey Kwasi. Estimating HIV incidence in the Akwa Ibom AIDS indicator survey (AKAIS), Nigeria using the limiting antigen avidity recency assay. J Int AIDS Soc. 2021 Feb;24(2):e25669. doi: 10.1002/jia2.25669. http://europepmc.org/abstract/MED/33619853 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Conan Nolwenn, Badawi Mahmoud, Chihana Menard L, Wanjala Stephen, Kingwara Leonard, Mambula Christopher, Ngugi Catherine, Okomo Gordon, Opollo Valarie, Salumu Leon, Nesbitt Robin, Szumilin Elisabeth, Huerga Helena. Two-fold increase in the HIV viral load suppression rate along with decreased incidence over six years in Ndhiwa sub-county, Kenya. Trop Med Int Health. 2021 Dec;26(12):1609–1615. doi: 10.1111/tmi.13688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Maman David, Chilima Benson, Masiku Charles, Ayouba Ahidjo, Masson Sophie, Szumilin Elisabeth, Peeters Martine, Ford Nathan, Heinzelmann Annette, Riche Benjamin, Etard Jean-François. Closer to 90-90-90. The cascade of care after 10 years of ART scale-up in rural Malawi: a population study. J Int AIDS Soc. 2016;19(1):20673. doi: 10.7448/IAS.19.1.20673. http://europepmc.org/abstract/MED/26894388 .20673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Scheer Susan, Nakelsky Shoshanna, Bingham Trista, Damesyn Mark, Sun Dan, Chin Chi-Sheng, Buckman Anthony, Mark Karen E. Estimated HIV Incidence in California, 2006-2009. PLoS One. 2013;8(2):e55002. doi: 10.1371/journal.pone.0055002. https://dx.plos.org/10.1371/journal.pone.0055002 .PONE-D-12-31414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maman David, Zeh Clement, Mukui Irene, Kirubi Beatrice, Masson Sophie, Opolo Valarie, Szumilin Elisabeth, Riche Benjamin, Etard Jean-François. Cascade of HIV care and population viral suppression in a high-burden region of Kenya. AIDS. 2015 Jul 31;29(12):1557–65. doi: 10.1097/QAD.0000000000000741. http://europepmc.org/abstract/MED/26244395 .00002030-201507310-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Moyo S, Gaseitsiwe S, Mohammed T, Pretorius Holme M, Wang R, Kotokwe KP, Boleo C, Mupfumi L, Yankinda EK, Chakalisa U, van Widenfelt E, Gaolathe T, Mmalane MO, Dryden-Peterson S, Mine M, Lebelonyane R, Bennett K, Leidner J, Wirth KE, Tchetgen Tchetgen E, Powis K, Moore J, Clarke WA, Lockman S, Makhema JM, Essex M, Novitsky V. Cross-sectional estimates revealed high HIV incidence in Botswana rural communities in the era of successful ART scale-up in 2013-2015. PLoS One. 2018 Oct 24;13(10):e0204840. doi: 10.1371/journal.pone.0204840. https://dx.plos.org/10.1371/journal.pone.0204840 .PONE-D-18-00719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grebe E, Welte A, Johnson LF, van Cutsem G, Puren A, Ellman T, Etard J, Consortium for the Evaluation and Performance of HIV Incidence Assays (CEPHIA) Huerga Helena. Population-level HIV incidence estimates using a combination of synthetic cohort and recency biomarker approaches in KwaZulu-Natal, South Africa. PLoS One. 2018 Sep 13;13(9):e0203638. doi: 10.1371/journal.pone.0203638. https://dx.plos.org/10.1371/journal.pone.0203638 .PONE-D-18-11983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Otecko Newton, Inzaule Seth, Odhiambo Collins, Otieno George, Opollo Valarie, Morwabe Alex, Were Kennedy, Ndiege Kenneth, Otieno Fredrick, Kim Andrea A, Zeh Clement. Viral and Host Characteristics of Recent and Established HIV-1 Infections in Kisumu based on a Multiassay Approach. Sci Rep. 2016 Nov 29;6:37964. doi: 