Abstract
Purpose:
Recently, a GGGGCC hexanucleotide repeat expansion in the C9ORF72 gene, located on chromosome 9p21 has been demonstrated to be the commonest cause of familial amyotrophic lateral sclerosis (ALS) and to account for 5 to 10 % of apparently sporadic ALS. Relatively little is known about the brain metabolism profile of patients carrying the expansion. Our aim was to identify the [18F]FDG PET profile in ALS patients with the C9ORF72 expansion (C9ORF72-ALS).
Methods:
Fifteen C9ORF72-ALS patients were compared with 12 patients with ALS and comorbid frontotemporal dementia (FTD) without the C9ORF72 expansion (ALS-FTD) and 30 cognitively normal patients with ALS without mutations of ALS-related genes (sALS). The three groups were then cross-matched to 40 neurologically normal controls. All patients underwent FDG PET within 4 months of diagnosis.
Results:
The C9ORF72-ALS patients compared with the sALS patients showed significant hypometabolism in the anterior and posterior cingulate cortex, insula, caudate and thalamus, the left frontal and superior temporal cortex, and hypermetabolism in the midbrain, bilateral occipital cortex, globus pallidus and left inferior temporal cortex. The ALS-FTD patients compared with the sALS patients showed more limited hypometabolic areas, including the orbitofrontal, prefrontal, anterior cingulate and insular cortex, and hypermetabolic areas, including the bilateral occipital cortex, the left precentral and postcentral cortex and superior temporal gyrus. The C9ORF72-ALS patients compared with the ALS-FTD patients showed hypometabolism in the left temporal cortex.
Conclusion:
ALS patients with the C9ORF72 hexanucleotide repeat expansion had a more widespread central nervous system involvement than ALS patients without genetic mutations, with or without comorbid FTD, consistent with their more severe clinical picture.
Keywords: Amyotrophic lateral sclerosis C9ORF72 gene FDG PET
Introduction
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disorder characterized by loss of cortical, bulbar and spinal motor neurons, leading to progressive palsy of voluntary muscles and ultimately death due to respiratory failure, usually within 3 years of onset [1]. While the majority of cases appears sporadically in the population, 10 % of patients have a positive family history of ALS or frontotemporal dementia (ALS-FTD), a related form of neurodegeneration [2, 3]. Recently, our group and others have demonstrated that a GGGGCC hexanucleotide repeat expansion in the C9ORF72 gene located on chromosome 9p21 is the commonest cause of familial ALS and also accounts for up to 10 % of apparently sporadic ALS [3–7]. Patients with thisC9ORF72 hexanucleotide repeat expansion are characterized clinically by an overlap phenotype consisting of ALS and FTD, sometimes including extrapyramidal and psychiatric signs (mainly delusions and hallucinations) [2, 3].
Relatively little is known about the anatomofunctional profile of patients carrying the pathogenetic expansion, especially those presenting with motor neuron dysfunction. Symmetric bilateral frontal and temporal cortical atrophy has been demonstrated using MRI in a cohort of 18 FTD or ALS-FTD patients carrying the pathogenetic expansion [8]. Magnetic resonance grey-matter voxel-based morphometry identified significant grey-matter atrophy in the right inferior and superior frontal gyrus, left anterior cingulate gyrus, and right precentral gyrus in four patients with FTD [2] and in ten patients with ALS-FTD carrying the pathogenetic expansion [9]. A PET study involving five patients with the C9ORF72 expansion presenting with a FTD phenotype revealed glucose hypometabolism in the frontal cortex and the anterior cingulate gyrus compared with an age-segmented normative database [9]. However, to date, there have been no studies examining the functional characteristics of ALS patients with the C9ORF72 hexanucleotide repeat expansion compared with both ALS patients without the mutation and controls.
The aim of our study was to address this gap in our knowledge by performing brain [18F]FDG PET in a series of ALS patients with the C9ORF72 hexanucleotide repeat expansion and to compare their scans with those of ALS-FTD and cognitively normal ALS patients without mutations of C9ORF72 or other ALS-related genes.
Methods
Patients and controls
A series of 195 ALS patients with probable, probable laboratory-supported or definite ALS according to the revised criteria of El Escorial [10], consecutively seen at the Turin ALS centre, participated in a cross-sectional study with FDG PET. Patients underwent PET within 4 months of diagnosis. Among these patients, we identified three groups: (1) 15 patients carrying the C9ORF72 expansion (C9ORF72-ALS), (2) 12 ALS patients with comorbid FTD who did not carry the expansion or any known ALS-related genetic mutation (ALS-FTD), and (3) 30 cognitively normal ALS patients who did not carry the expansion or any known ALS-related genetic mutation (sALS) and who were matched for age, gender, disease duration, and clinical phenotype to the C9ORF72-ALS patients.
Each group was compared with a neurologically normal control cohort consisting of 40 subjects who underwent 18F-FDG PET scanning and found to be negative for neoplastic diseases. Exclusion criteria for both ALS patients and control subjects were: the presence of major systemic illness, major vision disturbances, psychiatric illnesses, epilepsy, head trauma, parkinsonism, previous stroke or transitory ischaemic attack, brain masses, and current use of benzodiazepines or tricyclic antidepressants.
