Background:
In the United States, African American persons have the highest death rate and lowest survival rate of any racial or ethnic group for most types of cancer. Socioeconomic factors have been blamed for racial disparities in cancer outcomes (1). However, studies have shown that differences persist despite risk stratification for socioeconomic status and access to care, suggesting that patient comorbid conditions, differences in tumor biology, and underrepresentation of minorities in clinical trials may play a role. For example, certain types of cancer, such as multiple myeloma and prostate cancer, are more common in African American persons, yet landmark trials guiding management of these diseases included study populations that do not reflect the racial distribution of the actual disease population (2). Lack of trial participation by African American persons is thought to be related to lack of information regarding clinical trials; concern about trial conditions; dislike of the randomization required for trial participation; suspicion of health care providers' attitudes; and distrust of medical research related to historical events, such as the Tuskegee study (3).
Objective:
To examine whether these influences have affected participation of African American persons in pivotal trials of cancer medications submitted to the U.S. Food and Drug Administration (FDA) for approval.
Methods and Findings:
Data on participation were obtained from product labeling at Drugs@FDA. On the basis of publicly available FDA reviews, we assessed enrollment of African American persons in trials supporting 75 new oncologic drug approvals from 2014 to 2018. We calculated prevalence-corrected estimates for the participation of African American persons as the percentage of African American individuals among trial participants divided by the percentage of African American individuals among people with disease, which we designated as the “participation-to-prevalence ratio” (PPR). A PPR of 1.0 indicated identical representation of African American persons in the trial and disease populations, and we considered PPRs between 0.8 and 1.2 to indicate similar representation.
Between 2014 and 2018, a total of 61 763 patients enrolled in clinical trials that resulted in subsequent FDA approval for cancer drugs (Table). The proportion of African American persons enrolled in these trials was 7.44%. The calculated PPR for participation of African Americans in clinical trials that led to drug approval for all types of cancer combined was 0.31 (Figure). Underrepresentation of African Americans in these trials was consistent across major cancer subtypes, including breast cancer (PPR, 0.29), prostate cancer (PPR, 0.18), lung cancer (PPR, 0.15), and hematologic cancer (PPR, 0.12).
Table.
Characteristics of Trials From 2014 to 2018 that Led to Cancer Drug Approval by the FDA
| Cancer Subtype | FDA Approvals, n |
Trial Participants, n |
African American Participants, n |
|---|---|---|---|
| Solid cancer | |||
| Total | 45 | 49 349 | 3553 |
| Breast cancer | 9 | 22 075 | 1507 |
| Lung cancer | 8 | 6127 | 237 |
| Skin cancer | 8 | 5135 | 268 |
| Ovarian cancer | 4 | 1356 | 148 |
| Sarcoma | 2 | 1240 | 130 |
| Gastrointestinal cancer | 3 | 1520 | 74 |
| Others/multiple solid cancer | 11 | 11 896 | 1189 |
| Hematologic cancer | |||
| Total | 30 | 12 414 | 1033 |
| Leukemia | 17 | 6890 | 496 |
| Lymphoma | 5 | 756 | 200 |
| Multiple myeloma | 6 | 4155 | 314 |
| Myelodysplastic syndrome | 2 | 613 | 23 |
FDA = U.S. Food and Drug Administration.
Figure. Participation-to-prevalence ratio (PPR) of African Americans in clinical trials supporting U.S. Food and Drug Administration approval of cancer drugs from 2014 to 2018.
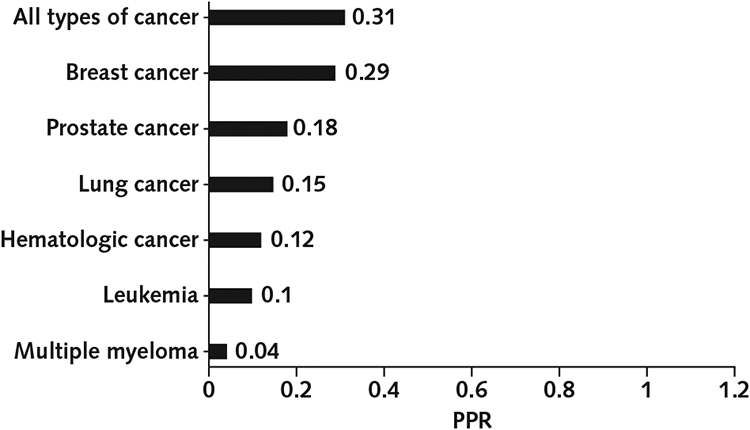
A PPR of 0.8 to 1.2 reflects similar representation of African American persons in the trial and disease populations. Hematologic cancer includes leukemia, lymphoma, and myelodysplastic syndrome. Results for multiple myeloma are expressed separately. Results for leukemia are expressed as part of hematologic cancer and also separately.