10.1038/srep37964. doi: 10.1038/srep37964.srep37964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Simmons R, Malyuta R, Chentsova N, Karnets I, Murphy G, Medoeva A, Kruglov Y, Yurchenko A, Copas A, Porter K, CASCADE Collaboration in EuroCoord HIV Incidence Estimates Using the Limiting Antigen Avidity EIA Assay at Testing Sites in Kiev City, Ukraine: 2013-2014. PLoS One. 2016 Jun 8;11(6):e0157179. doi: 10.1371/journal.pone.0157179. https://dx.plos.org/10.1371/journal.pone.0157179 .PONE-D-16-10229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Truong H, Fritz K, McFarland W, Hartogensis W, Fiamma A, Coates TJ, Morin SF. Recent HIV type 1 infection among participants in a same-day mobile testing pilot study in Zimbabwe. AIDS Res Hum Retroviruses. 2011 Jun;27(6):593–5. doi: 10.1089/aid.2010.0249. http://europepmc.org/abstract/MED/21087196 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Grebe Eduard, Busch Michael P, Notari Edward P, Bruhn Roberta, Quiner Claire, Hindes Daniel, Stone Mars, Bakkour Sonia, Yang Hong, Williamson Phillip, Kessler Debra, Reik Rita, Stramer Susan L, Glynn Simone A, Anderson Steven A, Williams Alan E, Custer Brian. HIV incidence in US first-time blood donors and transfusion risk with a 12-month deferral for men who have sex with men. Blood. 2020 Sep 10;136(11):1359–1367. doi: 10.1182/blood.2020007003. https://linkinghub.elsevier.com/retrieve/pii/S0006-4971(20)61732-3 .S0006-4971(20)61732-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Eshleman S, Hughes J, Laeyendecker O, Wang J, Brookmeyer R, Johnson-Lewis L, Mullis CE, Hackett J, Vallari AS, Justman J, Hodder S. Use of a Multifaceted Approach to Analyze HIV Incidence in a Cohort Study of Women in the United States: HIV Prevention Trials Network 064 Study. Journal of Infectious Diseases. 2013 Jan;207(2):223–31. doi: 10.1093/infdis/jis658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coates Thomas J, Kulich Michal, Celentano David D, Zelaya Carla E, Chariyalertsak Suwat, Chingono Alfred, Gray Glenda, Mbwambo Jessie K K, Morin Stephen F, Richter Linda, Sweat Michael, van Rooyen Heidi, McGrath Nuala, Fiamma Agnès, Laeyendecker Oliver, Piwowar-Manning Estelle, Szekeres Greg, Donnell Deborah, Eshleman Susan H, NIMH Project Accept (HPTN 043) study team Effect of community-based voluntary counselling and testing on HIV incidence and social and behavioural outcomes (NIMH Project Accept; HPTN 043): a cluster-randomised trial. Lancet Glob Health. 2014 May;2(5):e267–77. doi: 10.1016/S2214-109X(14)70032-4. https://linkinghub.elsevier.com/retrieve/pii/S2214-109X(14)70032-4 .S2214-109X(14)70032-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sexton Connie J, Costenbader Elizabeth C, Vinh Dang Thi Nhat, Chen Pai Lien, Hoang Tran Vu, Lan Nguyen Thi Hoang, Feldblum Paul, Kim Andrea, Giang Le Truong. Correlation of prospective and cross-sectional measures of HIV type 1 incidence in a higher-risk cohort in Ho Chi Minh City, Vietnam. AIDS Res Hum Retroviruses. 2012 Aug;28(8):866–73. doi: 10.1089/aid.2011.0221. [DOI] [PubMed] [Google Scholar]
- 133.Ministry of Health Malawi . Estimating HIV Incidence and Detecting Recent Infection Among Pregnant Adolescent Girls and Young Women in Malawi, 2017-2018: Final Report. Lilongwe, Malawi: Ministry of Health; 2019. Oct, [Google Scholar]
