LETTER 1
We read with interest about the investigations of Kampf et al. (2) concerning the antimicrobial activity of a new silver-containing polymer. The use of silver may be regarded as an effective non-resistance-inducing strategy to prevent device-related infections. In vitro and in vivo experiments have been performed during the recent years to develop potent antimicrobially acting silver-coated devices. Most of the authors claimed strong antimicrobial efficacy of silver-coated devices, and the industry launched several products on the market. However, randomized clinical studies showing statistically significant antimicrobial efficacy of silver-coated medical devices in high-risk populations of patients are rare, dealt with small numbers of patients only, and are controversial (6). The largest randomized controlled clinical study with 1,300 patients revealed no significant differences in infection rates between silver-coated and unmodified catheters (4), a finding which is in accordance with the observations made by Kampf et al. (2).
How does silver work? The silver cation (Ag+) is a highly reactive chemical structure which binds strongly to electron donor groups containing sulfur, oxygen, or nitrogen. Biological molecules generally contain all these components in the form of thio, amino, imidazole, carboxylate, and phosphate groups. Silver ions act by displacing other essential metal ions such as Ca2+ or Zn+. The binding of silver ions to bacterial DNA (3) may inhibit a number of important transport processes, such as phosphate and succinate uptake, and can interact with cellular oxidation processes as well as the respiratory chain. The Ag+-induced antibacterial killing rate is directly proportional to Ag+ concentrations, typically acting at multiple targets. The higher the silver ion concentration, the higher the antimicrobial efficacy. The release rate of unbound, free silver ion may also be correlated to the antimicrobial activity of thus-coated devices.
Kampf et al. neutralized the silver catheter by 5% horse serum. Recently, we showed decreased activity of silver ions as a result of the addition of albumin and NaCl to broth (5). MICs and minimal bactericidal concentrations (MBCs) for the bacterial strains tested (Escherichia coli ATCC 11229, Staphylococcus aureus ATCC 6538, and Staphylococcus epidermidis DSM 3269) increased with the addition of albumin to Mueller-Hinton (MH) broth, especially for MH broth containing albumin and NaCl (from 1 to 10 mg/ml). Very high MBCs were also observed for Ag ions in bovine serum (50-fold the broth MBC). This may be in accordance with the findings of Williams and Williams (7) who showed by microautoradiography that 3 mol of silver ions is bound specifically by 1 mol of albumin.
In an in vitro experiment, bacterial adhesion on polymeric devices was investigated in the presence of albumin and NaCl at physiological concentrations. Central venous catheters (nonimpregnated, aliphatic polyurethane, 1.7-mm diameter; B. Braun, Melsungen, Germany) were cut under sterile conditions into 1-cm pieces. These pieces were transferred into bacterial suspension (≈106 CFU/ml; E. coli ATCC 11229, S. aureus ATCC 6538, or S. epidermidis DSM 3269), remaining there for 2 h at 37°C. Afterwards, the catheter pieces were transferred into MH broth containing silver nitrate (for E. coli, 32 μg/ml; for S. aureus and S. epidermidis, 256 μg/ml), albumin (0.2%), and NaCl (0.9%).
Contaminated catheter pieces were incubated for 24 or 48 h at 37°C. Thereafter, the catheter segments were removed by means of sterile forceps, rinsed for 30 s under running tap water, transferred individually into 1 ml of physiological saline (containing 0.1% polysorbate 80), and agitated vigorously for 60 s. After agitation, the saline was diluted 1:10 or 1:100, and appropriate volumes of each dilution were pipetted onto the surface of tryptic soy agar supplemented with 5% sheep blood and incubated aerobically for 48 h at 37°C. After each incubation period grown colonies were counted and the number of adherent bacteria on catheter segments was calculated.
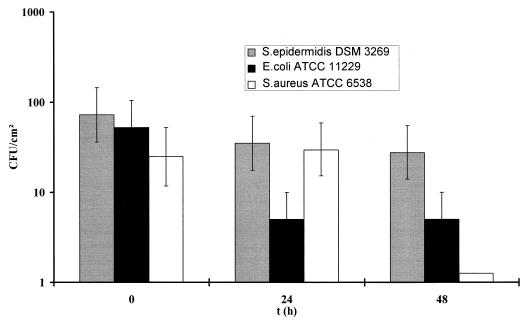
After 48 h of incubation, bacterial counts on the catheter surfaces were less than that for the control without silver nitrate but still measurable (Fig. 1).
FIG. 1.
Bacterial adhesion on polymeric surfaces in the presence of highly toxic and bactericidal concentrations of silver nitrate (for E. coli, 32 μg/ml; for S. aureus and S. epidermidis, 256 μg/ml). The broth (0.2% albumin, 0.9% NaCl) remained sterile after 24 h, whereas adherent bacteria survived.
There is no doubt that silver ions are remarkably active against a broad spectrum of bacteria. However, in an environment containing albumin and halide ions, the antibacterial activity of silver ions will be decreased as a result of specific absorption to albumin and precipitation into insoluble silver chloride crystals. Once attached to catheters, colonizing bacteria remain viable despite exposure even to high concentrations of antimicrobial substances (1). Therefore, silver-coated devices like those investigated by Kampf et al. may be clinically effective only when the concentration of free silver ions can be increased and when contact with albumin and Cl ions, as well as possible cytotoxic effects, is minimized. For reproducible inactivation of silver derivates we would like to recommend the addition of albumin (0.2%) and physiological NaCl (0.9%) to fluid as well as solid media.
REFERENCES
- 1.Elliott T S J. Plastic devices: new field for old microbes. Lancet. 1988;13:365–366. doi: 10.1016/s0140-6736(88)91168-3. [DOI] [PubMed] [Google Scholar]
- 2.Kampf G, Groß-Siestrup C, Wendt C, Martini H. Microbicidal activity of a new silver-containing polymer, SPI-ARGENT II. Antimicrob Agents Chemother. 1998;42:2440–2442. doi: 10.1128/aac.42.9.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Modak K, Fox C. Binding of silver sulfadiazine in the cellular components of Pseudomonas aeroginosa. Biochem Pharm. 1973;22:2392–2404. doi: 10.1016/0006-2952(73)90341-9. [DOI] [PubMed] [Google Scholar]
- 4.Riley D K, Classen D C, Stevens L E, Burke J P. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. Am J Med. 1995;98:349–358. doi: 10.1016/S0002-9343(99)80313-1. [DOI] [PubMed] [Google Scholar]
- 5.Schierholz J M, Wachol-Drebeck L, Lucas L, Pulverer G. Activity of silver ions in biological fluids. Zentbl Bakt. 1998;287:411–420. doi: 10.1016/s0934-8840(98)80178-3. [DOI] [PubMed] [Google Scholar]
- 6.Schierholz J M, Lucas L, Pulverer G. Silver coating of medical devices—a review. J Hosp Infect. 1998;40:257–262. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 7.Williams R L, Williams D F. Albumin adsorption on metal surfaces. Biomaterials. 1988;9:206. doi: 10.1016/0142-9612(88)90085-3. [DOI] [PubMed] [Google Scholar]