Genetic analysis
A repeat-primed PCR assay was used to screen for the presence of the GGGGCC hexanucleotide expansion in the first intron of C9ORF72 [6]. A repeat expansion of ≥30 was considered to be pathological. All patients were also screened for mutations in genes more commonly implicated in ALS in Caucasian populations, namely SOD1, FUS, TARDBP, OPTN and ANG [11].
Cognitive evaluation
FTD diagnosis was defined according to the criteria of Neary et al. [12]. ALS patients and controls underwent a detailed neuropsychological test battery [13] and were classified as having FTD, as having isolated executive impairment, or as being cognitively normal [14]. The following tests were applied: Frontal Systems Behavior Scale, using the Family form evaluated by a close relative (scores: normal ≤59, borderline 60 – 64; pathological ≥65) [15]; Mini Mental State Examination; Wisconsin Card Sorting Test; Trail Making A and B; Stroop Colour-Word Interference Test; letter and category fluency test; Wechsler Memory Scale–revised; Rey-Osterrieth Complex Figure Test; Token test; Wechsler Adult Intelligence Scale revised; and Raven’s Progressive Coloured Matrices. Depression and anxiety were evaluated with the Hospital Anxiety and Depression Scale.
18F-FDG PET imaging
Subjects fasted for at least 6 h prior to imaging. Before radiopharmaceutical injection, blood glucose was measured (<7.2 mmol/l in all cases). According to EANM guidelines [16] after a 20-min rest period in a silent and darkened room, with eyes closed and ears unplugged, subjects were injected with approximately 185 MBq of 18F-FDG through an intravenous cannula. PET/CT scanning was started approximately 40 min after injection and lasted another 10 min. A polycarbonate head holder was applied to reduce head movements during the scan.
The PET/CT scans were acquired using a Discovery ST-E System (General Electrics Medical Systems, Milwaukee, WI) with an axial resolution of 5.2 mm FWHM in three-dimensional mode. FDG PET/CT images were acquired in three dimensions as two sequential scans: a brain CT scan (thickness 3.75 mm, 140 kV, 60 – 80 mA) and a brain PET scan (one field of view of 30 transaxial centimetres). The PET scan was started immediately after the CT scan in order to use the CT data for attenuation correction of the PET data. Scatter correction was performed using the three-dimensional reconstruction algorithm. Data were collected in 128 × 128 matrices with reconstructed voxels of 2.34 × 2.34 × 2.00 mm.
Statistical analysis
A two-sample unpaired t-test was used to differentiate groups based on age and executive tests scores, while the chi-squared test was used to evaluate differences in gender distribution. Analyses were performed using age, gender and type of onset (bulbar vs. spinal) as covariates.
Using SPM2 (Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab 6.5 (Mathworks, Natick, MA), PET data were subjected to affine and nonlinear spatial normalization into MNI space. The spatially normalized set of images was then smoothed using an 8-mm isotropic gaussian filter to blur individual variations in gyral anatomy and to increase the signal-to-noise ratio. The resulting statistical parametric maps, SPM{t}, were transformed into normal distribution (SPM{z}) units. The SPM coordinates were corrected to match the Talairach coordinates by the subroutine implemented by Matthew Brett (http://brainmap.org/index.html). Brodmann areas (BAs) were then identified at a range of 0 to 3 mm from the corrected Talairach coordinates of the SPM output isocentres, after importing the corrected coordinates, by Talairach client (http://www.talairach.org/index.html).
In order to test the overall statistical significance of metabolic differences, one-way analysis of variance (ANOVA) was performed using the SPM2 f-contrast routine using all four groups as factors. Subsequently multiple unpaired t-tests between the four groups were performed using the “compare population: one-scan per subject” routine.
We used a p value threshold of <0.05 corrected for multiple comparisons with the False Discovery Rate (FDR) option, to explore SPM t-maps at the voxel level [6]. If statistical significance was not reached the threshold at the voxel level was explored at p < 0.001 uncorrected for multiple comparisons. This more liberal choice can be adopted to avoid type II errors attributable to over-conservative thresholds [17]. Given the exploratory nature of this analysis and considering the relatively low sensitivity of PET without repeated measures, higher thresholds could lead to false-negative results in PET studies.
The significance of the identified area was established at a p value of <0.05 corrected for multiple comparisons at the cluster level. Only clusters containing more than 125 voxels were considered to be significant based on the calculation of the partial volume effect (PVE) resulting from the spatial resolution of the PET camera (about double the FWHM).
Standard protocol approval and patient consent
The study design was approved by the institutional ethics committee. Patients and caregivers provided written informed consent.
Results
Demographics and clinical characteristics of the C9ORF72-ALS, the sALS and the ALS-FTD patients, and the neurologically normal controls are presented in Table 1. The three groups of ALS patients were fully comparable, with the obvious exception of FTD comorbidity. A detailed description of the C9ORF72-ALS patients is provided in the supplementary data (Table E-1). No ALS-FTD patients showed hallucinations or delusions and none had a positive family history of ALS or FTD. Cognitive test findings are presented in Table E-2.
Table 1.