- 134.Teixeira SL, Jalil CM, Jalil EM, Nazer SC, Silva SDCC, Veloso VG, Luz PM, Grinsztejn B. Evidence of an untamed HIV epidemic among MSM and TGW in Rio de Janeiro, Brazil: a 2018 to 2020 cross-sectional study using recent infection testing. J Int AIDS Soc. 2021 Jun 16;24(6):e25743. doi: 10.1002/jia2.25743. http://europepmc.org/abstract/MED/34132470 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Morineau G, Magnani R, Nurhayati A, Bollen L, Mustikawati DE. Is the BED capture enzyme immunoassay useful for surveillance in concentrated epidemics? The case of female sex workers in Indonesia. Southeast Asian J Trop Med Public Health. 2011 May;42(3):634–42. [PubMed] [Google Scholar]
- 136.Patel EU, Solomon SS, Lucas GM, McFall AM, Srikrishnan AK, Kumar MS, Iqbal SH, Saravanan S, Paneerselvam N, Balakrishnan P, Laeyendecker O, Celentano DD, Mehta SH. Temporal change in population-level prevalence of detectable HIV viraemia and its association with HIV incidence in key populations in India: a serial cross-sectional study. Lancet HIV. 2021 Sep;8(9):e544–e553. doi: 10.1016/S2352-3018(21)00098-9.S2352-3018(21)00098-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Hansoti B, Stead D, Eisenberg A, Mvandaba N, Mwinnyaa G, Patel EU, Parrish A, Reynolds SJ, Redd AD, Fernandez R, Rothman RE, Laeyendecker O, Quinn TC. A Window Into the HIV Epidemic from a South African Emergency Department. AIDS Research and Human Retroviruses. 2019 Feb;35(2):139–144. doi: 10.1089/aid.2018.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kim AA, Morales S, Lorenzana de Rivera I, Paredes M, Juarez S, Alvarez B, Liu X, Parekh B, Monterroso E, Paz-Bailey G. Short communication: HIV incidence among vulnerable populations in Honduras: results from an integrated behavioral and biological survey among female sex workers, men who have sex with men, and Garifuna in Honduras, 2006. AIDS Res Hum Retroviruses. 2013 Mar;29(3):516–9. doi: 10.1089/AID.2012.0032. [DOI] [PubMed] [Google Scholar]
- 139.Xu J, Tang W, Zou H, Mahapatra T, Hu Q, Fu G, Wang Z, Lu L, Zhuang M, Chen X, Fu J, Yu Y, Lu J, Jiang Y, Geng W, Han X, Shang H. High HIV incidence epidemic among men who have sex with men in china: results from a multi-site cross-sectional study. Infect Dis Poverty. 2016 Sep 05;5(1):82. doi: 10.1186/s40249-016-0178-x. https://idpjournal.biomedcentral.com/articles/10.1186/s40249-016-0178-x .10.1186/s40249-016-0178-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Solomon S, Mehta S, Srikrishnan A, Vasudevan C, McFall A, Balakrishnan P, Anand S, Nandagopal P, Ogburn EL, Laeyendecker O, Lucas GM, Solomon S, Celentano DD. High HIV prevalence and incidence among MSM across 12 cities in India. Aids. 2015 Mar;29(6):723–31. doi: 10.1097/qad.0000000000000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sane J, Heijman T, Hogema B, Koot M, van Veen Maaike, Götz Hannelore, Fennema J, Op de Coul E. Identifying recently acquired HIV infections among newly diagnosed men who have sex with men attending STI clinics in The Netherlands. Sex Transm Infect. 2014 Aug 28;90(5):414–7. doi: 10.1136/sextrans-2013-051420.sextrans-2013-051420 [DOI] [PubMed] [Google Scholar]
- 142.Forbi JC, Entonu PE, Mwangi LO, Agwale SM. Estimates of human immunodeficiency virus incidence among female sex workers in north central Nigeria: implications for HIV clinical trials. Trans R Soc Trop Med Hyg. 2011 Nov;105(11):655–60. doi: 10.1016/j.trstmh.2011.08.001.S0035-9203(11)00162-3 [DOI] [PubMed] [Google Scholar]