Discussion:
We conclude that African American persons are markedly underrepresented in clinical trials leading to FDA approval of cancer medications, and we believe that this discrepancy results in failed opportunities to understand cancer biology and the pharmacology of cancer medications. For example, a recent study identified increased activation of the “unfolded protein response” in African American patients with breast cancer compared with white patients, which may correlate with an increased prevalence of tamoxifen resistance in African American patients (4).
The NIH Revitalization Act of 1993 was established to ensure that women and members of minority groups are included as participants in clinical research. Several initiatives may help realize that goal. For example, in 2015, the FDA established Drug Trials Snapshots to provide consumers and health care professionals with information about who participated in clinical trials supporting FDA approval of new drugs (www.fda.gov/drugs/drug-approvals-and-databases/drug-trials-snapshots). In addition, a focus on recruitment strategies targeting African American participants, such as providing additional funds to sites enrolling more African American persons, may improve enrollment in clinical trials (3). Moreover, providing participants with better information about benefits to the community could increase enrollment (3). Finally, to the extent that bias affects the willingness of some researchers to offer clinical trial enrollment to minorities, a race-neutral approach to recruitment could increase the participation of minorities in clinical trials (5).
Our study has limitations. First, we included only studies that led to FDA approval. In addition, we could not adjust our results for any differences in age or other differences in the trial and disease populations.
Clinical trials have shaped the treatment paradigm for most types of cancer, and the results from these studies are generally applied equally to all races and ethnicities. Our study demonstrates that African American persons are underrepresented in trials leading to FDA approval of drugs for all cancer types. Further research is needed to understand this difference, its consequences, and how to address those consequences.
Footnotes
Disclosures: Disclosures can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M20-0410.
Reproducible Research Statement: Study protocol, statistical code, and data set: Available from Dr. Al Hadidi (hadidi@bcm.edu).
Presented in part at the 55th Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, 31 May to 4 June 2019; the Texas Society of Clinical Oncology 2019 Annual Conference, San Antonio, Texas, 14 September 2019; the 2019 Society of Hematologic Oncology Annual Meeting, Houston, Texas, 11 to 14 September 2019; and the San Antonio Breast Cancer Symposium 2019, San Antonio, Texas, 11 to 14 December 2019.
References
- 1.Bach PB, Schrag D, Brawley OW, et al. Survival of blacks and whites after a cancer diagnosis. JAMA. 2002;287:2106–2113. [PMID: 11966385] [DOI] [PubMed] [Google Scholar]
- 2.Zeng C, Wen W, Morgans AK, et al. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program in the United States, 1990 to 2010. JAMA Oncol. 2015;1:88–96. [PMID: 26182310] doi: 10.1001/jamaoncol.2014.161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rivers D, August EM, Sehovic I, et al. A systematic review of the factors influencing African Americans' participation in cancer clinical trials. Contemp Clin Trials. 2013;35:13–32. [PMID: 23557729] doi: 10.1016/j.cct.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 4.Eltayeb AE, Demas DM, Clarke R, et al. The unfolded protein response may contribute to racial disparity in endocrine responsiveness in breast cancer [Abstract]. In: Proceedings of the 106th Annual Meeting of the American Association for Cancer Research, Philadelphia, Pennsylvania, 18-22 April 2015. Cancer Res. 2015;75(15 Suppl). Abstract no. 1258. doi: 10.1158/1538-7445.AM2015-1258 [DOI] [Google Scholar]
- 5.Niranjan SJ, Martin MY, Fouad MN, et al. Bias and stereotyping among research and clinical professionals: perspectives on minority recruitment for oncology clinical trials. Cancer. 2020;126:1958–1968. [PMID: 32147815] doi: 10.1002/cncr.32755 [DOI] [PubMed] [Google Scholar]