Clinical characteristics of the C9ORF72-ALS, the sALS and the ALS-FTD patients, and the neurologically normal controls
| C9ORF72-ALS (n=15) | sALS (n=30) | ALS-FTD (n=12) | Controls (n=40) | p value* | |
|---|---|---|---|---|---|
|
| |||||
| Age at onset (years), mean (SD) | 58.6 (10.2) | 58.2 (10.6) | 64.8 (9.1) | 62.2 (14.1) | n.s. |
| Gender (women), n (%) | 4 (26.7 %) | 13 (43.3 %) | 6 (50 %) | 14 (35 %) | n.s. |
| Site of onset (bulbar), n (%) | 7 (46.7 %) | 13 (43.3 %) | 6 (50 %) | - | n.s. |
| Disease duration at time of PET (months), mean (SD) | 10.1 (7.3) | 12.1 (8.8) | 12.1 (8.6) | - | n.s. |
| ALSFRS-R score at time of PET, mean (SD) | 41.2 (4.5) | 42.3 (5.1) | 41.7 (4.9) | - | n.s. |
| FTD, n (%) | 10 (66.7 %) | 0 | 12 (100 %) | 0 | - |
| Isolated executive dysfunction, n (%) | 2 (13.3 %) | 0 | 0 | 0 | - |
Patient and control groups were compared with ANOVA
PET findings
One-way ANOVA showed a highly significant overall difference (p < 0.05 FDRcorrected) among the groups. C9ORF72-ALS vs. sALS.
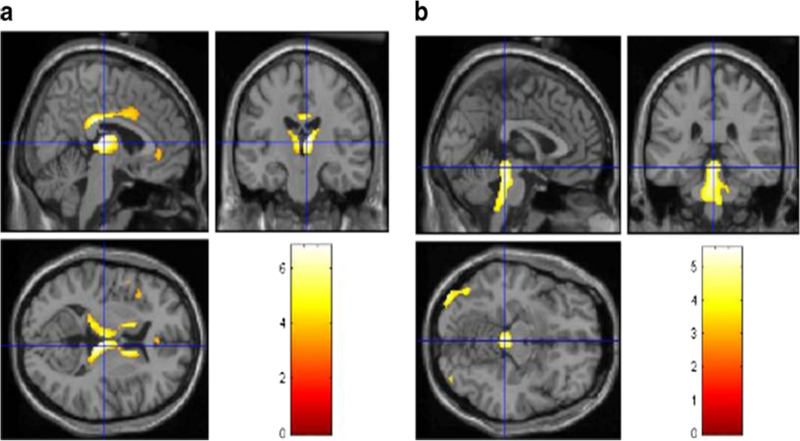
C9ORF72-ALS patients showed highly significant (p < 0.05 FDRcorrected) relative hypometabolism in the bilateral anterior and posterior cingulate cortex (BAs 23 and 24), insula (BA 13), caudate and thalamus, and the left frontal (BA 8) and superior temporal (BAs 22 and 38) cortices (Fig. 1a; Table E-3, Fig. E-1). There was also relative hypermetabolism (p < 0.05 FDRcorrected) in the C9ORF72-ALS patients in the midbrain, bilateral occipital cortex (BAs 17, 18 and 19), globus pallidus and the left middle (BA 39) and inferior temporal (BA 37) cortices (Fig. 1b; Table E-3, Fig. E-2).
Fig. 1.
PET findings in 15 C9ORF72-ALS patients compared with 30 sALS patients. Statistically significant differences (p < 0.05 FDR) are highlighted on a MRI T1 template. a Hypometabolism: top left sagittal view,top right coronal view. bottom left transverse view. Significant corrected clusters are seen in the bilateral anterior and posterior cingulate cortex, insula, caudate and thalamus, and the left frontal and superior temporal cortices. b Hypermetabolism: top left sagittal view, top right coronal view, bottom left transverse view. Significant corrected clusters are seen in the midbrain, bilateral occipital cortex, the globus pallidus, and the left middle and inferior temporal cortices.
ALS-FTD vs. sALS.
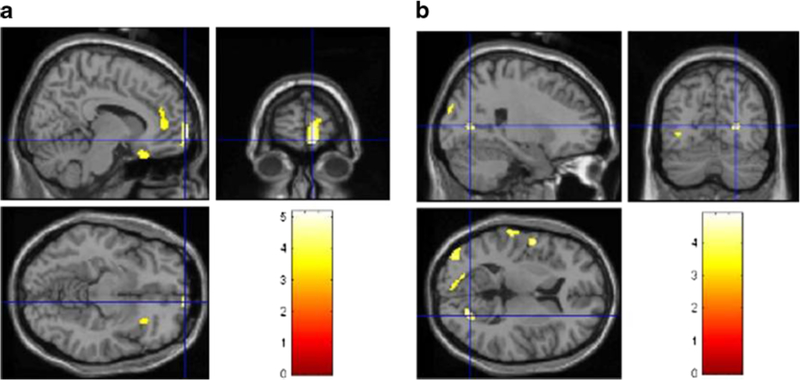
ALS-FTD patients showed relative hypometabolism (p < 0.001) in the right orbitofrontal (BAs 11 and 47), prefrontal (BAs 9 and 10), anterior cingulate (BA 32) and insular (BA 13) cortices (Fig. 2a; Table E-4, Fig. E-3). There was also relative hypermetabolism (p < 0.001) in ALS-FTD patients in the bilateral occipital (BAs 17, 18 and 19) cortex, in the left precentral and postcentral (BAs 6 and 43) cortices, and the superior temporal gyrus (BA 42) (Fig. 2b, Table E-4, Fig. E-4).
Fig. 2.