- 143.Combes SL, G-Yohannes A, Kidane A, Chen P, Aseffa A, Feldblum PJ, Shattuck D. HIV prevalence and incidence among women at higher risk of infection in Addis Ababa, Ethiopia. AIDS Res Hum Retroviruses. 2013 Mar;29(3):535–40. doi: 10.1089/AID.2012.0163. [DOI] [PubMed] [Google Scholar]
- 144.Zea MC, Olaya P, Reisen CA, Poppen PJ. MSM in Bogotá are living with HIV for extended periods without diagnosis or treatment. Int J STD AIDS. 2017 Aug 21;28(9):920–924. doi: 10.1177/0956462416681364. http://europepmc.org/abstract/MED/27872321 .0956462416681364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Woldesenbet S, Kufa-Chakezha T, Lombard C, Manda S, Cheyip M, Ayalew K, Chirombo B, Barron P, Diallo K, Parekh B, Puren A. Recent HIV infection among pregnant women in the 2017 antenatal sentinel cross-sectional survey, South Africa: Assay-based incidence measurement. PLOS ONE. 2021 Apr;16(4):17. doi: 10.1371/journal.pone.0249953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hu H, Liu X, Zhang Z, Xu X, Shi L, Fu G, Huan X, Zhou Y. Increasing HIV Incidence among Men Who Have Sex with Men in Jiangsu Province, China: Results from Five Consecutive Surveys, 2011-2015. Int J Environ Res Public Health. 2016 Aug 06;13(8):795. doi: 10.3390/ijerph13080795. https://www.mdpi.com/resolver?pii=ijerph13080795 .ijerph13080795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Solomon SS, Mehta SH, McFall AM, Srikrishnan AK, Saravanan S, Laeyendecker O, Balakrishnan P, Celentano DD, Solomon S, Lucas GM. Community viral load, antiretroviral therapy coverage, and HIV incidence in India: a cross-sectional, comparative study. Lancet HIV. 2016 Apr;3(4):e183–90. doi: 10.1016/S2352-3018(16)00019-9. http://europepmc.org/abstract/MED/27036994 .S2352-3018(16)00019-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Salustiano DM, Lima Kledoaldo Oliveira de, Cavalcanti AMS, Diaz RS, Lacerda HR. Comparison among the BED capture enzyme immunoassay test and AxSYM avidity index assay for determining recent HIV infection and incidence in two Voluntary Counselling and Testing Centres in Northeast Brazil. Braz J Infect Dis. 2014 Jul;18(4):449–53. doi: 10.1016/j.bjid.2014.03.001. https://linkinghub.elsevier.com/retrieve/pii/S1413-8670(14)00066-X .S1413-8670(14)00066-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang J, Xu J-J, Chu Z-X, Hu Q-H, Han X-X, Zhao B, Jiang Y-J, Geng W-Q, Shang H. Disparity of human immunodeficiency virus incidence and drug resistance in college student, non-student youth and older men who have sex with men: a cross-sectional study from seven major cities of China. Chinese medical journal. 2020 Dec;133(23):2778–86. doi: 10.1097/cm9.0000000000001161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xu Junjie, Smith M Kumi, Ding Guowei, Chu Jennifer, Wang Haibo, Li Qinghua, Chang Dongfang, Wang Guixiang, Shang Hong, Jiang Yan, Wang Ning. Drug use and sex work: competing risk factors for newly acquired HIV in Yunnan, China. PLoS One. 2013 Mar;8(3):e59050. doi: 10.1371/journal.pone.0059050. https://dx.plos.org/10.1371/journal.pone.0059050 .PONE-D-12-27998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rice B, de Wit M, Willis R, Hargreaves J, and all members of the MeSH Working Group . TRACE - Recency Learning Hub. London, UK: MeSH Consortium; 2019. Jul, [2022-01-21]. The feasibility and utility of HIV recent infection testing in a range of routine service-provision contexts. https://trace-recency.org/wp-content/uploads/2019/08/MeSH-report-on-HIV-recency-testing-in-routine-settings.pdf . [Google Scholar]
- 152.Robinson E, Moran J, O'Donnell K, Hassan J, Tuite H, Ennis O, Cooney F, Nugent E, Preston L, O'Dea S, Doyle S, Keating S, Connell J, De Gascun C, Igoe D. Integration of a recent infection testing algorithm into HIV surveillance in Ireland: improving HIV knowledge to target prevention. Epidemiol Infect. 2019 Jan;147:e136. doi: 10.1017/S0950268819000244. http://europepmc.org/abstract/MED/30869051 .S0950268819000244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Verhofstede Chris, Mortier Virginie, Dauwe Kenny, Callens Steven, Deblonde Jessika, Dessilly Géraldine, Delforge Marie-Luce, Fransen Katrien, Sasse André, Stoffels Karolien, Van Beckhoven Dominique, Vanroye Fien, Vaira Dolores, Vancutsem Ellen, Van Laethem Kristel. Exploring HIV-1 Transmission Dynamics by Combining Phylogenetic Analysis and Infection Timing. Viruses. 2019 Nov 26;11(12) doi: 10.3390/v11121096. https://www.mdpi.com/resolver?pii=v11121096 .v11121096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhang JF, Yao JM, Jiang J, Pan XH, Luo MY, Xia Y. Migration interacts with the local transmission of HIV in developed trade areas: A molecular transmission network analysis in China. Infect Genet Evol. 2020 Oct. :84. doi: 10.1016/j.meegid.2020.104376. [DOI] [PubMed] [Google Scholar]
- 155.Auvert Bertran, Taljaard Dirk, Rech Dino, Lissouba Pascale, Singh Beverley, Bouscaillou Julie, Peytavin Gilles, Mahiane Séverin Guy, Sitta Rémi, Puren Adrian, Lewis David. Association of the ANRS-12126 male circumcision project with HIV levels among men in a South African township: evaluation of effectiveness using cross-sectional surveys. PLoS Med. 2013;10(9):e1001509. doi: 10.1371/journal.pmed.1001509. https://dx.plos.org/10.1371/journal.pmed.1001509 .PMEDICINE-D-12-02855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rice Brian D, de Wit Mariken, Welty Susie, Risher Kathryn, Cowan Frances M, Murphy Gary, Chabata Sungai T, Waruiru Wanjiru, Magutshwa Sitholubuhle, Motoku John, Kwaro Daniel, Ochieng Benard, Reniers Georges, Rutherford George. Can HIV recent infection surveillance help us better understand where primary prevention efforts should be targeted? Results of three pilots integrating a recent infection testing algorithm into routine programme activities in Kenya and Zimbabwe. J Int AIDS Soc. 2020 Jun;23 Suppl 3:e25513. doi: 10.1002/jia2.25513. http://europepmc.org/abstract/MED/32602625 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Huang Yu-Ching, Huang Yen-Fang, Lin Min-Hau, Yang Jyh-Yuan, Liao Yu-Hsin, Lo Hsiu-Yun, Latkin Carl, Nelson Kenrad E. An outbreak of HIV infection among people who inject drugs linked to injection of propofol in Taiwan. PLoS One. 2019;14(2):e0210210. doi: 10.1371/journal.pone.0210210. https://dx.plos.org/10.1371/journal.pone.0210210 .PONE-D-18-23590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chauhan Chandar Kanta, Lakshmi P V M, Sagar Vivek, Sharma Aman, Arora Sunil K, Kumar Rajesh. Immunological markers for identifying recent HIV infection in North-West India. Indian J Med Res. 2020 Sep;152(3):227–233. doi: 10.4103/ijmr.IJMR_2007_18. http://www.ijmr.org.in/article.asp?issn=0971-5916;year=2020;volume=152;issue=3;spage=227;epage=233;aulast=Chauhan .IndianJMedRes_2020_152_3_227_298475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Braunstein Sarah L, Ingabire Chantal M, Geubbels Eveline, Vyankandondera Joseph, Umulisa Marie-Michèle, Gahiro Elysée, Uwineza Mireille, Tuijn Coosje J, Nash Denis, van de Wijgert Janneke H H M. High burden of