PET findings in 12 ALS-FTD patients compared with 30 sALS patients. Statistically significant differences (p < 0.001) are highlighted on a MRI T1 template. a Hypometabolism: top left sagittal view, top right coronal view, bottom left transverse view. Significant corrected clusters are seen in the right orbitofrontal, prefrontal, anterior cingulate and insular cortices. b Hypermetabolism: top left sagittal view, top right coronal view,bottom left transverse view. Significant corrected clusters are seen in the bilateral occipital cortex, in the left precentral and postcentral cortex and the superior temporal gyrus
C9ORF72-ALS vs. ALS-FTD.
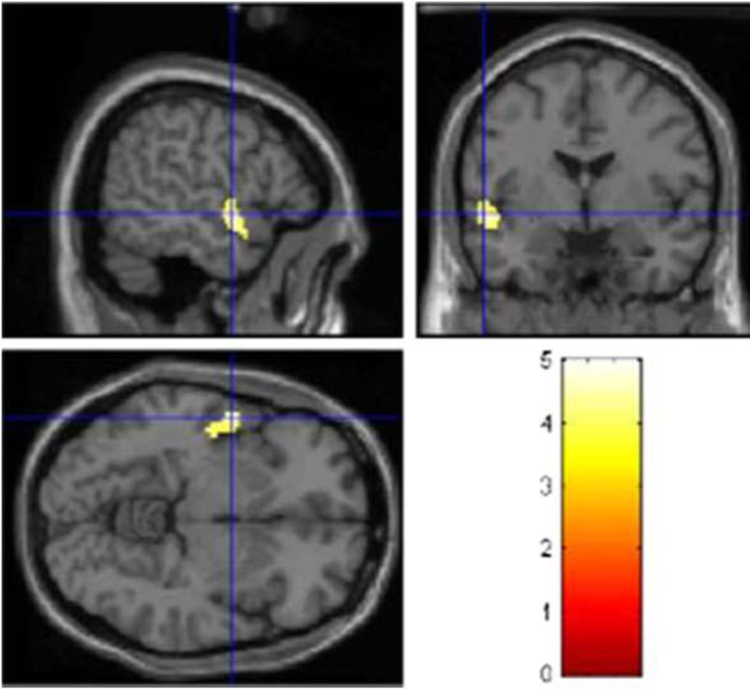
C9ORF72-ALS patients showed relative hypometabolism (p < 0.001) in the left temporal cortex (BA 22) (Fig. 3; Table E-5, Fig. E-5). No significant hypermetabolism was found in this comparison.
Fig. 3.
PET findings in 15 C9ORF72-ALS patients compared with 12 ALS-FTD patients. Statistically significant differences (p < 0.001) are highlighted on a MRI T1 template. Hypometabolism: top left sagittal view, top right coronal view, bottom left transverse view. Significant corrected clusters are seen in the left temporal cortex. No significant hypermetabolism was found in this comparison
Patients vs. controls.
C9ORF72-ALS patients showed a large hypometabolic area (p < 0.05 FDRcorrected) in the bilateral caudate and the thalamus as well as in the frontal cortex (BAs 6, 8, 9, 10, 32, 44, 45, 46) and insula (BA 13) (Fig. E-6, Table E-6). The same pattern (p < 0.001) was seen in ALS-FTD patients in that a hypometabolic area covered part of both frontal cortices (BAs 6, 8, 9, 44, 45, 47), though not as extensively as observed in the C9ORF72-ALS patients (Fig. E-8, Table E-7). C9ORF72-ALS patients showed hypermetabolism (p < 0.05 FDRcorrected) in the midbrain and the cerebellum, in several bilateral limbic regions (hippocampus, amygdale, insula and temporal pole) (Fig. E-7, Table E-5). In contrast, ALS-FTD patients only exhibited increased relative metabolism in the left cerebellum and midbrain (p < 0.001) (Fig. E-9, Table E-6).
sALS patients showed a largely diffuse relative hypometabolism (p < 0.05 FDRcorrected) in the bilateral occipitotemporoparietal and parietofrontal cortices, and hypermetabolism (p < 0.001) in the medial-superior temporal lobe (BAs 28 and 38, and hippocampus) (Figs. E-10 and E-11, Table E-7) as compared with non-ALS controls.
Discussion
We compared the glucose metabolism distribution in a series of 15 ALS patients carrying the GGGGCC hexanucleotide repeat expansion of the C9ORF72 gene, 30 cognitively normal ALS patients, 12 ALS-FTD patients and 40 non-ALS controls. Investigating neurodegenerative disorders by FDG PET contributes to the elucidation of their neurobiology by defining specific metabolic patterns for potential diagnostic use. Along with structural investigations, functional studies provide complementary information about disease’s staging and progress resulting in a better clinical characterization. This is being progressively achieved in, for example, Alzheimer’s Disease and mild cognitive impairment, in which FDG PET investigations provide reliable discriminations between the two groups of patients as well as between patients and controls [18, 19].