prevalent and recently acquired HIV among female sex workers and female HIV voluntary testing center clients in Kigali, Rwanda. PLoS One. 2011 Sep;6(9):e24321. doi: 10.1371/journal.pone.0024321. https://dx.plos.org/10.1371/journal.pone.0024321 .PONE-D-11-06731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Braunstein Sarah L, van de Wijgert Janneke H, Vyankandondera Joseph, Kestelyn Evelyne, Ntirushwa Justin, Nash Denis. Risk Factor Detection as a Metric of STARHS Performance for HIV Incidence Surveillance Among Female Sex Workers in Kigali, Rwanda. Open AIDS J. 2012;6:112–21. doi: 10.2174/1874613601206010112. http://europepmc.org/abstract/MED/23056162 .TOAIDJ-6-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zeh Clement, Inzaule Seth C, Ondoa Pascale, Nafisa Lillian G, Kasembeli Alex, Otieno Fredrick, Vandenhoudt Hilde, Amornkul Pauli N, Mills Lisa A, Nkengasong John N. Molecular Epidemiology and Transmission Dynamics of Recent and Long-Term HIV-1 Infections in Rural Western Kenya. PLoS One. 2016 Feb;11(2):e0147436. doi: 10.1371/journal.pone.0147436. https://dx.plos.org/10.1371/journal.pone.0147436 .PONE-D-15-30674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Min, Ma Yanling, Chen Huichao, Dai Jie, Luo Hongbing, Yang Chaojun, Dong Lijuan, Jin Xiaomei, Yang Min, Yang Li, Song Lijun, Jia Manhong, Song Zhizhong. Demographic characteristics and spatial clusters of recent HIV-1 infections among newly diagnosed HIV-1 cases in Yunnan, China, 2015. BMC Public Health. 2019 Nov 11;19(1):1507. doi: 10.1186/s12889-019-7557-8. https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-019-7557-8 .10.1186/s12889-019-7557-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kim Andrea A, Parekh Bharat S, Umuro Mamo, Galgalo Tura, Bunnell Rebecca, Makokha Ernest, Dobbs Trudy, Murithi Patrick, Muraguri Nicholas, De Cock Kevin M, Mermin Jonathan, 2007 KAIS study group Identifying Risk Factors for Recent HIV Infection in Kenya Using a Recent Infection Testing Algorithm: Results from a Nationally Representative Population-Based Survey. PLoS One. 2016;11(5):e0155498. doi: 10.1371/journal.pone.0155498. https://dx.plos.org/10.1371/journal.pone.0155498 .PONE-D-15-36782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Skaathun B, Pines HA, Patterson TL, Semple SJ, Pekar J, Harvey-Vera A. Recent HIV Infection among men who have sex with men and transgender women in Tijuana. Rev Saude Publica. 2020. :54. doi: 10.11606/s1518-8787.2020054002179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.El-Hayek C, Breschkin A, Nicholson S, Bergeri I, Hellard ME. Does Using a Bed Enzyme Immunoassay Test Enhance Current HIV Surveillance Practices? [Poster]. XVIII International AIDS Conference; 2010; Vienna, Austria. 2010. [Google Scholar]
- 166.Romero Anabel, González Victoria, Esteve Anna, Martró Elisa, Matas Lurdes, Tural Cristina, Pumarola Tomàs, Casanova Aurora, Ferrer Elena, Caballero Estrella, Ribera Esteve, Margall Núria, Domingo Pere, Farré Joan, Puig Teresa, Sauca M Goretti, Barrufet Pilar, Amengual M José, Navarro Gemma, Navarro Maria, Vilaró Josep, Ortín Xavier, Ortí Amat, Pujol Ferran, Prat Josep M, Massabeu Angels, Simó Josep M, Villaverde Carlos Alonso, Benítez Miguel Ángel, Garcia Isabel, Díaz Olga, Becerra Jasmina, Ros Rosa, Sala Roser, Rodrigo Isabel, Miró José M, Casabona Jordi, AERI Study group Identification of recent HIV-1 infection among newly diagnosed cases in Catalonia, Spain (2006-08) Eur J Public Health. 2012 Dec;22(6):802–8. doi: 10.1093/eurpub/ckr179.ckr179 [DOI] [PubMed] [Google Scholar]
- 167.Fearnhill Esther, Gourlay Annabelle, Malyuta Ruslan, Simmons Ruth, Ferns R Bridget, Grant Paul, Nastouli Eleni, Karnets Iryna, Murphy Gary, Medoeva Antonia, Kruglov Yuri, Yurchenko Alexander, Porter Kholoud, Concerted Action on SeroConversion to AIDS and Death in Europe (CASCADE) Collaboration in EuroCoord A Phylogenetic Analysis of Human Immunodeficiency Virus Type 1 Sequences in Kiev: Findings Among Key Populations. Clin Infect Dis. 2017 Oct 01;65(7):1127–1135. doi: 10.1093/cid/cix499. http://europepmc.org/abstract/MED/28575385 .3857834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Paz-Bailey G, Smith A, Masciotra S, Zhang W, Bingham T, Flynn C, German D, Al-Tayyib A, Magnus M, LaLota M, Rose C E, Owen S M. Early HIV Infections Among Men Who Have Sex with Men in Five Cities in the United States. AIDS Behav. 2015 Dec;19(12):2304–10. doi: 10.1007/s10461-015-1011-4. http://europepmc.org/abstract/MED/25680518 .10.1007/s10461-015-1011-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Smoleń-Dzirba Joanna, Rosińska Magdalena, Kruszyński Piotr, Bratosiewicz-Wąsik Jolanta, Janiec Janusz, Beniowski Marek, Bociąga-Jasik Monika, Jabłonowska Elżbieta, Szetela Bartosz, Porter Kholoud, Wąsik Tomasz J. Molecular epidemiology of recent HIV-1 infections in southern Poland. J Med Virol. 2012 Dec;84(12):1857–68. doi: 10.1002/jmv.23395. [DOI] [PubMed] [Google Scholar]
- 170.Welty S, Motoku J, Muriithi C, Rice B, de Wit M, Ashanda B, Waruiru W, Mirjahangir J, Kingwara L, Bauer R, Njoroge D, Karimi J, Njoroge A, Rutherford GW. Brief Report: Recent HIV Infection Surveillance in Routine HIV Testing in Nairobi, Kenya: A Feasibility Study. JAIDS. 2020 May;84(1):5–9. doi: 10.1097/qai.0000000000002317. [DOI] [PubMed] [Google Scholar]
- 171.Dennis Ann M, Murillo Wendy, de Maria Hernandez Flor, Guardado Maria Elena, Nieto Ana Isabel, Lorenzana de Rivera Ivette, Eron Joseph J, Paz-Bailey Gabriela. Social network-based recruitment successfully reveals HIV-1 transmission networks among high-risk individuals in El Salvador. J Acquir Immune Defic Syndr. 2013 May 01;63(1):135–41. doi: 10.1097/QAI.0b013e318288b246. http://europepmc.org/abstract/MED/23364512 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.de Wit Mariken M, Rice Brian, Risher Kathryn, Welty Susie, Waruiru Wanjiru, Magutshwa Sitholubuhle, Motoku John, Kwaro Daniel, Ochieng Benard, Reniers Georges, Cowan Frances, Rutherford George, Hargreaves James R, Murphy Gary. Experiences and lessons learned from the real-world implementation of an HIV recent infection testing algorithm in three routine service-delivery settings in Kenya and Zimbabwe. BMC Health Serv Res. 2021 Jun 22;21(1):596. doi: 10.1186/s12913-021-06619-6. https://bmchealthservres.biomedcentral.com/articles/10.1186/s12913-021-06619-6 .10.1186/s12913-021-06619-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Zhu Qiyu, JiKe Chunnong, Xu Chengdong, Liang Shu, Yu Gang, Wang Ju, Xiao Lin, Liu Ping, Chen Meibin, Guan Peng, Liu Zhongfu, Jin Cong. A New Strategy to Quantitatively Identify Hot-Spot Areas in Growth of New HIV Infections for Targeted Interventions. Front Public Health. 2021;9:680867. doi: 10.3389/fpubh.2021.680867. doi: 10.3389/fpubh.2021.680867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Avila-Ríos Santiago, García-Morales Claudia, Garrido-Rodríguez Daniela, Tapia-Trejo Daniela, Girón-Callejas Amalia Carolina, Mendizábal-Burastero Ricardo, Escobar-Urias Ingrid Yessenia, García-González Blanca Leticia, Navas-Castillo Sabrina, Pinzón-Meza Rodolfo, Mejía-Villatoro Carlos Rodolfo, Reyes-Terán Gustavo. HIV-1 drug resistance surveillance in antiretroviral treatment-naive individuals from a reference hospital in Guatemala, 2010-2013. AIDS Res Hum Retroviruses. 2015 Apr;31(4):401–11. doi: 10.1089/aid.2014.0057. [DOI] [PubMed] [Google Scholar]