In the present study a defined metabolic pattern of a genetically well-defined subgroup of ALS patients was found, encouraging the use of FDG PET in the clinical screening algorithm of this devastating neurodegenerative disorder. The C9ORF72-ALS patients had a unique distribution of metabolic activity when compared with the sALS patients, with relative hypometabolism in several areas of the limbic system (posterior and anterior cingulate cortex, insula, and temporal pole) and of the central structures (caudate and thalamus), while in the ALS-FTD patients less severe hypometabolism was limited to smaller areas of the insula and orbitofrontal, prefrontal and anterior cingulate cortices. As compared with the sALS patients, both the C9ORF72-ALS and ALS-FTD patients showed hypermetabolism in the occipital cortex which was more severe in the C9ORF72-ALS patients, that also showed significant hypermetabolism in the midbrain. When compared with the ALS-FTD patients, C9ORF72-ALS patients showed hypometabolism in the left temporal cortex. Temporal atrophy has previously been found in ALS-FTD patients [8] and might be exacerbated in C9ORF72-ALS patients accounting for the relative hypometabolism found in these patients.
We did not find a difference in the midbrain between the C9ORF72-ALS patients and ALS-FTD patients, possibly due to the relatively low number of subjects in the two groups that might have resulted in a subthreshold trend not reaching statistical significance. However, a previous study has shown different degrees of midbrain hypermetabolism in ALS patient subgroups [20], and this finding was confirmed in the present study in C9ORF72-ALS patients as compared with normal controls, underscoring its distinctive role as a biomarker in the most severe cases of ALS [21].
Although the C9ORF72 expansion has only recently been described, a pattern is beginning to emerge in terms of imaging characteristics in these patients. In accordance with our results, PET scanning involving five C9ORF72 carriers presenting with a FTD phenotype showed hypometabolism in the anterior cingulate, with mild to severe frontal cortical hypometabolism [8]; the anterior cingulate dysfunction was confirmed using perfusion single photon emission tomography [8].
Specific patterns of cortical and regional central nervous system (CNS) atrophy have also been described in C9ORF72-ALS patients based on MRI. Symmetric bilateral frontal and/or temporal cortical atrophy, associated with ventricular enlargement in serial scans, is a common finding in C9ORF72-ALS patients with a predominant FTD phenotype [8, 21]. Voxel-based morphometry analysis showed clusters of grey matter cortical atrophy in the right inferior and superior frontal gyri, the left anterior cingulate gyrus, and the right precentral gyrus in ALS and ALS-FTD patients with C9ORF72 mutation [2]. In contrast, similar analyses in FTD patients with the expansion revealed atrophy involving the bilateral thalamus, left opercular cortex, left orbitofrontal cortex and bilateral posterior cerebellum [22]. Diffusion tensor imaging analysis of FTD and ALS-FTD patients with the C9ORF72 mutation showed increased radial diffusivity and decreased fractional anisotropy bilaterally in the anterior thalamic radiations, uncinate fasciculus, anterior cingulum, anterior corpus callosum, right posterior corpus callosum, posterior inferior longitudinal fasciculus and superior longitudinal fasciculus [22]. A recent study in nine ALS patients with a C9ORF72 genotype showed anatomic involvement of cortical and subcortical regions, affecting fusiform, thalamic, supramarginal, orbitofrontal regions and Broca’s area [23].
Our study provides important novel information concerning PET imaging in C9ORF72 patients presenting with ALS. Such patients have a different pattern of impaired metabolism as compared with ALS patients without genetic mutations, either cognitively normal or with comorbid FTD. Hypometabolism involved specifically the limbic system and the central structures, while hypermetabolism was found in the midbrain. These changes were independent of site of onset, age and gender. We were able to identify this unique metabolic pattern in patients with the C9ORF72 expansion even though the number of patients included in the study, although much larger than in any other published study, was still relatively small.
These diffuse, highly significant and quite complex metabolic changes differentiate ALS patients with theC9ORF72 expansion from those without genetic mutations and from normal controls. The widespread CNS involvement observed in the PET scan is consistent with the more severe clinical picture observed among patients carrying the expansion [3], as well as with the findings of post-mortem studies showing more extensive neuropathological changes compared with ALS patients without the mutation [24, 25]. Of our 15 C9ORF72-ALS patients, 10 had a comorbid behavioural variant FTD, sometimes characterized by florid psychosis-like symptoms, while two patients had an isolated executive impairment and only three were cognitively normal. Interestingly, in our series of ALS patients with this mutation, several patients’ relatives showed FTD comorbid with psychotic symptoms or frank schizophrenia [2, 3, 7].
The hypometabolism of the bilateral frontal cortex found in the C9ORF72-ALS patients in comparison with both the sALS patients and the non-ALS controls is consistent with the presence of impairment of frontal functions typically observed in patients with FTD, and is more severe and extended than that observed in ALS-FTD without the expansion. However, hypometabolism of the anterior and posterior cingulate cortex has been related to behavioural symptoms, such as inability to detect errors, severe difficulty with resolving stimulus conflict, emotional instability and inattention [26], a symptom constellation usually observed in ALS-FTD as well as in patients with the C9ORF72 expansion [13]. Involvement of the anterior cingulate cortex and striatum has also been reported in patients with schizophrenia [27–29], and could represent the functional correlate of psychosis-like symptoms often those observed in ALS patients with the C9ORF72mutation.