- 175.Hauser A, Hofmann A, Meixenberger K, Altmann B, Hanke K, Bremer V, Bartmeyer B, Bannert N. Increasing proportions of HIV-1 non-B subtypes and of NNRTI resistance between 2013 and 2016 in Germany: Results from the national molecular surveillance of new HIV diagnoses. PLOS ONE. 2018 Nov;13(11):18. doi: 10.1371/journal.pone.0206234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Fogel J, Sivay M, Cummings V, Wilson E, Hart S, Gamble T, Laeyendecker O, Fernandez RE, Del Rio C, Batey DS, Mayer KH, Farley JE, McKinstry L, Hughes JP, Remien RH, Beyrer C, Eshleman SH. HIV drug resistance in a cohort of HIV-infected MSM in the United States. AIDS. 2020 Jan;34(1):91–101. doi: 10.1097/qad.0000000000002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.UNAIDS/WHO Working Group on Global HIV/AIDS and STI Surveillance . UNAIDS. Geneva, Switzerland: UNAIDS; 2015. [2022-01-18]. Monitoring HIV Impact Using Population-Based Surveys. https://www.unaids.org/sites/default/files/media_asset/JC2763_PopulationBasedSurveys_en.pdf . [Google Scholar]
- 178.ICAP, Columbia University Population-based HIV Impact Assessment: Guiding the Global HIV Response. ICAP. 2021. [2021-02-23]. https://phia.icap.columbia.edu/about .
- 179.ICAP . PHIA Project Countries. New York, NY: Columbia University; 2019. [2022-02-20]. https://phia.icap.columbia.edu/countries-overview/ [Google Scholar]
- 180.Truong HM, Kellogg TA, McFarland W, Louie B, Klausner JD, Philip SS, Grant RM. Sentinel surveillance of HIV-1 transmitted drug resistance, acute infection and recent infection. PLoS One. 2011;6(10):e25281. doi: 10.1371/journal.pone.0025281. https://dx.plos.org/10.1371/journal.pone.0025281 .PONE-D-11-05393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Godin A, Eaton JW, Giguère Katia, Marsh K, Johnson LF, Jahn A, Mbofana F, Ehui E, Maheu-Giroux M. Inferring population HIV incidence trends from surveillance data of recent HIV infection among HIV testing clients. AIDS. 2021 Nov 15;35(14):2383–2388. doi: 10.1097/QAD.0000000000003021. http://europepmc.org/abstract/MED/34261098 .00002030-900000000-96350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.The TRACE Initiative TRACE - Tracking with Recency Assays to Control the Epidemic. TRACE - Recency Learning Hub. 2021. [2021-01-31]. https://trace-recency.org .
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search sets and terms used for title, abstract, and MeSH terms/author keyword searches.
Search code.
Websites searched for eligible grey literature during the review.
Assessing the utility of HIV recency assays for surveillance purposes: systematic review protocol.
Sources identified during a systematic review of the literature (as described in the 'Methods' section) are organized below. Sources are ordered by (1) literature type (peer-reviewed vs gray), then (2) strength of evidence (highest to lowest), and then (3) last name of the first author (alphabetical).
Evidence included in this review, including the strength rating and topic of focus for each item.