Evidence of parkinsonism has been reported in about 35 % of patients with behavioural variant FTD or ALS-FTD carrying the C9ORF72 expansion, with a symmetric akinetic-rigid syndrome and, less frequently, posture and action tremor [8]. Similar parkinsonian features have been reported in patients with ALS or ALS-FTD with the same mutation, and parkinsonian syndromes are identified quite frequently in the pedigrees of their families [3, 25]. In the present series, three of the C9ORF72-ALS patients (20 %) had clear-cut parkinsonian symptoms not responsive to treatment with levodopa. The detection of the metabolic changes in the basal ganglia is consistent with the finding of extrapyramidal symptoms in these patients. Additionally, the involvement of the basal ganglia may also influence a patient’s cognition, both as a direct effect of the impairment, and also as part of circuits encompassing the basal ganglia and cortical structures [30], including the anterior cingulate cortex, dorsolateral prefrontal cortex, and supplementary motor area. In this regard, it is interesting to note that the C9ORF72 expansion has also been reported in patients clinically diagnosed with Alzheimer’s and idiopathic Parkinson’s disease [31, 32], in keeping with the widespread distribution of neuropathological defects observed with this mutation.
Apart from typical upper and lower motor neuron loss, pathology in ALS patients with the C9ORF72expansion is characterized by extensive involvement of extramotor areas, such as the frontal cortex, temporal cortex, hippocampus, substantia nigra, and cerebellar granule cells [8, 23–25]. Obviously, neuropathological examination represents the end-stage of the disease. However, our imaging findings were acquired early after the clinical diagnosis, but in patients with the C9ORF72 expansion still showed more widespread changes within the CNS than patients without the mutation, indicating that pathological changes are present in those with the C9ORF72 expansion from a relatively early stage of the disease. Patients with the C9ORF72 mutation had relative glucose hypermetabolism in the midbrain compared both with controls and to cognitively normal ALS with no mutations. This unique finding might be related to reactive astrocytosis or to a local increase in microglia [33, 34], and could be a relatively specific phenomenon in ALS patients [20].
Glutamate-induced glycolysis in astrocytic syncytium provides lactate as the substrate for neuronal firing and activates astrocytes by taking up glucose directly from intraparenchymal capillaries [35, 36]. In vivo evidence has been found by MRI spectroscopy that the glutamate excess in patients with ALS is proportional to the presence of dysarthria and dysphagia [37]. When neurons degenerate such a neurometabolic loop might cause increased 18F-FDG accumulation in ALS. Furthermore, glycogen synthesis in the brain is almost exclusively localized in astrocytes and is considered to be a mechanism providing additional energy substrate upon glutamatergic stimulation. All these phenomena may in turn result in the affected regions in an increase in the relative uptake of 18F-FDG due to the higher density of astrocytes and microglia in ALS patients than in controls.
A relevant issue in neuroimaging is the correction of metabolic data for PVE in potentially atrophic regions. In our study MRI scans of several patients were not available due to the difficulty that ALS patients may experience in undergoing an MRI scan. In fact only some of our patients could tolerate the MRI scan, and we felt inappropriate to correct the PET data for PVE only in a limited sample of patients. Using segmented MR images, Ibáñez et al. [38] corrected metabolic rates for glucose for PVE to evaluate the effect of atrophy on uncorrected values. In that study metabolic differences were confirmed after PVE correction and were not a mere artefact due to an increase in the cerebrospinal fluid space induced by the atrophy. Although neither age nor sex showed differences among the groups, we included them as covariates in the voxel analyses. This kept the variability under control and made the results more stable, primarily because both of these variables are known to have an impact on brain metabolism, irrespective of significant differences at the group level [39]
This study had some limitations. First, the use of a convenience group of subjects with a negative PET scan as a control group was suboptimal. However, these patients were specifically identified for this study and the same protocol and scanner were used in both patients and controls. Furthermore, as in other investigations, strict exclusion criteria were applied and the enrolment of these patients avoided exposure of healthy individuals to radiation [40]. Second, the finding of hypermetabolic areas could be an artefact of global mean normalization in the SPM procedure. However, we overcame this issue by allowing the normalization value to incorporate only those voxels with a value higher than the global mean and a post-hoc crossvalidation with higher thresholds has been performed that confirmed the present results [41].
In conclusion, we found that ALS patients with the C9ORF72 mutation are characterized by a pattern of glucose metabolism different from that in normal subjects and ALS patients with no genetic mutation, with relative hypometabolism in the thalamus, basal ganglia and limbic cortex and relative hypermetabolism in the occipital cortex, midbrain and bilateral cerebellum. These alterations are consistent with the unique symptom constellation in patients carrying the C9ORF72 hexanucleotide repeat expansion, and emphasize the ‘systemic’ nature of lesions related to this mutation even in the early clinical phases of the disorder.
Although the ALS subgroups were created on the basis of genetic and cognitive data, in none of them brain metabolism correlated significantly with any of the neuropsychological tests. This was possibly due to the small sample size but also to the lack of significant cognitive decline in some of the genetically impaired patients. In this respect the ability of 18F-FDG PET to demonstrate metabolic differences between such clinically similar patient groups might help to identify a pattern unique to patients with the C9ORF72 mutation useful in the diagnostic algorithm. Larger groups of patients bearing the same hexanucleotide repeat expansion are needed to confirm this hypothesis.
Supplementary Material
Acknowledgments
We thank the patients and the neurologically normal controls for their collaboration in this study. This work was supported in part by Compagnia di San Paolo, Programma Neuroscienze 2008–2009 (to M. Consuelo Valentini and A. Calvo), Ministero della Salute (Ricerca Sanitaria Finalizzata, 2010, grant RF-2010-2309849) (to A. Chiò) European Community’s Health Seventh Framework Programme (FP7/2007–2013 under grant agreement 259867) (to A. Chiò); The Intramural Research Programmes of the National Institutes of Health (NIH); National Institute on Aging (Z01-AG000949-02) (to Bryan J. Traynor).
Disclosures
Angelina Cistaro reports no disclosures.
Marco Pagani reports no disclosures.
Anna Montuschi reports no disclosures.
Andrea Calvo has received research support from the Ministry of Health (Ricerca Finalizzata), Regione Piemonte (Ricerca Finalizzata) and Compagnia di San Paolo.
Cristina Moglia reports no disclosures.
Antonio Canosa reports no disclosures.
Gabriella Restagno has received research support from the Ministry of Health (Ricerca Finalizzata), and Regione Piemonte (Ricerca Finalizzata).
Maura Brunetti reports no disclosures.
Bryan J. Traynor has a patent pending on the diagnostic and therapeutic uses of the C9ORF72hexanucleotide repeat expansion.
Flavio Nobili reports no disclosures.
Giovanna Carrara reports no disclosures.
Piercarlo Fania reports no disclosures.
Leonardo Lopiano has received research support from the Ministry of Health (Ricerca Finalizzata), and Regione Piemonte (Ricerca Finalizzata).
M. Consuelo Valentini reports no disclosures.
Adriano Chiò has received research support from the Ministry of Health (Ricerca Finalizzata), Regione Piemonte (Ricerca Finalizzata), and European Commission (7th Framework Program); he serves on the editorial advisory board of Amyotrophic Lateral Sclerosis; he serves on a scientific advisory board for Biogen Idec and Cytokinetics.
References
- 1.Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377:942–55. [DOI] [PubMed] [Google Scholar]
- 2.Byrne S, Elamin M, Bede P, Shatunov A, Walsh C, Corr B, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9ORF72 repeat expansion: a population-based cohort study. Lancet Neurol. 2012;11:232–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chiò A, Borghero G, Restagno G, Mora G, Drepper C, Traynor BJ, et al. Clinical characteristics of patients with familial amyotrophic lateral sclerosis carrying the pathogenic GGGGCC hexanucleotide repeat expansion of C9ORF72. Brain. 2012;135:784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, Boxer AL, Baker M, Rutherford NJ, et al. Expanded GGGGCC hexanucleotide repeat in noncoding region of C9ORF72 causes chromosome 9p-linked FTD and ALS. Neuron. 2011;72:245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Renton AE, Majounie E, Waite A, Simón-Sánchez J, Rollinson S, Gibbs JR, et al. A hexanucleotide repeat expansion in C9ORF72 is the cause of chromosome 9p21-linked ALS-FTD. Neuron. 2011;72:257–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majounie E, Renton AE, Mok K, Dopper EG, Waite A, Rollinson S, et al. Frequency of the C9ORF72 hexanucleotide repeat expansion in patients with amyotrophic lateral sclerosis and frontotemporal dementia: a cross-sectional study. Lancet Neurol. 2012;11:323–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sabatelli M, Conforti FL, Zollino M, Mora G, Monsurrò MR, Volanti P, et al. C9ORF72 hexanucleotide repeat expansions in the Italian sporadic ALS population. Neurobiol Aging. 1848;2012(33):e15–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boxer AL, Mackenzie IR, Boeve BF, Baker M, Seeley WW, Crook R, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. J Neurol Neurosurg Psychiatry. 2011;82:196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boeve BF, Boylan KB, Graff-Radford NR, DeJesus-Hernandez M, Knopman DS, Pedraza O, et al. Characterization of frontotemporal dementia and/or amyotrophic lateral sclerosis associated with the GGGGCC repeat expansion in C9ORF72. Brain. 2012;135:765–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks BR, Miller RG, Swash M, Munsat TL. World Federation of Neurology Research Group on Motor Neuron Diseases. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. [DOI] [PubMed] [Google Scholar]
- 11.Brown JA, Min J, Staropoli JF, Collin E, Bi S, Feng X, et al. SOD1, ANG, TARDBP and FUS mutations in amyotrophic lateral sclerosis: a United States clinical testing lab experience. Amyotroph Lateral Scler. 2012;13:217–22. [DOI] [PubMed] [Google Scholar]
- 12.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998;51:1546–54. [DOI] [PubMed] [Google Scholar]
- 13.Strong MJ, Grace GM, Freedman M, Lomen-Hoerth C, Woolley S, Goldstein LH, et al. Consensus criteria for the diagnosis of frontotemporal cognitive and behavioral syndromes in amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2009;10:131–46. [DOI] [PubMed] [Google Scholar]
- 14.Phukan J, Elamin M, Bede P, Jordan N, Gallagher L, Byrne S, et al. The syndrome of cognitive impairment in amyotrophic lateral sclerosis: a population-based study. J Neurol Neurosci Psychiatry. 2011;83:102–8. [DOI] [PubMed] [Google Scholar]
- 15.Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe): professional manual. Lutz, Fla, Psychological Assessment Resources, 2001. [Google Scholar]
- 16.Varrone A, Asenbaum S, Vander Borght T, Booij J, Nobili F, Någren K, et al. EANM procedure guidelines for PET brain imaging using [18F]FDG, version 2. Eur J Nucl Med Mol Imaging. 2009;36:2103–10. [DOI] [PubMed] [Google Scholar]
- 17.Oishi N, Udaka F, Kameyama M, Sawamoto N, Hashikawa K, Fukuyama H. Regional cerebral blood flow in Parkinson disease with nonpsychotic visual hallucinations. Neurology. 2005;65:1708–15. [DOI] [PubMed] [Google Scholar]
- 18.Bloudek LM, Spackman DE, Blankenburg M, Sullivan SD. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J Alzheimers Dis. 2011;26:627–45. [DOI] [PubMed] [Google Scholar]
- 19.Caroli A, Prestia A, Chen K, Ayutyanont N, Landau SM, Madison CM, et al. Summary metrics to assess Alzheimer disease-related hypometabolic pattern with 18F-FDG PET: head-to-head comparison. J Nucl Med. 2012;53:592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cistaro A, Valentini MC, Chiò A, Nobili F, Calvo A, Moglia C, et al. Brain hypermetabolism in amyotrophic lateral sclerosis: a FDG PET study in ALS of spinal and bulbar onset. Eur J Nucl Med Mol Imaging. 2012;39:251–9. [DOI] [PubMed] [Google Scholar]
- 21.Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranuline and sporadics. Brain. 2012;135:794–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bede P, Bokde ALW, Byrne S, Elamin M, McLaughlin RL, Kenna K, et al. A multiparametric MRI study of ALS stratified for the C9ORF72 genotype. Neurology. 2013;81:361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murray ME, DeJesus-Hernandez M, Rutherford NJ, Baker M, Duara R, Graff-Radford NR, et al. Clinical and neuropathologic heterogeneity of c9FTD/ALS associated with hexanucleotide repeat expansion in C9ORF72. Acta Neuropathol. 2011;122:673–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper-Knock J, Hewitt C, Highley JR, Brockington A, Milano A, Man S, et al. Clinico-pathological features in amyotrophic lateral sclerosis with expansions in C9ORF72. Brain. 2012;135:751–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001;935:107–17. [PubMed] [Google Scholar]
- 27.Fujimoto T, Takeuch K, Matsumoto T, Kamimura K, Hamada R, Nakamura K, et al. Abnormal glucose metabolism in the anterior cingulate cortex in patients with schizophrenia. Psych Res Neuroimaging. 2007;154:49–58. [DOI] [PubMed] [Google Scholar]
- 28.Patel NH, Vyas NS, Puri BK, Nijran KS, Al Nahhas A. Positron emission tomography in schizophrenia: a new perspective. J Nucl Med. 2010;51:511–20. [DOI] [PubMed] [Google Scholar]
- 29.Simpson EH, Kellendonk C, Kandel E. A possible role for the striatum in the pathogenesis of the cognitive symptoms of schizophrenia. Neuron. 2010;65:585–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grahn JA, Parkinson JA, Owen AM. The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res. 2009;199:53–60. [DOI] [PubMed] [Google Scholar]
- 31.Majounie E, Abramzon Y, Renton AE, Perry R, Bassett SS, Pletnikova O, et al. Repeat expansion in C9ORF72 in Alzheimer’s disease. N Engl J Med. 2012;366:283–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesage S, Le Ber I, Condroyer C, Broussolle E, Gabelle A, Thobois S, et al. C9ORF72 repeat expansions are a rare genetic cause of parkinsonism. Brain. 2013;136:385–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turner MR, Cagnin A, Turkheimer FE, Turkheimer F, Miller CCJ, Shaw CE. Evidence of widespread cerebral microglial activation in amyotrophic lateral sclerosis: an [11C](R)-PK11195 positron emission tomography study. Neurobiol Dis. 2004;15:601–9. [DOI] [PubMed] [Google Scholar]
- 34.Johansson A, Engler H, Blomquist G, Scott B, Wall A, Aquilonius SM, et al. Evidence for astrocytosis in ALS demonstrated by [11C]-(L)-deprenyl-D2-PET. J Neurol Sci. 2007;255:17–22. [DOI] [PubMed] [Google Scholar]
- 35.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci U S A. 1994;91:10625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2012;32:1152–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pioro EP, Majors AW, Mitsumoto H, Nelson DR, Ng TC. 1H-MRS evidence of neurodegeneration and excess glutamate + glutamine in ALS medulla. Neurology. 1999;53:71–9. [DOI] [PubMed] [Google Scholar]
- 38.Ibáñez V, Pietrini P, Alexander GE, Furey ML, Teichberg D, Rajapakse JC, et al. Regional glucose metabolic abnormalities are not the result of atrophy in Alzheimer’s disease. Neurology. 1998;50:1585–93. [DOI] [PubMed] [Google Scholar]
- 39.Pagani M, Dessi B, Morbelli S, Brugnolo A, Salmaso D, Piccini A, et al. MCI patients declining and not-declining at mid-term follow-up: FDG-PET findings. Curr Alzheimer Res. 2010;7:287–94. [DOI] [PubMed] [Google Scholar]
- 40.Alessandrini M, Pagani M, Napolitano B, Micarelli A, Bruno E, Chiaravalloti A, et al. Early and phasic cortical metabolic changes in vestibular neuritis onset. PLoS One. 2013;8(3):e57596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nobili F, Mazzei D, Dessi B, Morbelli S, Brugnolo A, Barbieri P, et al. Unawareness of memory deficit in amnestic MCI: FDG-PET findings. J Alzheimers Dis. 2010;22:993–1003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.